Abstract
Purpose.
To evaluate the effects of the activation of endogenous angiotensin-converting enzyme 2 (ACE2) using the compound diminazene aceturate (DIZE) in an experimental model of glaucoma in Wistar rats.
Methods.
DIZE (1 mg/kg) was administered daily, either systemically or topically, and the IOP was measured weekly. To examine the role of the Mas receptor in the effects of DIZE, the Ang-(1-7) antagonist A-779 was co-administered. Drainage of the aqueous humor was evaluated by using scintigraphy. The analysis of ACE2 expression by immunohistochemistry and the counting of retinal ganglion cells (RGCs) were performed in histologic sections. Additionally, the nerve fiber structure was evaluated by transmission electron microscopy.
Results.
The systemic administration and topical administration (in the form of eye drops) of DIZE increased the ACE2 expression in the eyes and significantly decreased the IOP of glaucomatous rats without changing the blood pressure. Importantly, this IOP-lowering action of DIZE was similar to the effects of dorzolamide. The antiglaucomatous effects of DIZE were blocked by A-779. Histologic analysis revealed that the reduction in the number of RGCs and the increase in the expression of caspase-3 in the RGC layer in glaucomatous animals were prevented by DIZE. This compound also prevented alterations in the cytoplasm of axons in glaucomatous rats. In addition to these neuroprotective effects, DIZE facilitated the drainage of the aqueous humor.
Conclusions.
Our results evidence the pathophysiologic relevance of the ocular ACE2/Ang-(1-7)/Mas axis of the renin–angiotensin system and, importantly, indicate that the activation of intrinsic ACE2 is a potential therapeutic strategy to treat glaucoma.
Keywords: angiotensin-(1-7), Mas receptor, renin-angiotensin system, ACE2 activation, glaucoma, eyes
Activation of intrinsic angiotensin-converting enzyme 2 lowered IOP without affect the systemic arterial pressure. This beneficial effect was dependent on Mas receptor, induction of neuroprotection, and facilitation of the drainage of the aqueous humor.
Introduction
Glaucoma is a group of optic neuropathies that have in common the progressive death of retinal ganglion cells (RGCs) and optical nerve damage resulting in visual loss.1,2 Elevated IOP is considered the most important risk factor associated with glaucoma, and many mechanisms underlie the development and progression of this disease. For instance, imbalances in the renin–angiotensin system (RAS) cascade are involved in glaucoma, as well as in several cardiovascular and renal diseases, including hypertension, heart failure, cardiac remodeling, ventricular hypertrophy, and chronic renal failure.3–6
Classically, the RAS is formed by a hormonal cascade, wherein renin cleaves circulating angiotensinogen from the liver to produce angiotensin (Ang) I. Subsequently, this peptide is converted into Ang II by angiotensin-converting enzyme (ACE). Ang II is a potent vasoconstrictor, growth modulator and proinflammatory peptide. However, this linear concept of the RAS has changed dramatically in the last several decades because new biologically active components have been incorporated into this system.7–9 In this context, the identification of the ACE2/Ang-(1-7)/Mas receptor axis should be noted.10 This axis is composed of the heptapeptide Ang-(1-7); ACE2, the main Ang-(1-7)–forming enzyme11,12; and the Ang-(1-7) receptor Mas.13 Increasing pathophysiologic importance has been attributed to this axis because it is able to elicit beneficial actions that oppose the various deleterious effects induced by Ang II acting on AT1 receptors.8,10 Therefore, ACE2 is a promising therapeutic target because it degrades Ang II to generate Ang-(1-7).6,14–18
Importantly, in addition to the circulating RAS, various members of this system have been identified in many organs, including the eyes.5 This discovery indicates that there are specific tissue-localized RASs that can regulate long term changes in these organs. Specifically, the expression of ACE2 was detected in both the ciliary bodies and retinas of pigs.18 In addition to ACE2, Ang-(1-7), and Mas were also observed in the eyes,5,19 indicating that the ACE2/Ang-(1-7)/Mas branch of the RAS is present in ocular tissues. Thus, in this current study, we hypothesized that the activation of intrinsic ACE2 might have a protective action against deleterious effects of glaucoma. To test this hypothesis, we treated glaucomatous rats with the compound diminazene aceturate (DIZE), an activator of endogenous ACE2. DIZE was identified by the virtual screening of chemical libraries based on the crystal structure of ACE2, and its effectiveness as an activator of ACE2 was confirmed by analysis of the cleavage of Ang II.20
Methods
Animals
Male Wistar rats weighing 180 to 220 g were obtained from the animal facility of the Faculty of Pharmacy (Federal University of Minas Gerais, Belo Horizonte, Brazil). The animals were housed in a temperature-controlled room (22–23°C) with a 12 to 12 hour light–dark cycle. Water and food were available ad libitum. The experimental protocols were performed in accordance with institutional guidelines approved by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais, Brazil (CETEA-UFMG), which are in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (protocol n°11/11). In addition, this study conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research.
Tonometry
IOP measurements were performed using a TonoPen XL applanation tonometer (Mentor, Norwell, MA) that was calibrated before use by an experienced tonometrist. To perform the measurements, unsedated animals were topically anesthetized by the instillation of 0.4% benoxinate hydrochloride and then carefully restrained with a small cloth. Three IOP readings (with SE less than 10%) were taken for each eye. The average of these three readings was considered the corresponding value of the IOP. The tonometrist was masked to the treatment, and an assistant performed the randomization process.
Systemic Blood Pressure Measurement
The mean arterial pressure (MAP) was evaluated by the tail-cuff method, which is a noninvasive computerized system for measuring the blood pressure (Kent Scientific Corporation, Torrington, CT). This tail-cuff blood pressure system utilizes volume-pressure recording technology to measure the rat's tail blood pressure. The animals were acclimated to the restraint and tail-cuff inflation for 1 day before the beginning of the experiments. The restraint platform was maintained at approximately 32°C to 34°C. For each session, the rat was placed in an acrylic box restraint, and the tail was inserted into a compression cuff that measured the blood pressure 15 times. The average of these values was calculated.
Induction of Glaucoma
Unilateral glaucoma was induced in the right eye by the injection of 30 μL hyaluronic acid (HA) (10 mg/mL) into the anterior chamber through the clear cornea, near the corneoscleral limbus, using a hypodermic needle (22 gauge) once per week for 4 or 5 weeks on the same calendar day and at the same time, according to the protocol of Moreno et al.21 The rats were anesthetized intramuscularly with ketamine (70 mg/kg) and xylazine (10 mg/kg). In addition, two drops of 0.4% benoxinate hydrochloride was instilled directly on the cornea as a topical anesthetic. No procedure was performed on the contralateral eye. Evaluation of the IOP was performed 1 day before the next HA injection.
Experimental Protocols
The animals were divided into four groups: (1) nonglaucomatous rats treated with vehicle (control: untreated nonglaucomatous rats), (2) nonglaucomatous rats treated with DIZE (control+DIZE: treated nonglaucomatous rats), (3) glaucomatous rats treated with vehicle (glaucoma: untreated glaucomatous rats), and (4) glaucomatous rats treated with DIZE (glaucoma+DIZE: treated glaucomatous rats). The experimental protocols were defined as follows:
Preventive and systemic protocol: unilateral induction of glaucoma once per week for 4 weeks (n = 8). IOP and MAP measurements were performed weekly on the day before each HA injection. The animals were systemically treated with DIZE daily (1 mg/kg diluted in saline; ∼0.5 mL per animal, once per day by gavage). The treatment was initiated at the time of the induction of glaucoma and lasted for 4 weeks (Fig. 1A).
Preventive and topical eye drop protocol: unilateral induction of glaucoma once per week for 4 weeks (n = 7–8). IOP and MAP measurements were performed weekly on the day before each HA injection. The animals were topically treated with DIZE daily (1 mg/kg diluted in saline; ∼0.02 mL per animal by instillation once per day). The treatment was initiated at the time of the induction of glaucoma and lasted for 4 weeks (Fig. 1B).
Treatment and topical eye drop protocol: unilateral induction of glaucoma once per week for 4 weeks (n = 7). IOP and MAP measurements were performed weekly on the day before each HA injection. The animals were topically treated with DIZE daily (1 mg/kg diluted in saline; ∼0.02 mL per animal by instillation once per day). The treatment was initiated after confirmation of an elevated IOP (i.e., 1 week after the first injection of HA) and lasted for 3 weeks (Fig. 1C).
Effects of DIZE versus dorzolamide: unilateral induction of glaucoma once per week for 5 weeks (n = 5–7). IOP and MAP measurements were performed weekly on the day before each HA injection. The animals were topically treated daily with DIZE (1 mg/kg diluted in saline; ∼0.02 mL per animal by instillation once per day) or 2% dorzolamide hydrochloride (∼5 μL). The treatments were initiated after confirmation of the elevated IOP (i.e., 1 week after the first injection of HA) and continued for 2 weeks after the treatments were terminated. Then, the IOP and MAP were measured for additional 2 weeks (Fig. 1D).
The role of Mas in the effects of DIZE: unilateral induction of glaucoma once per week for 3 weeks (n = 5). IOP and MAP measurements were performed weekly on the day before the HA injections. The animals were topically (instillation) treated with DIZE (1 mg/kg diluted in saline; ∼0.02 mL per animal once per day) daily. The treatment with DIZE and A-779, an antagonist of Mas, was initiated after the confirmation of the elevated IOP (i.e., 1 week after the first injection of HA) and lasted for 2 weeks. A-779 (120 μg/d) was administered using an osmotic minipump (ALZET Osmotic Pumps, Cupertino, CA) implanted subcutaneously (Fig. 1E).
Figure 1. .
Diagrammatic representation of the experimental protocols used in this study. (A) Preventive and systemic protocol; (B) preventive and topical eye drop protocol; (C) treatment and topical eye drop protocol; (D) effects of DIZE versus dorzolamide; and (E) the role of Mas in the effects of DIZE. IHC, immunohistochemistry; TEM, transmission electron microscopy.
Histologic Analysis
After enucleation, two small sagittal sections were made in the nasal and temporal sides of the eyes (n = 7), and the eyes were immediately immersed in Bouin's fluid for approximately 24 hours. Thereafter, they were dehydrated in increasing concentrations of ethanol (70%, 80%, 90%, 95%, and 100%), diaphanized in xylene, and embedded in Paraplast. Semiserial 6-μm sections (at 60-μm intervals) were obtained using a microtome (model HM335E; Microm, Inc., Minneapolis, MN). For histologic analysis and RGC counting, histologic sections were stained with hematoxylin-eosin (HE).
Immunohistochemical Analysis
To evaluate the ACE2 expression in the ocular structures (n = 6), we used an immunohistochemical technique. After enucleation, two small sagittal sections were made in the nasal and temporal sides of the eyes, and the eyes were immediately immersed in Bouin's fluid for approximately 24 hours. Thereafter, they were dehydrated in increasing concentrations of ethanol (70%, 80%, 90%, 95%, and 100%), diaphanized in xylene, and embedded in Paraplast (Sigma-Aldrich, St. Louis, MO). Semiserial 6-μm sections (at 60 μm of intervals) were obtained using a microtome. The histologic sections were diaphanized and hydrated in ethanol (100%, 95%, 90%, 80%, 70%, 50%, and 25%). Subsequently, peroxidase activity was blocked by incubation in 3% H2O2 for 15 minutes, and then, unspecific binding was blocked by incubation in a solution of 2% BSA containing 0.1% Tween 20 for 1 hour in a moist chamber. The sections were incubated with the primary antibody (polyclonal rabbit anti-ACE2, 1:500; Genetex, Inc., Irvine, CA) diluted in the blocking solution overnight at 4°C in a humid chamber. The sections were then incubated with the secondary antibody for 1 hour. Signal amplification was performed using a LSAB/DAKO streptavidin-biotin-peroxidase kit (Dako North America, Camarillo, CA) followed by incubation with 0.025% diaminobenzidine tetrahydrochloride hydrate and counterstaining with Harris' hematoxylin (Merck, Darmstadt, Germany). After images were obtained with a 40× objective, the ACE2 expression was quantified using Image Pro-Plus (Meyer Instruments, Inc., Houston, TX). For this analysis, 10 images of retinas per animal were obtained under the same conditions of light and contrast, and the results were expressed as the percentage of the area occupied by brown pixels, which represented ACE2 expression.
Immunofluorescence Analysis
To evaluate the expression of caspase-3 in the RGC layer (n = 4), we used immunofluorescence. Histologic sections were prepared as described above. The peroxidase activity was blocked by incubation in 3% H2O2 for 15 minutes, and then nonspecific binding was blocked by incubation in a solution of 2% BSA containing 0.1% Tween 20 for 1 hour in a moist chamber. The sections were incubated with the primary antibody (polyclonal rabbit anti-caspase-3, 1:500; Sigma-Aldrich) diluted in blocking solution overnight at 4°C in a moist chamber. The sections were then incubated with the secondary antibody (Alexa Fluor 488; Invitrogen, Eugene, OR) for 1 hour. Subsequently, the sections were stained with propidium iodide (1:50; Sigma-Aldrich). The stained slides were imaged using a confocal microscope (BX61, laser at 488 nm, 10X objective, 2× digital zoom; Olympus, Tokyo, Japan), and the images were rendered offline using the Volocity software (PerkinElmer, Waltham, MA). Images were captured using the Kaplan mode filter (10 times; FluoView software; Olympus).
Transmission Electron Microscopy
For ultrastructural evaluation of the retina, animals were enucleated, and frontal sections were taken behind the lens. The posterior segments of the eyes were immersed in a solution of 5% glutaraldehyde and 4% formaldehyde in sodium cacodylate buffer (0.1 M, pH 7.2) for 2 hours at room temperature (25°C) with constant stirring. After fixation, fragments of retinas close to the optic nerve were obtained from the posterior segments. Then, the samples were washed three times in sodium cacodylate buffer for 10 minutes and post fixed with a solution of osmium tetroxide in 1% sodium cacodylate buffer (0.1 M, pH 7.2) for 1 hour. Next, the material was washed again in sodium cacodylate buffer three times for 10 minutes each. The material was gradually dehydrated by immersion in 70%, 80%, and 90% ethanol once for 15 minutes each and twice in 100% ethanol of 15 minutes, and then immersion in propylene oxide twice for 15 minutes. The material was treated with propylene oxide/Epon resin (2:1) for 3 hours, followed by propylene oxide/Epon resin (1:1) overnight, and the next day, the material was placed in pure resin for 2 hours, followed by another 2 hours in a vacuum chamber. For polymerization, the material was placed in an oven at 60°C for 48 hours. Semithin sections were obtained with a glass knife on an ultramicrotome (Leica EM-UC6; Leica Microsystems, Wetzlar, Germany). These sections had a thickness of 250 to 300 nm and were stained with aqueous 1% toluidine blue. Ultrathin sections were also obtained using an ultramicrotome with diamond knives (Leica Microsystems) and these sections had a thickness of 70 to 90 nm and were placed on a 200 mesh copper screen. The screens were counterstained in a petri dish with aqueous saturated uranyl acetate and lead citrate for 10 minutes. After this procedure, the screens were washed in distilled water and placed in a drying oven at 37°C. The analysis was performed on a Tecnai G2-Spirit - FEI transmission electron microscope (FEI, Hillsboro, OR).
Scintigraphic Images
To evaluate the drainage of the aqueous humor, scintigraphic images of the animals (n = 5 in each group) were obtained using a gamma camera (Nuclide TM TH22; Mediso, Budapest, Hungary). The animals were anesthetized intramuscularly with ketamine (70 mg/kg) and xylazine (10 mg/kg), and two drops of 0.4% benoxinate hydrochloride were instilled directly onto the cornea as a topical anesthetic. After being anesthetized, the animals were placed in a lateral position on a gamma camera equipped with a low-energy collimator (Nuclide TM TH22; Mediso), and aliquots (50 μL) of sterile solution containing 3.7 megabecquerel of sodium pertechnetate (Na99mTcO4) were injected into the anterior chamber of the eyes. Five minute static planar images were acquired every 30 minutes for 3 hours using a 256 × 256 pixel matrix. The quantitative analysis of the images was performed in the regions of interest (ROIs; the eye, thyroid, and stomach) using the total counts of radioactivity, as previously described by Diniz et al.22
Statistical Analysis
All data were expressed as the mean ± SEM. The results regarding the effects of DIZE on the IOP, the morphometric analysis of the RGCs and the ACE2 expression levels were analyzed using one-way ANOVA followed by the Newman-Keuls posttest. The MAP and aqueous humor drainage data were analyzed using two-way ANOVA followed by the Bonferroni posttest. All these tests were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). The results were considered significant at P less than 0.05.
Results
ACE2 Expression in the Retina (Protocol 1)
The daily administration of DIZE for 4 weeks induced an increase in ACE2 expression in the inner segments of the photoreceptor layer, the outer and inner plexiform layers and the ganglion cell layer. The quantification of these results showed that the control and glaucomatous rats treated with DIZE had higher expression levels of ACE2 in the retinas compared with untreated animals (Fig. 2).
Figure 2. .
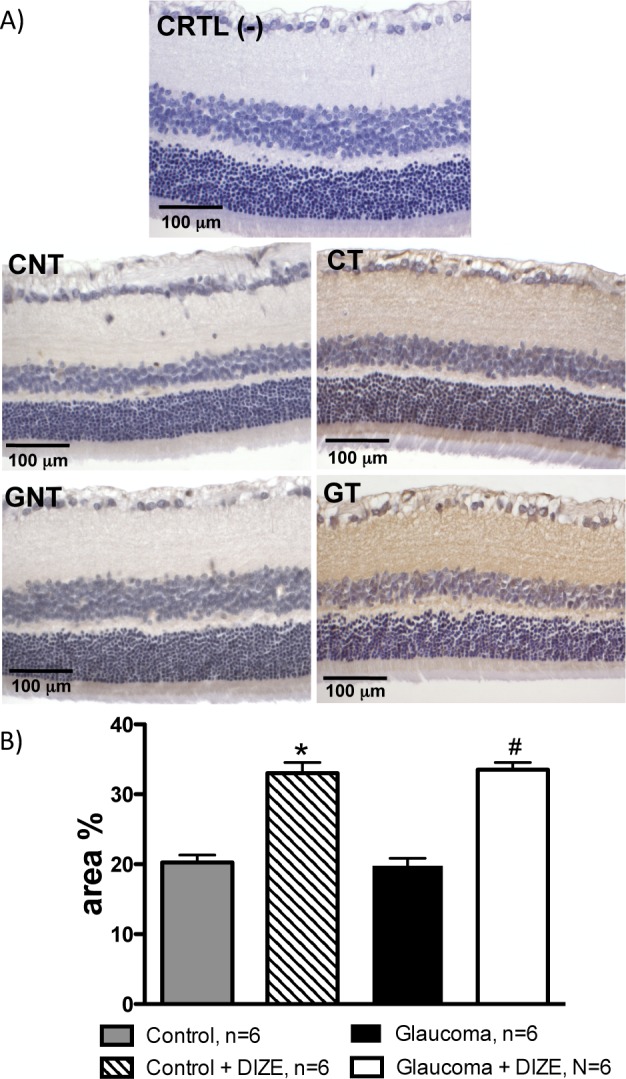
The administration of DIZE increased the expression of ACE2 in the retinas of control and glaucomatous rats. (A) Representative photomicrographs of the retinas of negative control (CRTL [-]), control untreated (CNT), control treated (CT), glaucoma untreated (GNT), and glaucoma treated (GT) animals. (B) Quantification of the ACE2 expression in the retinas of rats. *P < 0.001 versus control animals and #P < 0.001 versus glaucomatous rats. One-way ANOVA followed by the Newman-Keuls posttest.
Effects of the Administration of DIZE on the Prevention of Glaucoma (Protocols 1 and 2)
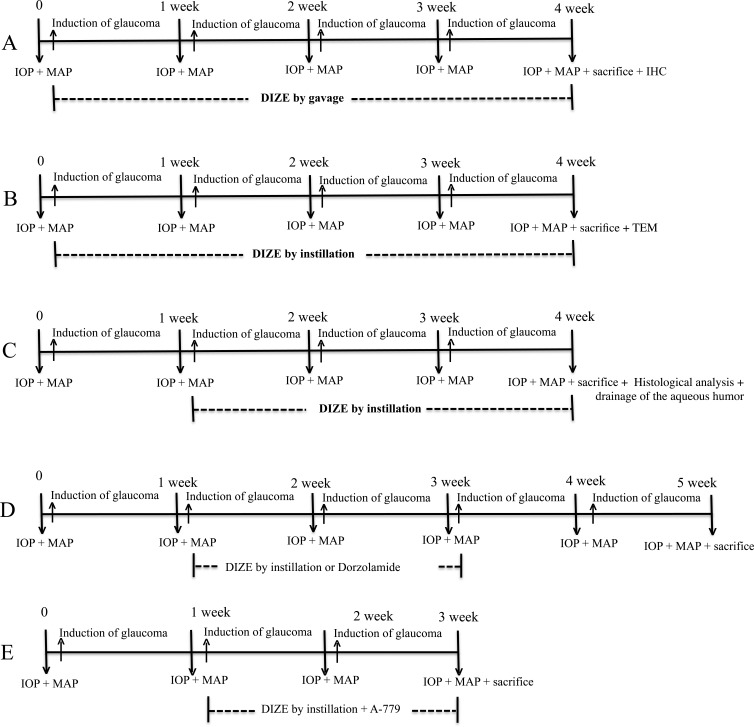
After observing that DIZE was able to increase the ACE2 expression in the retinas, we first evaluated whether this compound might prevent increases in the IOP. In this experiment, DIZE was systemically administered by gavage, and the treatment was initiated at the time of the glaucoma induction and continued for 4 weeks. The IOP was significantly higher in the untreated glaucomatous animals, and the treatment with DIZE was able to completely prevent the elevation of the IOP (27.9 ± 7.0 mm Hg vs. 11.2 ± 4.6 mm Hg in treated glaucomatous rats during the fourth week, Fig. 3A). To confirm these findings, we decided to apply DIZE topically to the eyes. Figure 3B shows that the IOP of the treated glaucomatous animals was lower than that of the untreated rats (27.1 ± 5.0 mm Hg vs. 8.7 ± 1.1 mm Hg in treated glaucomatous rats during the fourth week), indicating that, similar to the systemic approach, the topical application of DIZE was able to prevent increases in the IOP. Of note, the DIZE treatment did not change the MAP of the rats, demonstrating that the effects of this compound on the IOP were independent of reductions in the systemic blood pressure (Table).
Figure 3. .
Effects of DIZE on the prevention of glaucoma. (A) Preventive and systemic protocol and (B) preventive and topical eye drop protocol. The administration of DIZE was initiated at the same time as the induction of glaucoma was initiated and lasted for 4 weeks. *P < 0.05 versus control and #P < 0.05 versus glaucoma. One-way ANOVA followed by the Newman-Keuls posttest.
Table.
MAP (mm Hg) of Glaucomatous Rats Treated With DIZE or Left Untreated
|
Nontreated Animals |
Treated Animals |
|
| Preventive and systemic protocol, n = 8 | ||
| Wk 0 | 119.5 ± 7.5 | 120.5 ± 6.7 |
| Wk 1 | 128.5 ± 4.1 | 123.4 ± 2.2 |
| Wk 2 | 122.0 ± 5.6 | 113.0 ± 5.1 |
| Wk 3 | 118.4 ± 3.5 | 119.5 ± 4.5 |
| Wk 4 | 125.3 ± 4. 9 | 126.8 ± 3.9 |
| Preventive and topical eye drops protocol, n = 7–8 | ||
| Wk 0 | 117.0 ± 1.2 | 118.6 ± 3.8 |
| Wk 1 | 117.4 ± 2.5 | 119.4 ± 4.7 |
| Wk 2 | 116.4 ± 4.7 | 117.6 ± 6.1 |
| Wk 3 | 118.2 ± 4.9 | 120.4 ± 3.9 |
| Wk 4 | 116.2 ± 1.8 | 119.2 ± 2.6 |
| Treatment and topical eye drops protocol, n = 7 | ||
| Wk 0 | 117.3 ± 6.7 | 122.6 ± 2.1 |
| Wk 1 | 128.0 ± 4.1 | 131.0 ± 5.3 |
| Wk 2 | 127.5 ± 3.8 | 121.6 ± 7.0 |
| Wk 3 | 127.3 ± 6.4 | 131.0 ± 4.8 |
No significant differences were observed between the groups during the treatment period (two-way ANOVA followed by the Bonferroni posttest).
Effects of the Administration of DIZE on the Treatment of Glaucoma (Protocols 3 and 4)
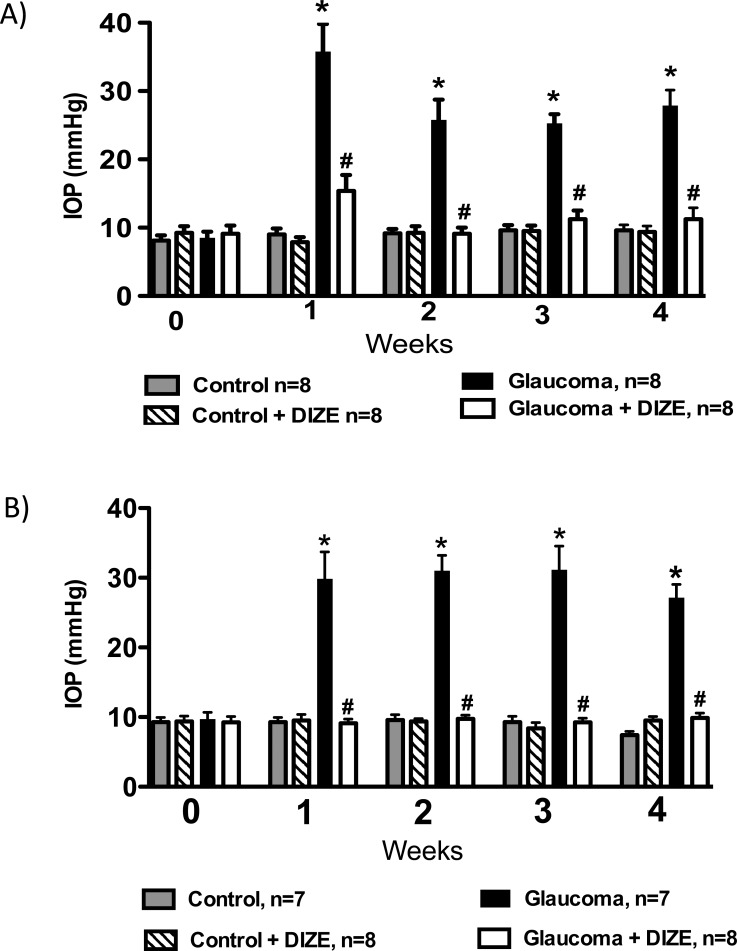
To test the ability of DIZE to reduce an established elevation of the IOP, we developed a protocol to treat glaucomatous rats. The administration of DIZE by instillation was initiated after confirmation of the elevated IOP (i.e., 1 week after the first injection of HA) and continued for 3 weeks. We found that even after the establishment of ocular hypertension, DIZE completely restored the IOP of the treated glaucomatous group (32.0 ± 1.2 mm Hg vs. 10.1 ± 0.4 mm Hg in treated glaucomatous rats during the fourth week, Fig. 4A). Importantly, the DIZE treatment did not change the MAP of the rats (Table). Thus, these findings demonstrate that the activation of ACE2 not only prevented increases in the IOP, but also reduced the IOP.
Figure 4. .
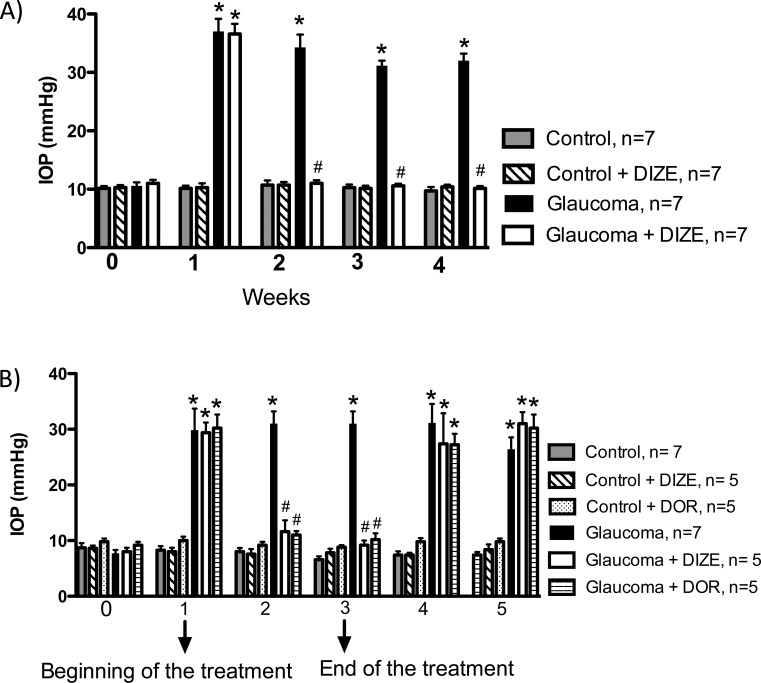
Effects of the administration of DIZE on the treatment of glaucoma. (A) Treatment and topical eye drop protocol. The administration of DIZE was initiated after confirmation of the elevated IOP (i.e., 1 week after the first injection of hyaluronic acid) and lasted for 3 weeks. (B) Comparison of the effects of DIZE and dorzolamide on the IOP. *P < 0.01 versus control and #P < 0.01 versus glaucoma. One-way ANOVA followed by the Newman-Keuls posttest.
Next, we compared the effects of DIZE with the effects of the commercially available antiglaucomatous drug dorzolamide. Interestingly, both drugs reduced the IOP by the same magnitude (DIZE: 11.6 ± 2.0 mm Hg and dorzolamide: 9.2 ± 0.8 mm Hg during the third week, Fig. 4B). Furthermore, after the discontinuation of the treatments (DIZE and dorzolamide), the IOP significantly increased to the levels observed before the initiation of the treatments (first week).
The Inhibition of Mas Abolishes the Protective Effects of DIZE (Protocol 5)
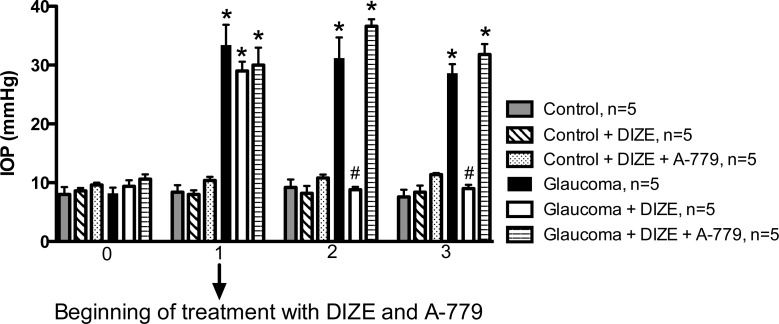
Our next aim was to determine the possible mechanisms involved in the hypotensive effects of DIZE on the IOP. To evaluate the participation of the Ang-(1-7)/Mas axis in the effects of DIZE, we used the Ang-(1-7) antagonist A-779. Figure 5 shows that A-779 was able to completely block the hypotensive effects of DIZE on the IOP. Therefore, the data suggests that the activation of intrinsic ACE2 increases the production of Ang-(1-7), which acts on Mas.
Figure 5. .
The role of Mas in the effects of DIZE on the IOP. The co-administration of A-779, a Mas antagonist, was able to block the effects of DIZE on the IOP. *P < 0.01 versus control and #P < 0.01 versus glaucoma. One-way ANOVA followed by the Newman-Keuls posttest.
DIZE Induces Neuroprotection in the Retinas of Glaucomatous Rats (Protocols 2 and 3)
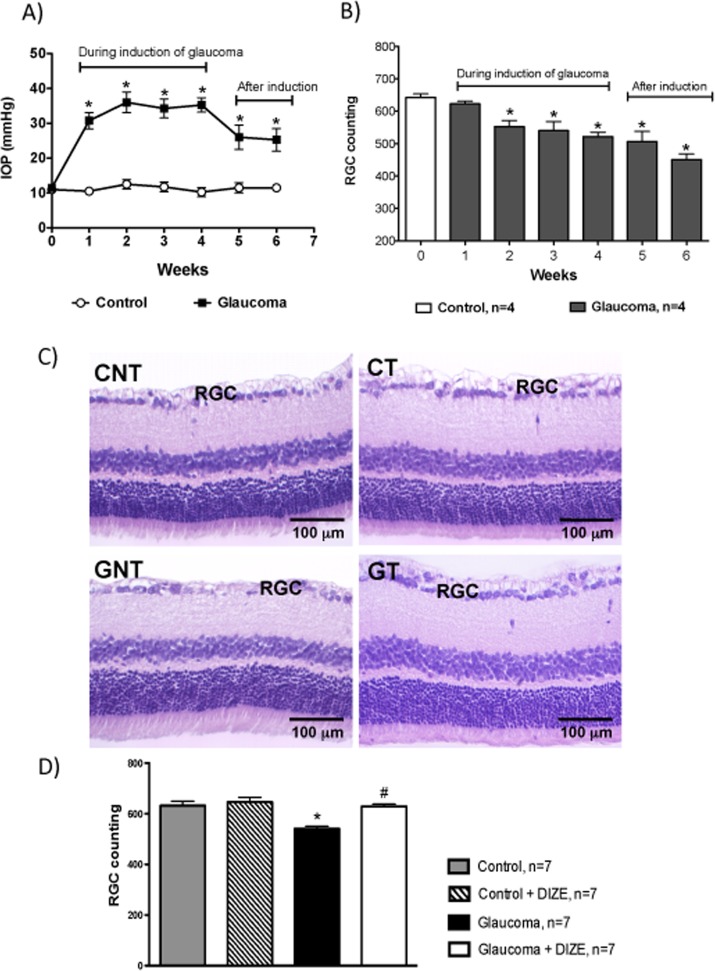
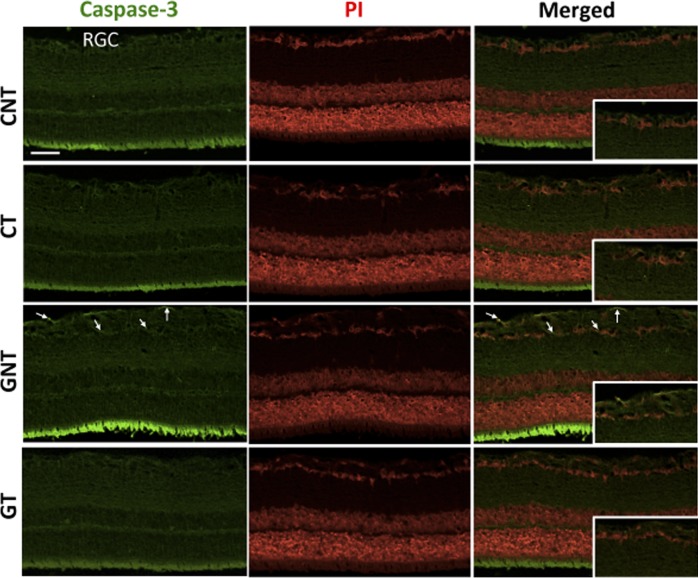
Histologic analysis showed that the glaucomatous rats exhibited an increase in the excavation of the optic nerve due to the severe loss of the neural fibers of RGCs and to a large reduction in neural fibers of the optic nerve. Treatment with DIZE prevented this histologic damage (Figs. 6A, 6B). Increases in the IOP were accompanied by reductions in the number of RGCs. This reduction started during the second week of glaucoma induction and lasted up to the sixth week. Of note, both the increases in the IOP and the reductions in the number of RGCs were not reversible after the induction of glaucoma was terminated (Figs. 7A, 7B). A smaller number of RGCs in the retinas of glaucomatous animals was not observed in glaucomatous animals treated with DIZE (561.5 ± 31.2 cells vs. 667 ± 28.9 cells in treated glaucomatous rats, Figs. 7C, 7D). Ultrastructural analysis revealed the presence of unmyelinated fibers of different diameters in the nerve fiber layer with different stages of axoplasm alterations in glaucomatous rats. The administration of DIZE prevented this damage (Fig. 8). Additionally, untreated glaucomatous rats exhibited an increase in the expression of caspase-3 in the RGC layer starting during the second week of glaucoma induction, and this increase was prevented by the administration of DIZE (Fig. 9, Supplementary Fig. S1). Altogether, these data indicate that DIZE induced a neuroprotective effect by decreasing the loss of neural fibers and RGCs, most likely by reducing the rate of apoptotic cell death.
Figure 6. .
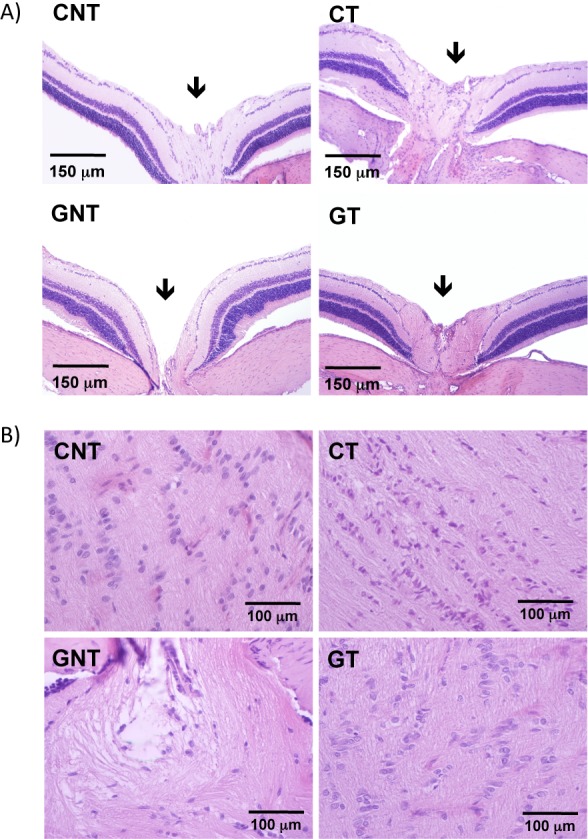
DIZE induced neuroprotection in the retinas of glaucomatous rats. (A) Representative photomicrographs of the excavation of the optic nerve (arrows). Note the exacerbation of the excavation in untreated glaucomatous animals (GNT) compared with all other groups. DIZE was able to prevent this effect (GT group). (B) Representative photomicrographs of longitudinal sections of the optic nerve. A large reduction in neural fibers was observed in untreated glaucomatous rats (GNT). Similarly, DIZE prevented this effect (GT group).
Figure 7. .
Histologic analysis of RGCs. (A) IOP during the 4 weeks of the induction of glaucoma followed by two additional weeks without induction. *P < 0.05 versus control. (B) Number of RGCs during the 4 weeks of the induction of glaucoma followed by two additional weeks without induction. *P < 0.05 versus control. (C) Representative photomicrographs of retinas showing the lower number of RGCs in untreated glaucomatous rats and the beneficial effect of DIZE on this parameter. (D) Quantification of RGCs in the retinas of rats. *P < 0.001 versus control and #P < 0.001 versus glaucoma. One-way ANOVA followed by the Newman-Keuls posttest.
Figure 8. .
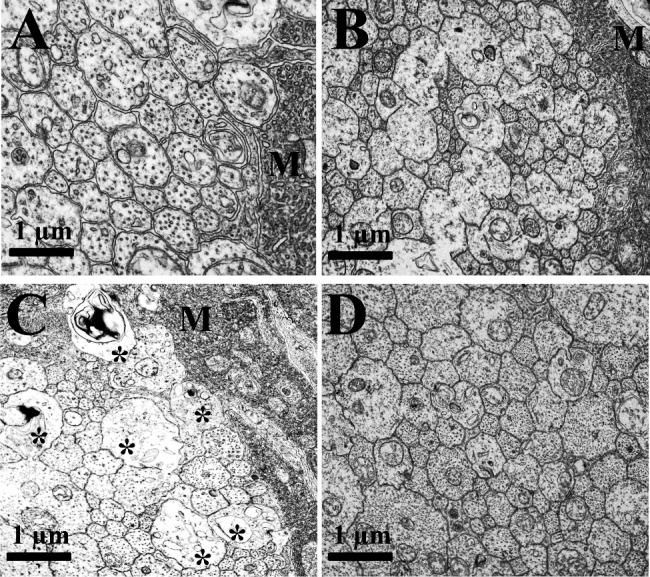
Transmission electron microscopy analysis of retinas showing the neural fiber layer. Representative photomicrographs of (A) control untreated, (B) control treated, (C) glaucoma untreated, and (D) glaucoma-treated animals. The glaucomatous rats had several unmyelinated fibers of different diameters partly involved with extensions of Müller cells (M) with axoplasmic alterations (*). These changes were not observed in glaucomatous animals treated with DIZE.
Figure 9. .
Immunofluorescence analysis showing the expression of caspase-3 in the RGC layer. Note the increased expression of caspase-3 in untreated glaucomatous animals (arrows) and the prevention of this effect by the administration of DIZE. PI, propidium iodide. Bar: 50 μm.
Effects of the Treatment With DIZE on the Drainage of Aqueous Humor (Protocol 3)
Another mechanism of action by which DIZE might reduce the IOP is facilitating the drainage of the aqueous humor. It was observed that the impairment of the drainage of the aqueous humor in glaucomatous rats was entirely restored by administering DIZE in the form of topical eye drops (Figs. 10A, 10B). Corroborating these findings, the level of technetium in the bloodstream and its uptake by the thyroid gland and stomach were higher in treated glaucomatous rats than in untreated animals (thyroid gland at 90 minutes: 179.4 ± 60.2 vs. 348.8 ± 29.9 in treated rats, and the stomach at 90 minutes: 1990.0 ± 102.3 vs. 4045.0 ± 147.9 in treated rats).
Figure 10. .
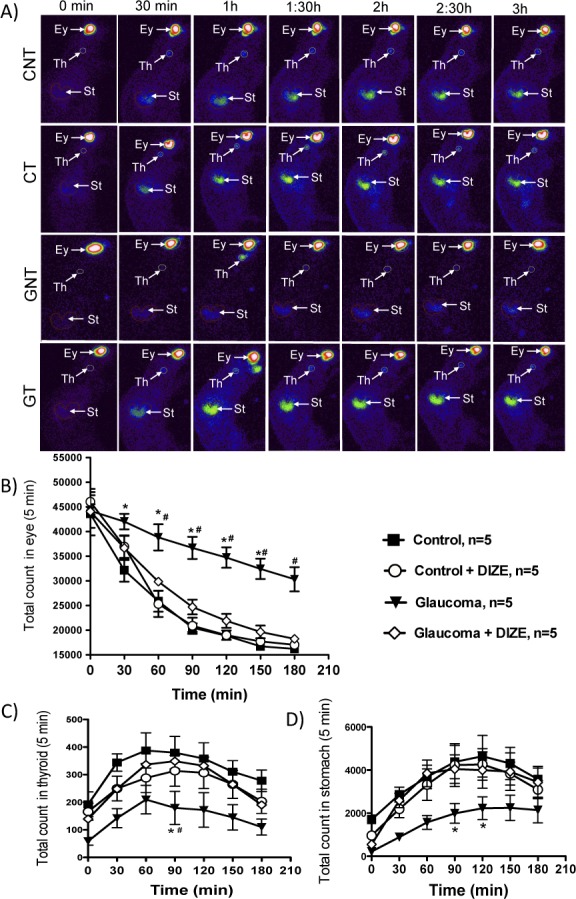
Effects of the treatment with DIZE on the drainage of aqueous humor. (A) Representative images of the aqueous humor drainage during the 3 hours of the experiment. Quantification of the (B) drainage of the aqueous humor and appearance of the technetium radioisotope in (C) the thyroid gland and (D) stomach. *P < 0.01 versus control and #P < 0.01 versus glaucoma+DIZE. Two-way ANOVA followed by the Bonferroni posttest. Ey, eye; Th, thyroid; St, stomach.
Discussion
The present study was designed to evaluate the effects of the activation of intrinsic ACE2 in an experimental model of glaucoma. Our main findings were that DIZE both prevented and reversed the elevation of the IOP induced by weekly injections of HA into the anterior chamber. These effects were probably mediated by Mas activation because the compound A-779 completely blunted the effects of DIZE. Furthermore, these antiglaucomatous effects of ACE2 activation involved the protection of neural fibers and RGCs and increases in the drainage of the aqueous humor.
The expression of ACE2 has been documented in both human19 and rodent retinas,23 and in the vitreous humor and ciliary body of porcine ocular tissues.18 In line with these reports, we found that ACE2 is expressed in the retinas of rats. Notably, treatment with DIZE significantly augmented the expression of ACE2 in the retinas. Increased ACE2 expression is a frequent finding when using ACE2 activators.24,25 This behavior strongly suggests that these compounds induce their beneficial effects, not only by forming Ang-(1-7) and/or degrading Ang II when acting as an ACE2 activator, but also through an unidentified mechanism that is able to induce the expression of ACE2. It is important to note that the effectiveness of DIZE as an activator of ACE2 was previously demonstrated by the analysis of the cleavage of Ang II.20
The reduction in the IOP due to the activation of intrinsic ACE2 was effective in our model of glaucoma. Importantly, the activation of intrinsic ACE2 not only prevented increases in the IOP but also reduced elevated IOPs. This finding is therapeutically relevant given the clinical routine. Of note, this ocular hypotensive effect induced by DIZE did not affect the MAP. The lack of an effect on the MAP is a desired feature for any antiglaucomatous drug because it indicates that the drug has high specificity, reducing potential side effects. To further explore the therapeutic potential of DIZE as an antiglaucomatous drug, we compared its effects with those of the commercially available antiglaucomatous drug dorzolamide. Both compounds elicited similar hypotensive effect, and when the treatments were stopped, comparable increases in the IOP were observed. Therefore, the activation of ACE2 has emerged as a promising therapeutic strategy to treat glaucoma.
Mechanistically, we demonstrated that the ability of DIZE to induce a reduction in the IOP occurs through the activation of the ACE2/Ang-(1-7)/Mas axis. The activation of this axis was evidenced by the observation that the Mas antagonist A-779 completely blocked the effects of DIZE on the IOP. In accordance with this finding, it has been reported that Mas is expressed in the ocular tissues of rats.5 Thus, because ACE2 is the main Ang-(1-7)–forming enzyme, our data suggest that the activation of intrinsic ACE2 increases the production of Ang-(1-7), which acts on Mas. Although we did not observe any significant effect on the eyes of normotensive rats, it has been reported that Ang-(1-7) reduces the IOP, possibly through a mechanism involving Mas, in normotensive rabbit eyes.26 This discrepancy observed between the normotensive eyes of rats and rabbits might be related to the species evaluated.
Neuroprotective mechanisms also underlie the effects of DIZE on glaucoma. Histopathologic changes in glaucomatous eyes are characterized by a reduction in the number of RGCs27 as a result of apoptosis28 and by the specific excavation of the optic nerve. In our study, the extensive death of RGCs, an increase in caspase-3 expression and the exacerbation of optic nerve excavation were observed in untreated glaucomatous animals. The activation of ACE2 was able to decrease the death of RGCs, reduce the caspase-3 expression, and prevent the excavation of the optic nerve. Therefore, because Ang II induces apoptosis,29 whereas Ang-(1-7) inhibits apoptosis, and because ACE2 has a dual beneficial role in the ocular RAS by degrading Ang II and generating Ang-(1-7), the antiglaucomatous effects of DIZE might be a consequence of these neuroprotective mechanisms.
To further explore the mechanisms of action by which DIZE decreases the IOP of glaucomatous rats, we evaluated the effects of this compound on the drainage of the aqueous humor. Our findings showed that the activation of intrinsic ACE2 was able to facilitate the drainage of the aqueous humor. The results obtained from the scintigraphic images showing higher radioactivity uptake by the thyroid and stomach of treated glaucomatous rats compared with nontreated animals are consistent with higher drainage of the aqueous humor to the blood stream. These data are in agreement with the results of the quantitative analysis (Figs. 9C, 9D). Although we did not investigate the molecular pathways underlying this effect of DIZE, possible mechanisms involve the release of nitric oxide (NO) and prostaglandins by Ang-(1-7).30–32 In the retinas, NO is a physiologic mediator present in rods, bipolar cells, amacrine cells, and ganglion cells.33 In addition, it promotes the relaxation of trabecular meshwork cells and ciliary muscle, and its production appears to be reduced in the context of POAG.30 Additionally, Vorwerk and colleagues34 observed RGC degeneration in the eyes of NO synthase-knockout mice. In relation to prostaglandins, indomethacin treatment abolished the IOP-lowering effect promoted by enalaprilat, indicating that prostaglandins may mediate, at least in part, the ocular hypotensive effect of enalaprilat.35
It should be mentioned that although we clearly demonstrated the participation of Mas, neuroprotection and the facilitation of drainage of the aqueous humor as mechanisms of action of DIZE, further experiments should be conducted, especially experiments using isolated retinal cells, to establish the precise molecular mechanisms by which DIZE induces its beneficial ocular effects in glaucomatous rats.
In summary, these results evidence the pathophysiologic relevance of the ocular ACE2/Ang-(1-7)/Mas axis of the RAS. Importantly, we found that the activation of intrinsic ACE2 had beneficial effects on glaucoma through a mechanism involving the Mas receptor, neuroprotection, and the drainage of the aqueous humor. Thus, our findings indicate that ACE2 is a potential therapeutic target in the treatment of glaucoma.
Supplementary Material
Acknowledgments
The authors thank the Centro de Microscopia/UFMG for the use of the transmission electron microscope.
Supported by grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG), and the National Institutes of Health (HL56921).
Disclosure: G. Foureaux, None; J.C. Nogueira, None; B.S. Nogueira, None; G.O. Fulgêncio, None; G.B. Menezes, None; S.O.A. Fernandes, None; V.N. Cardoso, None; R.S. Fernandes, None; G.P. Oliveira, None; J.R. Franca, None; A.A.G. Faraco, None; M.K. Raizada, None; A.J. Ferreira, None
References
- 1. Pang IH, Clark AF. Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 2007; 16: 483–505 [DOI] [PubMed] [Google Scholar]
- 2. Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010; 81: 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000; 107: 1287–1293 [DOI] [PubMed] [Google Scholar]
- 4. Luo BP, Brown GC. Update on the ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol. 2004; 15: 203–210 [DOI] [PubMed] [Google Scholar]
- 5. Vaajanen A, Lakkisto P, Virtanen I, et al. Angiotensin receptors in the eyes of arterial hypertensive rats. Acta Ophthalmol. 2009; 88: 431–438 [DOI] [PubMed] [Google Scholar]
- 6. Ferreira AJ, Bader M, Santos RAS. Therapeutic targeting of the angiotensin-converting enzyme 2/Angiotensin-(1-7)/Mas cascade in the reninangiotensin system: a patent review. Expert Opin Ther Pat. 2012; 22: 567–574 [DOI] [PubMed] [Google Scholar]
- 7. Santos RA, Ferreira AJ, Nadu AP, et al. Expression of an angiotensin-(1-7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics. 2004; 17: 292–299 [DOI] [PubMed] [Google Scholar]
- 8. Santos RA, Ferreira AJ, Simões E, Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008; 93: 519–527 [DOI] [PubMed] [Google Scholar]
- 9. Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006; 47: 515–521 [DOI] [PubMed] [Google Scholar]
- 10. Ferreira AJ, Santos RA. Cardiovascular actions of angiotensin-(1-7). Braz J Med Biol Res. 2005; 38: 499–507 [DOI] [PubMed] [Google Scholar]
- 11. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000; 87: E1–E9 [DOI] [PubMed] [Google Scholar]
- 12. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000; 275: 33238–33243 [DOI] [PubMed] [Google Scholar]
- 13. Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003; 100: 8258–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rojanapongpun P, Drance SM, Morrison BJ. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993; 77: 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danser AHJ, Derkx FHM, Admiraal PJJ, Deinum J, De Jong PTVM, Schalekamp MADH. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994; 35: 1008–1018 [PubMed] [Google Scholar]
- 16. Wagner J, Danser AHJ, Derkx FHM, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996; 80: 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savaskan E, Loffler KU, Meier F, Muller-Spahn F, Flammer J, Meyer P. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and AT1 receptor in human ocular tissues. Ophthalmic Res. 2004; 36: 312–320 [DOI] [PubMed] [Google Scholar]
- 18. Luhtala S, Vaajanen A, Oksala O, Valjakka J, Vapaatalo H. Activities of angiotensin-converting enzymes ACE1 and ACE2 and inhibition by bioactive peptides in porcine ocular tissues. J Ocul Pharmacol Ther. 2009; 25: 23–28 [DOI] [PubMed] [Google Scholar]
- 19. Senanayake Pd, Drazba J, Shadrach K, et al. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci. 2007; 48: 3301–3311 [DOI] [PubMed] [Google Scholar]
- 20. Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen. 2011; 16: 878–885 [DOI] [PubMed] [Google Scholar]
- 21. Moreno MC, Aldana Marcos HJ, Croxatto JO, et al. A new experimental model of glaucoma in rats through intracameral injections of hyaluronic acid. Exp Eye Res. 2005; 81: 71–80 [DOI] [PubMed] [Google Scholar]
- 22. Diniz SOF, Rezende CMF, Serakides R, et al. Scintigraphic imaging using technetium-99m-labeled ceftizoxime in an experimental model of acute osteomyelitis in rats. Nucl Med Commun. 2008; 29: 830–836 [DOI] [PubMed] [Google Scholar]
- 23. Tikellis C, Johnston CI, Forbes JM, et al. Identification of angiotensin converting enzyme 2 in the rodent retina. Curr Eye Res. 2004; 29: 419–427 [DOI] [PubMed] [Google Scholar]
- 24. Ferreira AJ, Shenoy V, Qi Y, et al. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol. 2011; 96: 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murça TM, Moraes PL, Capuruco CAB, et al. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul Pept. 2012; 177: 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaajanen A, Vapaatalo H, Kautiainen H, Oksala O. Angiotensin (1-7) reduces intraocular pressure in the normotensive rabbit eye. Invest Ophthalmol Vis Sci. 2008; 49: 2557–2562 [DOI] [PubMed] [Google Scholar]
- 27. Okisaka S, Murakami A, Mizukawa A, Junji I. Apoptosis in retinal ganglion cell decrease in human glaucomatous eyes. Jpn J Ophthalmol. 1997; 41: 84–88 [DOI] [PubMed] [Google Scholar]
- 28. Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999; 43: S151–S161 [DOI] [PubMed] [Google Scholar]
- 29. Hashizume K, Mashima Y, Fumayama T, et al. Genetic polymorphisms in the angiotensin II receptor gene and their association with open-angle glaucoma in a Japanese population. Invest Ophthalmol Vis Sci. 2005; 46: 1993–2001 [DOI] [PubMed] [Google Scholar]
- 30. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995; 36: 1765–1773 [PubMed] [Google Scholar]
- 31. Santos RAS, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept. 2000; 91: 45–62 [DOI] [PubMed] [Google Scholar]
- 32. Kostenis E, Milligan G, Christopoulos A, et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005; 111: 1806–1813 [DOI] [PubMed] [Google Scholar]
- 33. Neufeld AH. Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthalmol. 1999; 43: S129–S135 [DOI] [PubMed] [Google Scholar]
- 34. Vorwerk CK, Hyman BT, Miller JW, et al. The role of neuronal and endothelial nitric oxide synthase in retinal excitotoxicity. Invest Ophthalmol Vis Sci. 1997; 38: 2038–2044 [PubMed] [Google Scholar]
- 35. Shah GB, Sharma S, Mehta AA, Goyal RK. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J Cardiovasc Pharmacol. 2000; 36: 169–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.