Abstract
There are limited data on osteoarticular infections from resource-limited settings in Asia. A retrospective study of patients presenting to the Angkor Hospital for Children, Cambodia, January 2007–July 2011, identified 81 cases (28% monoarticular septic arthritis, 51% single-limb osteomyelitis and 15% multisite infections). The incidence was 13.8/100 000 hospital attendances. The median age was 7.3 years, with a male/female ratio of 1.9:1; 35% presented within 5 days of symptom onset (median 7 days). Staphylococcus aureus was cultured in 29 (36%) cases (52% of culture-positive cases); one isolate was methicillin-resistant (MRSA). Median duration of antimicrobial treatment was 29 days (interquartile range 21–43); rates of surgical intervention were 96%, and 46% of children had sequelae, with one fatality. In this setting osteoarticular infections are relatively common with high rates of surgical intervention and sequelae. Staphylococcus aureus is the commonest culturable cause, but methicillin-resistant S. aureus is not a major problem, unlike in other Asian centers.
Keywords: osteomyelitis, septic arthritis, pediatric, Asia
Introduction
Pediatric osteomyelitis and septic arthritis are severe infections, and diagnostic delay is associated with poor outcomes [1–3]. The incidence of pediatric osteomyelitis varies from 2.9 to 75 cases/100 000 individuals, and that of septic arthritis varies from 5 to 37 [4–9]. Unlike the increasing incidence of bone infection, the incidence of joint infection appears to be stable [10–12].
Specific data on pediatric osteoarticular infections from resource-limited Asian countries, such as Cambodia, are sparse [13–19]. Risk factors such as malnutrition, trauma and suboptimal vaccine coverage are widespread [20–22]. Diagnostic microbiology facilities are limited, and antimicrobial resistance is common [23]. This study characterized the epidemiology of osteoarticular infections in Cambodian children aged <16 years attending Angkor Hospital for Children (AHC), Siem Reap.
Material and Methods
Cases from January 2007–July 2011 were identified through hospital and laboratory records. Data collected included age, gender, residence, admission details, comorbidities, clinical presentation, laboratory investigations, surgical interventions, antibiotic treatment and outcomes. Weight-for-age Z-scores were calculated for children aged <5 years [24].
Disease episodes were defined as single treatment episodes, and relapses as recurrent disease episodes following improvement. Monoarticular septic arthritis described single joint infections; single-limb osteomyelitis included adjacent infected long bones within a limb and bone-plus-adjacent-joint involvement, and multisite infections included non-adjacent sites of infection, and/or non-musculoskeletal sites. Mandibular/foot infections were considered separately.
Data were analyzed using Stata 11.1 (StataCorp, TX, USA). Fisher’s exact and Kruskal–Wallis tests were used for comparisons between groups for categorical and continuous variables, respectively. Multivariable logistic regression was used to assess risk factors for binary outcomes.
Ethical approval was granted by the AHC Institutional Review Board and the Oxford Tropical Research Ethics Committee, UK.
Results
Of 81 patients, 60 (74%) had a single episode of osteoarticular infection, and 21 had primary episodes followed by relapse(s). The median age (range) was 7.3 years (0–14); boys were almost twice as commonly afflicted. Trauma was seen in 56% of cases, 41% of which was penetrating. Where vaccination status was known, 52 (74%) children had received age-appropriate vaccinations. Five of 23 (22%) under-5 s were moderately to severely undernourished.
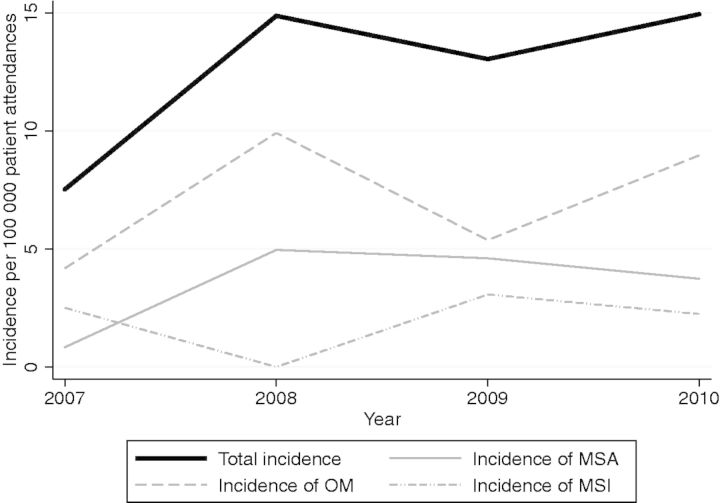
For primary episodes, details across clinical categories and symptoms are represented in Tables 1 and 2. There were no significant differences in white cell count (WCC), ESR or CRP between clinical groups. Crude incidence for non-relapses was 13.8/100 000 attendances; temporal changes by clinical group are depicted in Fig. 1. Median follow-up time (interquartile range [IQR]; range) was 28.5 days (0–140; 0–1339).
Table 1.
Demographic, admission and investigation results associated with clinical syndromes
| Monoarticular septic arthritis (n = 23) | Single-limb osteomyelitis with/without adjacent joint infection (n = 41) | Multisite infections (n = 12) | p-value | |
|---|---|---|---|---|
| Median age, years (IQR) | 6.7 (1.1–11.2) | 6.4 (3.1–9.5) | 9.25 (6.15–11.85) | 0.21 |
| Male, n (%) | 15 (65) | 29 (71) | 5 (42) | 0.17 |
| Median duration of symptoms, days (IQR) | 5 (2–28) | 21 (6–45) | 5.5 (3–6) | 0.003 |
| Positive blood culture, n (% of total patients sampled) | 5 (56) | 4 (50) | 10 (91) | 0.10 |
| Staphylococcus aureus infection, n (%) | 4 (17) | 26 (63) | 12 (100) | <0.001 |
| Median length of stay, days (IQR) | 12 (4–13) | 13 (8–16) | 28 (19–40) | <0.001 |
| Number admitted to intensive care (%) | 0 | 1 (3) | 7 (58) | <0.001 |
| Median length of stay in intensive care, days (IQR) | 1 | 8.5 (4–10) | 0.13 |
Cases of mandibular (n = 2), calcaneal (n = 2) and metatarsal osteomyelitis (n = 1) have been excluded from this analysis.
Table 2.
Symptoms associated with particular clinical syndromes
| Symptom (%) | Monoarticular septic arthritis (n = 22)a | Single-limb osteomyelitis with/without adjacent joint infection (n = 41) | Multisite infections (n = 12) | p-value |
|---|---|---|---|---|
| Fever, n (%) | 19 (86) | 30 (73) | 11 (100) | 0.11 |
| Bone/joint pain, n (%) | 21 (95) | 35 (85) | 10 (91) | 0.60 |
| Decreased movement, n (%) | 18 (82) | 28 (68) | 9 (82) | 0.50 |
| Erythema, n (%) | 5 (23) | 21 (51) | 7 (64) | 0.04 |
| Swelling, n (%) | 14 (64) | 37 (90) | 11 (100) | 0.009 |
| Respiratory, n (%) | 1 (5) | 3 (7) | 8 (73) | <0.001 |
Cases of mandibular (n = 2), calcaneal (n = 2) and metatarsal osteomyelitis (n = 1) have been excluded from this analysis.
aClinical details not available for one case.
Fig. 1.
Changes in incidence by clinical syndrome, 2007–2010. MSA, monoarticular septic arthritis; OM, single-limb osteomyelitis (includes single-limb osteomyelitis with adjacent joint involvement); MSI, multisite infection. No observed changes were statistically significant.
Many patients sought treatment before attendance at our institution: 48% had been reviewed in a private clinic and/or by traditional healers, and 24% had taken antibiotics.
Monoarticular septic arthritis
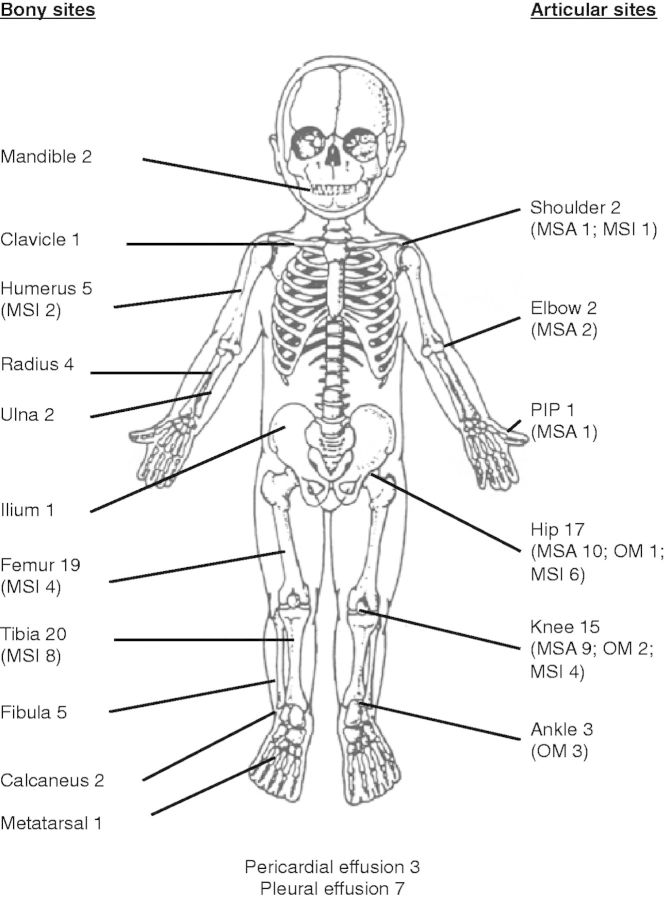
Forty-eight percent of cases presented within 5 days of symptom onset; 68% had fever, joint pain and decreased movement. The hip was involved in 43%, and the knee in 39% of cases (Fig. 2). Microbiological results are presented in Table 3.
Fig. 2.
Prevalence of anatomical site involved in osteoarticular infections. MSA, monoarticular septic arthritis; OM, single-limb osteomyelitis (includes single-limb osteomyelitis with adjacent joint involvement); MSI, multisite infection; PIP, proximal interphalangeal joint.
Table 3.
Demographic and microbiological details for sample-positive monoarticular septic arthritis cases (n = 13)
| Patient characteristics | Age (years) | Gender | Causative organism | Sites of culture positivity |
Susceptibility results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | Synovial fluid | AMP | AMC | CHL | CIP | CRO | ERY | GEN | OXA | PEN | SXT | ||||
| 1 | 1.1 | F | Haemophilus influenzae | H. influenzae | Gram-negative bacilli on microscopy; no growth on culture | R | S | S | S | R | S | ||||
| 2 | 0.7 | M | H. influenzae | Sample not taken | H. influenzae | S | S | S | S | S | R | ||||
| 3 | 14.2 | M | Streptococcus pyogenes | Gram-positive cocci on microscopy; no growth on culture | S. pyogenes | S | S | S | |||||||
| 4 | 9.9 | F | Beta-hemolytic streptococcusa | Beta-hemolytic streptococcus | Negative | S | S | S | |||||||
| 5 | 9.1 | F | Salmonella enterica Typhi | S. Typhi | Negative | R | R | Rb | S | R | |||||
| 6 | 11.8 | F | Staphylococcus aureus | S. aureus | Gram-positive cocci on microscopy; no growth on culture | S | R | S | Sc | R | S | ||||
| 7 | 7.8 | M | S. aureus | Negative | S. aureus | S | S | S | S | R | S | ||||
| 8 | 6.7 | F | S. aureus | Negative | S. aureus | S | S | S | S | R | S | ||||
| 9 | 13.1 | F | S. aureus | Not done | S. aureus | S | R | S | S | R | S | ||||
| 10 | 0.8 | M | Unspecified gram-negative bacillus | Not done | Gram-negative bacilli on microscopy; no growth on culture | Susceptibility testing not done | |||||||||
| 11 | 1.8 | M | Mixed growth | Not done | No Gram stain results; Mixed growth of Enterobacter aerogenes, Streptococcus pneumoniae and an unspeciated gram-negative bacillus | Susceptibility testing not done | |||||||||
| 12 | 9.1 | M | Unspecified gram-positive bacillus | Gram-positive bacilli on microscopy; no growth on culture | Susceptibility testing not done | ||||||||||
| 13 | 14 | F | Unspecified Gram-positive bacillus | Gram-positive bacillus on microscopy of two aspirates; growth of unidentified Gram-positive bacillus on culture | Susceptibility testing not done | ||||||||||
Antimicrobial abbreviations as follows: AMP, ampicillin; AMC, co-amoxiclav; CHL, chloramphenicol; CIP, ciprofloxacin; CRO, ceftriaxone; ERY, erythromycin; GEN, gentamicin; OXA, oxacillin; PEN, penicillin; SXT, co-trimoxazole.
aLancefield grouping not done.
bReduced susceptibility to ciprofloxacin.
cMethicillin susceptibility inferred from oxacillin susceptibility.
Aspiration/drainage was performed in 21 of 22 cases, with 55% having multiple aspirations; five cases required subsequent arthrotomies. Six had arthrotomies without preceding aspiration. The median (IQR) duration of antimicrobial treatment was 27 days (10–29), with 10 days of intravenous treatment. Neither hip joint involvement nor arthrotomy was associated with sequelae (p = 0.66, 1.0).
Single-limb osteomyelitis
On presentation, the median (IQR) duration of symptoms was 17.5 days (6–60). Forty-six percent presented acutely (<14 days of symptoms), 37% subacutely (≥14 ≤90) and 17% with chronic osteomyelitis (>90). The femur (37% of cases) was most commonly affected (Fig. 2).
A higher mean admission WCC was associated with acute presentations (21.1 vs. 11.7 × 109/l; p < 0.001). Mean values for WCC, ESR and CRP were non-significantly higher in those with sequelae.
Fifty percent of admission blood cultures were positive. Bacterial pathogens were cultured from 25 bone/pus samples from 37 individuals: 22 with Staphylococcus aureus alone (susceptibilities in Table 4), one with Haemophilus influenzae and two with mixed infections (one S. aureus/Escherichia coli; one S. aureus/beta-hemolytic streptococcus). No methicillin-resistant S. aureus (MRSA) was isolated. One case was positive for acid-fast bacilli on microscopy.
Table 4.
Antimicrobial susceptibilities of S. aureus isolates in osteomyelitis cases (n = 24)
| Susceptibility pattern | Number of cases (%) |
|---|---|
| Fully susceptible to first line antibioticsa | 1 (4) |
| Resistant to penicillin | 9 (38) |
| Resistant to penicillin + erythromycin | 6 (25) |
| Resistant to penicillin + co-trimoxazole | 5 (21) |
| Resistant to penicillin + erythromycin + co-trimoxazole | 2 (8) |
| Resistant to erythromycin | 1 (4) |
aDefined as penicillin, erythromycin, gentamicin, ciprofloxacin, trimethoprim, oxacillin.
All but two patients had surgery. The median duration of antimicrobial therapy (IQR) was 30 days (25–43) with 12 days of intravenous therapy.
Both mandibular cases were associated with poor dentition; one had health care-associated MRSA osteomyelitis. The three cases with foot osteomyelitis all occurred post-puncture wounds.
Multisite infections
Multisite infections were invariably associated with S. aureus. They included most cases (7/8) admitted to intensive care and the only fatality.
Sequelae
Excluding foot and mandibular cases, 35 (46%) children had sequelae (Table 5), of which 20 were relapses. The only risk factor for sequelae was the use of antimicrobial therapy for >30 days [adjusted odds ratio 6.4 (95% CI 1.6–25.5), p = 0.008].
Table 5.
Summary of relapse and outcome data for patients with particular clinical syndromes
| Outcome | Number of patients by clinical group |
||
|---|---|---|---|
| Monoarticular septic arthritis | Single-limb osteomyelitis ± adjacent joint infection | Multisite infections | |
| Outcomes at final follow-up in patients without relapse (n = 56) | |||
| Decreased movement (%) | 3 (13) | 7 (24) | 2 (40) |
| Residual pain (%) | 0 | 1 (3) | 0 |
| Persistent fever (%) | 1 (5) | 0 | 0 |
| Fever and decreased movement (%) | 1 (5) | 0 | 0 |
| Death (%) | 0 | 0 | 1 (20) |
| Considered cured (%) | 17 (77) | 21 (72) | 2 (40) |
| Outcomes at final follow-up in patients with at least one relapse (n = 20) | |||
| Decreased movement | 0 | 4 [+1]a (38) | 2 (33) |
| Limb swelling | 0 | 2 [+1]a (24) | 0 |
| Considered cured | 1 (100) | 5 (38) | 4 (67) |
Cases of mandibular (n = 2), calcaneal (n = 2) and metatarsal osteomyelitis (n = 1) have been excluded from this analysis.
aNumbers in square brackets denote status at last visit; however, further follow-up was planned for these two patients beyond the study end period.
Of the cases known to have relapsed, 15 relapsed once and five had multiple relapses. Final outcomes are shown in Table 5.
Discussion
This study is one of the larger, recent, single-center series of pediatric osteoarticular infection, and the only study, to our knowledge, from Cambodia. Interstudy comparisons of incidence are difficult, as denominators are frequently different [5–7, 25]; however, osteoarticular infections represent a relatively common surgical problem locally, accounting for approximately 1 in 50 surgical admissions.
Staphylococcus aureus was the most commonly cultured pathogen, but MRSA was not a major problem, unlike in other regional studies [13, 14]. Six percent of cases cultured H. influenzae, demonstrating the importance of empirical cover for this in the absence of adequate vaccination.
More than 95% of patients required surgical intervention, and rates of infectious sequelae were high, although we did not find any associations with published risk factors for poor outcomes [15, 26]. Delays in presentation and inappropriate preliminary treatment are likely to be contributing factors. A further concern is the use of low-dose/low-frequency oral antibiotic regimens in treatment to improve compliance. Studies of suitable regimens in our setting are needed.
Study limitations include incomplete follow-up; this and the sample size have made it unfeasible to model risk factors for relapse. Diverse treatment approaches were used, making it difficult to identify optimal regimens. We did not look for Kingella kingae, which is an important pathogen elsewhere [27, 28]. Nevertheless, we demonstrate relevant features of these infections in Cambodian children, which can be used as a baseline for modifications to treatment approaches and the monitoring of epidemiological trends.
Funding
This work was supported by the Wellcome Trust of Great Britain and the Li Ka Shing–University of Oxford Global Health Program.
Acknowledgements
Authors acknowledge the help of Dr William Housworth, the executive director, and all staff at AHC, including health care staff and the medical records department. We thank Mr Sun Sopheary for his assistance in the extraction of data from the AHC electronic database, and Dr Terry Donald of the Women and Children’s Hospital, Adelaide, South Australia for his kind permission to use the picture of the pediatric skeleton.
References
- 1.Faust SN, Clark J, Pallett A, et al. Managing bone and joint infection in children. Arch Dis Child. 2012;97:545–53. doi: 10.1136/archdischild-2011-301089. [DOI] [PubMed] [Google Scholar]
- 2.Kang SN, Sanghera T, Mangwani J, et al. The management of septic arthritis in children: systematic review of the English language literature. J Bone Joint Surg Br. 2009;91:1127–33. doi: 10.1302/0301-620X.91B9.22530. [DOI] [PubMed] [Google Scholar]
- 3.Nunn TR, Cheung WY, Rollinson PD. A prospective study of pyogenic sepsis of the hip in childhood. J Bone Joint Surg. 2007;89:100–6. doi: 10.1302/0301-620X.89B1.17940. [DOI] [PubMed] [Google Scholar]
- 4.Riise ØR, Kirkhus E, Handeland KS, et al. Childhood osteomyelitis—incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr. 2008;8:45. doi: 10.1186/1471-2431-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blyth MJ, Kincaid R, Craigen MA, et al. The changing epidemiology of acute and subacute haematogenous osteomyelitis in children. J Bone Joint Surg Br. 2001;83:99–102. doi: 10.1302/0301-620x.83b1.10699. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie WJ. The epidemiology of acute haematogenous osteomyelitis of childhood. Int J Epidemiol. 1985;14:600–6. doi: 10.1093/ije/14.4.600. [DOI] [PubMed] [Google Scholar]
- 7.Labbé JL, Peres O, Leclair O, et al. Acute osteomyelitis in children: the pathogenesis revisited? Orthop Traumatol Surg Res. 2010;96:268–75. doi: 10.1016/j.otsr.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Riise ØR, Handeland KS, Cvancarova M, et al. Incidence and characteristics of arthritis in Norwegian children: a population-based study. Pediatrics. 2008;121:e299–306. doi: 10.1542/peds.2007-0291. [DOI] [PubMed] [Google Scholar]
- 9.Lavy CB, Peek AC, Manjolo G. The incidence of septic arthritis in Malawian children. Int Orthop. 2005;29:195–6. doi: 10.1007/s00264-005-0643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8:175–81. doi: 10.1586/eri.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gafur OA, Copley LA, Hollmig ST, et al. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008;28:777–85. doi: 10.1097/BPO.0b013e318186eb4b. [DOI] [PubMed] [Google Scholar]
- 12.Yagupsky P, Bar-Ziv Y, Howard CB, et al. Epidemiology, etiology, and clinical features of septic arthritis in children younger than 24 months. Arch Pediatr Adolesc Med. 1995;149:537–40. doi: 10.1001/archpedi.1995.02170180067010. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi Y, Togawa M, Shiomi M. Septic arthritis and acute hematogenous osteomyelitis in childhood at a tertiary hospital in Japan. Pediatr Int. 2009;51:371–6. doi: 10.1111/j.1442-200X.2008.02740.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen WL, Chang WN, Chen YS, et al. Acute community-acquired osteoarticular infections in children: high incidence of concomitant bone and joint involvement. J Microbiol Immunol Infect. 2010;43:332–8. doi: 10.1016/S1684-1182(10)60051-5. [DOI] [PubMed] [Google Scholar]
- 15.Sukswai P, Kovitvanitcha D, Thumkunanon V, et al. Acute hematogenous osteomyelitis and septic arthritis in children: clinical characteristics and outcomes study. J Med Assoc Thai. 2011;94(Suppl 3):S209–16. [PubMed] [Google Scholar]
- 16.Kim SG, Jang HS. Treatment of chronic osteomyelitis in Korea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:394–8. doi: 10.1067/moe.2001.117810. [DOI] [PubMed] [Google Scholar]
- 17.Muangchan C, Nilganuwong S. The study of clinical manifestation of osteoarticular tuberculosis in Siriraj Hospital, Thailand. J Med Assoc Thai. 2009;92(Suppl 2):S101–9. [PubMed] [Google Scholar]
- 18.Tsai MH, Huang YC, Chiu CH, et al. Nontyphoidal Salmonella bacteremia in previously healthy children: analysis of 199 episodes. Pediatr Infect Dis J. 2007;26:909–13. doi: 10.1097/INF.0b013e318127189b. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel DA, Shrestha OP, Rajbhandary T, et al. Epidemiology of surgical admissions to a children’s disability hospital in Nepal. World J Surg. 2010;34:954–62. doi: 10.1007/s00268-010-0487-3. [DOI] [PubMed] [Google Scholar]
- 20.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–8. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 21.United Nations Children’s Fund. Cambodia—Statistics. http://www.unicef.org/infobycountry/cambodia_statistics.html (13 July 2012, date last accessed)
- 22.National Institute of Statistics (Cambodia) Ministry of Health (Cambodia) and ICF Macro. Cambodia Demographic and Health Survey 2010-2011 (CDHS). Calverton, USA: ICF Macro, 2011. [Google Scholar]
- 23.Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37:291–5. doi: 10.1016/j.ijantimicag.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 24.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and Development. Geneva: World Health Organisation; 2006. p. 312. http://www.who.int/childgrowth/en/ (13 July 2012, date last accessed) [Google Scholar]
- 25.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–8. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 26.Lavy CB. Septic arthritis in Western and sub-Saharan African children—a review. Int Orthop. 2007;31:137–44. doi: 10.1007/s00264-006-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis. 2004;4:358–67. doi: 10.1016/S1473-3099(04)01046-1. [DOI] [PubMed] [Google Scholar]
- 28.Chometon S, Benito Y, Chaker M, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. 2007;26:377–81. doi: 10.1097/01.inf.0000259954.88139.f4. [DOI] [PubMed] [Google Scholar]