Abstract
JC virus DNA in the cerebrospinal fluid provides the laboratory confirmatory diagnosis of progressive multifocal leukoencephalopathy in patients whose clinical symptoms and MRI findings are consistent with PML. The LMMN, NIH made the confirmatory laboratory diagnosis in 35 MS patients treated with natalizumab. Thirteen patients had 3 or more CSF samples taken from weeks to months following PML diagnosis. Seven of the 13 patients demonstrated persistence of JCV DNA in the CSF even though all patients experienced IRIS, immune reconstitution inflammatory syndrome and 11 patients had plasma exchange and 2 had immunoabsorption. Specific anti-JCV antibody was measured in plasma/sera samples from 25 of the 35 patients. Most of the samples showed moderate to high or rising antibody levels from the time of PML diagnosis. However, plasma from 1 patient at or near the time of PML diagnosis had a titer considered seronegative and 2 other plasma samples from patients had titers considered at baseline for seropositivity. In several PML cases, viral persistence and neurological deficits have continued for several years indicating that once initiated, JCV infection may not entirely clear even with IRIS.
Immune modulatory therapies such as natalizumab (Tysabri@; Biogen/Idec.com) show an increased risk of PML compared with other immune altering treatments.1 As of June 7, 2010, BiogenIdec reports 55 cases of PML in MS patients on natalizumab with an approximate incidence of 1 per 1000, incidence increasing with dosing (medinfo.biogenidec.com). In a recent report, Clifford and colleagues outlined the clinical status in 28 cases describing immune reconstitution in most patients following plasma exchange (PLEX) recommending increased clinical vigilance and monitoring of patients beyond 3 years.2 It was anticipated that CNS inflammatory reactions (IRIS) in these patients would clear JCV infection since many of these patients had an ‘early’ diagnosis of PML. However, we analyzed CSF samples from 35 PML patients, (1 in the Sentinel trial3 and 34 post-marketing), that showed 7 of these patients sustained viral persistence in the CSF for weeks to months after PLEX and/or IA and IRIS. One of these cases4 has been followed with periodic, detectable viral DNA found in the CSF 3 years after PML diagnosis. Six patients’ CSF samples showed decreasing viral load with 4 resulting in undetectable viral load.
Methods
qPCR Assay for detection of viral DNA in CSF
The Laboratory of Molecular Medicine and Neuroscience, LMMN, established and maintains CLIA validated and certified real time, quantitative polymerase chain reaction assays, qPCR, for the detection of JC Virus genome in clinical samples.5 The assay targets a highly conserved region of the viral genome that codes for the amino terminal region of the multifunctional T protein, that is required for successful viral infection. The limit of detection of the assay is 10 copies of the viral genome per milliliter based on standards of viral DNA and testing samples that are available from the NIH6. CSF is sent to the LMMN frozen, thawed just prior to assay in which 200ul are used to extract and concentrate template to 25ul.7 Samples are sent to the LMMN from centers that treat patients on a voluntary basis. There is no protocol or study that requires testing at the LMMN. Consequently, not all samples from PML patients have been analyzed at the LMMN.
ELISA assay for the determination of specific antibodies to JC Virus
The viral VP1 gene that codes for the capsid protein has been cloned into a baculovirus vector that is used to infect insect cells for large scale production of the major capsid protein that self assembles into a virus like particle and to which both antibody and T cell responses are directed.8,9,10Purified recombinant VP1 is used to coat wells in plastic plates as the target antigen in standard ELISA format using 4 fold serial dilutions of serum or plasma.11 The titer of the antibody is reported as the dilution of the sample that achieves 0.05 absorption at 450nm optical density reading above controls and reported under CLIA process as well. Only IgG has been measured in the data reported here. The antibody titer has been benchmarked against the hemagglutination inhibition (HI) assay previously used to determine serological status.12 Antibody titers at ≥2560 are considered a baseline level for seropositive and those below i.e. ≥160-640, are considered seronegative.
Results
Viral DNA in CSF of MS patients with PML
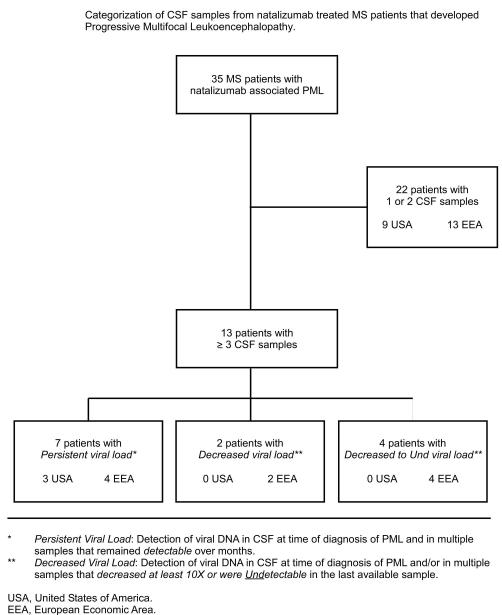
Figure 1 shows the distribution of 35 MS patients on natalizumab whose CSFs were assayed for viral DNA and single or serial plasma (or sera) were also available for antiviral antibody testing. Clinical descriptions of 20 patients have been reported recently as referenced in Tables 1A and 1B that also includes treatment history that accompanied the initial sample.2 The LMMN provided the initial laboratory diagnosis of PML in 21 case and confirmed PML in 14 others. Over the course of weeks to months, 13 of these patients had 3 or more CSF samples tested for JCV DNA following their clinical progress after discontinutation of natalizumab, plasma exchange (PLEX) or immunabsorption (IA) to reduce the pharmacological effects of natalizumab and initiation of immune reconstitution inflammatory syndrome (IRIS) that all patients developed. Table 1A shows 7 of these patients demonstrated viral persistence as defined as consistent detection of viral DNA in the CSF over months. One of the first cases of PML diagnosed from the Sentinel trail4 has had CSFs tested since 2005 to 2008, over 3 years, with detectable viral DNA 3 years after PML diagnosis, Table 1A patient 6. This patient has periodic neurological deficits that may be attributable to viral reactivation and persistent JCV infection (manuscript in preparation). In a retrospective review of longitudinal plasma samples on this patient from Jan, 2003, July 2003, Dec 2003 and Aug 2004, there was an indication of viremia for years prior to PML occurrence in Dec 2004 based on the assay used at the time in which only one of duplicate samples was positive.13 An AIDS associated case of PML not related to natalizumab therapy had JCV DNA detected in the CSF at the time of PML diagnosis and then 5 years later (2300c/ml). This patient had a history of seizures and headaches that were attributed to HIV-1 disease. CSF samples were not taken until recently (J. Rumbaugh, personal communication). Multiple CSF samples from 2 other patients, Table 1A numbers 8,9, showed a decrease of viral load over weeks from an extraordinarily high viral load of 3×106 and 1×106 that decreased to 1×103 and 1×104 in 4 and 2 months respectively.. CSFs in 4 patients, Table 1A 10-13, resulted in undetectable viral load in the last sample that was taken. No other CSF samples are available to determine whether any of these 6 patients have had viral DNA reappear in the CSF.
Figure 1.
Flow diagram showing number of MS patients treated with natalizumab who developed PML and their associated geographic location.
Table 1A.
JC Virus DNA copy number in ≥ 3 CSF samples and plasma/serum including antiviral antibody
| Patient (ref # LN) |
Treatment History |
Sample collection date # |
CSF viral copies |
Plasma/serum viral copies/ antibody titer |
|
|---|---|---|---|---|---|
| Persistent Viral Load | |||||
| EEA | |||||
|
| |||||
| 1 | PLEX, CDV | 6/15/09* | 415c/ml | NA* | |
| 7/3/09 | 745c/ml | NA | |||
| 9/4/09 | 201c/ml | NA | |||
|
| |||||
| 2 (#4) |
IA, mefloquine | 11/26/08 | 69c/ml | ||
| 12/5/08 | 215c/ml | 426c/ml s | 2,560 | ||
| 1/19/09 | 117c/ml | 19c/ml s | 10,240 | ||
| 1/28/09 | 181c/ml | 329c/ml s | 40,960 | ||
| 3/12/09 | 48c/ml | 61c/ml s | 163,840 | ||
| 4/24/09 | 26c/ml | Und p | 163,840 | ||
| Und s | |||||
|
| |||||
| 3 (#9) |
IA, IVIG, mirtazipine |
4/17/09 | 68c/ml | ||
| 5/13/09 | 50c/ml | ||||
| 6/10/09 | 134c/ml | ||||
| 7/22/09 | 3879c/ml | 2640c/ml s | 40,960 | ||
| 10/14/09 | 89c/ml | 79c/ml s | 2,621,440 | ||
|
| |||||
| 4 | Plex, | 9/29/09 | 35c/ml | ||
| (#18) | mefloqine, | 10/12/09 | 66c/ml | 49c/ml p | 40,960 |
| mirtazipine | 30c/ml s | ||||
| 10/21/09 | 155c/ml | 295c/ml p | 163,840 | ||
| 396c/ml s | |||||
| 11/4/09 | 81c/ml | 87c/ml p | 2,261,440 | ||
| 111c/ml s | |||||
| 11/18/09 | 75c/ml | 148c/ml p | 2,261,440 | ||
| 95c/ml s | |||||
| 12/8/09 | 21c/ml | 27c/ml p | 2,261,440 | ||
| 3/8/10 | 12c/ml | Und p | 2,261,440 | ||
| Und s | |||||
|
| |||||
| 5 | PLEX, | 11/26/09 | 41c/ml | NA | |
| (#5) | mefloquine | 12/7/09 | 126c/ml | NA | |
| 12/16/09 | 227c/ml | NA | |||
| USA | |||||
|
| |||||
| 64 | PLEX, Ara C, | 3/2/05 | 6,050c/ml | 2500c/ml s | 40,960 |
| CDV | 3/29/05 | 2,275c/ml | und s | 655,360 | |
| 9/20/05 | 42c/ml | und s | 655,360 | ||
| 6/11/08 | 37c/ml | 14c/ml p | |||
| 9/12/08 | 26c/ml | 108c/ml p | |||
|
| |||||
| 7 | PLEX | 10/9/09 | 40,646c/ml | und s | 10,240 |
| (#23) | 11/3/09 | 101,900c/ml | |||
| 11/17/09 | 769,000c/ml | ||||
| Decreased Viral Load | |||||
| EEA | |||||
|
| |||||
| 8 | PLEX | 4/7/09 | 96,436c/ml | 463c/ml p | 10,240 |
| (#6) | 275c/ml s | ||||
| 4/21/09 | 601,825c/ml | 12,487c/ml p | 40,960 | ||
| 10,812c/ml s | 5/5/09 | 2,241,062c/ml | 10,900c/ml p | 40,960 | |
| 8,350c/ml s | 5/29/09 | 3,482,575c/ml | 18,012c/ml p | 40,960 | |
| 21,875c/ml s | |||||
| 7/16/09 | 13,825c/ml | 4,063c/ml p | 10,485,760 | ||
| 9/3/09 | 1,114c/ml | 1,100c/ml p | 10,485,760 | ||
| 1.374c/ml s | |||||
| USA | |||||
|
| |||||
| 9 | PLEX, | 5/18/09 | 1,220,175c/ml | NA | |
| (#7) | mefloquine | 6/2/09 | 444,325c/ml | NA | |
| 7/7/09 | 9,937c/ml | NA | |||
| Decreased to Undetected Viral Load | |||||
| EEA | |||||
|
| |||||
| 105 | PLEX, IA, | 7/24/08 | 53c/ml | NA | |
| (#1) | mefloquine | 8/15/08 | 10c/ml | NA | |
| 8/28/08 | Und | 12c/ml p | 163,840 | ||
|
| |||||
| 11 | PLEX, | 10/9/2009 | 11c/ml | NA | |
| (#28) | mefloquine | 1/8/2010 | 26c/ml | 6c/ml s | 655,360 |
| 3/16/2010 | und | und s | 163,840 | ||
|
| |||||
| 12 | PLEX, IVIG, | 12/22/09 | 51c/ml | NA | |
| mefloquine, | 1/26/09 | 39c/ml | NA | ||
| mirtazipine | 2/25/09 | 70c/ml | NA | ||
| 3/25/10 | 9c/ml | ||||
| 4/12/10 | und | ||||
|
| |||||
| 13 | PLEX, | 9/9/09 | 1387c/ml | 28c/ml p | 40,960 |
| (#15) | mefloquine, | 18c/ml s | 655,360 | ||
| mirtazipine | 10/7/09 | 169c/ml | 26c/ml p | 655,360 | |
| 1/29/10 | Und | und p/s | |||
First date indicates sample at time of PML diagnosis.
NA: Sample(s) Not Available. P plasma; s serum. Antibody titers are ≥ reported value
Und: Undetected in qPCR assay
(#) refers to patient number and information in reference # 2
EEA European Economic Area; USA United States of America
PLEX: plasma exchange; IA: immune absorption; CDV: cidofovir; Ara C: cytosine arabinoside
Table 1B.
JC Virus DNA copy number in 1 or 2 CSF samples and plasma/serum including antiviral antibody
| Patient (ref # LN) |
Treatment History |
Sample collection date # |
CSF viral copies |
Plasma/serum viral copies/ antibody titer |
|
|---|---|---|---|---|---|
| EEA | |||||
|
| |||||
| 14 | IA | 1/15/09* | 92c/ml | 54c/ml s* | 40,960 |
| (#8) | 1/28/09 | 250c/ml | und p | 10,240 | |
| 32c/ml s | 10,240 | ||||
|
| |||||
| 15 | No history | 5/29/09 | 18c/ml | ||
| 6/4/09 | 762c/ml | ||||
|
| |||||
| 16 | PLEX, | 7/24/09 | 297c/ml | ||
| (#14) | mefloquine | 7/29/09 | und p | 640 | |
| 7/29/09 | 85c/ml s | 160 | |||
|
| |||||
| 17 | PLEX, mefloquine, | 9/24/09 | 122c/ml | ||
| (#17) | mirtazipine, CDV | ||||
|
| |||||
| 18 | PLEX | 10/7/09 | 2,927c/ml | und p | 2,560 |
| (#21) | und s | ||||
|
| |||||
| 19 | PLEX, IA | 12/1/09 | 197 | und p | 10,240 |
| und s | |||||
|
| |||||
| 20 | No history | 11/19/09 | 57c/ml | ||
|
| |||||
| 21 | PLEX | 1/15/10 | 1,424c/ml | ||
| 1/19/10 | 4,448c/ml p | 163,840 | |||
|
| |||||
| 22 | PLEX | 2/16/10 | 31c/ml | und p | 163,840 |
| und s | |||||
|
| |||||
| 23 | PLEX, mefloquine | 2/25/10 | 38,005c/ml | 239c/ml s | 10,240 |
|
| |||||
| 24 | PLEX | 1/21/10 | 12c/ml | ||
| 3/4/10 | 157c/ml p | 12,621,440 | |||
| 3/2/10 | 58c/ml s | ||||
|
| |||||
| 25 | IA, mirtazipine | 4/28/10 | 91c/ml | 25c/ml p | |
|
| |||||
| 26 | PLEX | 9/30/10 | 2374c/ml | 9c/ml s | ≥163,840 |
| 11/16/09 | 2574c/ml | 131c/ml p | ≥40,960 | ||
| USA | |||||
|
| |||||
| 27 | PLEX, mefloquine | 10/28/08 | 34,500c/ml | ||
| (#3) | 2,900c/ml p | ||||
|
| |||||
| 28 | PLEX, mefloquine, | 7/23/09 | 322c/ml | ||
| (#11) | levetiracetam | 9/2/09 | 2,988c/ml | ||
|
| |||||
| 29 | PLEX, | 8/4/09 | 3,600,000c/ml | ||
| (#12) | miretazipine | ||||
|
| |||||
| 30 | PLEX, mirtazipine | 10/2/09 | 257c/ml | ||
| (#19) | |||||
|
| |||||
| 31 | PLEX, mefloquine | 10/22/09 | 39c/ml | ||
| (#24) | 1/8/10 | 1,081c/ml | |||
| 2/23/10 | 163,840 | ||||
|
| |||||
| 32 | PLEX, mirtazipine | 10/26/09 | 90,125c/ml | ||
| (#26) | |||||
|
| |||||
| 33 | PLEX | 3/1/10 | 8,925c/ml | ||
| 4/19/10 | 613c/ml | 628c/ml p | 2,621,440 | ||
| 511c/ml s | |||||
|
| |||||
| 34 | No history | 4/21/10 | 380c/ml | und s | 40,960 |
|
| |||||
| 35 | PLEX | 4/23/10 | 21,736c/ml | ||
(#) refers to patient number and information in reference # 2
First date indicates sample at time of PML diagnosisM
NA: Sample(s) Not Available
Und: Undetected in qPCR assay
EEA: European Economic Area; USA United States of America
c/ml: viral DNA copies per ml
NA: Sample(s) Not Available
all antibody titers are ≥ the value reported
p: plasma s: serum
PLEX: plasma exchange; IA: immune absorption; CDV: cidofovir; Ara C: cytosine arabinoside
Table 1B shows the data from 22 patients with only 1 or 2 CSF samples taken at the time of suspected PML and a few weeks later. No further CSF samples have been available to assess whether any of these PML patients have reactivated or persistent infection. Fig 2 shows a box plot of the distribution of viral copy number from all 35 patients’ initial CSF at time of PML diagnosis with a mean approximating 500 copies with an upper margin of 9000 c/ml and a lower margin of 90c/ml. Interestingly, 57% of samples fell below the mean while 43 % were above. 13 samples or 37% had fewer than 90 c/ml at the time of PML diagnosis, a low copy number but should be within the limits of a qPCR assay coupled with ≥ 40% DNA template extraction efficiency.
Figure 2.
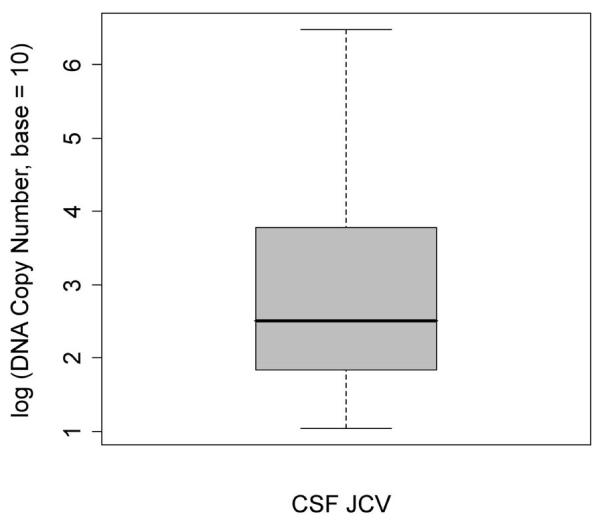
Box plot of the distribution of viral copy number in 35 CSF samples taken at or near the time of PML diagnosis: median = 2.508; 25th percentile = 1.794, 75th percentile = 3.866, minimum value = 1.041, maximum value = 6.477. The data are normally distributed about the mean (p-value=0.3137).
Viral DNA and antibody titers in plasma/serum
Table 1A also shows that 9 patients had plasma/serum samples available in whom 8 patients were viremic at multiple time points. However, all patients had moderate to rising antibody titers to JCV even in the viral persistent group that reached the highest titer measured, 10×106, indicating robust humoral immune responses in PML patients. One PML patient with a CSF viral load of 297c/ml, Table 1B (16), had a negative serum antibody titer at the time of diagnosis, 160 to 640, but was also viremic, 85 c/ml, and had high copy number in the urine, 27,212 c/ml. This patient was also reported as being HIV-1 sero-positive. Two other PML patients in Tables 1A (2) and Table 1B (18) had antibody levels at baseline for seropositivity, 2560, with CSF viral loads of 2900 and 69 c/ml. This patient’s antibody titer increased to ≥163,840 over 5 months with decreasing viral copy number in the CSF and plasma in Table 1A, (2).
Table 2 shows data from a series of CSF and plasma samples from MS patients from the AFFIRM and SENTINEL clinical studies that were assayed as controls from non-PML samples. None of the non-PML patients had viral genome copies in the CSF. Antibody titers determined by the LMMN ELISA certified assay showed seropositives in 65% of MS patients, 90% of Crohn’s Disease and 80% of rheumatoid arthritis patients.
TABLE 2.
JCV status of Plasma or CSF Samples from Patients in the AFFRIM/SENTINEL Trial
| ELISA ASSAY on Plasma or Serum for anti JCV antibody titers | ||||
|---|---|---|---|---|
| MS* | Crohn’s | RA | NK | Total |
| (214) | (40) | (5) | -- | (259) |
| 65% + | 90% + | 80% + | -- | 69.9% + |
| 35% − | 10% − | 20% − | -- | 30.1% − |
| 2560-10,240” | 10,240-40,960” | 10,240 | -- | |
| qPCR ASSAY on CSF | ||||
| (346) | (34) | (4) | (4) | (388) |
| 0/346 | 0/34 | 0/4 | 0/4 | 0/388 |
“ Average antibody titer in ELISA assay using 4x serial dilutions.
MS, multiple sclerosis; RA, rheumatoid arthritis; NK, diagnosis unknown. -- Not applicable.
Discussion
The availability of clinical samples from PML patients at the time of laboratory diagnosis and follow up for extended periods of time has revealed viral persistence that can occur for years. This observation might be important if not critical when evaluating new clinical episodes of neurological deficits and may warrant periodic assessment for viral genome in the CSF as well as in the plasma. Perhaps surprising in some PML patients is that viral persistence occurs even after IRIS and in the presence of high antibody titers to JCV. The observation that 2 PML patients had antibody titers to JCV at the time of diagnosis that were baseline as seropositive and 1 PML patient’s sample was seronegative in the LMMN ELISA assay suggests a closer look on the use of antibody levels for either PML risk assessment14 or as a prognostic indicator. A cross reference analysis of identical samples tested in several laboratories experienced with the ELISA assay might clarify the interpretation of antibody titers that define serological status. At this point, presence of and rise in antibody levels may be a valuable indication of virus exposure and active infection respectively. It is noteworthy that of 8 PML patients with multiple CSF samples and plasma/sera in Table 1A, antibody titers increased from the time of PML diagnosis to extremely high titers and in a few cases reached to levels at 106. This would indicate that PML patients have the ability to respond to JCV infection, evidenced also as viremia in these patients, with a robust humoral immune response. A clear assumption would be that JCV eludes antibody dependent clearance of JCV since these patients not only responded to infection but also were sero-positive before PML. Assessment of patients’ cell mediated immune responses to multiple viral antigens and immune surveillance remains a key feature then in determining the ability to prevent PML or limit infection after PML occurs15.
It is not known whether the origin of CSF viral DNA during persistence is derived from the brains of PML patients or virus periodically enters the brain from the periphery. It is anticipated that PML will continue to occur in natalizumab treated patients as in other patients on therapies that modulate the immune system. In many if not all of these PML patients regardless of resolution of acute disease, clinical signs of neurological disability suggests long term pathological consequences of PML.
Acknowledgement
The Laboratory of Molecular Medicine and Neuroscience, NINDS, receives support from the Division of Intramural Research, NINDS. There is no cost for the assays nor does the LMMN receive any form of compensation. All reports on sample tests go directly to the Centers and physicians who send the samples. The authors express appreciation to all LMMN members, to K. Johnson, Ph.D., Senior Statistician, NINDS for data analysis in figure 2, to the staff at the Centers that provided the samples and the cooperation of Biogen/Idec in their acquisition.
Footnotes
None of the authors have any conflicts of interest.
References
- 1.Major EO. Progressive Multifocal Leukoencephalopathy in patients on immunomodulatory therapies. Ann Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 2.Clifford D, De Luca A, Simpson DM, et al. Natalizumab associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons learned from 28 cases. Lancet Neuro. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 3.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langer-Gold A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Eng J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 5.Linda H, von Heijne A, Ryschkewitsch C, et al. Diagnosis and treatment of progressive multifocal leukoencephalopathy after natalizumab monotherapy in a person with multiple sclerosis. N Engl J Med. 2009;361:1081–1087. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health Office of Technology Transfer Federal Register Announcement. qPCR assay for Detection of JC Virus. 2009 Jul 6; HHS Reference No. E-152-2009/0. http://ott.od.nih.gov.
- 7.Ryschkewitsch C, Jensen P, Hou J, et al. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121(2):217–221. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Lenz P, Day PM, Pang YY, et al. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. 8. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Melenhorst J, Hensel N, et al. T cell responses to peptide fragments of the BKV T antigen-implications for cross reactivity of immune response to JC Virus. J of Gen Virol. 2006;87:2951–2960. doi: 10.1099/vir.0.82094-0. [DOI] [PubMed] [Google Scholar]
- 10.Koralnik IJ, Du Pasquier RA, Kuroda MJ, et al. Association of prolonged survival in HLA-A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol. 2002;168:499–504. doi: 10.4049/jimmunol.168.1.499. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton R, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition (HI) and enzyme immunoassay (EIA) for JC Virus and BK Virus. J Clin Micro. 2000;38:105–109. doi: 10.1128/jcm.38.1.105-109.2000. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowles W, Pipkin P, Andrews N, Vyse A, Minor P, Brown D, Miller E. Population based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J of Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 13.Rudick R, O’Connor P, Polman C, et al. Effects of natalizumab treatment on the presence of JC Virus DNA in blood and urine in Multiple Sclerosis patients. American Academy of Neurology Annual Meeting. 2010. S31.002.
- 14.Gorelik L, Bixler S, Lerner M, et al. Evaluation of the incidence of anti-JCV antibodies in a cohort of natalizumab-treated MS patients. American Academy of Neurology Annual Meeting. 2010. S31.003.
- 15.Ransohoff RM. Natalizumab and PML. Nat. Neurosci. 2005;8:1275. doi: 10.1038/nn1005-1275. [DOI] [PubMed] [Google Scholar]