Abstract
5α-reductase inhibitors (5α-RIs), including finasteride and dutasteride, are commonly used medical therapies for benign prostatic hyperplasia (BPH). Many studies reported that preoperative 5α-RI had impact on intraoperative haemorrhage during surgery for BPH, but it was still in controversial. So, we conducted a systematic review of the effects and mechanisms of 5α-RIs on intraoperative bleeding for BPH. MEDLINE, EMBASE, the Cochrane Controlled Trail Register of Controlled Trials and the reference lists of retrieved studies were searched in the analysis. Sixteen publications involving 15 different randomized controlled trials (RCTs) and a total of 1156 patients were used in the analysis, including 10 RCTs for finasteride and five RCTs for dutasteride. We found that preoperative finasteride treatment decreases microvessel density (MVD) in resected prostate specimens. Total blood loss, blood loss per gram of resected prostate tissue and decreases in haemoglobin were all greatly reduced in the finasteride group as compared to controls. Dutasteride appeared to have no effect on bleeding. This meta-analysis shows that preoperative finasteride treatment could decrease intraoperative haemorrhage during surgery for BPH. Preoperative dutasteride had no effect on intraoperative haemorrhage, but further high-quality prospective studies are still needed to confirm this observation.
Keywords: 5α-reductase inhibitor, benign prostate hyperplasia, haemorrhage, meta-analysis, microvessel density
Introduction
5α-reductase inhibitors (5α-RIs), including finasteride and dutasteride, are commonly used medical therapies for benign prostatic hyperplasia (BPH). Finasteride, a type II 5α-RI, blocks the conversion of testosterone to dihydrotestosterone. Inhibition of 5α-reductase reduces the concentration of dihydrotestosterone in the prostate, which results in a decreased volume of the prostate, improved urinary flow and a decline in the incidence of acute urinary retention and the need for surgery.1 Recently, several studies have demonstrated that finasteride interferes with angiogenesis in the prostate, which results in tissue regression.2 Finasteride is also efficacious in decreasing gross haematuria caused by BPH that can lead to clot retention and the need for blood transfusion when prolonged.3, 4 However, the ability of finasteride to decrease blood loss during surgical interventions for BPH and its mechanisms of action remain controversial.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Although one meta-analysis focused on the effects of preoperative finasteride on reducing bleeding during transurethral resection of prostate (TURP),16 the number of experiments included was insufficient and the underlying mechanisms for these treatment effects were not considered.
Dutasteride, a new member of 5α-RI group, offers the most complete inhibition of 5α-reductase because it blocks both type I and II receptors;17 however, its effects on intraoperative bleeding during treatment for BPH and its mechanisms remain controversial, and to date no meta-analysis of these effects has been conducted.18, 19, 20, 21, 22
The goal of the present study was to perform a meta-analysis to evaluate the effects of finasteride and dutasteride on intraoperative bleeding during transurethral management of BPH, which will resolve some of the remaining controversies over the use of these drugs.
Materials and methods
Inclusion criteria
Randomized controlled trials (RCTs) that met the following criteria were included: (i) the study referred to the effect of preoperative 5α-RIs on bleeding during the intraoperative management of BPH and alterations of microvessel density (MVD) within the resected prostatic specimens; (ii) the study provided sufficient data for analysis, including the mean values and the standard deviations (s.d.s) of the MVDs and blood loss volumes; and (iii) the full text of the study could be accessed. If these inclusion criteria were not met, the studies were excluded from the analysis.
Search strategy
MEDLINE (from 1966 to June 2010), EMBASE (from 1974 to June 2010), the Cochrane Controlled Trail Register of Controlled Trials and the reference lists of retrieved studies were searched to identify RCTs that referred to the effects of preoperative treatment with 5α-RIs on bleeding during the intraoperative management of BPH and also the mechanism of action for these drugs. The following search terms and acronyms were used: finasteride, dutasteride, bleeding, TURP, MVD and BPH.
Trial selection
Two reviewers independently scanned the search results for potentially relevant studies and retrieved the full text of these articles. In the event that they had been published in more than one location, experimental data were used only once. Together, the authors discussed each of the RCTs that were included and opted to exclude studies that either failed to meet the inclusion criteria or could not be agreed upon by the authors. A flow chart of study selection is presented in Figure 1.
Figure 1.
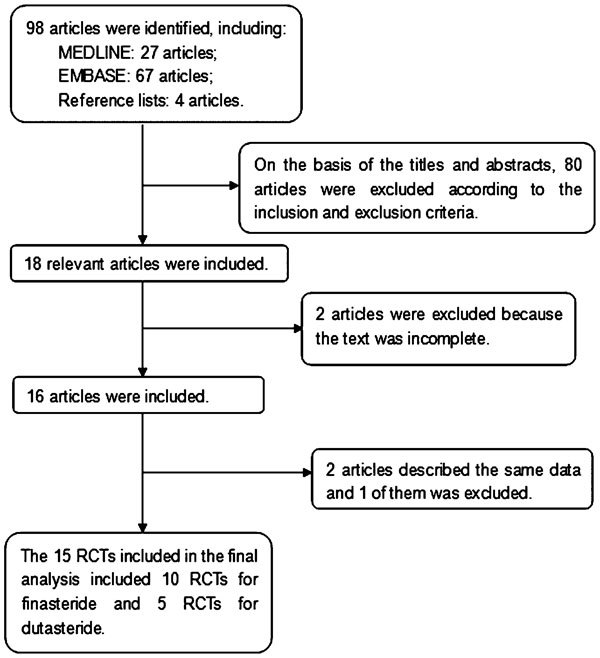
Flow chart illustrating the numbers of studies included in the meta-analysis. RCT, randomized controlled trial.
Quality assessment
The methodological quality of each study was assessed according to how patients were allocated to the arms of the study, concealment of allocation procedures, blinding and data loss due to attrition. The studies were then classified qualitatively according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.2.23 Based on the quality-assessment criteria, each study was rated and assigned to one of the following three broad quality categories: A: if all quality criteria were adequately met, the study was deemed to have a low risk of bias; B: if one or more of the quality criteria was only partially met or unclear, the study was deemed to have a moderate risk of bias; or C: if one or more of the criteria were not met, inadequately met, or not included, the study was deemed to have a high risk of bias. Sensitivity analyses were then performed on the basis of whether these quality factors were adequate, inadequate or unclear. Differences were resolved by discussion among the authors.
Data extraction
Two reviewers independently extracted data from the published papers for analysis. The following information was collected: (i) the name of the first author and the publication year; (ii) the study design and sample size; (iii) duration and type of preoperative drug treatment; (iv) the source of patients; (v) indicators of blood loss during treatment including total blood loss, blood loss/weight, haemoglobin (Hb) alterations and Hb alteration/weight; and (vi) MVD of the resected prostate specimens. Disagreements were resolved by consultation with an additional author.
Statistical analysis
The meta-analysis of comparable data was carried out using Review Manager 5.0.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). Due to the large number of plots, we combined the six forest plots into two plots using Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA, USA; Figures 2 and 3). We referred to these funnel plots for the meta-analysis, but they did not provide any evidence of a publication bias (Figure 4). Egger's and Macaskill's tests were also used to identify potential publication bias in the studies, and both tests confirmed that there was no publication bias (P=0.7586 and P=0.5240, respectively; Table 1).
Figure 2.
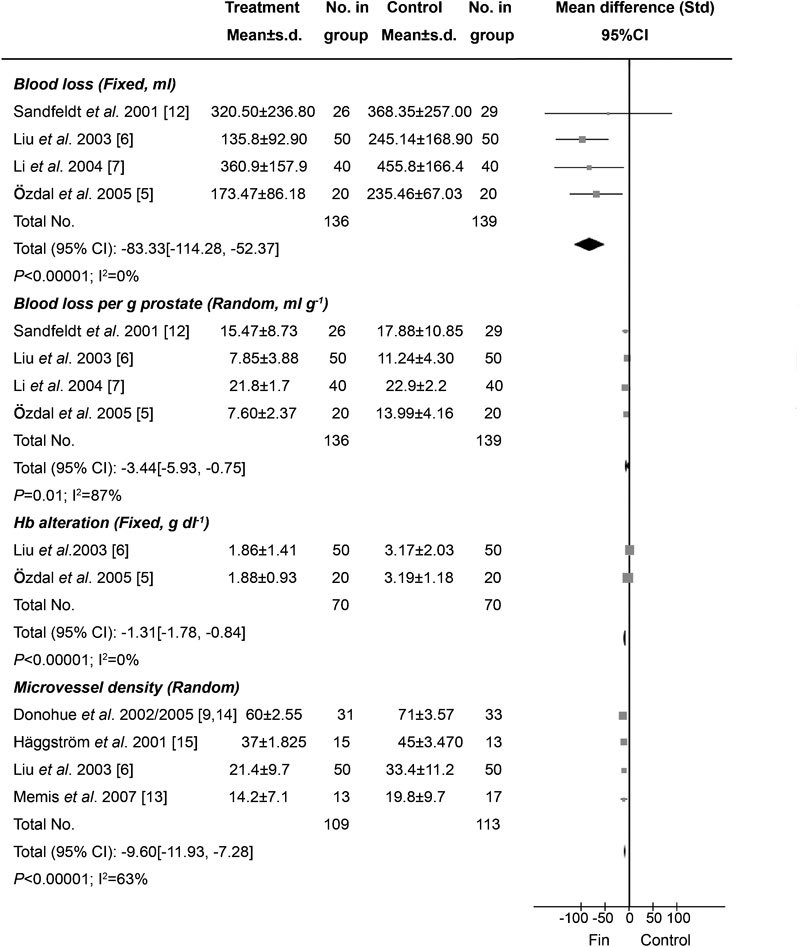
Effects of preoperative finasteride treatment on bleeding during TURP and its mechanisms. The volume of prevented blood loss ranged from 47.85 to 109.34 ml (P<0.00001), the volume of blood loss prevented per gram of resected prostate tissue ranged from 1.1 to 6.39 ml g−1 (P=0.01) (the range of prevented blood loss volume and blood loss volume per gram of resected prostate tissue were calculated on the reciprocal mean of different trials). Decreases in Hb were significantly less in the finasteride group (P<0.00001). Microvessel density in the finasteride group was lower than that in the control group (P<0.00001). CI, confidence interval; Hb, haemoglobin; TURP, transurethral resection of prostate.
Figure 3.
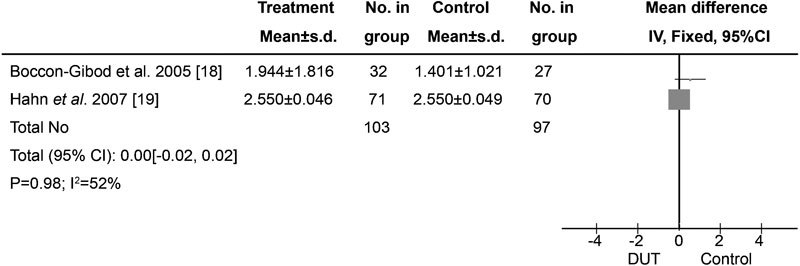
Effects of preoperative dutasteride on bleeding during transurethral interventions for BPH. No effect of preoperative dutasteride treatment on bleeding during transurethral intervention for BPH was found (P=0.98). BPH, benign prostatic hyperplasia; CI, confidence interval; DUT, dutasteride.
Figure 4.
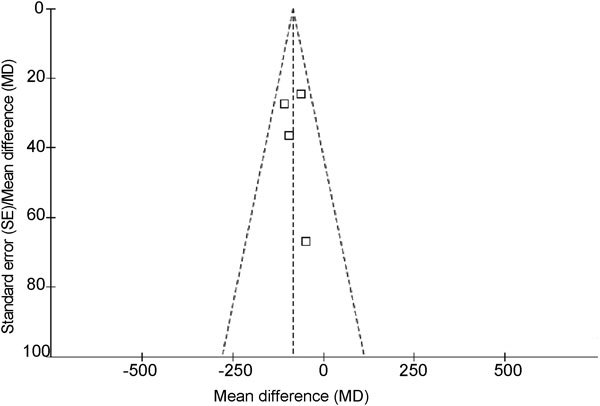
Funnel plot of the studies represented in our meta-analysis. This funnel plot provided us with a qualitative estimation of publication bias of the studies, and no evidence of bias was found.
Table 1. Egger's test and Macaskill's test of the studies included in the meta-analysis.
| Test | Std_eff/ Eff_size | Coefficient | Standard error | t | P>|t| | 95% confidence interval | |
|---|---|---|---|---|---|---|---|
| Egger's test | Slope | −0.94508 | 0.96382 | 0.35 | 0.4302 | −3.167664348 | 1.2775001936 |
| Bias | 1.36786 | 3.88901 | NA | 0.7586 | −7.600202806 | 10.335930666 | |
| Macaskill's test | Sample size | −0.00507 | 0.00662 | −0.77 | 0.5240 | −0.0203339 | 0.01019807 |
Abbreviation: NA, not available.
Results were expressed as the mean difference for continuous outcomes with 95% confidence intervals. The standard mean difference was used if the outcome data were not recorded in a consensus method. A ‘fixed-effects' statistical model was used if there was no conspicuous heterogeneity, and a ‘random-effects' model was used if heterogeneity was detected. Tests for heterogeneity were performed using Chi-square tests with significance level set at P<0.1. A sensitivity analysis was performed to explore whether the heterogeneity was a result of low study quality, and the lowest-quality trials were excluded.
Results
Characteristics of individual studies
The database search and reference lists of retrieved studies found 98 potential articles to be used in our meta-analysis. Based on the inclusion and exclusion criteria, 80 articles were excluded after simply reading the titles and abstracts of the articles and two articles were excluded because they lacked a full text. In all, 16 articles with 15 RCTs were included in the analysis, with 10 RCTs5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 for finasteride and 5 RCTs18, 19, 20, 21, 22 for dutasteride (Figure 1). The trials in these articles had been conducted in 13 different countries across Europe, North America and Asia. A majority (60%, 9/15) of the studies we analysed had a sample size larger than 50; the remaining 40% (6/15) had a sample size smaller than 50, but larger than 25. Baseline characteristics of the 15 individual studies included in our meta-analysis are listed in Table 2.
Table 2. Baseline characteristics and basic data of the 15 individual studies included in the present meta-analysis.
| Citation | Design | Trial | Control | Sample size | Age (years) | Duration of drugs | Source of study/patient cohort | Publication language | Research contents | P values | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | |||||||||
| Özdal et al.5 (2005) | RCT | FIN | No | 20 | 20 | 66.9 | 66.3 | 4 weeks | Ankara Numune Education and Research Hospital, Ankara, Turkey | English | Blood loss; blood loss per gram prostate; Hb alteration | P<0.05; P<0.0001;P<0.0001 |
| Liu et al.6 (2003) | RCT | FIN | No | 50 | 50 | 68.9 | 68.4 | 2 weeks | West China Hospital, Sichuan University, Chengdu, China | Chinese | Blood loss; blood loss per gram prostate; Hb alteration; MVD | P<0.05; P<0.05;P<0.05 |
| Li et al.7 (2004) | RCT | FIN | No | 40 | 40 | 70.7 | 72.1 | 1-2 weeks | Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China | Chinese | Blood loss; blood loss per gram prostate | P<0.05; P<0.05 |
| Lekas et al.8 (2006) | RCT | FIN | No | 88 | 90 | 68.6 | 68.8 | 25.3 weeks | General Hospital of Nikea and Sismanoglio Hospital, Greece | English | Blood loss; MVD | P<0.001; P<0.001 |
| Donohue et al.9, 14 (2002/2005) | RCT | FIN | Pla | 31 | 33 | 69.9 | 70.2 | 2 weeks | Good Hope Hospital, Birmingham, UK | English | Hb alteration; MVD | P>0.05; P<0.01 |
| Berardinis et al.10 (2005) | RCT | FIN | Pla | 100 | 100 | 68.0 | 69.0 | 8 weeks | University ‘La Sapienza' Policlinico Umberto, Italy | English | MVD | P<0.05 |
| Lund et al.11 (2005) | RCT | FIN | Pla | 16 | 17 | 66.5 | 67.0 | 12 weeks | Viborg Hospital, Viborg, Skejby Hospital and Aarhus University Hospital, Denmark | English | Blood loss | P>0.05 |
| Sandfeldt et al.12 (2001) | RCT | FIN | Pla | 26 | 29 | 69.0 | 68.0 | 12 weeks | Huddinge University Hospital, Karolinska Institute, Sweden andWycombe Hospital, UK | English | Blood loss; blood loss per gram prostate; MVD | P>0.05; P<0.002; P>0.05 |
| Memis et al.13 (2007) | RCT | FIN | No | 13 | 17 | 65.0 | 64.0 | 4 weeks | Numune Education and Research Hospital, Ankara, Turkey | English | MVD | P<0.05 |
| Häggström et al.15 (2002) | RCT | FIN | Pla | 15 | 13 | NM | NM | 12 weeks | Umea University, Sweden, Aarhus University Hospital, Denmark, and Sahlgrenska University Hospital, Goteborg, Sweden | English | MVD | P>0.05 |
| Boccon-Gibod et al.18 (2005) | RCT* | DUT | Pla | 32 | 27 | NM | NM | 2 weeks | Service d'Urologie, Hôpital Bichat, Paris, France | French | Hb alteration per gram prostate | P<0.05 |
| Hahn et al.19 (2007) | RCT* | DUT | Pla | 71 | 70 | 67.0 | 66.0 | 4 weeks | 23 centres in six countries (Denmark, Finland, Sweden, Norway, The Netherlands and UK) | English | Hb alteration per gram prostate; MVD | P>0.05; P>0.05 |
| Tuncel et al.20 (2009) | RCT | DUT | No | 27 | 21 | 68.1 | 67.7 | 5 weeks | Ankara Numune Research and Training Hospital, Ankara, Turkey | English | Blood loss; blood loss/weight; Hb alteration; MVD | P=0.124; P=0.130; P=0.912; P=0.401 |
| Ku et al.21 (2009) | RCT | DUT | No | 21 | 20 | 72.0 | 69.0 | 2-4 weeks | Seoul National University Hospital, Seoul, Korea | English | MVD | P=0.754 |
| Bepple et al.22 (2009) | RCT | DUT | Pla | 30 | 29 | 66.0 | 66.0 | 12 weeks | Eastern Virginia Medical School, Norfolk, VA, USA | English | Blood loss | P=0.14 |
Abbreviations: DUT, dutasteride; FIN, finasteride; MVD, microvessel density; NM, not mentioned; No, no drug was used; Pla, placebo; PV, green-light photoselective vaporisation of the prostate; RCT, randomized controlled trial; RCT*, multicentre randomized controlled trial; TURP, transurethral resection of the prostate.
Quality of individual studies
Among the studies included in the analysis, three described the randomisation processes that they had employed.8, 11, 15 Seven out of 15 studies used blinding methods,5, 9, 10, 14, 18, 19, 21 including four double-blinded RCTs.10, 12, 18, 19 One of these 15 studies performed a power calculation to assess the optimal sample size,11 and one used an intention-to-treat analysis.22 The quality levels of the 15 identified studies ranged from A to C.
Clinical outcome after preoperative finasteride treatment
Blood loss during management of BPH
Six studies of the effects of preoperative finasteride on total blood loss during surgery for BPH were identified, which involved 486 participants (240 in the treatment group and 246 in the control group); conclusions differed across studies.5, 6, 7, 8, 11, 12 No significant differences were found with respect to the resection weights of the samples. Two of the six articles were not included in the meta-analysis because they failed to report SD.8, 11 In total, only four trials were included in the meta-analysis, representing a cohort of 275 participants (136 in the treatment group and 139 controls) (Figure 2).5, 6, 7, 12 Among the four included studies, three RCTs supported the notion that preoperative finasteride could decrease bleeding during transurethral interventions for BPH,5, 6, 7 whereas one study did not.12 According to our analysis, no heterogeneity was found among the three trials (P=0.56), and thus a fixed-effects model was chosen for the analysis. Based on our analysis, the volume of blood loss saved ranged from 47.85 to 109.34 ml (P<0.00001) (Figure 2).
Blood loss per gram of resected prostate tissue
Four studies on the effects of preoperative finasteride on blood loss per gram of resected prostate tissue during surgery for BPH were identified for a total of 275 participants (136 in the treatment group and 139 controls) (Figure 2).5, 6, 7, 12 The volume of blood loss per gram of resected prostate tissue ranged from 1.1 to 6.39 ml g−1 (P=0.01),5, 6, 7 and no conspicuous difference was observed in the remaining study12 (Figure 2). Heterogeneity existed among the three trials as determined using the random-effects model (P<0.0001).
Hb change after surgery
Three studies were identified changes in Hb, including 208 patients overall (102 in the treatment group and 106 in control) (Figure 2).5, 6, 14 However, one trial was not included in the meta-analysis because it lacked SD values.14 There was no heterogeneity between the remaining two trials and therefore, the fixed-effects model was used (P=1.00). According to the analysis, the decrease in Hb in the finasteride group was significantly lower than in the control group (P<0.00001).
Mechanisms of finasteride action
Seven studies involving 655 participants referred to the MVD of the prostate tissues. However, only four of these seven studies presented SD values6, 13, 14, 15 and were included in the meta-analysis (Figure 2). In three experiments, the MVD of the resected prostate tissue was lower in the treatment group than in the control group;6, 14, 15 no differences were found in the remaining one study.13 Overall, 222 participants (109 in the treatment group and 113 controls) were included in the meta-analysis. Heterogeneity did not exist among the studies (P=0.04), so a random-effects model was chosen. The analysis revealed that the MVD of the resected prostate specimens was significantly lower in the finasteride group than in the control group (P<0.00001).
Clinical outcome with preoperative dutasteride
Five RCTs involving two multicentre trials were identified. Only two RCTs referred to changes in Hb per gram of resected prostate.18, 19 No heterogeneity was detected among the studies (P=0.15), and no significant differences were found between the treatment and control groups (P=0.98; Figure 3). In the study conducted by Tuncel et al.,20 total blood loss, blood loss/weight and Hb changes did not differ between the treatments and control groups (P=0.124, 0.130 and 0.912, respectively). In addition, no difference in blood loss volume between treatment and control groups was found by Bepple et al.22 (P=0.14).
MVD after treatment with dutasteride
Three studies that referred to MVD were identified.19, 20, 21 In the studies conducted by Tuncel et al.20 and Ku et al.,21 the mean MVD of the resected prostate specimens were not significantly different between the treatment and control groups (P=0.401 and 0.754, respectively), and a similar outcome was also observed in Hahn et al.19
Discussion
5α-RIs, including finasteride and dutasteride, are commonly used medical therapies for BPH and offer similar effects for treating lower urinary tract symptoms.24 Bleeding as a complication of the transurethral management of BPH is common both during and after surgery. The effects of finasteride and dutasteride on such bleeding and its mechanisms have been well studied, but the conclusions remain equivocal. The ability of preoperative finasteride to decrease blood loss has been observed in many studies,5, 6, 7, 8, 9, 10 but these effects are not found in all RCTs.11, 12, 13, 14, 15 For example, in the study conducted by Boccon-Gibod et al.,18 preoperative treatment with dutasteride decreased bleeding during treatment for BPH, but these findings were not consistent across studies.19, 20, 21, 22
Our meta-analysis reviewed 15 contemporary RCTs involving 1156 participants (580 who were treated with 5α-RIs and 576 patients in the control group). The results of this meta-analysis suggest that preoperative treatment with finasteride for 2–4 weeks could decrease bleeding during transurethral resection for BPH, possibly because finasteride appears to decrease MVD of prostate tissue. In contrast, dutasteride administered before BPH treatment has no impact on MVD of prostate specimens or intraoperative bleeding.
Finasteride, a type II 5α-RI, is a well-known inhibitor of the conversion of testosterone to dihydrotestosterone. Reduced expression of vascular endothelial growth factor (VEGF), a potent stimulator of angiogenesis, has been reported after finasteride treatment.25 One of the possible mechanisms for the relationship between VEGF and finasteride is that the former is an androgen-controlled growth factor.26 MVD is an indicator of vascularity and thus serves as a surrogate marker for bleeding.27 Based on our analysis of 10 RCTs that pertained to finasteride, we found that preoperative finasteride could significantly decrease MVD of the prostate tissue and reduce bleeding during BPH treatment. One possibility is that finasteride administered preoperatively for a duration from 2 weeks to 4 months reduces the expression of VEGF. In a study conducted by Liu et al.,6 a similar effect was observed with only 1–2 weeks of preoperative finasteride treatment.
Dutasteride offers the most complete blockade of 5α-reductase because it antagonizes both type I and II receptors. In theory, dutasteride should produce effects similar to finasteride. However, in our analysis, we did not find any differences between dutasteride treatment and control groups (P=0.98). It may be that the sample sizes of the trials were not large enough to generate enough data for detecting significant effects. Furthermore, the distinct pharmacological functions of each of the two 5α-RIs may be another factor that requires further research. Further high-quality studies are still needed to identify whether preoperative dutasteride actually affects the amount of bleeding during transurethral surgery for BPH.
We found that preoperative finasteride treatment for 2–4 weeks decreased bleeding during transurethral resection for BPH, and we suggested that this effect could be due to the ability of finasteride to decrease expression of VEGF and MVD in prostate tissue. Our results show that preoperative dutasteride had no effect on bleeding during transurethral procedures for BPH. As with all meta-analyses, it is important to consider certain caveats that accompany the findings. Publication bias may influence the results because negative data are less likely to be published. However, some of the published papers that we consulted contained negative results,11, 12, 13, 15 and our funnel plot does not provide any evidence of publication bias. Our results from the Egger's and Macaskill's tests also confirms that there has been no publication bias (P=0.7586 and P=0.5240, respectively; Table 1). The quality of the experiments used for the meta-analysis varied, and for some analyses, the sample sizes were still not large enough to obtain significant results. Additionally, several of the RCTs did not offer SD values, which may be because the data were skewed in their distribution because experiments were not sufficiently powered. According to the quality-assessment scale that we developed, the quality of individual studies in the meta-analysis was variable; quality appears to be the main reason for heterogeneity among these studies, and this heterogeneity probably arose from several factors. Firstly, there were important differences in the adequacy of the randomisation process, blinding methodology, control group and the duration for which the drugs were used preoperatively. Secondly, study outcomes may have been measured by different methods. Thirdly, the level of the operators varied in the trials. Finally, potential selection biases could have influenced the homogeneity of the groups, and relatively small sample sizes limited statistical power for identifying true associations. When heterogeneity among individual studies is taken into account, this meta-analysis will be crucial for assessing the effects of 5α-RIs on changes to MVD in prostate specimens and blood loss volumes during treatment of BPH. In consideration of the limited quality of the studies included in the analysis, we suggest that further experimental work should be rigorously designed, which includes allocation generation (e.g., random number tables, computer-generated random numbers, coin tossing and shuffling), allocation concealment (e.g., central randomisation; serially numbered, opaque and sealed envelopes), blinding method and use of intention-to-analysis. In addition, further high-quality prospective studies are needed to identify the mechanisms by which 5α-RIs exert their actions.
Author contributions
YZ designed the research, interpreted the data and revised the paper. HTZ, XXP and CCY performed the data extraction, conducted the meta-analysis and drafted the paper. All of the authors approved the submitted and final versions of the manuscript.
Acknowledgments
Financial support for our studies from the Research Fund of Capital Medical Development (No. 2009-2069) and Urological Backbone Fund of Beijing Municipal Health Bureau (No. 2009-3-15) is gratefully acknowledged.
The authors declare that they have no competing financial interests.
References
- Marks LS, Partin AW, Dorey FJ, Gormley GJ, Epstein JI, et al. Long-term effects of finasteride on prostate tissue composition. Urology. 1999;53:574–80. [PubMed] [Google Scholar]
- Pareek G, Shevchuk M, Armenakas NA, Vasjovic L, Hochberg DA, et al. The effect of finasteride on the expression of vascular endothelial growth factor and microvessel density: a possible mechanism for decreasing prostate bleeding in treated patients. J Urol. 2003;169:20–3. doi: 10.1016/S0022-5347(05)64025-6. [DOI] [PubMed] [Google Scholar]
- Puchner PJ, Miller MI. The effects of finasteride on hematuria associated with benign prostatic hyperplasia: a preliminary report. J Urol. 1995;154:1779–82. [PubMed] [Google Scholar]
- Sieber PR, Rommel FM, Huffnagle HW, Breslin JA, Agusta VE, et al. The treatment of gross hematuria secondary to prostatic bleeding with finasteride. J Urol. 1998;159:1232–3. [PubMed] [Google Scholar]
- Özdal Ö LÖ, zden C, Benli K, Gokkaya S, Bulut S, et al. Effect of short-term finasteride therapy on preoperative bleeding in patients who were candidates for transurethral resection of the prostate (TUR-P): a randomized controlled study. Prostate Cancer Prostatic Dis. 2005;8:215–8. doi: 10.1038/sj.pcan.4500818. [DOI] [PubMed] [Google Scholar]
- Liu XD, Yang YR, Lu YP, Zhang XH, Li FY, et al. Preoperative finasteride on decreasing operative bleeding during transurethral resection of prostate. Chin J Urol. 2003;24:694–6. [Google Scholar]
- Li GH, He ZF, Yu DM, Li XD, Chen ZD. Effect of finasteride on intraoperative bleeding and irrigating fluid absorption during transurethral resection of prostate: a quantitative study. J Zhejiang Univ (Med Sci) 2004;33:258–60. doi: 10.3785/j.issn.1008-9292.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Lekas AG, Lazaris AC, Chrisofos M, Papatsoris AG, Lappas D, et al. Finasteride effects on hypoxia and angiogenetic markers in benign prostatic hyperplasia. Urology. 2006;68:436–41. doi: 10.1016/j.urology.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Sharma H, Abraham R, Natalwala S, Thomas DR, et al. Transurethral prostate resection and bleeding: a randomized, placebo controlled trial of the role of finasteride for decreasing operative blood loss. J Urol. 2002;168:2024–6. doi: 10.1016/S0022-5347(05)64287-5. [DOI] [PubMed] [Google Scholar]
- Berardinis ED, Antonini G, Busetto GM, Gentile V, Silverio FD, et al. Reduced intraoperative bleeding during transurethral resection of the prostate: evaluation of finasteride, vascular endothelial growth factor, and CD34. Curr Prostate Rep. 2005;6:123–7. [Google Scholar]
- Lund L, Ernst-Jensen KM, Tørring N, Nielsen JE. Impact of finasteride treatment on perioperative bleeding before transurethral resection of the prostate: a prospective randomized study. Scand J Urol Nephrol. 2005;39:160–2. doi: 10.1080/00365590510007694. [DOI] [PubMed] [Google Scholar]
- Sandfeldt L, Bailey DM, Hahn RG. Blood loss during transurethral resection of the prostate after 3 months of treatment with finasteride. Urology. 2001;58:972–6. doi: 10.1016/s0090-4295(01)01408-x. [DOI] [PubMed] [Google Scholar]
- Memis A, Ozden C, Ozdal OL, Guzel O, Han O, et al. Effect of finasteride treatment on suburethral prostatic microvessel density in patients with hematuria related to benign prostate hyperplasia. Urol Int. 2008;80:177–80. doi: 10.1159/000112610. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Hayne D, Karnik U, Thomas DR, Foster MC. Randomized, placebo controlled trial showing that finasteride reduces prostatic vascularity rapidly within 2 weeks. BJU Int. 2005;96:1319–22. doi: 10.1111/j.1464-410X.2005.05849.x. [DOI] [PubMed] [Google Scholar]
- Häggström S, Tørring N, Møller K, Jensen E, Lund L, et al. Effects of finasteride on vascular endothelial growth factor—a placebo controlled randomized study in BPH patients. Scand J Urol Nephrol. 2002;36:182–7. doi: 10.1080/003655902320131848. [DOI] [PubMed] [Google Scholar]
- Zhu YC, Zhong L, Wei Q. Meta-analysis of finasteride for perioperative bleeding in patients undergoing transurethral resection of prostate. Chin J Evid-based Med. 2008;8:456–60. [Google Scholar]
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–41. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- Boccon-Gibod L, Valton M, Ibrahim H, Comenducci A. Effect of dutasteride on reduction of intraoperative bleeding related to transurethral resection of the prostate. Prog Urol. 2005;15:1085–9. [PubMed] [Google Scholar]
- Hahn RG, Fagerström T, Tammela TL, Trip OV, Beisland HO, et al. Blood loss and postoperative complications associated with transurethral resection of the prostate after pretreatment with dutasteride. BJU Int. 2007;99:587–94. doi: 10.1111/j.1464-410X.2006.06619.x. [DOI] [PubMed] [Google Scholar]
- Tuncel A, Ener K, Han O, Nalcacioglu V, Aydin O, et al. Effects of short-term dutasteride and Serenoa repens on perioperative bleeding and microvessel density in patients undergoing transurethral resection of the prostate. Scand J Urol Nephrol. 2009;43:377–82. doi: 10.3109/00365590903164498. [DOI] [PubMed] [Google Scholar]
- Ku JH, Shin JK, Cho MC, Myung JK, Moon KC, et al. Effect of dutasteride on the expression of hypoxia-inducible factor-1a,vascular endothelial growth factor and microvessel density in rat and human prostate tissue. Scand J Urol Nephrol. 2009;43:445–53. doi: 10.3109/00365590903337896. [DOI] [PubMed] [Google Scholar]
- Bepple JL, Barone BB, Eure G. The effect of dutasteride on the efficacy of photoselective vaporization of the prostate: results of a randomized, placebo-controlled, double-blind study (DOP trial) Urology. 2009;74:1101–4. doi: 10.1016/j.urology.2009.06.067. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Chichester; John Wiley & Sons, Ltd; 2009. Cochrane Handbook for Systematic Reviews of Interventions 5.0.2. [Google Scholar]
- Kaplan S, Chung D, Lee R, Melamed S, Te A. A 5 year study of the use 5-alpha reductase inhibitors in men with benign prostatic hyperplasia: finasteride has equal efficacy and prostate volume reduction but has less sexual side effects and breast enlargement than dutasteride. J Urol. 2010;183:e692–3. [Google Scholar]
- Monti S, Sciarra F, Adamo MV, Toscano V, Trotta MC, et al. Prevalent decrease of the EGF content in the periurethral zone of BPH tissue induced by treatment with finasteride or f lutamide. J Androl. 1997;18:488–94. [PubMed] [Google Scholar]
- Haggstrom S, Lissbrant IF, Bergh A, Damber JE. Testosterone induces vascular endothelial growth factor synthesis in the ventral prostate in castrated rats. J Urol. 1999;161:1620–5. [PubMed] [Google Scholar]
- Foley SJ, Bailey DM. Microvessel density in prostatic hyperplasia. BJU Int. 2000;85:70–3. doi: 10.1046/j.1464-410x.2000.00322.x. [DOI] [PubMed] [Google Scholar]


