In a recent Opinion article Shen and Ji discussed the hypothesis that metabolites of curcumin, specifically vanillin and ferulic acid, could account for its striking poly-pharmacology as well as for the enigmatic low levels of curcumin in animal and human plasma upon dietary administration [1]. Testing of this hypothesis requires that the degradation products are positively identified such that the correct products are considered for activity. Here, we contest the widely held notion that vanillin, ferulic acid, and feruloylmethane are abundant products of the non-enzymatic degradation of curcumin.
The instability of curcumin in buffers of neutral-alkaline pH is well recognized [2–4]. A paper on characterization of the degradation products was published by Wang and coworkers in 1997 [5]. With >200 citations according to the Thomson Reuters Web of Knowledge the identification of vanillin, ferulic acid, and feruloylmethane as the products of non-enzymatic degradation of curcumin rests almost squarely on a single study [5]. But what has really been shown in this paper?
To begin with, Wang and co-workers explicitly state in the abstract that “vanillin, ferulic acid, feruloyl methane were identified as minor degradation products” (Quotations in this and the next paragraph are cited from [5].) This conclusion was based on RP-HPLC analyses of degradation reactions of curcumin in 0.1 M phosphate buffer pH 7.2 at 37°C for 2 h. The first HPLC solvent used gave a major peak at 2.4 min retention time that co-eluted with a standard of vanillin (Figs. 3A, B in [5]). Two small peaks (≈8% and ≈2% relative abundance) eluting at 3.3 min and 4.3 min were identified as ferulic acid and feruloylmethane based on comparison with the retention time of authentic standards. Using a second, less strongly eluting solvent and a longer HPLC column the authors found that “the vanillin peak separated into two peaks. A major unknown peak was eluted at the retention time just next to vanillin. Vanillin was the smaller and the unknown compound the larger peak (Fig. 3C, D)”. In this chromatogram ferulic acid appeared to be absent and feruloylmethane was only a very small and broad peak. Chromatography was monitored using a fixed-wavelength UV detector set at 280 nm, so the peak sizes are skewed in favor of vanillin and ferulic acid – whereas, as it turns out, the major product has only little absorbance at 280 nm and is underestimated. The authors performed additional GC-MS analyses that supported formation of the cleavage products but, again, no information on their abundance was given. Longer incubation of curcumin in buffer (12–24 h) shifted the balance toward a moderate increase of vanillin at the expense of the major product [5].
What about the major product? The authors characterized the product using LC-ESI-MS but found it “difficult to get large amount and high purity of this compound” for NMR analysis [5]. The product was thus tentatively identified as trans-6-(4′-hydroxy-3′-methoxyphenyl)-2,4-dioxo-5-hexenal based on a perceived molecular weight of 248 – even though the ESI-MS spectrum (Fig. 5A in [5]) showed several prominent ions at much higher m/z values. Well aware of their limited data the authors pointed out that “further studies are needed to confirm the unidentified components.” Thus, the paper by Wang [5] shows that vanillin, ferulic acid, and feruloylmethane are, at best, minor byproducts whereas the major degradation product of curcumin was not identified conclusively.
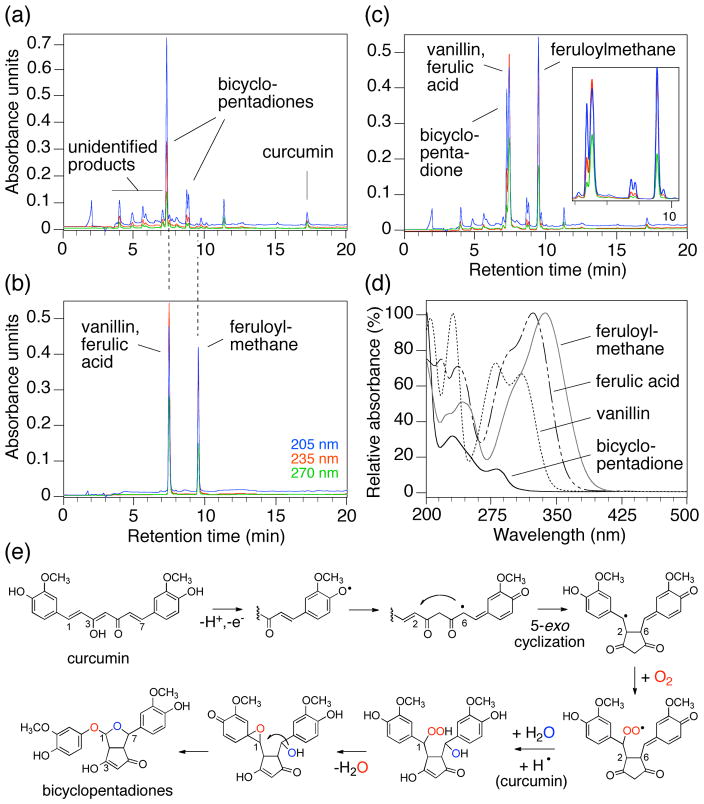
We have performed similar degradation reactions as described in [5] and analyzed the products using RP-HPLC with diode array detection (Fig. 1) [6]. One major peak was detected; vanillin, ferulic acid, and feruloylmethane were close to undetectable or absent. LC-ESI-MS analysis of the major product (and its configurational isomers) gave a molecular weight of 400, indicating insertion of oxygen (MW 32) during degradation of curcumin (MW 368) [6]. MS-MS experiments gave fragment ions identical to ions shown by Wang indicating that the major degradation products in [5] and our analyses were identical. We have isolated and identified this product using 1D and 2D homo- and heteronuclear NMR experiments. The product is a bicyclopentadione derivative of curcumin with two atoms of oxygen inserted into the heptadienone chain connecting the phenolic rings [6]. The same bicyclopentadione has been identified previously as the product of soybean lipoxygenase-catalyzed transformation of curcumin [7]. Although the mechanism of transformation was not elucidated at the time it has become clear that oxidative transformation is a major pathway of peroxidase- and oxygenase-mediated degradation of curcumin [6]. Using 14C-labeled curcumin we have identified several additional, highly polar metabolites that we are in the process of characterizing. Some of the more stable reaction intermediates we have isolated are the likely source for vanillin, formed by alternate degradation pathways during transformation to the bicyclopentadione (O.N. Gordon et al., unpublished).
Figure 1.
The degradation products of curcumin. (a) RP-HPLC analysis of a degradation reaction of curcumin (50 μM in phosphate buffer pH 7.5 for 2 h), (b) authentic standards of vanillin, ferulic acid (co-eluting), and feruloylmethane, (c) co-chromatography of the samples in (a) and (b). The inset shows an expanded view of the peaks eluting between 7 and 10 min. Chromatograms were recorded at 205 nm (blue), 235 nm (red), and 270 nm (green) and are shown in the same scale. RP-HPLC conditions were similar to [6]. (d) UV spectra of the bicyclopentadione, vanillin, ferulic acid, and feruloylmethane. (e) The proposed mechanism for the autoxidation of curcumin is initiated by H-abstraction from one of the phenolic hydroxyls. The phenoxyl radical moves into the carbon chain leaving a quinone methide that will eventually be quenched by addition of water from the buffer. The radical at C-6 performs a 5-exo-cyclization with the 1,2-double bond to give the cyclopentadione ring and locating the carbon-centered radical at C-1. Addition of molecular oxygen (O2) gives a peroxyl radical that is reduced to the hydroperoxide by abstracting a hydrogen from another curcumin molecule propagating the autoxidation chain reaction. The hydroperoxide loses water and rearranges to a spiro-epoxide; hydrolysis of the epoxide by the (water-derived) C-7 hydroxyl gives the final bicyclopentadione product. Only one of the two oxygens in the final product is derived from air, and the other is derived from water [6].
The autoxidative transformation of curcumin is a unique reaction among bioactive dietary polyphenols. Is it relevant for understanding its poly-pharmacology? Analyses of cultured RAW264.7 macrophage-like cells incubated with curcumin gave the bicyclopentadione as one of two major transformation products, the other being the reduced hexahydrocurcumin metabolite [6]. Certainly, for understanding the effects of curcumin in cultured cells, the products and intermediates resulting from autoxidative transformation should be considered as possible mediators of biological activity. The quinone methide intermediate, for example, is a highly reactive electrophilic species predicted to have strong propensity for reaction with protein cysteine residues [8]. Michael addition of this species with the regulatory Cys-179 of IκB kinase β could account for inhibition of the NF-κB pathway that is one of the major targets of the anti-inflammatory activity of curcumin [9].
The degradation of curcumin in aqueous buffer at physiological pH is an autoxidation. It is also the direct consequence of its action as an antioxidant. A radical chain reaction leads to the stable incorporation of oxygen into curcumin, resulting in the dioxygenated bicyclopentadione product [6]. Vanillin, ferulic acid, and feruloylmethane are not abundant degradation products of curcumin. They may very well be bioactive compounds in their own right [1], but should not be used to determine whether metabolites of curcumin account for its poly-pharmacology.
Acknowledgments
This work was supported in part by pilot awards to C.S. from the Vanderbilt Institute of Chemical Biology, the Vanderbilt DDRC (P30DK058404) and the NCI SPORE in GI Cancer (5P50CA095103). O.N.G. acknowledges support by training grant 2T32GM07628 from the National Institutes of Health.
References
- 1.Shen L, Ji HF. The pharmacology of curcumin: is it the degradation products? Trends Mol Med. 2012;18:138–144. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Tønnesen HH, Karlsen J. Studies on curcumin and curcuminoids. V Alkaline degradation of curcumin. Z Lebensm Unters Forsch. 1985;180:132–134. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- 3.Tønnesen HH, Karlsen J. Studies on curcumin and curcuminoids. VI Kinetics of curcumin degradation in aqueous solution. Z Lebensm Unters Forsch. 1985;180:402–404. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer E, et al. Studies on the stability of turmeric constituents. J Food Engin. 2003;56:257–259. [Google Scholar]
- 5.Wang YJ, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 6.Griesser M, et al. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J Biol Chem. 2011;286:1114–1124. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider C, et al. 2-[(4″-Hydroxy-3′-methoxy)-phenoxyl]-4-(4″-hydroxy-3″-methoxyphenyl)-8-hydroxy-6-oxo-3-oxabicyclo[3.3.0]-7-octene: unusual product of the soybean lipoxygenase-catalyzed oxygenation of curcumin. J Mol Catalysis B: Enzymatic. 1998;4:219–227. [Google Scholar]
- 8.Thompson DC, et al. Biological and toxicological consequences of quinone methide formation. Chem Biol Interact. 1993;86:129–162. doi: 10.1016/0009-2797(93)90117-h. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharm. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]