We report a case of AIDS presenting as varicella-zoster virus (VZV) meningomyeloradiculitis associated with human immunodeficiency virus (HIV) quasispecies compartmentalization within the cerebrospinal fluid (CSF), and a CSF viral load that was 1 log higher than in peripheral blood. Prolonged antiviral therapy for both VZV and HIV type 1 was associated with partial resolution.
Keywords: human immunodeficiency virus, viral quasispecies, central nervous system compartmentalization, varicella-zoster virus, meningomyeloradiculitis
Abstract
We report a case of AIDS presenting as varicella-zoster virus (VZV) meningomyeloradiculitis associated with human immunodeficiency virus (HIV) quasispecies compartmentalization within the cerebrospinal fluid (CSF), and a CSF viral load that was 1 log higher than in peripheral blood. Prolonged antiviral therapy for both VZV and HIV type 1 was associated with partial resolution.
Viral diversity is an important feature of human immunodeficiency virus (HIV) pathogenesis. Within an individual, HIV exists as a population of related yet distinct viral variants termed viral quasispecies [1]. These quasispecies evolve as an adaptation to selection pressures such as antiviral therapy and/or cellular microenvironment, and can lead to changes in key virologic properties including cellular tropism and coreceptor utilization [2]. Viral quasispecies are not evenly distributed throughout the body, and analysis of their diversity suggests that there may be cell type and/or tissue-specific viral variants that may exist within an infected individual [3]. The term compartmentalization has been attributed to the restriction of viral quasispecies between cells or tissues [4]. One compartment of great interest is the central nervous system (CNS), where there have been reports of selective replication of distinct viral quasispecies leading to resistance confined to the CNS [5], as well as an association between increased cerebrospinal fluid (CSF) viral load and HIV dementia [6].
It is not yet known whether quasispecies compartmentalization in the brain and CSF is associated with other CNS infections in patients with HIV. This patient population does, however, have an increased risk of developing reactivation of varicella-zoster virus (VZV) infection that can lead to serious neurological complications due to subclinical extension of VZV into the CNS. These CNS complications include postherpetic neuralgia, ophthalmic zoster with contralateral hemiparesis, acute or chronic encephalitis, myelitis, polyradiculitis, motor neuropathies, and cranial nerve palsies [7, 8], as well as immune reconstitution inflammatory syndrome (IRIS) [9].
We report a case of AIDS presenting as VZV meningomyeloradiculitis, which was associated with an HIV type 1 (HIV-1) load in the CSF that was approximately 1 log higher than that in peripheral blood, and with HIV viral quasispecies compartmentalization within the CSF. The patient's neurological syndrome was progressive. A prolonged course of antiviral therapy for both VZV and HIV-1 was associated with stabilization and partial resolution.
CASE REPORT
A 50-year-old African American man presented with a 4-day history of left-sided chest pain and progressive left leg weakness resulting in multiple falls. Two days prior to admission, he noted a painful vesicular rash on the left side of his chest, but denied having any fever, headache, visual disturbances, seizures, neck stiffness, or incontinence. He had a 15-pack-year smoking history, and reported remote intranasal cocaine use, as well as having unprotected intercourse with multiple female partners. His physical exam revealed oropharyngeal thrush, a vesicular rash in the left T4 dermatome, and 0/5 strength in the left lower extremity in association with an increased left patellar reflex and an extensor plantar response on the left. Temperature and pinprick sensation were decreased in the right leg, and torso below T5.
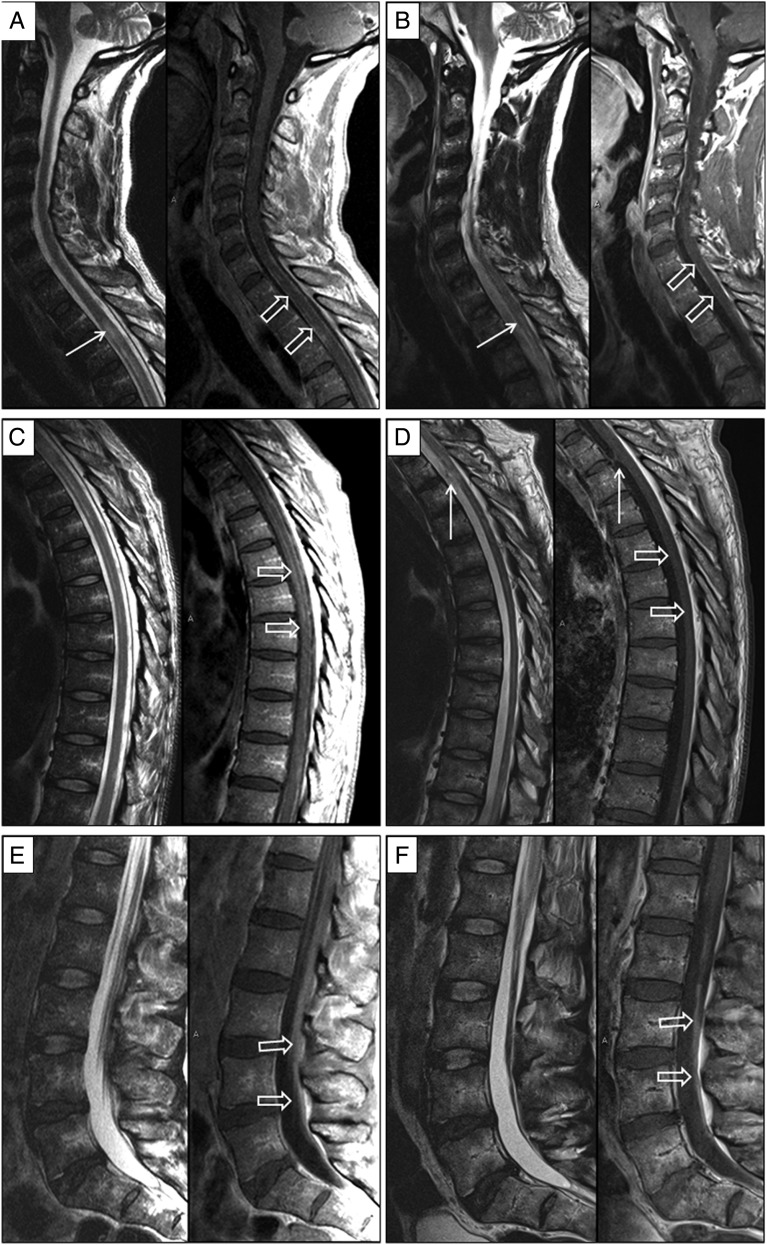
Magnetic resonance imaging (MRI) of the spine showed a focal, centrally located intramedullary hyperintensity on T2-weighted images, at the T3 level that was associated with slight cord expansion and subtle enhancement. There was also marked diffuse meningeal thickening and enhancement involving the cervical and thoracic cord as well as the cauda equina nerve roots (Figure 1). Laboratory testing was significant for a positive HIV 1/2 rapid antibody screen, a CD4+ T-cell count of 35 cells/μL, and an HIV-1 load of 14 163 copies/mL. Plasma HIV-1 viral sequence analysis for reverse transcriptase (RT) and protease resistance mutations was negative. Additional blood tests included negative rapid plasma reagin, cytomegalovirus polymerase chain reaction (PCR), Toxoplasma serologies, and serum antigen for Cryptococcus. A blood PCR for Epstein-Barr virus was positive at 350 copies/mL. The CSF contained 132 white blood cells (WBCs)/mm3 (27% neutrophils and 60% lymphocytes), 130 red blood cells/mm3, 486 mg/dL of protein, and 63 mg/dL of glucose. Cytomegalovirus, herpes simplex virus, and John Cunningham virus PCRs were all negative in the CSF, and the Venereal Disease Research Laboratory test was nonreactive. VZV PCR was positive in the CSF. The CSF HIV-1 load was found to be 108 982 copies/mL.
Figure 1.
Magnetic resonance imaging findings at presentation and after treatment. Sagittal T2-weighted and sagittal enhanced T1-weighted images of the cervical, thoracic, and lumbosacral spine upon presentation (A, C, and E respectively) and after treatment (B, D, and F respectively). Thickening and enhancement of the meningeal covers (hollow arrows in A and C) is noted upon presentation. This improves significantly on the follow-up examination (hollow arrows in B and D). Parenchymal cord involvement with abnormal T2-hyperintense lesion at T3 level noted upon presentation also improves significantly on follow-up (solid arrows in A and B). Enhancement of the cauda equina nerve roots (hollow arrows in E) is later replaced by nerve root clumping and enhancement (hollow arrows in F) suggesting the development of chronic arachnoiditis. Note the development of adhesions along the ventral aspect of the cord at T3–T4 level (white solid arrows in D).
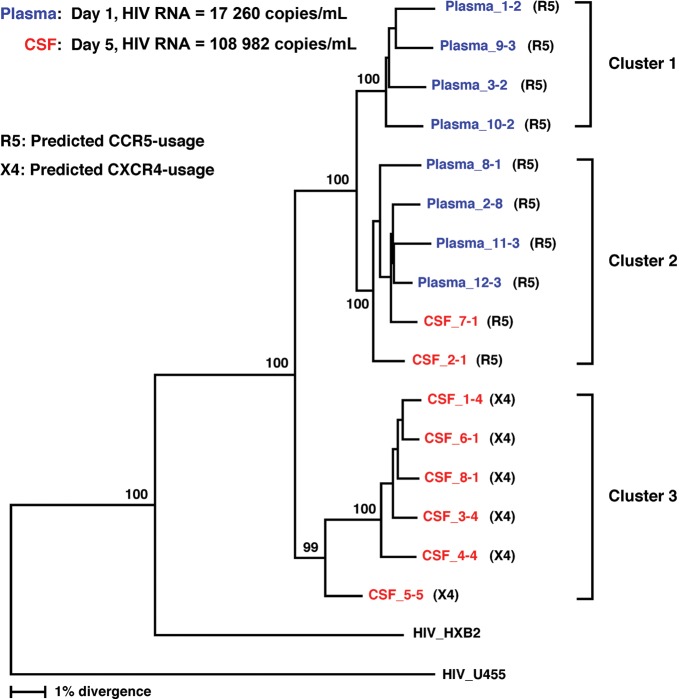
Given the significant discrepancy in HIV-1 load between the plasma and CSF, phylogenetic analysis of the HIV-1 envelope (env) gene using cell-free virion RNA as a template from plasma and CSF samples was performed as previously described [10], except that the following 2 sets of primers were used to amplify full-length envelope genes: +5954 (sense) 5′-GGCTTAGGCATCTCCTATGGCAGGAAGAA-3′ and –9146 (antisense) 5′-CTGCCAATCAGGGAAGTAGCCTTGTGT-3′ in a first-round reaction; +6202 (sense) 5′-AGAAAGAGCAGAAGACAGTGGCAATGA-3′ and –9068 (antisense) 5′-TAGCCCTTCCAGTCCCCCCTTTTCTTTTA-3′ in a second-round reaction. HIV-1 sequences from the patient clustered into 3 phylogenetically distinct groups: one group consisting of plasma virus sequences, a second group containing plasma and CSF virus sequences, and a third group consisting exclusively of CSF virus sequences. The compartmentalization of HIV-1 between the peripheral blood and spinal cord is represented by separate clusters for plasma, and CSF sequences in the phylogenetic tree (Figure 2). Moreover, the major separations observed in the phylogenetic tree were associated with predicted CXC chemokine receptor type 4 (CXCR4) versus CC chemokine receptor type 5 (CCR5) usage of the virus [11]. Based on the Web PSSM analysis (a bioinformatics tool for predicting HIV-1 coreceptor usage), the viral sequences clustering in the CSF showed CXCR4 use, whereas the viral sequences predominating in the plasma predicted CCR5 use.
Figure 2.
Phylogenetic analysis of human immunodeficiency virus type 1 (HIV-1) env sequences derived from cerebrospinal fluid (CSF) and plasma samples. Phylogenetic relationships among the full-length envelope gene sequences were estimated using the neighbor-joining method. Bootstrap percentile values from 1000 replications are shown at nodes defining major grouping of sequences. Statistical support of 50% or greater is shown. HIV-1 U455 was used as an outgroup. Cluster 1 contains plasma sequences, cluster 2 contains plasma and CSF sequences, and cluster 3 contains CSF sequences. The majority of CSF sequences predict CXCR4 usage, while all plasma sequences predict CCR5 usage.
Soon after admission, the patient was started on antiretroviral therapy (emtricitabine, tenofovir, efavirenz), intravenous acyclovir, and methylprednisolone. Following 7 days of treatment, he experienced progression of his neurologic impairment with an increase in CSF pleocytosis (132–306 WBCs/mm3). VZV DNA, however, was no longer detected in his CSF by PCR. Thus, after 14 days of acyclovir, he was switched to famciclovir. Nevertheless, follow-up MRI showed several new T2-hyperintense lesions in the thoracic cord with persistent abnormal cord meningeal and cauda equina nerve root enhancement. The patient was therefore restarted on intravenous acyclovir for an additional 4 weeks. He was then switched to valacyclovir for 8 weeks followed by an additional 8 weeks of valacyclovir at prophylactic doses. His follow-up MRI at the end of his treatment course showed significant improvement with almost complete resolution of the meningeal enhancement, as well as decreased size and conspicuity of the cord lesions. There was, however, persistent evidence of cauda equina nerve root clumping and enhancement suggestive of irreversible arachnoiditis (Figure 1). Follow-up plasma HIV loads eventually became <50 copies/mL, and CSF HIV loads declined from 108 982 copies/mL to 211 copies/mL (Table 1). The patient is currently much improved neurologically and is ambulating with a walker.
Table 1.
Measured Plasma and Cerebrospinal Fluid HIV Loads (Copies/mL)
| Day |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | 12 | 14 | 20 | 34 | 56 | 89 | 166 | 318 | |
| Plasma | 14 163 | 17 260 | 136 | 348 | 69 | 149 | 64 | <50 | <50 | <50 | ||
| Cerebrospinal fluid | 108 982 | 649 | 1569 | |||||||||
DISCUSSION
To our knowledge, this is the first report of a case of HIV quasispecies compartmentalization in the CNS, in a patient presenting with VZV meningomyeloradiculitis and incomplete Browne-Séquard syndrome. Our patient improved significantly with a prolonged course of acyclovir followed by valacyclovir, and prompt initiation of antiretroviral therapy.
Herpes zoster and its related neurological complications are more common in the HIV-infected population, especially when CD4+ T-cell counts are <100 cells/μL [7, 12]. Such complications are often diagnosed based on the detection of VZV DNA in the CSF and a temporal association with the appearance of skin lesions. However, zoster reactivation can occur in the absence of any skin manifestations [7, 13–15]. We reviewed reported cases of VZV myelitis or myeloradiculitis in patients with HIV/AIDS (Table 2). Seven of 14 reported patients died, most of whom were from before the era of combined antiretroviral therapy. In at least 9 out of the 14 cases, patients were treated with antivirals (mainly intravenous acyclovir) with or without a short course of steroids. CSF HIV loads were not reported in any of the cases.
Table 2.
Reported Cases of Varicella-Zoster Virus Myelitis or Myeloradiculitis in Patients With HIV
| Reference/Year Reported | Age at Presentation, Sex | On ARVs at Diagnosis | CD4+ Count (Cells/μL) | Clinical Syndrome | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Present case | 50, M | No | 35 | Meningomyeloradiculitis with partial Brown-Séquard syndrome | Methylprednisolone × 1 wk | Partial neurological recovery |
| Thoracic zoster | Acyclovir IV × 6 wk | |||||
| Valacyclovir × 8 wk | ||||||
| Antiretrovirals | ||||||
| [20] 2010 | 24, M | Yes | NR | Myeloradiculitis of the cauda equina | Acyclovir IV × 3 wk | Survived with normalization of CSF |
| Disseminated rash | ||||||
| [23] 2009 | 41, F | Yes | 155 | Necrotizing myelitis with meningoencephalitis resulting in bulbar palsy and respiratory failure | Dexamethasone IV | Died |
| Six vesicular lesions on anterior chest | Acyclovir IV | |||||
| Ceftriaxone IV | ||||||
| Benzylpenicillin | ||||||
| [13] 2004 | 48, M | Yes | 10 | Multilevel transverse myelitis | Methylprednisolone × 3 d | Partial neurological recovery |
| No rash | Valacyclovir | |||||
| [24] 1996 | 31, M | No | 6 | Myelitis (T8 level) | Acyclovir IV | Signed out against medical advice |
| Thoracic zoster | ||||||
| [24] 1996 | 47, M | NR | NR | Myelitis (T3–T5 level) | Acyclovir IV × 7 d | Partial neurological recovery |
| Trigeminal zoster | Famciclovir | |||||
| Dexamethasone IV × 7 d | ||||||
| Foscarnet × 3 d | ||||||
| Clindamycin × 2 d | ||||||
| Pyrimethamine × 2 d | ||||||
| Leucovorin × 2 d | ||||||
| [25] 1996 | 29, M | Yes | 16 | Myelopathy (T10 level) with urinary retention and no findings on spinal MRI | Acyclovir IV × 21 d | Complete neurological recovery |
| Thoracic zoster | Acyclovir oral | |||||
| [25] 1996 | 35, F | Yes | 5 | Bilateral palsies of cranial nerves VII and VIII resulting in deafness | Acyclovir IV × 35 d | Partial neurological recovery |
| Encephalopathy | Acyclovir oral | |||||
| Brown-Séquard syndrome | Withdrawal of antiretroviral | |||||
| Cervical zoster | ||||||
| [14] 1995 | 35, M | Yes | 32 | Diffuse necrotic myeloradiculitis | NR | Died |
| No rash | ||||||
| [26] 1994 | 40, M | Yes | 239 | Ascending myelopathy (T5 level) with complete paraplegia, urinary retention and no findings on spinal MRI | Acyclovir oral | Survived without any neurological recovery |
| Thoracic zoster | Died | |||||
| [27] 1994 | 42, M | No | 3 | Meningomyeloradiculitis | Gancyclovir IV | |
| (C4–C7 level) | Syphilis treatment | |||||
| Ophthalmic zoster | ||||||
| [27] 1994 | 40, M | No | NR | Myeloradiculitis | NR | Died |
| Thoracic zoster | ||||||
| [15] 1994 | 41, M | No | 20 | Cervical myelitis | Prednisone | Died |
| No rash | Acyclovir oral | |||||
| [22] 1993 | 30, M | No | 290 | Ventriculitis and meningomyeloradiculitis (T5 level) | Antiretrovirals | Died |
| No rash | ||||||
| [28] 1991 | 36, M | NR | NR | Thoracic transverse myelitis | NR | Died |
| Thoracic zoster |
Abbreviations: ARV, antiretrovirals; CSF, cerebrospinal fluid; IV, intravenous; MRI, magnetic resonance imaging; NR, not reported.
HIV infects the CNS during acute primary infection, and compartmentalizes within this site by infecting macrophages and microglia [16]. The CNS is an important reservoir for long-term viral persistence (and potential neurocognitive impairment), partly due to the relatively poor CNS penetration of many antiretrovirals, as well as the fact that drug resistance can evolve independently within the CNS [17]. In fact, studies have shown that there can be CSF HIV escape with reported CSF viral loads as high as 12 885 copies/mL while plasma viral loads remain suppressed due to combined antiretroviral therapy [18, 19]. In our patient, however, the discrepancy between the CSF and plasma viral loads was present prior to beginning combined antiretroviral therapy, during a severe episode of zoster reactivation. These findings suggest that CNS coinfection with VZV may have played a role in the enhanced HIV replication in the CSF.
The primary determinant of HIV macrophage–mediated neurotropism is genetic variation of the viral env gene. In our patient, phylogenetic analysis of env sequences demonstrated a first viral cluster containing plasma sequences, a second viral cluster containing both plasma and CSF sequences, and a third viral cluster consisting of CSF sequences. Interestingly, the clustering pattern was associated with predicted coreceptor usage: the first and second clusters with CCR5 usage, and the third cluster with CXCR4 usage. However, consistent with previous reports [13], the predicted coreceptor usage did not distinguish viruses in CSF from those in plasma.
Tissue-specific compartmentalization of HIV-1 variants in the brain has been demonstrated in a number of studies [13, 20–22]. The CSF is a compartment that bridges the brain and the periphery. Thus, it is plausible that HIV-1 variants in the CSF can be genetically similar to those in the brain and the periphery. In fact, there has been a report demonstrating a close genetic relationship between HIV-1 variants in CSF and plasma [20]. This intermingling of CSF and plasma HIV-1 sequences was also observed in our patient. These findings suggest that the CSF compartment was infiltrated by HIV-1 from the periphery (represented by the second cluster) and subsequently underwent local amplification of the HIV-1 variants (resulting in the formation of the third cluster), possibly driven by the selective pressure that was unique to the CSF compartment. From a clinical standpoint, viral load discrepancies between the plasma and CSF should, at the very least, prompt sequencing of the RT, protease, and integrase portions of the genome to assist in clinical decision making, and the selection of the appropriate antiretroviral regimen.
Our patient presented with a severe neurological complication of zoster reactivation in the setting of HIV load discrepancy between the plasma and CSF with quasispecies compartmentalization in the CNS. He required a prolonged course of intravenous acyclovir and was started on antiretrovirals early in his course in order to achieve a significant resolution of his neurological symptoms. It is possible that the initial worsening of the patient's neurological status may have been due to IRIS, especially in the context of initiation of ART 1 week prior. However, the patient was already on IRIS treatment doses of steroids at the time that ART was initiated, and they did not appear to be of benefit in this setting. Moreover, the CSF VZV PCR had become negative, and the CSF HIV load was increased relative to the plasma viral load.
It is unclear whether localized HIV replication in the CNS played a role in the severity of the zoster reactivation in this patient or whether concomitant VZV infection facilitated CNS HIV replication. Future phylogenetic analyses of HIV sequences in the CSF during zoster reactivation or other acute CNS infections may be of interest in better understanding the potential synergy between these 2 viruses.
Notes
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy of the US government.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lauring AS, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6:e1001005. doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackard JT. HIV compartmentalization: a review on a clinically important phenomenon. Curr HIV Res. 2012;10:133–42. doi: 10.2174/157016212799937245. [DOI] [PubMed] [Google Scholar]
- 3.van't Wout AB, Ran LJ, Kuiken CL, Kootstra NA, Pals ST, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–96. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickle DC, Shriner D, Mittler JE, Frenkel LM, Mullins JI. Importance and detection of virus reservoirs and compartments of HIV infection. Curr Opin Microbiol. 2003;6:410–6. doi: 10.1016/s1369-5274(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 5.Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol. 2004;78:10133–48. doi: 10.1128/JVI.78.18.10133-10148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritola K, Robertson K, Fiscus SA, Hall C, Swanstrom R. Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. J Virol. 2005;79:10830–4. doi: 10.1128/JVI.79.16.10830-10834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De La Blanchardiere A, Rozenberg F, Caumes E, et al. Neurological complications of varicella-zoster virus infection in adults with human immunodeficiency virus infection. Scand J Infect Dis. 2000;32:263–9. doi: 10.1080/00365540050165893. [DOI] [PubMed] [Google Scholar]
- 8.Gnann JW., Jr Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91–8. doi: 10.1086/342963. [DOI] [PubMed] [Google Scholar]
- 9.Newsome SD, Nath A. Varicella-zoster virus vasculopathy and central nervous system immune reconstitution inflammatory syndrome with human immunodeficiency virus infection treated with steroids. J Neurovirol. 2009;15:288–91. doi: 10.1080/13550280902913610. [DOI] [PubMed] [Google Scholar]
- 10.Imamichi H, Degray G, Dewar RL, et al. Lack of compartmentalization of HIV-1 quasispecies between the gut and peripheral blood compartments. J Infect Dis. 2011;204:309–14. doi: 10.1093/infdis/jir259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MA, Coetzer M, van't Wout AB, Morris L, Mullins JI. A reliable phenotype predictor for human immunodeficiency virus type 1 subtype C based on envelope V3 sequences. J Virol. 2006;80:4698–704. doi: 10.1128/JVI.80.10.4698-4704.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vafai A, Berger M. Zoster in patients infected with HIV: a review. Am J Med Sci. 2001;321:372–80. doi: 10.1097/00000441-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ohagen A, Devitt A, Kunstman KJ, et al. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol. 2003;77:12336–45. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meylan PRA, Miklossy J, Iten A, et al. Myelitis due to varicella-zoster virus in an immunocompromised patient without a cutaneous rash. Clin Infect Dis. 1995;20:206–8. doi: 10.1093/clinids/20.1.206. [DOI] [PubMed] [Google Scholar]
- 15.Manian FA, Kindred M, Fulling KH. Chronic varicella-zoster virus myelitis without cutaneous eruption in a patient with AIDS: report of a fatal case. Clin Infect Dis. 1995;21:986–8. doi: 10.1093/clinids/21.4.986. [DOI] [PubMed] [Google Scholar]
- 16.Bagasra O, Lavi E, Bobroski L, et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–85. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 18.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well-controlled plasma viral load. AIDS. 2012;26:1765–74. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 20.Strain MC, Letendre S, Pillai SK, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol. 2005;79:1772–88. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korber BT, Kunstman KJ, Patterson BK, et al. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–81. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang S, Vinters HV, Akashi T, O'Brien WA, Chen IS. HIV-1 env sequence variation in brain tissue of patients with AIDS-related neurologic disease. Acquir Immune Defic Syndr. 1991;4:1082–92. [PubMed] [Google Scholar]
- 23.Chang CC, McLean C, Vujovic O, et al. Fatal acute varicella-zoster virus hemorrhagic meningomyelitis with necrotizing vasculitis in an HIV-infected patient. Clin Infect Dis. 2009;48:372–3. doi: 10.1086/595894. [DOI] [PubMed] [Google Scholar]
- 24.de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–31. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 25.Lionnet F, Pulik M, Genet P, et al. Myelitis due to varicella-zoster virus in two patients with AIDS: successful treatment with acyclovir. Clin Infect Dis. 1996;22:138–40. doi: 10.1093/clinids/22.1.138. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Tortosa E, Gadea I, Gegundez MI, et al. Development of myelopathy before herpes zoster rash in a patient with AIDS. Clin Infect Dis. 1994;18:810–2. doi: 10.1093/clinids/18.5.810. [DOI] [PubMed] [Google Scholar]
- 27.Gray F, Belec L, Lescs MC, et al. Varicella-zoster virus infection of the central nervous system in the acquired immune deficiency syndrome. Brain. 1994;117(Pt 5):987–99. doi: 10.1093/brain/117.5.987. [DOI] [PubMed] [Google Scholar]
- 28.Devinsky O, Cho ES, Petito CK, Price RW. Herpes zoster myelitis. Brain. 1991;114(Pt 3):1181–96. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]