Abstract
We describe two cases of fungal granulomatous interstitial nephritis (GIN) presenting as acute kidney injury (AKI). Increased serum creatinine was detected in Patient 1 after chemotherapy for pharyngeal cancer and in Patient 2 after steroid pulse therapy for bronchial asthma. Renal histology of both patients revealed GIN. Polymerase chain reaction (PCR)-based detection of fungal DNA sequences from kidney tissue demonstrated Trichosporon laibachii and Candida albicans, respectively. When AKI occurs in an immunocompromised host, differential diagnosis of fungal interstitial nephritis should be considered. Furthermore, PCR-based detection of fungal DNA sequences from renal specimens can be useful for rapid diagnosis.
Keywords: acute kidney injury, fungal granulomatous interstitial nephritis, polymerase chain reaction, trichosporon
Introduction
Granulomatous interstitial nephritis (GIN) is a rare histological diagnosis found through biopsy of native and transplanted kidneys [1–6]. Although the more common causes are drug therapy (9–45%) and sarcoidosis (9–29%) [1–5], many published studies have demonstrated that GIN can be induced by infection. Mycobacteria and fungal infections have been reported as the main infectious causes of GIN, especially in immunocompromised patients [1, 2, 5, 6, 7–16]. In such patients, GIN was reported to be one of the causes of acute kidney injury (AKI) [7–10, 11–15]. The identification of the causative agents of invasive fungal infections is critical for guiding antifungal therapy. However, the current culture-based phenotypic methods are insensitive and slow.
We report two cases of GIN presenting as AKI. Polymerase chain reaction (PCR)-based detection of fungal DNA sequences from kidney tissue revealed Trichosporon laibachii and Candida albicans, respectively.
Case reports
Patient 1
Clinical history
A 73-year-old Japanese man was admitted to the otolaryngology department for the treatment of pharyngeal cancer. Laboratory tests revealed a serum creatinine level of 0.8 mg/dL (71 µmol/L) and an estimated glomerular filtration rate of 72 mL/min/1.73 m2 (1.2 mL/s/1.73 m2) with no abnormality in urinalysis.
Six courses of a chemotherapy regimen including docetaxel (28 mg/day) and cisplatin (7 mg/day) were given over 5 days, and, concomitantly, a total of 68 Gy of radiation was administered during 2 months. Patient's serum creatinine was 0.9 mg/dL (80 µmol/L) at the end of chemotherapy. One month after the completion of chemotherapy and radiation therapy, the patient's body temperature rose to 40°C. Although chest X-ray showed no consolidation in the lungs, antibiotics (piperacillin sodium and teicoplanin) were started because sputum cultures were found to be positive for Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. Two days later, the patient was referred to our nephrology department because of elevated serum creatinine and oliguria. Laboratory results were: creatinine, 8.1 mg/dL (716 µmol/L); serum albumin, 2.1 g/dL (21 g/L); and C-reactive protein (CRP), 11.1 mg/dL. The white blood cell count was 14.3 × 103/µL (14.3 × 109/L) with 70% neutrophils. The hemoglobin level was 8.2 g/dL (82 g/L). The serum level of β-d-glucan was high (>600 pg/mL); however, antigen titers for Cryptococcus (the Eiken latex agglutination test; Eiken, Tokyo, Japan), Aspergillus (Platelia ELISA kit; Fujirebio, Tokyo, Japan) and Candida (Platelia ELISA kit; Fujirebio, Tokyo, Japan) were negative.
Urinalysis showed no proteinuria, 5–9 red blood cells/high-power field and 5–9 white blood cells/high-power field. Abdominal computed tomography (CT) showed markedly enlarged kidneys bilaterally. The central venous line via the internal jugular vein was removed because catheter infection was suspected. Blood and urine cultures were negative; however, culture of the tip of the central venous catheter was positive for C. albicans. Piperacillin and teicoplanin were continued and fluconazole was added for C. albicans. Hemodialysis was started 1 week later, and a biopsy of the patient's left kidney was performed 10 days later after consultation.
Histologic findings of kidney biopsy
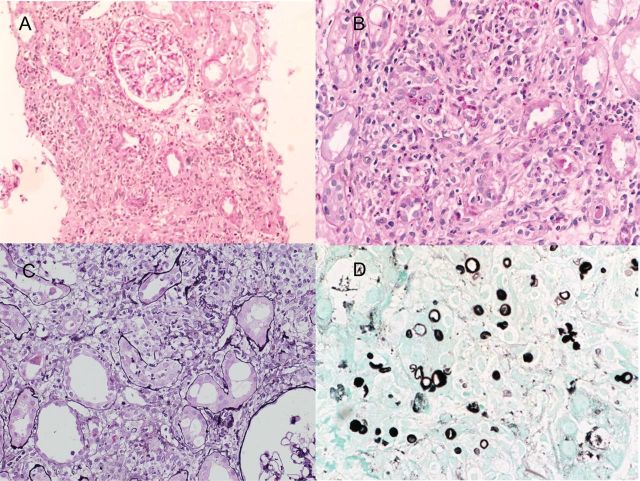
Microscopic findings showed severe interstitial cell infiltration and fibrosis with granulomatous formation. Inflammatory cells included mononuclear, epithelioid and giant cells. Most of the glomeruli were highly collapsed. Grocott's methenamine silver (GMS) stain showed multiple small, rounded, yeast-like features in the interstitium (Figure 1).
Fig. 1.
(A) Low-power view showing diffuse and severe interstitial cell infiltration. Glomeruli are well preserved [periodic acid-Schiff (PAS), original magnification ×150]. (B and C) Low-power view showing granulomatous interstitial inflammation with mononuclear and epithelioid cells. In the interstitium, there were focal isolated multinucleated Langhans giant cells (PAS and periodic acid silver methenamine [PASM], original magnification ×200). (D) High-power view of GMS stain showing multiple small, rounded yeast-like features in the interstitium. These organisms were diagnosed as Trichosporon laibachii by PCR-based detection (GMS, original magnification, ×400).
Patient 2
Clinical history
A 23-year-old Japanese man was admitted to another hospital for a severe bronchial asthma attack. The patient had a medical history of asthma for more than 10 years; however, no medication had been prescribed. On the day of admission, as his respiratory status worsened, he was intubated and mechanical ventilation was started. Methylprednisolone pulse therapy (1 g/day for 3 days) was administered. Two weeks after admission, the patient's renal function deteriorated and hemodialysis was initiated. Abdominal CT demonstrated enlargement of both kidneys. At the same time, the patient's body temperature rose to 37.5°C. Antifungal therapy (fluconazole, 400 mg on alternate days) was started because C. albicans was detected by blood culture. One month after admission, the patient was transferred to our hospital.
Physical examination showed a body temperature of 38.0°C and blood pressure of 133/66 mmHg. Coarse crackles were auscultated in both lungs. Eye examination showed no endophthalmitis and no abnormalities of fundus. Laboratory results were: creatinine, 3.0 mg/dL (265 µmol/L); serum albumin, 1.3 g/dL (13 g/L); and CRP, 13.8 mg/dL. The white blood cell count was 17.7 × 103/µL (17.7 × 109/L) with 72% neutrophils. The hemoglobin level was 7.1 g/dL (71 g/L). The serum level of β-d-glucan was high (>1200 pg/mL), and a titer of Candida antigen was positive. Urinalysis showed 3 (+) proteinuria, too many red blood cells/high-power field and 50–99 white blood cells/high-power field. Abdominal CT showed markedly enlarged kidneys bilaterally. Chest X-ray demonstrated diffuse consolidations and pleural effusions in both lungs. Echocardiography revealed no valvular abnormalities, including vegetation. Sputum and blood cultures were positive for P. aeruginosa. Antibiotics (imipenem/cilastatin, 0.25 g/day) were started. The antifungal medication was changed from fluconazole to amphotericin B because of prolonged elevation of β-d-glucan (>1200 pg/mL). One month after admission, the patient was withdrawn from mechanical ventilation. Despite improvement of the pulmonary infection, the decline in renal function and elevation of β-d-glucan continued. Two months after admission, CT showed multiple low-density areas in both kidneys. Scintigraphy with gallium citrate Ga67 showed strong radionuclide uptake in both kidneys. These findings were considered multiple renal abscesses. Three months after admission, we decided to perform bilateral nephrectomy because eradication of organisms was regarded impossible without the removal of the whole tissue.
Histologic findings of removed kidneys
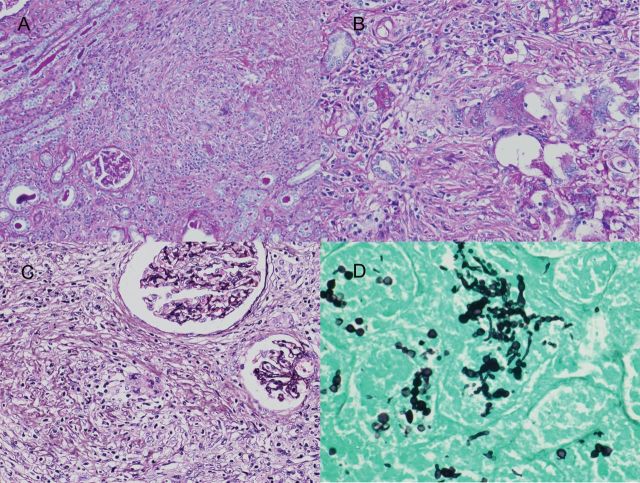
Macroscopic inspection of the patient's removed kidneys found multiple abscesses, mainly in the subcapsular cortical area. Microscopic examination revealed severe interstitial cell infiltration and fibrosis with granulomatous formation. Inflammatory cells included lymphocytes, plasma cells and multinucleated histiocytes. Most of the glomeruli were highly collapsed. The GMS stain showed multiple small, rounded, yeast-like features and branching filamentous features in the interstitium, including at the center of the abscess formation (Figure 2).
Fig. 2.
(A) Low-power view showing diffuse and severe interstitial cell infiltration (PAS, original magnification ×100). (B and C) Low-power view showing granulomatous interstitial inflammation with mononuclear and epithelioid cells. There were focal isolated multinucleated Langhans giant cells in the interstitium. Glomeruli are collapsed (PAS and periodic acid silver methenamine, original magnification ×200). (D) High-power view of GMS stain showing multiple small, rounded yeast-like features and branching filamentous features in the interstitium. These organisms were diagnosed as Candida albicans by PCR-based detection (GMS, original magnification, ×400).
Molecular biologic analysis of cases
An appropriate area of formalin-fixed, paraffin-embedded tissue (FFPE) blocks was selected aligned with GMS stain and a 2 mg of segment was excised and minced using a clean disposable scalpel. DNA was extracted using Genomic DNA purification Kit (Qiagen GmbH, Hilden, Germany). The internal transcribed spacer (ITS) region was amplified with the PCR assay using the universal primer set of the ITS region of the fungi [17]. Nucleotide sequence analysis and a BLAST (Basic Local Alignment Search Tool) search of the European Molecular Biology Laboratory (EMBL) and GenBank® databases revealed 100% homology with T. laibachii in Patient 1 and C. albicans in Patient 2.
Discussion
GIN is a rare histological diagnosis that is discovered in <1% of biopsied native and transplanted kidneys [1–6]. GIN caused by infection accounts for 6.6% of the total cases of GIN [1–5]. Mycobacteria and fungi are the main etiologies of the infections. Occurrences of GIN secondary to fungal infections, including Histoplasma, Candida and Cryptococcus, have been found primarily in immunocompromised patients and have been described in a few reports [1, 2, 5–16].
The identification of the causative agents of invasive fungal infections is critical for guiding antifungal therapy. However, the current culture-based phenotypic methods are insensitive and slow, requiring considerable expertise for correct morphological identification of less common or unusual fungi [18]. While histopathologic examination can prove the presence of invasive fungal infections by demonstrating fungal elements, identification of the specific fungus at the genus or species level based on morphologic characteristics is limited [19]. In recent years, PCR techniques performed with deparaffinized tissue sections have been used to try to improve the detection and identification of pathogens [20, 21]. PCR-based detection of fungal DNA sequences can be rapid, sensitive and specific. The ITS regions of fungal ribosomal DNA are highly variable sequences of great importance in distinguishing fungal species by PCR analysis. In previous studies [20, 21], the yield of PCR and sequencing in identifying fungal DNA from FFPE tissue specimens has ranged from 60 to 68%.
We have encountered two patients with GIN presenting as AKI. In Patient 1, a high serum level of β-d-glucan and a positive catheter culture for C. albicans suggested invasive Candida infection. However, PCR-based detection of fungal DNA sequences from the patient's kidney tissue revealed T. laibachii. To our knowledge, this is the first report of T. laibachii causing GIN. In this case, there is a conflict concerned with positive central venous catheter tip culture of C. albicans and positive PCR results of T. laibachii in renal tissues. Although renal biopsy specimens were repeatedly examined by PCR many times, only DNA sequence of T. laibachii, not C. albicans, was detected. These results demonstrate that both organisms would have existed; one was C. albicans of catheter origin and the other was T. laibachii of unknown origin. Unfortunately, this patient died from pneumonia of unknown origin, despite strong antifungal therapy including amphotericin B. In Patient 2, a high serum level of β-d-glucan and a positive blood culture for C. albicans also suggested invasive Candida infection. Accordingly, PCR-based detection from the patient's removed kidneys demonstrated C. albicans. This patient recovered from sepsis caused by C. albicans and continued regular hemodialysis at an outpatient clinic. Basically, repeated cultures of specimens, including blood, sputum, urine and others or tests of serum antigen detection, are gold standards for the diagnosis of fungal infection. PCR-based detection of fungal DNA sequences is not always easy in a clinical setting because it requires a molecular biological technique. Moreover, contamination of other species of fungus does not completely prevent. However, such trial to detect causative organisms should be made in order to rescue immunocompromised patients.
In summary, we have reported two cases of fungal GIN presenting as AKI. DNA sequencing of the PCR products from biopsied and resected kidney tissue demonstrated concordance with T. laibachii and C. albicans, respectively. When AKI occurs in an immunocompromised host, differential diagnosis of fungal interstitial nephritis should be considered. Furthermore, PCR-based detection of fungal DNA sequences from renal specimens can be useful for rapid diagnosis and treatment.
Conflict of interest statement
None declared.
Acknowledgements
We thank Dr Yoshihiko Kameoka and Tsutomu Koyama (Department of Emergency Medicine, The Jikei University Kashiwa Hospital) for extraordinary clinical contribution to Case 2. We also thank Dr Sadayori Hoshina (Department of Laboratory Medicine, The Jikei University School of Medicine) and Dr Yutaka Yamaguchi (Yamaguchi's Pathology Laboratory) for appropriate advice for molecular biological methods and pathological diagnosis.
References
- 1.Mignon F, Méry JP, Mougenot B, et al. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp. 1984;13:219–245. [PubMed] [Google Scholar]
- 2.Viero RM, Cavallo T. Granulomatous interstitial nephritis. Hum Pathol. 1995;26:1347–1353. doi: 10.1016/0046-8177(95)90300-3. doi:10.1016/0046-8177(95)90300-3. [DOI] [PubMed] [Google Scholar]
- 3.Bijol V, Mendez GP, Nosé V, et al. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol. 2006;14:57–63. doi: 10.1177/106689690601400110. doi:10.1177/106689690601400110. [DOI] [PubMed] [Google Scholar]
- 4.Joss N, Morris S, Young B, et al. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007;2:222–230. doi: 10.2215/CJN.01790506. doi:10.2215/CJN.01790506. [DOI] [PubMed] [Google Scholar]
- 5.Pasquet F, Chauffer M, Karkowski L, et al. Granulomatous interstitial nephritis: A retrospective study of 44 cases. Rev Med Interne. 2010;31:670–676. doi: 10.1016/j.revmed.2010.04.012. doi:10.1016/j.revmed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Meehan SM, Josephson MA, Haas M. Granulomatous tubulointerstitial nephritis in the renal allograft. Am J Kidney Dis. 2000;36:E27. doi: 10.1053/ajkd.2000.17735. doi:10.1053/ajkd.2000.17735. [DOI] [PubMed] [Google Scholar]
- 7.Ahuja TS, Remmers A, Rajaraman S, et al. Acute renal failure in a patient with AIDS: histoplasmosis-induced granulomatous interstitial nephritis. Am J Kidney Dis. 1998;32:E3. doi: 10.1053/ajkd.1998.v32.pm10074611. [DOI] [PubMed] [Google Scholar]
- 8.Nasr SH, Koscica J, Markowitz GS, et al. Granulomatous interstitial nephritis. Am J Kidney Dis. 2003;41:714–719. doi: 10.1053/ajkd.2003.50143. doi:10.1053/ajkd.2003.50143. [DOI] [PubMed] [Google Scholar]
- 9.Adams AL, Cook WJ. Granulomatous interstitial nephritis secondary to histoplasmosis. Am J Kidney Dis. 2007;50:681–685. doi: 10.1053/j.ajkd.2007.06.022. doi:10.1053/j.ajkd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Qian Q, Humayun H, Humayun Y, et al. Granulomatous interstitial nephritis associated with disseminated histoplasmosis in an immunocompetent patient. Am J Kidney Dis. 2011;58:1018–1021. doi: 10.1053/j.ajkd.2011.08.022. doi:10.1053/j.ajkd.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Hill-Edgar AA, Nasr SH, Borczuk AC, et al. A rare infectious cause of renal allograft dysfunction. Am J Kidney Dis. 2002;40:1103–1107. doi: 10.1053/ajkd.2002.37092. doi:10.1053/ajkd.2002.37092. [DOI] [PubMed] [Google Scholar]
- 12.Chung S, Park CW, Chung HW, et al. Acute renal failure presenting as a granulomatous interstitial nephritis due to cryptococcal infection. Kidney Int. 2009;76:453–458. doi: 10.1038/ki.2008.494. doi:10.1038/ki.2008.494. [DOI] [PubMed] [Google Scholar]
- 13.David VG, Korula A, Choudhrie L, et al. Cryptococcal granulomatous interstitial nephritis and dissemination in a patient with untreated lupus nephritis. Nephrol Dial Transplant. 2009;24:3243–3245. doi: 10.1093/ndt/gfp293. doi:10.1093/ndt/gfp293. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay AG, Olesnicky L, Pirani CL. Acute tubulo-interstitial nephritis from candida albicans with oliguric renal failure. Clin Nephrol. 1985;24:310–314. [PubMed] [Google Scholar]
- 15.Benoit G, Mandalenakis N, Faucher C, et al. Candidemia and granulomatous tubulointerstitial nephritis. Nephrol Ther. 2008;4:278–279. doi: 10.1016/j.nephro.2008.03.008. doi:10.1016/j.nephro.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Ozdemir BH, Sar A, Uyar P, et al. Posttransplant tubulointerstitial nephritis: clinicopathological correlation. Transplant Proc. 2006;38:466–469. doi: 10.1016/j.transproceed.2005.12.050. doi:10.1016/j.transproceed.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 17.White TJ, Bruns T, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al., editors. PCR Protocols: A Guide to Methods and Applications. San Diego, CA: Academic Press; 1989. pp. 315–322. [Google Scholar]
- 18.Alexander BD, Pfaller MA. Contemporary tools for the diagnosis and management of invasive mycoses. Clin Infect Dis. 2006;43:S15–S27. doi:10.1086/504491. [Google Scholar]
- 19.Procop GW, Wilson M. Infectious disease pathology. Clin Infect Dis. 2001;32:1589–1601. doi: 10.1086/320537. doi:10.1086/320537. [DOI] [PubMed] [Google Scholar]
- 20.Bialek R, Konrad F, Kern J, et al. PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J Clin Pathol. 2005;58:1180–1184. doi: 10.1136/jcp.2004.024703. doi:10.1136/jcp.2004.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau A, Chen S, Sorrell T, et al. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J Clin Microbiol. 2007;45:380–385. doi: 10.1128/JCM.01862-06. doi:10.1128/JCM.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]