Kaposi sarcoma and lymphoma rates were highest immediately after antiretroviral therapy (ART) initiation, particularly among patients with low CD4 cell counts, whereas other cancers increased with time on ART. Calendar year of ART initiation was not associated with subsequent cancer incidence.
Keywords: HIV-associated malignancies, AIDS-defining cancer, non-AIDS-defining cancer, combination antiretroviral therapy
Abstract
Background
Cancer is an important cause of morbidity and mortality in individuals infected with human immunodeficiency virus (HIV), but patterns of cancer incidence after combination antiretroviral therapy (ART) initiation remain poorly characterized.
Methods
We evaluated the incidence and timing of cancer diagnoses among patients initiating ART between 1996 and 2011 in a collaboration of 8 US clinical HIV cohorts. Poisson regression was used to estimate incidence rates. Cox regression was used to identify demographic and clinical characteristics associated with cancer incidence after ART initiation.
Results
At initiation of first combination ART among 11 485 patients, median year was 2004 (interquartile range [IQR], 2000–2007) and median CD4 count was 202 cells/mm3 (IQR, 61–338). Incidence rates for Kaposi sarcoma (KS) and lymphomas were highest in the first 6 months after ART initiation (P < .001) and plateaued thereafter, while incidence rates for all other cancers combined increased from 416 to 615 cases per 100 000 person-years from 1 to 10 years after ART initiation (average 7% increase per year; 95% confidence interval, 2%–13%). Lower CD4 count at ART initiation was associated with greater risk of KS, lymphoma, and human papillomavirus–related cancer. Calendar year of ART initiation was not associated with cancer incidence.
Conclusions
KS and lymphoma rates were highest immediately following ART initiation, particularly among patients with low CD4 cell counts, whereas other cancers increased with time on ART, likely reflecting increased cancer risk with aging. Our results underscore recommendations for earlier HIV diagnosis followed by prompt ART initiation along with ongoing aggressive cancer screening and prevention efforts throughout the course of HIV care.
While the distribution of cancer types within human immunodeficiency virus (HIV)-infected populations has changed due to advances in treatment and demographic changes, malignancies remain an important cause of morbidity and mortality [1–4]. Cancer incidence trends in the HIV population have been thoroughly examined across calendar time [1, 5–7]. However, incidence rates among HIV patients are not well described across time relative to initiation of combination antiretroviral therapy (ART), even though most cancers in developed countries are diagnosed after ART initiation [8–10].
Given the many changes that can occur after ART initiation, including immune reconstitution, HIV replication, aging, and ongoing exposure to carcinogens, cancer incidence over time following ART initiation is expected to be dynamic. Additionally, improvements in access to HIV testing and linkage to care as well as changes in the timing of ART initiation may impact the timing and incidence of cancer diagnoses in treated patients. In a large, diverse US cohort of HIV-infected patients in care, the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS), we evaluated trends in cancer incidence rates over time after ART initiation to inform cancer screening and prevention efforts for the HIV population in the ART era. We further identified patient characteristics associated with these trends that may provide insights into the etiology of cancers occurring in HIV-infected populations.
METHODS
Study Population
We conducted this study in the CNICS cohort, which includes >25 000 HIV-infected adults in routine care at 8 university-affiliated CFARs from 1995 to the present. The methodology and characteristics of the CNICS cohort are described in detail elsewhere [11]. In brief, CNICS is a dynamic observational cohort with approximately 1400 new patients enrolling and 13% of existing patients leaving care each year. CNICS captures extensive and comprehensive electronic medical data, including demographics, medications, comorbid diagnoses, and laboratory values [11]. Diagnoses of cancer are verified through a standardized process, including detailed record abstraction and adjudication of malignancies [12].
For our study, we included patients who initiated ART with a known start date between 1 January 1996–30 August 2011, and had a CD4 count and HIV RNA measurement at ART initiation. Baseline CD4 count and HIV RNA measures were the values most proximal to, but no more than 12 months before, ART initiation. ART was defined as concurrent initiation of at least 3 different antiretrovirals. For our primary analyses, we included patients with prior exposure to 1 or 2 nucleoside/nucleotide agents (mono- and dual ART, respectively) or who had an unknown antiretroviral history prior to initiating ART. We did not analyze person-time prior to ART initiation; however, we did assess effects of prior therapy exposure on our results, and conducted sensitivity analyses in which only antiretroviral-naive patients at ART initiation were included.
Patients were followed from ART initiation to the first of the following: cancer diagnosis, death, loss to follow-up (defined as 12 months without a clinical visit), or administrative censoring at the last date of cancer ascertainment for each clinic site (range of 31 May 2010–31 August 2011). Follow-up time was administratively censored at 10 years after ART initiation, at which point <10% of the patients remained under observation. Only the first cancer diagnosis after ART initiation was included as an event. In our primary analyses, we included all patients initiating ART without regard to cancer events prior to ART initiation. To assess the influence of including patients with a previous history of cancer, we performed sensitivity analyses in which patients with cancer diagnoses prior to ART initiation were excluded.
Statistical Analysis
Incidence rates (IRs) were estimated as the total number of cancer diagnoses within a time interval divided by the total number of person-years contributed within the same interval. Overall cancer incidence rates and incidence rates within 6-month and 1-year time intervals following ART initiation were calculated for specific cancers as well as for clinically meaningful predefined groups of cancers. Specifically, we assessed incidence rates using different categorizations including AIDS-defining cancers (ADCs; including Kaposi sarcoma [KS], non-Hodgkin lymphoma, and cervical cancer); non-AIDS-defining cancers (NADCs); lymphomas; human papillomavirus (HPV)–related cancers (including cervical, anal, squamous cell oral cavity/pharynx, vaginal/vulvar, and penile cancer); virus-related cancers (including all ADCs, all HPV-related cancers, Hodgkin lymphoma, and liver cancer); and virus-unrelated cancers.
Poisson regression was used to contrast incidence rates between time intervals and to estimate trends in incidence rates across time following ART initiation. Baseline clinical characteristics were examined with Cox regression to identify predictors of cancer incidence following ART initiation. Cancers were grouped by common etiology and similar incidence patterns over time when assessing associations with baseline characteristics. Multivariable analyses were conducted with adjustment for all baseline demographic and clinical predictors determined to be associated with cancer incidence based on a priori knowledge. Two separate adjustment sets were used: one with CD4 count at ART initiation, and one with nadir CD4 count any time prior to ART. These 2 variables were not included simultaneously as they were highly correlated, but were included separately to examine whether choice of CD4 measure affected other estimates differentially, especially for patients with prior antiretroviral exposure. Incidence rates across time were also stratified by CD4 count at ART initiation (≥200 or <200 cells/mm3), age (≥45 or <45 years), and prior exposure to antiretrovirals. Changes in the associations of these predictors with cancer incidence were assessed visually and through the evaluation of interaction terms with log(time) in regression models.
RESULTS
Baseline Characteristics
Of 25 337 patients enrolled in CNICS at the time of this analysis, 11 485 were observed to initiate ART between 1996 and 2011 at a CNICS site with pre-ART CD4 count and HIV RNA levels available. Of these 11 485 patients, 20% were female, 43% white, 41% black, and 11% Hispanic (Table 1). Median age at ART initiation was 38 years (interquartile range [IQR], 32–45). Median year of ART initiation was 2004 (IQR, 2000–2007), and 28% of patients had exposure to antiretrovirals prior to combination ART initiation. At ART initiation, median CD4 count was 202 cells/mm3 (IQR, 61–338 cells/mm3) and median HIV RNA was 4.8 log10 copies/mL (IQR, 4.3–5.3 copies/mL). Most patients initiated a protease inhibitor–based regimen (47%) or a nonnucleoside reverse transcriptase inhibitor–based regimen (42%). In addition, 5% initiated a triple-nucleos(t)ide reverse transcriptase inhibitor regimen, 4% initiated a regimen with a protease inhibitor and a nonnucleoside reverse transcriptase inhibitor, and 2% initiated regimens with another anchor drug, including an integrase inhibitor, fusion inhibitor, or entry inhibitor. The median length of follow-up after ART initiation was 3.1 years (IQR, 1.4–6.2 years), and 9.5% of patients remained in follow-up at 10 years post-ART.
Table 1.
Demographic and Clinical Characteristics of 11 485 Patients Initiating Combination Antiretroviral Therapy in the Centers for AIDS Research Network of Integrated Clinical Systems, 1996–2011
| Characteristic | No. of Patients |
|---|---|
| Total | 11 485 |
| Female sex | 2304 (20.1%) |
| Age, median (IQR) | 38 (32–45) |
| Race | |
| White | 4933 (43.0%) |
| Black | 4677 (40.7%) |
| Hispanic | 1273 (11.1%) |
| Other/unknown | 602 (5.2%) |
| Injection drug user | 2156 (18.8%) |
| Men who have sex with men | 6328 (55.1%) |
| Antiretroviral exposure prior to first ART | 3207 (27.9%) |
| ART initiation year, median (IQR) | 2004 (2000–2007) |
| ART regimen type | |
| PI | 5443 (47.3%) |
| NNRTI | 4835 (42.1%) |
| ≥3 NRTIs | 570 (5.0%) |
| NNRTI + PI | 441 (3.8%) |
| Othera | 196 (1.7%) |
| CD4 count, cells/mm3, median (IQR) | 202 (61–338) |
| HIV RNA level, log10 copies/mL, median (IQR) | 4.8 (4.3–5.3) |
Abbreviations: ART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Includes regimens with an integrase inhibitor, fusion inhibitor, or entry inhibitor.
Cancer Incidence and Timing
During the 46 318 person-years of follow-up after ART initiation, 457 cancer diagnoses were observed with an incidence rate (IR) of 987 cases per 100 000 person-years (95% confidence interval [CI], 900–1081 cases per 100 000 person-years; Table 2). The overall incidence of ADCs was similar to that of NADCs (ADC IR, 515 per 100 000 person-years [95% CI, 454–584 cases per 100 000 person-years]; NADC IR, 466 per 100 000 person-years [95% CI, 408–533 cases per 100 000 person-years]). The most common ADC was KS with an IR of 304 per 100 000 person-years (95% CI, 258–358 cases per 100 000 person-years), whereas the most common NADC was anal cancer with an IR of 69 per 100 000 person-years (95% CI, 49–98 cases per 100 000 person-years). Among women, the most common NADC was breast cancer (IR, 128 per 100 000 person-years [95% CI, 75–221 cases per 100 000 person-years]).
Table 2.
Cancer Incidence Rates Within 10 Years After Combination Antiretroviral Therapy Initiation, Centers for AIDS Research Network of Integrated Clinical Systems, 1996–2011
| Incidence Rate per 100 000 Person-years (95% CI) |
||||
|---|---|---|---|---|
| Cancer Type | Total No. | 0–10 y | 0–6 mo | 6 mo–10 y |
| Overall | 457 | 987 (900–1081) | 2405 (2030–2848) | 793 (711–884) |
| ADC | 241 | 515 (454–584) | 1881 (1554–2278) | 330 (279–390) |
| KS | 143 | 304 (258–358) | 1342 (1071–1683) | 164 (129–208) |
| NHL non-CNS | 76 | 161 (128–201) | 357 (230–553) | 134 (103–175) |
| NHL CNS | 19 | 40 (26–63) | 160 (84–308) | 24 (13–44) |
| Cervicala | 3 | 30 (10–92) | … | … |
| NADC | 216 | 466 (408–533) | 520 (362–749) | 459 (398–530) |
| Virus-related NADCs | 89 | 192 (156–237) | 251 (149–424) | 184 (147–231) |
| Squamous cell anal | 32 | 69 (49–98) | 72 (27–191) | 69 (47–100) |
| Hodgkin lymphoma | 27 | 58 (40–85) | 144 (72–287) | 47 (30–73) |
| Liver | 17 | 37 (23–59) | 18 (3–127) | 39 (24–64) |
| Squamous cell oral cavity/pharynx | 9 | 19 (10–37) | … | … |
| Otherb | 4 | 9 (3–23) | … | … |
| Virus-unrelated NADCs | 127 | 274 (230–326) | 269 (162–447) | 275 (228–331) |
| Lung | 26 | 56 (38–82) | 54 (17–167) | 56 (38–85) |
| Prostatea | 20 | 54 (35–83) | 22 (3–159) | 58 (37–91) |
| Breasta | 13 | 128 (75–221) | 177 (44–708) | 122 (68–221) |
| Melanoma | 10 | 22 (12–40) | … | … |
| Colorectal | 8 | 17 (9–35) | … | … |
| Kidney | 5 | 11 (5–26) | … | … |
| Otherc | 45 | 93 (69–125) | … | … |
| Lymphomasd | 122 | 259 (217–309) | 660 (479–912) | 205 (165–253) |
| HPV-related cancerse | 48 | 104 (78–138) | 108 (48–140) | 103 (76–140) |
Only overall incidence rates were calculated for cancer types with ≤10 cases.
Abbreviations: ADC, AIDS-defining cancer; CI, confidence interval; CNS, central nervous system; HPV, human papillomavirus; KS, Kaposi sarcoma; NADC, non-AIDS-defining cancer; NHL, non-Hodgkin lymphoma.
a Cervical cancer and breast cancer incidence calculated only among women. Prostate cancer incidence calculated only among men.
b Other virus-related cancers include penile, vaginal, and vulvar.
c Other virus-unrelated cancers include bladder, brain, esophagus, larynx, leukemia, multiple myeloma, ovary, pancreas, peritoneum, small intestine, soft tissue, stomach, testicular, uterus, or non–squamous cell oral cavity/pharynx.
d Lymphomas included NHL non-CNS, NHL CNS, and Hodgkin lymphoma.
e HPV-related cancers included cervical, squamous cell anal (which includes squamous cell anorectal cancer), squamous cell oral cavity/pharynx, penile, vaginal, and vulvar cancer.
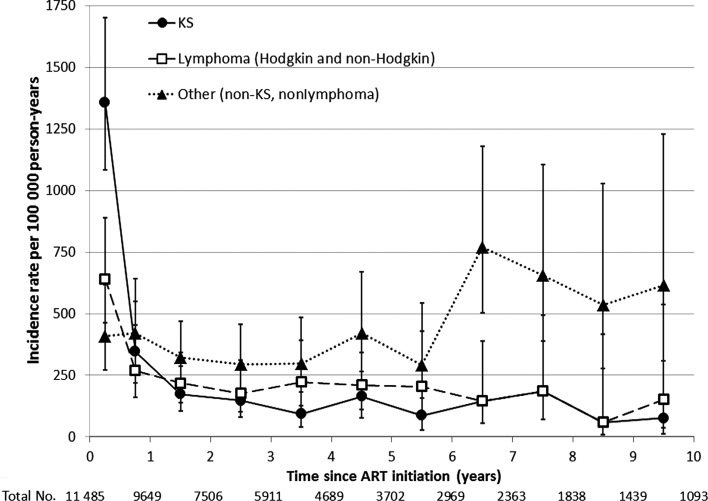
The timing of cancer incidence after ART initiation differed between cancer types. The incidence of KS was particularly high within the first 6 months after ART initiation (IR, 1342 cases per 100 000 person-years [95% CI, 1071–1683 cases per 100 000 person-years]), but decreased dramatically thereafter (IR for second 6 months, 348 per 100 000 person-years [95% CI, 219–552 cases per 100 000 person-years]), and stabilized at a low rate (IR for 6 months–10 years, 164 per 100 000 person-years [95% CI, 129–208 cases per 100 000 person-years]) (Table 2, Figure 1). The incidence of lymphomas (both Hodgkin and non-Hodgkin) was also higher in the first 6 months after ART initiation (IR, 660 per 100 000 person-years [95% CI, 479–912 cases per 100 000 person-years]) compared to 6–12 months after ART initiation (IR, 269 per 100 000 person-years [95% CI, 160–455 cases per 100 000 person-years]), but the absolute change in incidence was smaller than for KS. By contrast, the incidence of other cancers appeared to increase with time after ART initiation. The incidence of all non-KS, non-lymphoma cancers combined increased by 7% each year from ART initiation (95% CI, 2%–13%; P = .009), from 416 to 615 cases per 100 000 person-years in years 1 and 10 after ART initiation, respectively.
Figure 1.
Incidence of first cancer across time following initiation of combination antiretroviral therapy (ART), Centers for AIDS Research Network of Integrated Clinical Systems, 1996–2011. After ART initiation, incidence rates were estimated in the first 6 months, the second 6 months, and every year thereafter. The vertical lines extending from each incidence rate estimate represent the 95% confidence interval. Listed below the x-axis are the total numbers of patients remaining in follow-up at the end of each year. Solid line with circles, Kaposi sarcoma incidence; dashed line with squares, lymphoma incidence; dotted line with triangles, incidence of non-Kaposi sarcoma, nonlymphoma cancers. Abbreviations: ART, combination antiretroviral therapy; KS, Kaposi sarcoma.
Predictors of Cancer Incidence and Time Modification of Predictors
Several characteristics at the time of ART initiation were associated with subsequent cancer incidence. Older age increased risk for all cancers except KS in bivariable analyses, and associations appeared the same in multivariable analyses (Table 3). Nonlymphoma, HPV-unrelated NADCs were most strongly associated with older age (adjusted hazard ratio [AHR] for 10-year difference in age, 2.33 [95% CI, 1.97–2.74]). Associations of older age with cancer risk were consistent throughout time following ART initiation (P for interaction with time >.05 for all cancers).
Table 3.
Patient Characteristics at Combination Antiretroviral Therapy Initiation Associated With Incidence of First Cancer Stratified by Cancer Type, Centers for AIDS Research Network of Integrated Clinical Systems, 1996–2011
| Characteristic | Hazard Ratio (95% CI) |
|
|---|---|---|
| Bivariable | Multivariablea | |
| Kaposi sarcoma | ||
| Age at ART initiation (per 10-y increase) | 1.00 (.84–1.19) | 0.99 (.82–1.21) |
| Calendar year of starting ART | 1.03 (.99–1.07) | 1.01 (.97–1.07) |
| Prior mono- or dual therapy | 1.00 (.70–1.43) | 1.34 (.91–1.99) |
| CD4 count, per 100 cells/mm3 | 0.64 (.56–.73) | 0.63 (.54–.73) |
| Viral load, per log10 copies/mL | 1.79 (1.42–2.27) | 1.31 (1.01–1.70) |
| Lymphomas | ||
| Age at ART initiation, per 10-y increase | 1.29 (1.07–1.55) | 1.30 (1.07–1.58) |
| Calendar year of starting ART | 0.98 (.93–1.03) | 0.97 (.91–1.02) |
| Prior mono- or dual therapy | 0.95 (.64–1.41) | 0.86 (.55–1.33) |
| CD4 count, per 100 cells/mm3 | 0.81 (.72–.91) | 0.80 (.71–.91) |
| Viral load, per log10 copies/mL | 1.07 (.85–1.36) | 0.85 (.65–1.11) |
| HPV-related cancers | ||
| Age at ART initiation, per 10-y increase | 1.33 (.99–1.79) | 1.41 (1.04–1.93) |
| Calendar year of starting ART | 0.96 (.88–1.04) | 1.00 (.91–1.10) |
| Prior mono- or dual therapy | 2.33 (1.32–4.13) | 2.67 (1.41–5.04) |
| CD4 count, per 100 cells/mm3 | 0.83 (.69–0.99) | 0.80 (.65–0.97) |
| Viral load, per log10 copies/mL | 1.13 (.78–1.65) | 1.05 (.69–1.61) |
| Nonlymphoma, HPV-unrelated NADCs | ||
| Age at ART initiation, per 10-y increase | 2.34 (2.01–2.74) | 2.33 (1.97–2.74) |
| Calendar year of starting ART | 1.01 (.96–1.06) | 1.00 (.95–1.06) |
| Prior mono- or dual therapy | 1.19 (.85–1.69) | 1.15 (.78–1.70) |
| CD4 count, per 100 cells/mm3 | 0.99 (.91–1.07) | 0.99 (.89–1.09) |
| Viral load, per log10 copies/mL | 0.95 (.77–1.18) | 0.93 (.73–1.19) |
Abbreviations: ART, combination antiretroviral therapy; CI, confidence interval; HPV, human papillomavirus; NADC, non-AIDS-defining cancer.
a Multivariable analyses adjusted for other covariates listed as well as Centers for AIDS Research Network of Integrated Clinical Systems study site, sex/men who have sex with men, race, and injection drug use.
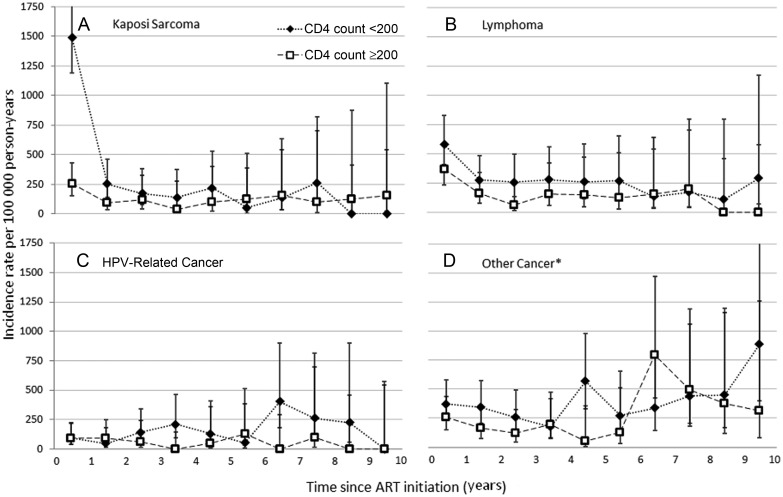
CD4 count at ART initiation was also an independent predictor of cancer. In both bivariable and multivariable analyses, lower CD4 count was associated with higher incidence of all cancer groups except the nonlymphoma, HPV-unrelated NADCs. Lower CD4 count was a particularly strong predictor of KS (AHR per 100 cells/mm3 increase, 0.63 [95% CI, .54–.73]). The pattern of a high incidence of KS within the first 6 months followed by a steep decline was only seen among those with a CD4 count <200 cells/mm3 at ART initiation (Figure 2, P for interaction of CD4 count with time = .002). By contrast, those with a CD4 count ≥200 cells/mm3 had low incidence of KS throughout time after ART initiation (IR, 0.1 per 100 person-years [95% CI, .1–.2 cases per 100 000 person-years]). Low CD4 count was also associated with high incidence of lymphomas and HPV-related cancers, but for these cancers, associations with CD4 count were consistent across time (both P for interaction with time >.20; Figure 2).
Figure 2.
Cancer incidence across time following initiation of combination antiretroviral therapy (ART) stratified by CD4 count at ART initiation, Centers for AIDS Research Network of Integrated Clinical Systems, 1996–2011. Graphs divided by cancer type: A, Kaposi sarcoma; B, lymphoma; C, human papillomavirus–related cancer; D, other cancers. Dotted lines with diamonds, incidence rates among those with CD4 counts <200 cells/mm3 at ART initiation; dashed lines with squares, incidence rates among those with CD4 counts ≥200 cells/mm3 at ART initiation. *Other cancer includes lung, liver, prostate, breast, melanoma, colorectal, kidney, bladder, brain, esophagus, larynx, leukemia, multiple myeloma, ovary, pancreas, peritoneum, small intestine, soft tissue, stomach, testicular, uterus, or non–squamous cell oral cavity/pharynx. Abbreviations: ART, combination antiretroviral therapy; HPV, human papillomavirus.
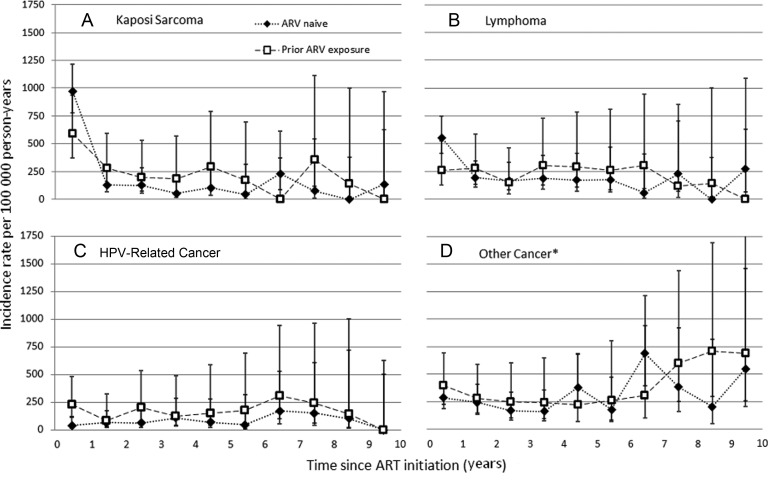
Prior exposure to antiretrovirals was associated with higher incidence of HPV-related cancer following ART initiation. This association persisted even when nadir CD4 count was included in the model in place of CD4 count at ART initiation. Across all follow-up time, prior antiretroviral exposure was not associated with incidence of other cancers. However, patients with prior antiretroviral exposure had a lower incidence of lymphoma compared to antiretroviral-naive patients in the first year following ART initiation (AHR, 0.41 [95% CI, .19–.93]), but a higher incidence after 1 year (P for interaction with time <.05; Figure 3).
Figure 3.
Cancer incidence across time following initiation of combination antiretroviral therapy (ART) stratified by antiretroviral history at ART initiation, Centers for AIDS Research Network of Integrated Clinical Systems, 1996–2011. Graphs divided by cancer type: A, Kaposi sarcoma; B, lymphoma; C, human papillomavirus–related cancer; D, other cancers. Dotted lines with diamonds, incidence rates among those who were antiretroviral naive at ART initiation; dashed lines with squares, incidence rates among those with prior exposure to mono- or dual therapy or an unknown antiretroviral history at ART initiation. *Other cancer includes lung, liver, prostate, breast, melanoma, colorectal, kidney, bladder, brain, esophagus, larynx, leukemia, multiple myeloma, ovary, pancreas, peritoneum, small intestine, soft tissue, stomach, testicular, uterus, or non–squamous cell oral cavity/pharynx. Abbreviations: ART, combination antiretroviral therapy; ARV, antiretroviral; HPV, human papillomavirus.
Other characteristics examined were weaker predictors of cancer incidence. Higher level of HIV RNA pre-ART was associated with higher KS incidence after adjusting for CD4 count, but not with any other cancer. No statistically significant associations were found between calendar year of ART initiation and incidence of any cancer in bivariable analysis. After accounting for variables such as age and CD4 count at ART initiation, there was no association between calendar year and any cancer (Table 3). The lack of association was robust to parameterization of calendar year as a continuous or categorical variable.
In sensitivity analysis where 489 patients with cancer diagnoses prior to ART initiation were excluded, similar incidence rates and incidence trends were observed and the same predictors of cancer incidence were identified (data not shown). Similarly, our findings were consistent in a subset analysis conducted among 8278 patients who were antiretroviral naive at ART initiation. In this subset, cancer incidence rates and trends were similar to the full study population, and factors associated with higher risk were also consistent in both unadjusted and adjusted analyses (data not shown).
DISCUSSION
This study of 11 485 patients from a multisite US clinical cohort of HIV-infected patients revealed distinct patterns of cancer incidence following ART initiation. This likely reflects varying etiologic contributions of aging, immunosuppression, and prior antiretroviral exposure, to the occurrence of specific cancer types. By contrast, cancer incidence did not appear to change over calendar time among ART initiators, suggesting that the incremental improvements in ART regimens during the modern ART era have not had dramatic effects on cancer incidence.
The most dramatic change in incidence after ART initiation was seen for KS, which had a higher incidence than all other cancers combined in the first 6 months and a steep decline thereafter. Notably, this pattern was seen exclusively in those initiating ART with a CD4 count <200 cells/mm3. A similar pattern has been noted in ART initiators in the Swiss HIV Cohort Study [13]. The HIV/AIDS Cancer Match Study found that severe immunosuppression at AIDS diagnosis was strongly associated with risk of a new KS diagnosis in the 4–9 months after AIDS diagnosis and less strongly thereafter [14]. This finding and prior research demonstrating higher KS incidence among those with lower current CD4 counts [8, 15–17] are consistent with an early risk driven by more severe immunosuppression. In addition to severe immunosuppression increasing the risk of KS development, these individuals are more likely to experience more rapid immune reconstitution that may unmask previously subclinical KS, a phenomenon referred to as immune reconstitution inflammatory syndrome (IRIS) [18–21].
Lymphomas showed a pattern similar to KS, but with a lower incidence in the first 6 months and a more gradual decrease in incidence thereafter. A decrease in incidence after 6 months was seen for both non-Hodgkin lymphoma and Hodgkin lymphoma. Individuals with no prior antiretroviral exposure and those with lower CD4 counts at ART initiation had higher lymphoma incidence within the first 6 months. Similar to KS, these patient groups may be more likely to develop lymphoma-associated IRIS events after ART initiation. An IRIS effect for lymphoma was hypothesized after a study in France showed a similar incidence pattern when looking at Hodgkin lymphoma after ART [22]. It is also possible that early symptoms of subclinical KS or lymphoma may have led to the diagnosis of HIV and prompt initiation of ART, with documentation of the definitive cancer diagnosis shortly after starting ART. Although 1 lymphoma cases and 9 KS cases were observed within the first week after ART initiation, these early cases do not completely account for the higher incidence in the first 6 months. Regardless, these findings highlight the importance of early HIV diagnosis and timely ART initiation before individuals reach advanced immunosuppression.
All other cancers combined showed an increasing trend over time following ART initiation with a lower incidence within the first 6 months and a significant trend toward higher incidence over time. This increase is likely a consequence of increasing cancer incidence with advancing age, as noted in the general population. Within this group of cancers that excluded KS and lymphoma, HPV-related cancers showed a strong association with CD4 count at ART initiation and higher incidence in those with prior ARV exposure, which may reflect longer duration of HIV infection. As HIV infection can increase the risk of HPV infection, lag time may occur before the increased risk is manifested as increased cancer diagnosis rates in patients with a longer duration of HIV infection [23–26]. It is also possible that adjustment for nadir CD4, a risk factor for HPV-associated cancers [7, 9, 26, 27], was inadequate if patients with prior antiretroviral exposure experienced their nadir CD4 before entry into a CNICS clinic. We did not observe an association between CD4 count at ART initiation and incidence of cancers that are neither AIDS-defining or associated with viral infections. Others have shown some virus-unrelated NADCs to be associated with the extent of immunosuppression [16, 28]. A larger sample size may be needed to observe an association between low CD4 count and virus-unrelated NADCs; however, our data suggest that the relationship is not particularly strong.
Previous studies have described changing trends in cancer incidence within the HIV population as a whole [5, 7, 29]; however, in our population of ART initiators no changes in cancer incidence were observed over calendar time. Our study included more recent calendar years of follow-up than previous studies, and 75% of patients initiated ART in the year 2000 or later. Trends identified in the larger HIV population are likely indicative of increased uptake of ART, whereas more recent changes in the potency or durability of first-line ART regimens [30] may have little impact on cancer incidence. This emphasizes the continued need for cancer screening and prevention measures in the HIV population, regardless of continued improvements in ART. For instance, increased HPV vaccination and anal pap smear screening may help prevent anal cancer, one of the most common malignancies in this population.
Our sample size was considerable; however, there were not enough incident cancer cases to conduct separate analyses for all specific cancer types. Although we aimed to categorize cancers in etiologically meaningful ways, there may be differing trends for cancers grouped together that were undetectable in our study, particularly within the heterogeneous group of nonlymphoma, non-HPV-related NADCs. Additionally, even within specific cancer types, malignancies may have different etiologic origins. Such is the case with oral cavity/pharynx cancers and non-Hodgkin lymphomas, in which only a proportion are linked to viral coinfection (HPV and Epstein-Barr virus, respectively). Despite this limitation, such groupings were necessary to discern meaningful trends and associations and can be used to guide more detailed analyses in the future.
This study was notable for the following strengths: (1) a large and representative HIV-infected clinical population; (2) detailed laboratory and antiretroviral information; and (3) well-validated cancer diagnoses. The current study does not account for changes or discontinuations in ART after initiation, nor does it account for differences in the immunologic or virologic response to ART. In a future study we will examine how these changes after ART initiation may impact cancer incidence to expand on these findings and further our understanding of cancer incidence trends over time in this population.
We showed distinct patterns of cancer incidence after ART initiation that differed by cancer type and were modified by several baseline patient characteristics. These findings highlight the importance of cancer screening and cancer prevention efforts throughout the course of HIV clinical care for those cancers for which evidence-based interventions exist. Currently, robust cancer screening and prevention guidelines have not been established that address the specific needs of HIV-infected individuals. Within the first year after ART initiation, KS and lymphomas are the largest sources of cancer morbidity. After the first year of ART initiation, HPV-associated cancer (particularly anal cancer) and other NADCs become more common. These results suggest that screening for HPV-associated cancers and certain NADCs should be prioritized once patients are on stable ART.
Notes
Acknowledgments. We thank the patients, principal investigators, coinvestigators, and research staff at participating CFAR Network of Integrated Clinical Systems sites at the following institutions: Case Western Reserve University; University of Alabama at Birmingham; University of California at San Francisco; University of Washington; University of California at San Diego; Fenway Community Health Center of Harvard University; University of North Carolina at Chapel Hill; and Johns Hopkins University. In particular, we thank Peggie Griffith of the Data Management Core at the University of Washington and Donna Porter of the Administrative Core at the University of Alabama at Birmingham for their assistance.
Financial support. This work was supported by the National Institutes of Health (5T32AI007001-35 to E. L. Y., R24 AI067039, and P30-AI50410). The funding sources did not participate in the study design; collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Potential conflicts of interest. S. N. has received grant support from Pfizer, Bristol-Myers Squibb, and Merck. J. J. E is a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, ViiV, and Janssen, and has received institutional research support from GlaxoSmithKline, Bristol-Myers Squibb, and Merck. All other authors report no potential conflicts.
The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 2.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–62. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–30. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 4.Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis. 2010;51:957–62. doi: 10.1086/656416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103:416–22. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pipkin S, Scheer S, Okeigwe I, Schwarcz S, Harris DH, Hessol NA. The effect of HAART and calendar period on Kaposi's sarcoma and non-Hodgkin lymphoma: results of a match between an AIDS and cancer registry. AIDS. 2011;25:463–71. doi: 10.1097/QAD.0b013e32834344e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 8.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:491–9. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao C, Leyden WA, Xu L, et al. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS. 2012;26:2223–31. doi: 10.1097/QAD.0b013e32835935b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–55. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschi S, Maso LD, Rickenbach M, et al. Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. Br J Cancer. 2008;99:800–4. doi: 10.1038/sj.bjc.6604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 15.Petoumenos K, van Leuwen M, Vajdic C, et al. Cancer, immunodeficiency and antiretroviral treatment: results from the Australian HIV Observational Database (AHOD) HIV Med. 2013;14:77–84. doi: 10.1111/j.1468-1293.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–9. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst Monogr. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe HW, De Stavola BL, Carpenter LM, Porter K, Cox DR. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS. 2011;25:1395–403. doi: 10.1097/QAD.0b013e3283489c8b. [DOI] [PubMed] [Google Scholar]
- 19.Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J Clin Oncol. 2005;23:5224–8. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 20.Feller L, Anagnostopoulos C, Wood NH, Bouckaert M, Raubenheimer EJ, Lemmer J. Human immunodeficiency virus-associated Kaposi sarcoma as an immune reconstitution inflammatory syndrome: a literature review and case report. J Periodontol. 2008;79:362–8. doi: 10.1902/jop.2008.070225. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis. 2012;54:424–33. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanoy E, Rosenberg PS, Fily F, et al. HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood. 2011;118:44–9. doi: 10.1182/blood-2011-02-339275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23:875–85. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bower M, Powles T, Newsom-Davis T, et al. HIV-associated cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr. 2004;37:1563–5. doi: 10.1097/00126334-200412150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crum-Cianflone NF, Hullsiek KH, Marconi VC, et al. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24:535–43. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piketty C, Selinger-Leneman H, Grabar S, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22:1203–11. doi: 10.1097/QAD.0b013e3283023f78. [DOI] [PubMed] [Google Scholar]
- 28.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116:5306–15. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 29.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 30.Willig JH, Abroms S, Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–60. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]