Abstract
Friend virus (FV) infection of mice induces the expansion and activation of regulatory T cells (Tregs) that dampen acute immune responses and promote the establishment and maintenance of chronic infection. Adoptive transfer experiments and the expression of Neuropilin 1 indicate that these cells are predominantly natural Tregs rather than virus-specific conventional CD4+ T cells that converted into induced Tregs. Analysis of Treg TCR Vβ chain usage revealed a broadly distributed polyclonal response with a high proportionate expansion of the Vβ5+ Treg subset, which are known to be responsive to endogenous retrovirus-encoded superantigens. In contrast to the major population of Tregs, the Vβ5+ subset expressed markers of terminally differentiated effector cells, and their expansion was associated with the level of the antiviral CD8+ T cell response rather than the level of FV infection. Surprisingly, the expansion and accumulation of the Vβ5+ Tregs was IL-2 independent but dependent upon TNFα. These experiments reveal a subset-specific Treg induction by a new pathway.
1 Introduction
CD4+ regulatory T cells (Tregs) are a subset of T cells with immunosuppressive properties essential for both the prevention of autoimmune diseases in healthy individuals (1) and the prevention of immunopathological damage during immune responses to infectious agents (2). Suppression of immune responses by Tregs can also delay or prevent microorganism clearance and facilitate persistence (3–7). A role for Treg-mediated immunosuppression during viral infections was first described in mice infected with Friend virus (FV) (8), and roles for Tregs in human infections with viruses such as HCV (9–13), HBV (14) and HIV (15–17) are well-documented. In the FV model, acute retroviral infection is associated not only with the expansion of immune cells necessary for the resolution of fulminant disease, but also with the expansion and activation of immunosuppressive CD4+, Foxp3+, regulatory T cells (18). This expansion and activation of Tregs dampens acute virus-specific immune responses, particularly CD8+ T cell responses (19–21), and contributes to the establishment and maintenance of long-term chronic FV infections (7).
To develop therapeutics to modulate Treg responses to viral infection, it is critical to fully understand the characteristics of the responding Tregs and the factors that induce them. Tregs are classified into two general categories based on their developmental lineage and protein expression patterns (22, 23). “Natural” Tregs (nTregs) are generated through selection on self antigens in the thymus and constitutively express the high affinity alpha chain of the IL-2 receptor (CD25)(24), neuropilin 1 (Nrp1) (25, 26), and forkhead transcription factor (Foxp3)(27–29). The other major class of Foxp3-expressing Tregs, termed “adaptive” or “induced” (iTregs), are generated from conventional T cells in the periphery in response to antigenic stimulation (30–33), inflammation and TGF-beta signaling (34–38), or oral antigen tolerization regimes (23, 39–41). Although the immunosuppressive T cells in the FV model were originally called “virus-induced” Tregs (8), this designation predated the current and common usage of the term “induced” and the lineage of the Tregs responding to FV infection has not been determined until now. We investigated this question using adoptive transfer experiments designed to detect either expansion of nTregs or conversion of conventional CD4+ T cells.
The breadth of the Treg response was investigated by examining the TCR Vβ chain usage of Tregs expanding during FV infection. It was recently shown that infection with lymphocytic choriomeningitis virus (LCMV) clone 13, which causes persistent infections, preferentially expanded Tregs using the TCR Vβ5 chain (42). Vβ5 usage by CD4+ T cells is revealing because these cells recognize known self-antigens, specific superantigens (Sags) encoded by endogenous mouse mammary tumor viruses (Mtvs) (43). When Vβ5+ TCR from conventional CD4+ T cells bind to certain MHC class molecules (eg. H-2Ab) complexed with Mtv Sags (eg. Mtv 8, 9) during thymic selection, they are deleted leaving very few Vβ5+ CD4+ T cells in the repertoire (43, 44). In contrast, Vβ5+ Tregs are relatively resistant to clonal deletion by endogenous superantigens during thymic selection (45). We found that during infection with FV, both Vβ5+ and Vβ5− Tregs expanded, but the expansion of Vβ5+ Tregs was proportionately higher in comparison to V5β − Tregs. Furthermore, we observed a number of significant differences between Vβ5+ and Vβ5− Treg subsets indicating that the Vβ5+ subset is activated and expands via distinct mechanisms.
Materials and Methods
Mice, viruses, and tissue harvest
Unless otherwise noted, mice were female (C57BL/10 x A.BY)F1 (abbreviated Y10) (H-2b/b, Fv1b, Rfv3r/s) bred at the Rocky Mountain Laboratories (RML) (Hamilton, MT) or C57BL/6 mice (abbreviated B6) purchased from Harlan, Germany or Jackson Laboratories. Mice were used between 8 and 24 weeks of age at the beginning of the experiments. For CD4+ adoptive transfer experiments, Foxp3 eGFP (hereafter called Foxp3GFP) mice (46) or Foxp3GFP mice carrying a TCR Vβ chain specific for an FV envelope epitope (47) (hereafter called CD4.TCR Tg.Foxp3GFP) mice were bred at RML. CD8+ T cell adoptive transfer experiments utilized TCR transgenic mice specific for the DbGagL FV epitope (48) expressing a Thy1.1+ congenic marker (hereafter called CD8.TCR Tg), which were bred at RML. The Friend virus (FV) stock used in these experiments was FV complex containing replication competent B-tropic Friend murine leukemia helper retrovirus (F-MuLV), replication defective polycythemia-inducing spleen focus-forming retrovirus, and no lactate dehydrogenase-elevating virus (LDV) (49). Mice were FV infected by i.v. injection of 0.5 ml phosphate-buffered balanced salt solution (PBBS) containing 6000 spleen focus-forming units (SSFU) of FV complex in Y10 mice and 20,000 SFFU in more resistant B6 mice. The LDV infection was given as a 1-to-50 dilution of sera injected i.v. in 0.1 ml PBBS, generated as previously described (49). Anesthetized mice were perfused with heparinized PBS to displace blood from the tissues. Hepatocytes were removed from liver homogenates using a 35% Percoll gradient. Euthanization was done by cervical dislocation of animals under deep isoflurane anesthesia. Mice were treated in accordance with the regulations and guidelines of the Animal Care and Use Committee of the Rocky Mountain Laboratories and the National Institutes of Health (Bethesda, MD) and of the Animal Care and Use Committee of the University of Essen and according to German animal rights law.
Surface and intracellular staining antibodies and flow cytometry
The antibodies (Abs) used for cell staining were purchased from BD Pharmingen, BioLegend or eBioscience, unless otherwise noted. The Abs used for surface staining were: Alexa700-, Pacific Blue-, Pacific Orange-anti-CD4; Pacific Blue-anti-CD8; Pacific Blue-or PerCP-Cy5.5-anti-Thy1.1 (CD90.1); APC-anti-neuropilin 1 (FAB566A) (R&D); Biotin-, FITC-or PE-anti-Vβ4, Vβ5, Vβ6, Vβ8, Vβ11, Vβ14; PerCP-Streptavidin; PerCP-Cy5.5-or PE-anti-CD69; PerCP-Cy5.5-, APC-or PE-anti-CD25; PerCP-anti-CD43; PE-anti-CD11a; APC-anti-KLRG1; and eFluor450-anti-CD127. Intracellular Foxp3 staining was performed according to the manufacturer’s recommendation using FITC-, PE-, PeCy7-, or Alexa700-anti-Foxp3 (FJK-16s) and the Foxp3 staining kit from eBioscience. For intracellular Ki-67 staining, cells were surfaced stained before fixation and permeabilization using reagents from the eBioscience Foxp3 kit and then stained with FITC-, PE-or PerCP-1 Cy5.5-anti-Ki-67. The five-to seven-color flow cytometric data were collected with an LSRII or FACSCanto II (BD Biosciences) and analyzed using FlowJo (Tree Star).
Infectious centers assay
Lymphocyte dilutions were plated onto susceptible Mus dunni cells (grown to approximately 20% confluency), incubated for 2 days at 37°C and 5% CO2, fixed with 95% ethanol, stained with F-MuLV envelope-specific mAb 720 overnight at 4°C and then developed with peroxidase-conjugated goat anti-mouse IgG and aminoethylcarbazole substrate (50). Foci were identified and counted.
Cell sorting, cell tracer labeling and adoptive transfers
For Treg conversion experiments, splenocytes from naïve Foxp3GFP or CD4.TCR Tg.Foxp3GFP mice were enriched using anti-CD4 paramagnetic beads and the Miltenyi MACS system following the manufacturer’s recommendations. Cells were then stained with Alexa700-or Pacific Orange-anti-CD4 and then sorted on a FACSAria (BD Biosciences) to greater than 95% pure populations of CD4+GFP− or CD4+GFP+ T cells. Cells from the CD4.TCR Tg.Foxp3GFP mice were additionally labeled with CellTrace™ violet (Invitrogen) following the recommendations. Between 7–19×106 CD4+GFP− cells and 1–2×106 CD4+GFP+ cells were transferred into Y10 recipients by i.v. injection in 0.5 ml PBBS. For the transfer of FV-specific CD8+ T cells, mice were adoptively transferred i.v. with 500, 5000, 50,000 or 500,000 Miltenyi MACS bead enriched cells from the spleens of naïve CD8.TCR Tg mice (51). Control mice were not given CD8+ T cells. The same day the recipients were infected with FV as described above.
CD8+ T cell depletions and blocking IL-2 or TNFα in vivo
Naïve mice were depleted of CD8+ T cells by three 0.5 ml intraperitoneal injections of approximately 300 μg anti-CD8 tissue culture supernatant (clone 169.4) given every other day. The mice were infected with FV 2 days following the third and final depletion treatment. Anti-CD8 treatment achieved greater than 98% depletion of CD8+ T cells in 0 the blood and spleen at 2 weeks post-infection (wpi) (data not shown). The mice were euthanized for analysis at 2wpi. For IL-2 blocking studies, Y10 mice were injected i.p. every other day starting from the time of FV infection with 50 μg each of functional grade JES6-5H4 (rat IgG2b) and JES6-1A12 (rat IgG2a) anti-IL-2 mAbs (BioXCell) until tissue harvest at 2 wpi. Control mice were concurrently given seven i.p. injections of 100 μg of Rat IgG. For TNFα blocking studies, B6 mice were injected i.p. every other day with 200 μg of functional grade XT3.11 (BioXCell) starting from the time of FV infection until tissue harvest at 12 days post-infection (dpi) for a total of 6 injections.
RT-PCR
Spleens from naïve and 1 wpi Y10 or B6 mice were first enriched for CD11c+ cells then CD19+ cells using two subsequent Miltenyi MACS bead enrichment procedures. Total RNA extraction was performed using the RNeasy kit (Qiagen) with DNase treatment according to the manufacturer’s instructions. cDNA was generated using the Superscript VILO cDNA Synthesis kit (Invitrogen) according to the manufacturer’s instructions. Semi-quantitative PCR for Mtv9 expression was performed using Platinum Quantitative PCR Supermix (Invitrogen) with forward primer (5′-CAACCGCAGTCAAAGAACAG-3′), reverse primer (5′-GCCACCACCTGTCTCCTATT-3′) and MGB probe (5′FAM-CAAGGACTATCGGCCACAGGCC-MGB-3′) with reaction conditions of 50°C for 2 min and 95°C for 2 min. followed by 40 cycles of 95°C for sec and 69°C for 30 sec. Semi-quantitative PCR for Mtv Sag expression was performed using the SYBR GreenER qPCR Supermix (Invitrogen) with forward primer (5′-ATCGCCTTTAAGAAGGACGCCTTCT-3′) and reverse primer (5′-GCAAAGCAGAGCTATGCC-3′) (42) with reaction conditions of 50°C for 2 min and 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 69°C for 1 min. These reactions were performed in quadruplicate on an ABI 7900 HTS Real Time PCR System (Applied Biosystems). Ct values were collected for GAPDH and Mtv genes during the log phase of the cycle. Expression levels were normalized to GAPDH for each sample (ΔCt = Ct gene of interest-Ct GAPDH). PCR efficiencies (10(−1/slope)-1) where slope = log [template] by Cq) for GAPDH from naïve mice was 1.18, GAPDH from 7 dpi was 1.08; for Mtv9 from naïve mice was 1.49, for Mtv9 from 7dpi mice was 1.38; for Mtv (42) from naïve mice was 0.75, and Mtv from 7 dpi mice was 0.90. The slopes of the curves were similar with no statistically significant differences. All data used were from less than 40 rounds of amplification. No Mtv or Mtv9 PCR amplification was detectable using templates from control Mtv null mice (52).
Results
FV infection expands natural Tregs
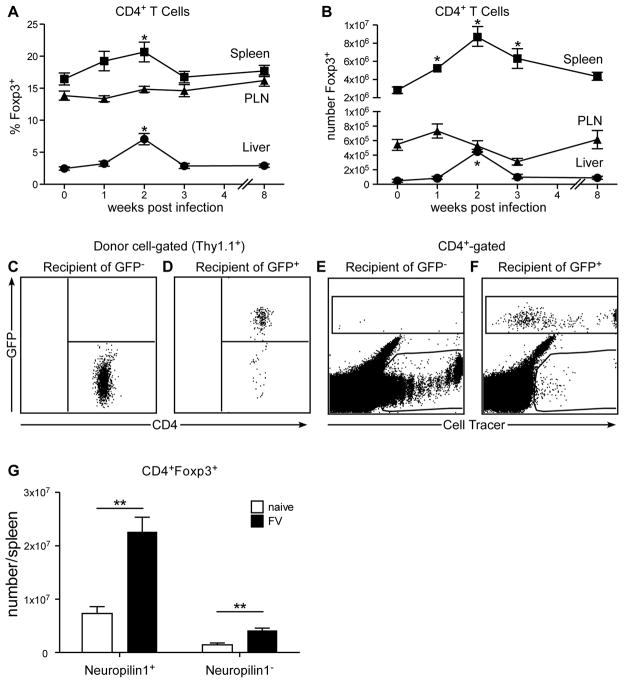
To confirm previous results (20), acute FV infection induced the expansion of CD4+, Foxp3+ regulatory T cells (Tregs) as indicated by increases in both the proportions (Fig. 1A) and absolute numbers (Fig. 1B) of these cells in spleen and liver peaking at two weeks post-infection. The spleen and liver are two organs heavily infected by FV. In contrast, peripheral lymph nodes, which do not get heavily infected with FV or infiltrated with virus-specific lymphocytes (20), displayed no significant expansion of Tregs (Figure 1A, B). Thus, the induction of Tregs correlated with the tissue specificity of FV infection.
Figure 1. FV infection induces expansion of natural Tregs.
Kinetics of the change in A) percent and B) absolute number of CD4+ T cells expressing Foxp3+ cells in the spleen (squares), peripheral lymph nodes (PLN) (triangles) and liver (circles) following FV infection. Bars represent the mean and standard error of the mean (SEM) of 13–17 mice from 7 independent experiments per time point. The statistically significant increases from naïve to post-FV infection in the spleen and liver are indicated (*) (One-Way ANOVA w/Dunnett’s multiple comparisons post-test). Mice received an adoptive transfer of FACS sorted C) Thy1.1+ CD4+ GFP− or D) Thy1.1+ CD4+ GFP+ cells from naïve Foxp3GFP reporter mice and recipients were infected with FV. At 14 dpi the spleens of recipient mice were analyzed by flow cytometry for GFP expression in Thy1.1+ donor cells. Dot plots are representative of 3 independent experiments. In a similar experiment, mice were infected with FV and given CellTrace™ violet-labeled E) CD4+ GFP− or F) CD4+ GFP+ cells from naïve CD4.TCR Tg.Foxp3GFP reporter mice. At 7 dpi the spleens were analyzed by flow cytometry for GFP and CellTrace™ violet expression. At 2wpi, splenic Tregs were analyzed for expression of G) surface Neuropilin 1 and absolute numbers of each subset were calculated on a per spleen basis. Differences between naïve and infected were statistically different: 320% increase for Nrp1+ Tregs, P = .0064 (unpaired t test).
To determine whether the Tregs that expanded after FV infection originated from thymus-derived natural Foxp3+ cells (nTregs) or converted into Tregs from Foxp3− conventional CD4+ T cell precursors in the periphery (iTregs), Foxp3GFP reporter mice were utilized for adoptive transfer experiments. In these reporter mice, all Foxp3+ Tregs express GFP (46). Genetically labeled (Thy1.1+) CD4+ T cells from the spleens of the reporter mice were FACS sorted to obtain >95% pure CD4+GFP− or CD4+ GFP+ cells, which were then adoptively transferred into recipients infected with FV at the time of transfer. At 2 wpi no detectable conversion of GFP negative donor cells into GFP positive cells was observed (Figure 1C). Thus the Foxp3 promoter was silent in the conventional CD4+ donor T cells indicating that conversion to Tregs had not occurred during FV infection. As a positive control for GFP expression in Tregs, GFP+ (Foxp3+) donor cells were also transferred. Since Tregs are a small subset, fewer cells were transferred, but their GFP signal was easily detectable at 2 wpi (Figure 1D). To maximize the chances of observing conversion of FV-specific conventional CD4+ T cells into Tregs during infection, the above experiment was repeated using CD4.TCR Tg.Foxp3GFP reporter mice expressing an FV-specific CD4+ T cell receptor (TCR) beta chain transgene (47). These mice do not have an FV-specific TCRα transgene, but they possess a polyclonal repertoire with an expanded component of FV-specific CD4+ T cells, about 50% of which express Vα2 (47). Before transfer the donor cells were labeled to measure their in vivo proliferation by fluorescent label dilution. At 1-week post-FV infection there was significant proliferation of conventional (GFP−) CD4+ T cells in response to FV infection (Figure 1E), but again there was no conversion to GFP expression. It was previously shown in this transgenic model that the Treg repertoire was devoid of TCRs reactive with FV (47), and this was confirmed in our experiments (data not shown). Despite exclusion of FV-specific TCRs from donor Tregs, the adoptively transferred GFP+ Tregs showed significant proliferation in response to FV infection (Figure 1F). This result suggested that factors other than virus-specificity induced nTreg expansion.
To confirm that FV infection induced nTreg, we used Neuropilin 1 (Nrp1), which was recently described as a marker distinguishing natural Tregs from induced Tregs (25, 26). Small numbers of Nrp1− Tregs were found in naive mice, and a slight albeit significant expansion of these cells was observed following FV infection (Figure 1G, right). However, the vast majority of Treg expansion occurred in the Nrp1+ nTreg subpopulation (Figure 1G, left). Together with the adoptive transfer experiments, the results indicate that the expansion of Tregs in response to FV infection was predominantly due to the proliferation of nTregs rather than the conversion of conventional CD4+ T cells into iTregs.
Expansion of Vβ5+Tregs during acute FV infection
To examine the breadth of T cell receptor usage by Tregs during FV infection, CD4+ Foxp3+ T cells from spleens were analyzed for expression of 14 different TCR Vβ chains at peak expansion (two weeks post-FV infection). These 14 Vβ’s represented ~70% of all TCR Vβ chains used by CD4+ Tregs in both naïve and infected mice. Expansion of all TCR Vβ chains tested was observed in response to FV infection (Fig. 2A). Tregs expressing Vβ5 had the largest proportional increase at 2 wpi (Fig. 2B) and showed the largest fold increase in absolute cell numbers (6.5 fold, Fig. 2C), representing 6.7% of the total Treg expansion in the spleen. The remaining Vβ5− Tregs increased approximately three fold (Fig. 2D). Vβ5+ Treg expansion was even more striking in the liver where the increase was over 30 fold (Fig. 2E) compared to 8.6 fold for the Vβ5− Tregs (Fig. 2F). In contrast to FV infection and previous results with LCMV infection (42), infection of mice with Lactate dehydrogenase-elevating virus (49) caused relatively little expansion of Vβ5+ Tregs (Fig. 2G). Thus large increases in Vβ5+ Tregs are not induced by all viral infections in mice.
Figure 2. Expansion of Tregs in spleen following FV infection.
A) The absolute numbers and B) proportions of TCR Vβ subsets expressed by CD4+ Foxp3+ Tregs from the spleens of naïve mice (white bars) and FV-infected mice (2wpi) (black bars). Bars represent the mean and standard deviation from 5 mice. The calculated absolute numbers of C) Vβ5+ and D) Vβ5− CD4+Foxp3+ Tregs in the spleens and E) Vβ5+ and F) Vβ5− Tregs in the livers of naïve (white bars) and FV infected mice (black bars) at 0, 1, 2, 4 and 8 wpi. G) The kinetics of CD4+ Foxp3+ T cells expressing Vβ5 from the spleens (squares) and livers (circles) following LDV infection. Data are the mean and SEM of mice from 5 independent experiments. For Panel A, all Treg subsets have significant increases in absolute number (P < 0.05 by t test). For Panel B, only the increase in Vβ5+ Tregs was significant (P < 0.05 by t test). For Panel G, a significantly greater proportion of Vβ5+ Tregs was observed after FV-infection compared to day 0 for the spleen where indicated (*) (One-Way ANOVA with Dunnett’s multiple comparisons post-test).
The phenotypes of Vβ5+and Vβ5− Tregs
To further characterize the phenotype and activation status of Vβ5+ and Vβ5− Tregs during FV infection, these subsets were analyzed by flow cytometry for dual expression of the early activation marker, CD69, and the cell proliferation marker, Ki-67. The proportion of CD69+ Ki-67+ cells in both Vβ5+ and Vβ5− Treg subsets from spleen and liver were significantly higher at 2 wpi compared to naïve mice (Figure 3A, B). Thus the expansion of Tregs in these organs was accompanied by activation and proliferation markers, indicating that the increased proportions of Tregs were not simply the result of redistribution of the cells due to trafficking.
Figure 3. Vβ5+ and Vβ5− Tregs express an activated phenotype following FV infection.
Dual expression of surface CD69 (activation) and intracellular Ki-67 (cell division) on A) Vβ5+ and B) Vβ5− CD4+Foxp3+ Tregs from the spleens (SPL) and livers (LIV) of naïve (white bars) and FV-infected (2 wpi) (black bars) mice. Data are the mean and SEM of 13–15 mice. The percentage of C) Vβ5+ and D) Vβ5− CD4+Foxp3+ Tregs that express high levels of CD25 in naïve (white bars) and FV-infected (2 wpi) (black bars) mice. Data are the mean and SEM of 6–11 mice. All differences between naïve and FV-infected mice in panels A, B and C were significant (P < 0.05 by t test). E) Representative FACS dot plots showing the dual expression profiles of CD25 and intracellular Ki-67 on naïve and FV infected (2wpi) splenic CD4+Foxp3+ Tregs. Numbers represent the mean percentage and SEM of cells in each quadrant from 9–10 mice. F) The percentage positive (left panels) and mean fluorescent intensity (MFI) (right panels) of indicated markers on CD4+Foxp3+ Tregs from naïve and FV infected mice at 12 dpi. Data are from 7 independent experiments using from 5–17 mice (* P < 0.05, ** P < 0.01, ***P < 0.001, NS = not significant by One-Way ANOVA with Dunnett’s multiple comparisons post-test). G) Splenic Tregs were analyzed for expression of Neuropilin 1. Absolute numbers of each subset were calculated on a per spleen basis for Vβ5+ (left) and Vβ5− (right) Tregs in the spleens of naïve (white bars) and FV infected mice (black bars) at 2 wpi (** P < 0.01, ***P < 0.001 by unpaired t test).
IL-2 is critical for the survival and function of Tregs (53, 54) and the high affinity alpha chain of the IL-2R receptor (CD25) is constitutively expressed on nTregs. Consistent with previous results (55), the basal level of CD25 expression on Tregs showed tissue specific differences in naïve mice, with more CD25hi cells in the spleen compared to the liver (Fig. 3C, D). Interestingly, there were significantly lower proportions of CD25hi cells in the Vβ5+ subset from both spleen and liver at 2 wpi compared to naïve mice (Fig. 3C). Analysis of Ki-67 co-expression indicated that CD25 expression was significantly reduced in actively dividing (Ki-67+) splenic Tregs (Fig. 3E). Thus the decreased proportion of CD25hi cells in the Vβ5+ Treg subset was most likely a reflection of their active division during FV infection.
Multiparameter flow cytometric analysis was used to determine if any additional markers differentiated Vβ5+ and Vβ5− Treg subpopulations. Significantly higher proportions of the Vβ5+ Treg subset from infected mice expressed the activation marker CD43 (the activation-associated glycoform (56, 57)) and CD11a (a co-stimulatory signaling molecule upregulated upon antigen recognition (58)) compared to Vβ5− Tregs (Fig. 3F). Additionally more Vβ5+ Tregs had high expression of KLRGI (a marker for terminally5 differentiated effector T cells (59)) and decreased expression of CD127 (IL-7R, a marker for memory T cells (60)) (Fig. 3F). This expression pattern in the Vβ5+ Tregs is the phenotype of terminally-differentiated, short-lived effector T cells (61). Further, the Vβ5+ subset expressed Nrp1 (25, 26), indicating their nTreg origin (Fig. 3G). Thus both Vβ5+ and Vβ5− Tregs became activated during acute FV infection, yet the Vβ5+ subset appeared more differentiated, possibly due to stronger activation by Sag.
Lack of induction of Mtv sag in dendritic cells or B cells
It was recently reported that CD11c+ dendritic cells up-regulated Mtv sag expression during chronic LCMV Clone 13 infection, and that both Mtv sag expression and dendritic cells were required for LCMV-induced expansion of Vβ5+ Tregs (42). To determine if FV infection also up-regulated Mtv sag expression in dendritic cells, CD11c+ cells were enriched by magnetic bead columns and analyzed for Mtv sag mRNA expression by semi-quantitative RT-PCR. Two sets of primers were used to amplify Mtv transcripts relevant to Vβ5 TCR binding: one set was specific for Mtv9 sag (Fig. 4A); the other set had more broad Mtv sag reactivity, and was identical to the primers used in the LCMV study (Fig. 4B). We examined the cells at 1 wpi since Treg expansion was already evident at 1 wpi (Fig. 1B), peaked at 2 wpi, and we reasoned that sag presentation would necessarily precede peak expansion. Mtv sag expression was detected in dendritic cells from uninfected control mice, but in contrast to LCMV Clone 13 infection, FV infection resulted in decreased sag mRNA expression relative to Gapdh (Fig. 4A, B). Purified B cells, which are also MHC class II positive and express endogenous Mtvs also lacked increased Mtv sag mRNA levels after FV infection (Fig. 4A, B). Total Mtv expression in unfractionated cells was also decreased following infection (data not shown). Thus, the mechanism involved in expansion of Vβ5+ Tregs during FV infection appeared to be different than that in LCMV Clone 13 infection.
Figure 4. The relative expression of Mtv is not up-regulated in FV-infected mice.

Expression of Mtv mRNA relative to Gapdh mRNA in cells that were bead-enriched for CD11c+ (DC’s) or CD19+ (B cells). Cells were enriched from spleens of naïve (white bars) and FV-infected (1wpi) (black bars) Y10 mice using A) primers designed specifically for the Mtv9 Sag region or B) intragenic env and LTR sequence primers as previously described (42). Bars represent data from 4 mice run in quadruplicate with SEM. (* P < 0.05, ***P < 0.001 by unpaired t test).
CD8+ T cells drive the expansion of Vβ5+ Tregs
Treg expansion during FV infection occurs shortly after a massive activation and proliferation of virus-specific effector CD8+ T cells (18). Therefore, we determined if CD8+ T cell responses influenced Treg expansion. As an initial approach, CD8+ T cells were depleted from mice prior to FV infection, and splenic CD4+ Foxp3+ Tregs from non-depleted or depleted mice were analyzed at the time of peak expansion (2 wpi). At 2wpi splenic CD8+ T cells were depleted by > 98%. Interestingly, CD8 depletion abrogated the disproportionate expansion of the Vβ5+ Treg subset (Fig. 5A), and a significant loss rather than expansion of the Vβ5+ Treg subset was observed (Fig. 5B). By contrast, the expansion of the other Treg Vβ subsets analyzed at 2 wpi was not significantly affected by CD8 depletion (Fig. 5C).
Figure 5. CD8+ T cell depletion abrogates Vβ5+ Treg expansion during infection.
A) The percentage of splenic CD4+Foxp3+ Tregs expressing the indicated TCR Vβ chains in naïve (white bars), FV infected CD8 intact (black bars) or FV infected CD8 depleted (gray bars) mice at 2 wpi (* P < 0.05). The average absolute numbers of B) Vβ5+ and C) Vβ5− CD4+Foxp3+ Tregs per spleen from the CD8 intact (black bars) and CD8 depleted (gray bars) mice. D) Infectious centers assay data from the spleens of these mice. Data are the mean and SEM from 9 pooled mice combining 3 independent experiments. E) Expression of Mtv9 sag mRNA from bead-purified CD11c+ cells from the naïve (white bar), FV-infected (1 wpi) (black bar) or FV-infected/CD8+ depleted (gray bar) mice. Bars represent the mean and SEM from 4 individual B6 mice.
Since the CD8+ T cell response is critical for recovery from FV infection (62) the levels of infection were significantly increased in CD8-depleted mice (Fig. 5D). The lack of Vβ5+ Treg expansion in mice with high virus loads indicated that increased infection levels did not induce expansion of the Vβ5+ Treg subset. Increased virus loads also did not drive expansion of the non Vβ5+ Tregs (Fig. 5C). It is possible that deletion of CD8+ DCs had an effect in this experiment, but Mtv Sag expression in dendritic cells from CD8-depleted mice was equivalent to non-depleted mice (Fig. 5E). Furthermore, we have previously shown that this depletion regimen results in less than 50% depletion of CD8+ DCs (20). These results suggested that the Vβ5+ Treg subset was not only phenotypically distinct from the bulk population of Tregs, but appeared uniquely dependent on the CD8+ T cell response to induce its expansion.
To further examine the influence of CD8+ T cells on Vβ5+ Treg expansion we performed adoptive transfers of titrated numbers of FV-specific CD8+ T donor cells from CD8.TCR Tg mice (51) into FV-infected mice. Increasing the number of transferred cells resulted in higher levels of engraftment (Fig. 6A), and engraftment levels above 20% resulted in significantly decreased infection (Fig. 6B). In spite of decreased infection levels, the proportion of Tregs expressing Vβ5+ Tregs significantly increased (Fig. 6C). These results confirmed that the expansion of the Vβ5+ Treg subset required the CD8+ T cell response rather than high levels of FV infection.
Figure 6. FV-specific CD8+ T cells induce expansion of Vβ5+ Tregs during infection.

Mice were adoptively transferred with 0, 500, 5000, 50000, or 500000 bead purified CD8+ T cells from the spleens of naïve CD8.TCR Tg mice and then infected with FV. At 1 wpi A) the percentage of CD8+ T cells expressing the Thy1.1+ donor marker were analyzed and B) the infectious centers (IC) were determined from the spleens of these mice. C) Additionally, splenic CD4+Foxp3+ Tregs were analyzed for the proportions of Vβ5+ Tregs. Data are from 8–11 mice from 3 independent experiments. An asterisk indicates that data in the column are significantly different from the data in the no transfer control (P < 0.05 by One-Way Anova with Tukey’s post test for multiple comparisons).
Vβ5+ Treg expansion is IL-2 independent
A recent report defined a role for CD8+ T cells in promoting Treg expansion by production of IL-2 (63). To determine if the effect of CD8+ T cells on the Vβ5+ Treg subset was due to IL-2 production, IL-2 blocking antibodies were administered to mice for the first two weeks after infection. Since IL-2 is critical for the development and survival of Tregs (64–66) it was not surprising that blocking IL-2 function during the first two weeks of FV infection significantly decreased the absolute number of both total Tregs (Fig. 7A) and Vβ5− Tregs (Fig. 7B). Interestingly, the expansion of Tregs in the Vβ5+ subset was not significantly reduced when IL-2 was blocked (Fig. 7C). IL-2 blockade did not significantly alter FV infection levels (Fig. 7D), nor did it affect the expansion of activated (CD43+) CD8+ T cells (Fig. 7E). Moreover, the proportions of tetramer-positive CD8+ T cells specific for the H-2Db–restricted F-MuLV glycosylated Gag immunodominant epitope (48) were statistically equivalent at 2 wpi regardless of IL-2 blockade (data not shown). Thus, blocking IL-2 did not significantly reduce the number of activated CD8+ T cells at 2 wpi, and the CD8+ T cells induced Vβ5+ Treg expansion. Surprisingly, the expansion of the Vβ5+ subset of Tregs, in contrast to the overall Treg population, was uniquely independent of IL-2, possibly due to the distinct activation mechanisms initiated by Sag recognition (67–69).
Figure 7. Vβ5+ Treg expansion in FV infection is IL-2 independent.
A) The absolute number of CD4+ cells expressing Foxp3 in naïve mice (white bars), FV-infected mice (2 wpi) (black bars) and mice infected with FV and treated with IL-2 blocking antibody treatment (gray bars). The absolute number of splenic CD4+Foxp3+ T cells expressing B) Vβ5− and C) Vβ5+ at 2 wpi. D) Infection levels in the spleen as measured by infectious center assays. There was no significant difference between the groups by Student’s t test. E) The absolute number of activated (CD43+) CD8+ T cells in mice from treated and untreated groups as indicated.
Vβ5+ Treg expansion dependence on TNFα
To further investigate the mechanism of CD8+ T cell-controlled Vβ5+ Treg expansion, the roles of two CD8 effector molecules, perforin and granzyme B, were analyzed by infecting perforin-null and granzyme B-null mice with FV. Analysis of the Treg compartment for Vβ5+ Treg expansion following FV infection showed no significant effect in these knockout mouse strains (data not shown).
Tregs have been reported to express tumor necrosis factor receptor 2 (TNFRII), which has important consequences for their function (70). Since CD8+ T cells can be a significant source of TNFα, CD8+ T cell-production of this cytokine was analyzed by intracellular cytokine staining. Significantly higher numbers CD8+ T cells produced TNFα after FV infection than before infection (Fig. 8A). To examine the role of TNFα on FV-induced Treg induction TNFα blocking antibodies were administered to FV infected mice during the first two weeks of infection. Anti-TNFα treatments significantly reduced expression of the proliferation marker Ki-67 on Vβ5+ Tregs (Fig. 8B, left panel) yet produced no effect on the Vβ5− Tregs (Fig. 8B, right panel). Furthermore, blocking TNFα significantly reduced the proportion of fully differentiated (KLRG1+) Vβ5+ Tregs (Fig. 8C). Expansion of the Vβ5+ Tregs at 2 wpi was significantly diminished by TNFα blockade (Fig. 8D), although the treatment did not reduce levels completely back to the basal levels observed in naïve mice. Thus, the expansion of Vβ5+ Tregs induced by FV12 infection was partially dependent on TNFα. TNFα blockade also produced a significant increase in virus loads indicating an anti-viral function for this molecule (Fig. 8D). These results provide further evidence that viral titers were not the main driving force behind Vβ5+ Treg expansion.
Figure 8. Vβ5+ Treg expansion in FV infection is TNFα-dependent.
A) The absolute number of CD8+ T cells producing TNFα was calculated from the proportion of cells that produced TNFα in a 5 hour in vitro intracellular cytokine assay. Cells are from mice at 12 dpi. Expression of intracellular Ki-67 (B) and KLRG-1 (C) on Vβ5+ (left) and Vβ5− (right) CD4+Foxp3+ Tregs from the spleens of naïve mice (white bars), FV-infected mice (black bars) or FV-infected/anti-TNFα-treated mice (gray bars), respectively. D) The proportion of Vβ5+ Tregs in the spleens of the same mice. E) Infection levels in the spleens as measured by infectious center assays. Data are the means with SEMs from 2 independent experiments. (* P < 0.05, ** P < 0.01, ***P < 0.001 by unpaired t test)
Discussion
Our experiments provide several lines of evidence indicating that the expansion of Tregs during acute FV infection is due to the activation and proliferation of the existing pool of natural, presumably self-specific Tregs (71), rather than the conversion of conventional, virus-specific CD4+ T cells into iTregs. Expression of fourteen different T cell receptor Vβ chains was assayed, and all showed significant expansion (Fig. 2A). This result differed from the virus-specific, conventional CD4+ T cell population where no expansion of Vβ4+ T cells or disproportionate expansion of Vβ5+ cells was observed (data not shown). The present data as well as previous results (47) indicate that the Tregs responding to FV infection are not virus-specific and respond to different stimuli than conventional CD4+ T cells.
What drives Treg expansion if not viral antigen? One possibility is that the inflammatory response during viral infection is an important factor. We found that the expansion of the majority of Tregs was IL-2 dependent, but the Vβ5+ Treg subset was uniquely IL-2 independent (Fig. 7). Interestingly, our results indicate that the CD8+ T cell response to FV infection is a major factor driving expansion of the Vβ5+ Treg subset (Fig. 5, 6). A predominant Vβ5+ Treg response was recently observed in LCMV Clone 13 infection and was associated with increased expression of endogenous Mtv sag mRNA by dendritic cells (42). In FV infection increase of Mtv sag expression was not observed, either in dendritic cells or in B cells where Mtv expression is highest and inducible by LPS (72–75) (Fig 4A and B). Additionally, increased Mtv sag RNA levels were not observed in unfractionated splenocytes, T cells, erythroblasts, or non-dendritic cell fractions in repeated experiments (data not shown). Thus expansion of the Vβ5+ Treg subset in FV infection is not dependent on increased expression of Mtv sag, suggesting that basal expression levels are sufficient as a first signal stimulus to Vβ5+ Tregs. The lack of increased Mtv sag expression during FV infection may be a reason why infection with LCMV Clone 13 induced a stronger Treg response than FV, yielding approximately 25% Vβ5+ Tregs compared to about 7% Vβ5+ Tregs in FV infection
In the LCMV studies selective depletion of DCs by diphtheria toxin abrogated Vβ5+ Treg expansion and implicated DCs as important mediators of the Treg response. However, since LCMV-specific CD8+ T cell responses are dependent on DCs (76) the depletion of DCs would also block CD8+ T cell expansion, and possibly TNFα responses. In light of our current results demonstrating dependence of Vβ5+ Treg expansion on CD8+ T cell responses and TNFα, the effect of DC ablation on Vβ5+ Tregs in the LCMV study might have been indirect rather than direct.
Interestingly, only a minimal Vβ5+ Treg response was observed during LDV infection (Fig. 2G), although both LDV and FV produce comparable CD8+ T cell responses (49). LDV also induces polyclonal B cell activation (77). It is possible that some aspect of the immune response to LDV infection suppresses Vβ5+ Treg expansion, but FV/LDV co13 infections induce a strong Treg response (78) including disproportionate Vβ5+ Treg expansion (data not shown). Suppression of the response would be expected to be a dominant effect. Predominant cytokines produced by the CD8+ T cells might differ between these viral infections to affect the levels of Treg expansion. Further comparative studies will be needed to resolve this issue and reveal mechanisms of Vβ5+ Treg induction other than TNFα.
With regard to the involvement of CD8+ T cell-produced cytokines in driving Vβ5+ Treg expansion, it was recently reported that IL-2 production by CD8+ T cells can be important in promoting Treg expansion (63, 79). This role makes logical sense since IL-2 is produced by responding CD8+ T cells, and is important for the survival and activation of Tregs. When we blocked IL-2 with neutralizing antibodies, expansion of the bulk population of Vβ5− Tregs, which is not dependent on CD8+ T cells (Fig. 5C), was significantly reduced (Fig. 7B), whereas the Vβ5+ Tregs maintained their expansion (Fig. 7C). Thus, in FV infection the expansion of the bulk population of Vβ5− Tregs is dependent on IL-2 and independent of CD8+ T cells. In contrast, CD8+ T cell responses clearly affected expansion of the Vβ5+ Treg subset (Fig. 5,6), which responded in an IL-2 independent manner (Fig. 7). IL-2-independent expansion of Tregs is a unique finding that likely relates to the fact that Sag stimulation of T cells delivers a very potent signal through a mechanism distinct from cognate antigen recognition (67–69). Furthermore, bacterial Sags stimulate the expansion of T cells even in the absence of IL-2 (80). Thus our findings are consistent with a role for both Sag recognition and CD8+ T cell responses in the expansion of Vβ5+ Tregs. Sag recognition might provide the first induction signal and TNFα the second.
Previous reports demonstrated that Treg responses play a significant role in inhibiting immune responses to both acute and chronic FV infection (7, 19). It is now evident that this Treg response is complex, with at least two distinct subsets of responding cells with differing requirements for IL-2, TNFα and CD8+ T cells. Further experiments will be required to determine if the suppressive effect of the Vβ5+ Tregs is proportionate with its subpopulation size, or whether the unique activation mechanisms for these cells affect their function. Although blockade of TNFα partially prevented expansion of Vβ5+ Tregs, improved CD8+ T cell responses were not observed, and the FV infection was significantly worse (Fig. 8E). This result was likely due to loss of the pluripotent antiviral effects of TNFα.
In an effort to investigate the biological significance of Vβ5+ Tregs during FV infection, these cells were selectively depleted prior to infection using Vβ5-specific antibodies. Although antibody-mediated depletions were successful and effects were observed, significant numbers of Vβ5+ CD8+ T cells were depleted in addition to Vβ5+ Tregs, making interpretation of the results impossible. Thus the role of Vβ5+ Tregs in FV disease remains unclear. Nevertheless, endogenous retroviruses encoding Sags have been shown to influence susceptibility to diseases induced by both viral and bacterial pathogens (81). Thus, Vβ5+ Tregs may play a more dominant role in certain human and animal diseases. Our results suggest that the distinct mechanisms of induction of these cells would require different therapeutic modalities to control them.
Acknowledgments
This work was supported by the Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institute of Health, USA, the German Research Foundation (TRR60 Project B4 and GRK1045-2), and by the UK Medical Research Council (U117581330).
References
- 1.Sakaguchi S, Powrie F, Ransohoff RM. Re-Establishing Immunological Self-Tolerance in Autoimmune Disease. Nature Medicine. 2012;18:54–58. doi: 10.1038/nm.2622. [DOI] [PubMed] [Google Scholar]
- 2.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ Regulatory T Cells Control the Severity of Viral Immunoinflammatory Lesions. Journal of immunology. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 3.Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting Edge: Regulatory T Cells Prevent Efficient Clearance of Mycobacterium Tuberculosis. Journal of immunology. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 4.Kaparakis M, Laurie KL, Wijburg O, Pedersen J, Pearse M, van Driel IR, Gleeson PA, Strugnell RA. CD4+ CD25+ Regulatory T Cells Modulate the T-Cell and Antibody Responses in Helicobacter-Infected Balb/C Mice. Infect Immun. 2006;74:3519–3529. doi: 10.1128/IAI.01314-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-Like Receptor 2 Suppresses Immunity against Candida Albicans through Induction of Il-10 and Regulatory T Cells. Journal of immunology. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T Cell Responses Develop in Parallel to Th Responses and Control the Magnitude and Phenotype of the Th Effector Population. Journal of immunology. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 7.Dietze KK, Zelinskyy G, Gibbert K, Schimmer S, Francois S, Myers L, Sparwasser T, Hasenkrug KJ, Dittmer U. Transient Depletion of Regulatory T Cells in Transgenic Mice Reactivates Virus-Specific CD8+ T Cells and Reduces Chronic Retroviral Set Points. Proc Natl Acad Sci U S A. 2011;108:2420–2425. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ Regulatory T Cells Induced by Chronic Retroviral Infection. Proc Natl Acad Sci USA. 2001;98:9226–9230. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. T Cells with a CD4+CD25+ Regulatory Phenotype Suppress in Vitro Proliferation of Virus-Specific CD8+ T Cells During Chronic Hepatitis C Virus Infection. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T Cells Suppress in Vitro Proliferation of Virus-Specific CD8+ T Cells During Persistent Hepatitis C Virus Infection. J Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of Hcv-Specific T Cells without Differential Hierarchy Demonstrated Ex Vivo in Persistent Hcv Infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired Effector 24 Function of Hepatitis C Virus-Specific CD8+ T Cells in Chronic Hepatitis C Virus Infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald AJ, Duffy M, Brady MT, McKiernan S, Hall W, Hegarty J, Curry M, Mills KH. CD4 T Helper Type 1 and Regulatory T Cells Induced against the Same Epitopes on the Core Protein in Hepatitis C Virus-Infected Persons. J Infect Dis. 2002;185:720–727. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- 14.Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, Bertoletti A. Modulation of the CD8+-T-Cell Response by CD4+ CD25+ Regulatory T Cells in Patients with Hepatitis B Virus Infection. J Virol. 2005;79:3322–3328. doi: 10.1128/JVI.79.6.3322-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, Jackson R, O’Shea A, Roby G, Kovacs C, Connors M, Migueles SA, Fauci AS. CD25+ Regulatory T Cells Isolated from Hiv-Infected Individuals Suppress the Cytolytic and Nonlytic Antiviral Activity of Hiv-Specific CD8+ T Cells in Vitro. AIDS Res Hum Retroviruses. 2007;23:438–450. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- 16.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, Planta M, McGlaughlin M, Jackson R, Ziegler SF, Fauci AS. CD25(+)CD4(+) Regulatory T Cells from the Peripheral Blood of Asymptomatic Hiv-Infected Individuals Regulate CD4(+) and CD8(+) Hiv-Specific T Cell Immune Responses in Vitro and Are Associated with Favorable Clinical Markers of Disease Status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of Hiv-Specific T Cell Activity by Lymph Node CD25+ Regulatory T Cells from Hiv-Infected Individuals. Proc Natl Acad Sci U S A. 2007;104:3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. Kinetics of CD8+ Effector T Cell Responses and Induced CD4+ Regulatory T Cell Responses During Friend Retrovirus Infection. Eur J Immunol. 2006;36:2658–2670. doi: 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- 19.Zelinskyy G, Dietze K, Sparwasser T, Dittmer U. Regulatory T Cells Suppress Antiviral Immune Responses and Increase Viral Loads During Acute Infection with a Lymphotropic Retrovirus. PLoS Pathog. 2009;5:e1000406. doi: 10.1371/journal.ppat.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelinskyy G, Dietze KK, Husecken YP, Schimmer S, Nair S, Werner T, Gibbert K, Kershaw O, Gruber AD, Sparwasser T, Dittmer U. The Regulatory T-Cell Response During Acute Retroviral Infection Is Locally Defined and Controls the Magnitude and Duration of the Virus-Specific Cytotoxic T-Cell Response. Blood. 2009;114:3199–3207. doi: 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- 21.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, Evans LH, Peterson KE, Yang G, Hasenkrug KJ. Functional Impairment of CD8(+) T Cells by Regulatory T Cells During Persistent Retroviral Infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 22.Curotto de Lafaille MA, Lafaille JJ. Natural and Adaptive Foxp3+ Regulatory T Cells: More of the Same or a Division of Labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Bluestone JA, Abbas AK. Natural Versus Adaptive Regulatory Cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing Il-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 25.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 Distinguishes Natural and Inducible Regulatory T Cells among Regulatory T Cell Subsets in Vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ. Neuropilin 1 Is Expressed on Thymus-Derived Natural Regulatory T Cells, but Not Mucosa-Generated Induced Foxp3+ T Reg Cells. J Exp Med. 2012;209:1723–1742. S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T Cell Lineage Specification by the Forkhead Transcription Factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 29.Khattri R, Cox T, Yasayko SA, Ramsdell F. An Essential Role for Scurfin in CD4+CD25+ T Regulatory Cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 30.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of Regulatory T Cells with Known Specificity for Antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 31.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of Foxp3+ Regulatory T Cells in the Periphery of T Cell Receptor Transgenic Mice Tolerized to Transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 32.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25-T Cells Generate CD25+Foxp3+ Regulatory T Cells by Peripheral Expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 33.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential Development of Interleukin 2-Dependent Effector and Regulatory T Cells in Response to Endogenous Systemic Antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen ZM, O’Shaughnessy MJ, Gramaglia I, Panoskaltsis-Mortari A, Murphy WJ, Narula S, Roncarolo MG, Blazar BR. Il-10 and Tgf-Beta Induce Alloreactive CD4+CD25-T Cells to Acquire Regulatory Cell Function. Blood. 2003;101:5076–5083. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of Peripheral CD4+CD25-Naive T Cells to tory T Cells by Tgf-Beta Induction of Transcription Factor oxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting Edge: Tgf-Beta Induces a Regulatory Phenotype in CD4+CD25-T Cells through Foxp3 Induction and Down-Regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 37.Rao PE, Petrone AL, Ponath PD. Differentiation and Expansion of T Cells with Regulatory Function from Human Peripheral Lymphocytes by Stimulation in the Presence of Tgf-{Beta} J Immunol. 2005;174:1446–1455. doi: 10.4049/jimmunol.174.3.1446. [DOI] [PubMed] [Google Scholar]
- 38.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A Functionally Specialized Population of Mucosal Cd103+ Dcs Induces Foxp3+ Regulatory T Cells Via a Tgf-Beta and Retinoic Acid-Dependent Mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral Tolerance in the Absence of Naturally Occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills KH, McGuirk P. Antigen-Specific Regulatory T Cells--Their Induction and Role in Infection. Semin Immunol. 2004;16:107–117. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral Tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punkosdy GA, Blain M, Glass DD, Lozano MM, O’Mara L, Dudley JP, Ahmed R, Shevach EM. Regulatory T-Cell Expansion During Chronic Viral Infection Is Dependent on Endogenous Retroviral Superantigens. Proc Natl Acad Sci U S A. 2011;108:3677–3682. doi: 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blish CA, Gallay BJ, Turk GL, Kline KM, Wheat W, Fink PJ. Chronic Modulation of the Tcr Repertoire in the Lymphoid Periphery. immunology. 1999;162:3131–3140. 22. [PubMed] [Google Scholar]
- 44.Tomonari K, Fairchild S, Rosenwasser OA. Influence of Viral Superantigens on V Beta-and V Alpha-Specific Positive and Negative Selection. Immunol Rev. 1993;131:131–168. doi: 10.1111/j.1600-065x.1993.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 45.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T Cells: Expression of Il-2r Alpha Chain, Resistance to Clonal Deletion and Il-2 Dependency. Int Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 46.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector Th17 and Regulatory T Cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 47.Antunes I, Tolaini M, Kissenpfennig A, Iwashiro M, Kuribayashi K, Malissen B, Hasenkrug K, Kassiotis G. Retrovirus-Specificity of Regulatory T Cells Is Neither Present nor Required in Preventing Retrovirus-Induced Bone Marrow Immune Pathology. Immunity. 2008;29:782–794. doi: 10.1016/j.immuni.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Qin H, Chesebro B, Cheever MA. Identification of a Gag-Encoded Cytotoxic T-Lymphocyte Epitope from Fbl-3 Leukemia Shared by Friend, Moloney, and Rauscher Murine Leukemia Virus-Induced Tumors. J Virol. 1996;70:7773–7782. doi: 10.1128/jvi.70.11.7773-7782.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robertson SJ, Ammann CG, Messer RJ, Carmody AB, Myers L, Dittmer U, Nair S, Gerlach N, Evans LH, Cafruny WA, Hasenkrug KJ. Suppression of Acute Anti-Friend Virus CD8+ T-Cell Responses by Coinfection with Lactate Dehydrogenase-Elevating Virus. J Virol. 2008;82:408–418. doi: 10.1128/JVI.01413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson MN, Miyazawa M, Mori S, Caughey B, Evans LH, Hayes SF, Chesebro B. Production of Monoclonal Antibodies Reactive with a Denatured Form of the Friend Murine Leukemia Virus Gp70 Envelope Protein: Use in a Focal Infectivity Assay, Immunohistochemical Studies, Electron Microscopy and Western Blotting. Journal of virological methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 51.Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, Kokot NC, Lerner CG, Sather BD, Huseby ES, Greenberg PD. CD8+ T Cell Tolerance to a Tumor-Associated Antigen Is Maintained at the Level of Expansion Rather Than Effector Function. J Exp Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhadra S, Lozano MM, Dudley JP. Conversion of Mouse Mammary Tumor Virus to a Lymphomagenic Virus. Journal of Virology. 2005;79:12592–12596. doi: 10.1128/JVI.79.19.12592-12596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic Maintenance of Natural Foxp3(+) CD25(+) CD4(+) Regulatory T Cells by Interleukin (Il)-2 and Induction of Autoimmune Disease by Il-2 Neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers L, Messer RJ, Carmody AB, Hasenkrug KJ. Tissue-Specific Abundance of Regulatory T Cells Correlates with CD8+ T Cell Dysfunction and Chronic Retrovirus Loads. J Immunol. 2009;183:1636–1643. doi: 10.4049/jimmunol.0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AT, Federsppiel B, Ellies LG, Williams MJ, Burgener R, 20Duronio V, Smith CA, Takei F, Ziltener HJ. Characterization of the Activation-Associated Isoform of CD43 on Murine T Lymphocytes. J Immunol. 1994;153:3426–3439. [PubMed] [Google Scholar]
- 57.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic Regulation of T Cell Immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 58.McDermott DS, Varga SM. Quantifying Antigen-Specific CD4 T Cells During a Viral Infection: CD4 T Cell Responses Are Larger Than We Think. Journal of immunology. 2011;187:5568–5576. doi: 10.4049/jimmunol.1102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased Expression of the Nk Cell Receptor Klrg1 by Virus-Specific CD8 T Cells During Persistent Antigen Stimulation. Journal of Virology. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective Expression of the Interleukin 7 Receptor Identifies Effector CD8 T Cells That Give Rise to Long-Lived Memory Cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 61.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and Genomic Profiling of Effector CD8 T Cell Subsets with Distinct Memory Fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zelinskyy G, Myers L, Dietze KK, Gibbert K, Roggendorf M, Liu J, Lu M, Kraft AR, Teichgraber V, Hasenkrug KJ, Dittmer U. Virus-Specific CD8+ T Cells Upregulate Programmed Death-1 Expression During Acute Friend Retrovirus Infection but Are Highly Cytotoxic and Control Virus Replication. Journal of immunology. 2011;187:3730–3737. doi: 10.4049/jimmunol.1101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ Regulatory T Cells Control CD8+ T-Cell Effector Differentiation by Modulating Il-2 Homeostasis. Proc Natl Acad Sci U S A. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR. Il-2 Receptor Signaling Is Essential for the Development of Klrg1+ Terminally Differentiated T Regulatory Cells. Journal of immunology. 2012;189:1780–1791. doi: 10.4049/jimmunol.1103768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A Function for Interleukin 2 in Foxp3-Expressing Regulatory T Cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 66.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 Signaling Is Required for CD4(+) Regulatory T Cell Function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. Binding of Superantigen Toxins into the Cd28 Homodimer Interface Is Essential for Induction of Cytokine Genes That Mediate Lethal Shock. PLoS Biol. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamoyska R. Superantigens: Supersignalers? Sci STKE. 2006;2006:pe45. doi: 10.1126/stke.3582006pe45. [DOI] [PubMed] [Google Scholar]
- 69.Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, Tsoukas CD, McCormick JK, Madrenas J. Bacterial Superantigens Bypass Lck-Dependent T Cell Receptor Signaling by Activating a Galpha11–21 Dependent, Plc-Beta-Mediated Pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Oppenheim JJ. The Phenotypic and Functional Consequences of Tumour Necrosis Factor Receptor Type 2 Expression on 24 CD4(+) Foxp3(+) Regulatory T Cells. Immunology. 2011;133:426–433. doi: 10.1111/j.1365-2567.2011.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous Activation of Autoreactive CD4+ CD25+ Regulatory T Cells in the Steady State. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waanders GA, Lees RK, Held W, MacDonald HR. Quantitation of Endogenous Mouse Mammary Tumor Virus Superantigen Expression by Lymphocyte Subsets. Eur J Immunol. 1995;25:2632–2637. doi: 10.1002/eji.1830250934. [DOI] [PubMed] [Google Scholar]
- 73.Halpern MS, Ewert DL, Flores LJ, Lin KY, England JM. Endogenous Retroviral Envelope Antigen in Plasma Cells. Journal of immunology. 1981;127:698–702. [PubMed] [Google Scholar]
- 74.Alberto BP, Callahan LF, Pincus T. Evidence That Retrovirus Expression in Mouse Spleen Cells Results from B Cell Differentiation. Journal of immunology. 1982;129:2768–2772. [PubMed] [Google Scholar]
- 75.King LB, Lund FE, White DA, Sharma S, Corley RB. Molecular Events in B Lymphocyte Differentiation. Inducible Expression of the Endogenous Mouse Mammary Tumor Proviral Gene, Mtv-9. Journal of immunology. 1990;144:3218–3227. [PubMed] [Google Scholar]
- 76.Probst HC, van den Broek M. Priming of Ctls by Lymphocytic 42 Choriomeningitis Virus Depends on Dendritic Cells. Journal of immunology. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- 77.Plagemann PG, Rowland RR, Even C, Faaberg KS. Lactate Dehydrogenase-Elevating Virus: An Ideal Persistent Virus? Springer Semin Immunopathol. 1995;17:167–186. doi: 10.1007/BF00196164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C, Hasenkrug KJ. Cd137 Costimulation of CD8+ T Cells Confers Resistance to Suppression by Virus-Induced Regulatory T Cells. J Immunol. 2008;180:5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kish DD, Gorbachev AV, Fairchild RL. CD8+ T Cells Produce Il-2, Which Is Required for Cd(4+)CD25+ T Cell Regulation of Effector CD8+ T Cell Development for Contact Hypersensitivity Responses. J Leukoc Biol. 2005;78:725–735. doi: 10.1189/jlb.0205069. [DOI] [PubMed] [Google Scholar]
- 80.Jin H, Gong D, Adeegbe D, Bayer AL, Rolle C, Yu A, Malek TR. Quantitative Assessment Concerning the Contribution of Il-2rbeta for Superantigen-Mediated T Cell Responses in Vivo. Int Immunol. 2006;18:565–572. doi: 10.1093/intimm/dxh398. [DOI] [PubMed] [Google Scholar]
- 81.Bhadra S, Lozano MM, Payne SM, Dudley JP. Endogenous Mmtv Proviruses Induce Susceptibility to Both Viral and Bacterial Pathogens. PLoS Pathog. 2006;2:e128. doi: 10.1371/journal.ppat.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]