Abstract
Prostate cancer is one of the most common malignancies in men. Previous research has determined that androgen deprivation therapy (ADT) may be accompanied by an unfavourable metabolic profile. In this prospective study, 133 men were recruited, including 46 prostate cancer patients who had undergone bilateral orchiectomy and been on flutamide (the ADT group), 37 men with prostate cancer who had undergone radical prostatectomy (the non-ADT group) and 50 normal control subjects (the control group). All subjects were followed for at least 12 months. From baseline to 3 months, men in the ADT group had increased levels of fasting serum insulin and low-density lipoprotein compared to the other two groups (P<0.05). No obvious changes were found in the other parameters (P>0.05). After 12 months, men in the ADT group had increased levels of waist circumference, fasting serum insulin and glucose, total cholesterol, high-density lipoprotein and low-density lipoprotein compared to the other two groups (P<0.05). Additionally, the morbidity rate of metabolic syndrome in the ADT group was higher (P<0.05) compared to the other two groups. ADT through surgical castration for men with prostate cancer may be associated with unfavourable metabolic changes. The benefits of the therapy should be balanced prudently against these risks.
Keywords: androgen deprivation therapy, bilateral orchiectomy, metabolic changes, metabolic syndrome, prostate cancer
Introduction
Prostate cancer is the fifth most common malignancy worldwide and the second most common malignancy in men, with 679 000 new cases in 2002. These cases accounted for 19% and 5.3% of new cancer cases in developed and developing countries (11.7% overall), respectively, making regional difference one of its major characteristics.1 In 2002, the incidence rate of prostate cancer was 1.6 per 100 000 in China. Although this rate is lower compared with that in the United States and other Western countries, it is continuously increasing, especially in China's urban areas. In Shanghai, the rate has increased from 1.6 per 100 000 in 1973 to 7.7 per 100 000 in 2000.2 The prognosis of prostate cancer is relatively good; 86% of prostate cancer cases are diagnosed as local or regional disease and have a 5-year relative survival rate close to 99%.3 Even with respect to advanced disease, the 5-year survival rate is 20.8%, which also seems to be better than breast cancer (17.0%), colorectal cancer (5.3%) and lung cancer (1.5%).4
Since Huggins et al.5 demonstrated the androgen dependence of prostate cancer, androgen deprivation therapy (ADT) has been increasingly applied in the treatment for prostate cancer. ADT with gonadotropin-releasing hormone agonist or bilateral orchiectomy is the mainstay of treatment for metastatic and recurrent prostate cancer6 and may be beneficial to men with locally advanced disease.7, 8, 9 Although ADT offers important clinical benefits, it is associated with a variety of complications such as hot flushes, anaemia, sexual dysfunction, changing body composition, osteoporosis and fractures, and increased risk for diabetes and cardiovascular diseases.10, 11, 12 Some of the complications have negative effects on quality of life assessments, while others may induce serious problems including myocardial infarction and sudden cardiac death.13 A long-term study revealed that 53% of the deaths were not attributable to prostate cancer. Of these cases, cardiovascular problems are the predominant causes.14 Because the prognosis of prostate cancer is favourable, physicians should be prudent when considering ADT because of the complications and therefore follow the patients more closely. In this study, we investigated metabolic changes in men with prostate cancer undergoing ADT through bilateral orchiectomy.
Materials and methods
Subjects and study design
This was a prospective study (n=133) conducted in a university-affiliated hospital. Forty-six men with newly diagnosed prostate cancer were recruited when they were about to commerce ADT according to clinical guidelines for the treatment of prostate cancer (the ADT group). All of these patients underwent bilateral orchiectomy and were being treated with flutamide (250 mg, three times daily; Fudan Forward Co., Ltd, Shanghai, China). Thirty-seven men with prostate cancer underwent radical prostatectomy were also recruited (the non-ADT group). Men in the non-ADT group received no further intervention except the surgery. The control group was composed of 50 normal males. All subjects completed a baseline evaluation. They returned to hospital every 3 months for follow-up and were studied for at least 12 months.
Exclusion criteria
Men were excluded from this study if they had any of the following: liver function tests or serum creatinine levels over twice the upper limit of normal, a history of any form of hypogonadism before the study, a history of thyroid diseases, a history of diabetes mellitus or cardiovascular diseases, and metastatic prostate cancer.
Outcome measures
The 133 men completed a baseline evaluation before initiating the treatment. All of them returned to hospital every 3 months for further evaluation after an overnight fast. Early in the morning of each visit, fasting subjects were weighted while wearing a hospital gown using a platform scale. Their blood pressure was measured with a mercurial sphygmomanometer, and blood samples were collected. Fasting serum insulin, fasting blood glucose, fasting cholesterol, triglycerides, high-density lipoprotein and low-density lipoprotein were measured in Shanghai Renji Hospital clinical laboratories. The International Diabetes Federation definition was used to diagnose metabolic syndrome.15
Statistical analysis
All data were analysed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). The normality of the analysed data was corroborated using the Shapiro–Wilk test. When parametric analysis was possible, the data were expressed as mean±s.d., and one-way analysis of variance was used for comparison among the three groups. The Chi-square test was used for nonparametric analysis. P<0.05 was considered statistically significant.
Results
All subjects completed a baseline evaluation before initiating their treatment. Age, testosterone, waist circumference and the levels of fasting serum insulin, glucose and lipids were comparable between the three groups (Table 1). No significant differences were found at baseline (P>0.05</tblref>). From baseline to 3 months, a significant decrease in testosterone was observed in men undergoing ADT (P<0.05). Additionally, they showed increased levels of fasting serum insulin and low-density lipoprotein compared with the other two groups (P<0.05). No significant changes were found in the other parameters (Table 2).
Table 1. Baseline evaluation of the three groups.
| ADT group (n=46) | Non-ADT group (n=37) | Control group (n=50) | P value* | |
|---|---|---|---|---|
| Age (years) | 70.9±5.4 | 69.5±3.9 | 71.7±4.9 | 0.114 |
| Testosterone (ng dl−1) | 449±152 | 409±145 | 425±133 | 0.518 |
| Waist circumference (cm) | 86.6±6.4 | 85.6±6.6 | 86.1±5.8 | 0.762 |
| Fasting insulin (mU l−1) | 15.21±4.67 | 16.25±4.02 | 14.45±3.43 | 0.127 |
| Fasting glucose (mmol l−1) | 5.23±0.55 | 5.35±0.52 | 5.20±0.45 | 0.375 |
| Total cholesterol (mmol l−1) | 4.82±0.49 | 4.80±0.53 | 4.65±0.44 | 0.204 |
| Triglyceride (mmol l−1) | 1.55±0.42 | 1.47±0.36 | 1.40±0.29 | 0.138 |
| HDL (mmol l−1) | 1.32±0.23 | 1.28±0.28 | 1.25±0.19 | 0.366 |
| LDL (mmol l−1) | 3.03±0.56 | 2.87±0.56 | 3.08±0.43 | 0.165 |
Abbreviations: ADT, androgen deprivation therapy; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
One-way analysis of variance was used to compare parameters at baseline among the three groups.
Table 2. Evaluation of the three groups after 3 months.
| ADT group (n=46) | Non-ADT group (n=37) | Control group (n=50) | P value* | ||||
|---|---|---|---|---|---|---|---|
| 3 months | Change | 3 months | Change | 3 months | Change | ||
| Testosterone (ng dl−1) | 39±19 | −410±151 | 386±131 | −23±72 | 458±156 | 33±71 | <0.001 |
| Waist circumference (cm) | 87.2±6.9 | 0.6±3.1 | 85.4±5.5 | −0.2±2.5 | 86.3±6.2 | 0.3±2.7 | 0.373 |
| Fasting insulin (mU l−1) | 22.47±5.64 | 7.26±2.50 | 16.89±4.06 | 0.64±2.96 | 15.68±3.22 | 1.23±2.63 | <0.001 |
| Fasting glucose (mmol l−1) | 5.39±0.69 | 0.16±0.47 | 5.58±0.47 | 0.23±0.34 | 5.34±0.54 | 0.14±0.56 | 0.689 |
| Total cholesterol (mmol l−1) | 5.14±0.67 | 0.32±0.47 | 4.95±0.67 | 0.15±0.36 | 4.82±0.51 | 0.17±0.31 | 0.070 |
| Triglyceride (mmol l−1) | 1.60±0.42 | 0.05±0.32 | 1.55±0.41 | 0.08±0.37 | 1.43±0.31 | 0.03±0.23 | 0.762 |
| HDL (mmol l−1) | 1.41±0.31 | 0.09±0.24 | 1.29±0.22 | 0.01±0.27 | 1.35±0.25 | 0.10±0.21 | 0.192 |
| LDL (mmol l−1) | 3.21±0.48 | 0.18±0.45 | 3.02±0.65 | 0.15±0.69 | 2.97±0.55 | −0.11±0.45 | 0.012 |
Abbreviations: ADT, androgen deprivation therapy; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*One-way analysis of variance was used to compare “change from baseline” among the three groups.
After 12 months of treatment, men in the ADT group had decreased testosterone and higher levels of waist circumference, fasting serum insulin and glucose, total cholesterol, high-density lipoprotein and low-density lipoprotein compared with the other two groups (P<0.05; Table 3).
Table 3. Evaluation of the three groups after 12 months.
| ADT group (n=46) | Non-ADT group (n=37) | Control group (n=50) | P value* | ||||
|---|---|---|---|---|---|---|---|
| 12 months | Change | 12 months | Change | 12 months | Change | ||
| Testosterone (ng dl−1) | 25±9 | −424±149 | 356±127 | −53±66 | 394±129 | −31±68 | <0.001 |
| Waist circumference (cm) | 88.5±6.3 | 1.8±3.4 | 85.9±6.0 | 0.3±2.8 | 86.7±5.7 | 0.7±2.3 | 0.035 |
| Fasting insulin (mU l−1) | 26.99±6.94 | 11.78±3.91 | 18.02±3.97 | 1.77±3.48 | 16.88±3.09 | 2.43±2.94 | <0.001 |
| Fasting glucose (mmol l−1) | 6.11±0.75 | 0.88±0.68 | 5.46±0.51 | 0.11±0.51 | 5.52±0.37 | 0.32±0.44 | <0.001 |
| Total cholesterol (mmol l−1) | 5.32±0.61 | 0.50±0.44 | 4.86±0.68 | 0.06±0.38 | 4.97±0.46 | 0.32±0.29 | <0.001 |
| Triglyceride (mmol l−1) | 1.59±0.42 | 0.04±0.32 | 1.62±0.52 | 0.15±0.40 | 1.49±0.31 | 0.09±0.25 | 0.344 |
| HDL (mmol l−1) | 1.51±0.42 | 0.19±0.23 | 1.38±0.31 | 0.10±0.29 | 1.32±0.24 | 0.07±0.20 | 0.048 |
| LDL (mmol l−1) | 3.46±0.72 | 0.43±0.72 | 3.09±0.53 | 0.22±0.43 | 3.14±0.48 | 0.06±0.41 | 0.003 |
Abbreviations: ADT, androgen deprivation therapy; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*One-way analysis of variance was used to compare “change from baseline” among the three groups.
At baseline, 9 out of 46 patients in the ADT group met the criteria for metabolic syndrome compared to 5 out of 37 patients in the non-ADT group and 8 out of 50 subjects in the control group (χ2=0.561, P=0.755). However, after 12 months of treatment, components of metabolic syndrome in the ADT group, including waist circumference and fasting blood glucose, were higher than those in the other two groups. Seventeen out of 46 patients in the ADT group met the criteria for metabolic syndrome compared with 6 out of 37 patients in the non-ADT group and 9 out of 50 subjects in the control group (χ2=6.438, P=0.040).
Men undergoing ADT had a significantly higher morbidity rate of metabolic syndrome compared with other two groups (P<0.05) (Figure 1).
Figure 1.
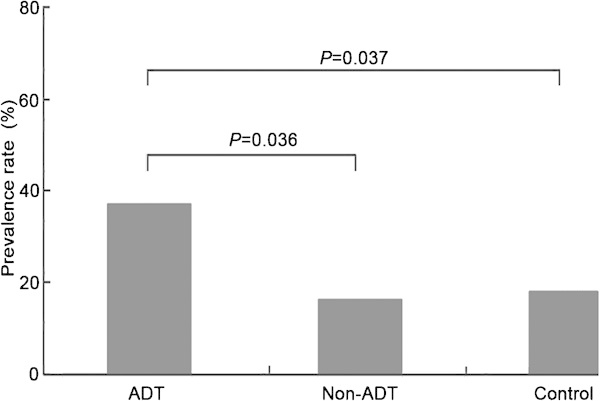
The prevalence rate of metabolic syndrome in the three groups after 12 months of treatment. ADT, androgen deprivation therapy.
Discussion
The benefits of ADT have been well-received for select patients. Over the past few decades, the use of ADT has been greatly expanded, while relatively little attention has been paid to its side effects.16 Men with prostate cancer have higher rates of non-cancer mortality than do men in the general population, and some of the excess non-cancer deaths may be treatment-induced.17 In men undergoing ADT, metabolic changes are observed often, which may lead to an increased risk of type 2 diabetes, cardiovascular diseases and metabolic syndrome and might contribute to the increase of non-cancer deaths.18
A review of the literature suggests that male hypogonadism is accompanied by an increased risk of insulin resistance and diabetes mellitus.19, 20, 21 Ding et al.22 performed a systematic review of 43 studies including 6427 men and found that total testosterone levels of men with type 2 diabetes were significantly lower than men without diabetes. Men with prostate cancer who are undergoing ADT are excellent examples of hypogonadism; therefore, they might have insulin resistance and hyperglycemia as well. In our study, increased fasting serum insulin, a marker of insulin resistance, was observed in the ADT group. From baseline to 3 months, insulin level in the ADT group rose by 47.7%, and it continued to increase afterwards. Twelve months later, the elevation of fasting blood glucose in the ADT group was then significant. Other studies also confirmed this connection between ADT, insulin resistance and hyperglycemia.13, 23, 24 Although metabolic changes are sometimes neglected in the early phase, they can start as early as 3 months after treatment. Fasting insulin is a more sensitive index compared to fasting blood glucose. Therefore, physicians should pay more attention to fasting insulin after the commencement of ADT and consider taking necessary measures when the insulin level is too high. Furthermore, waist circumference in the ADT group was higher than in the other two groups after 12 months of treatment (P<0.05). All of these observations suggest that ADT for prostate cancer may lead to the development of insulin resistance and type 2 diabetes.
The role of androgen in development of cardiovascular diseases has been attractive. Decreased testosterone may lead to unfavourable changes in the lipid profile. In the ADT group, low-density lipoprotein levels increased as early as 3 months after the commencement of ADT. After 12 months, elevated levels of total cholesterol, high-density lipoprotein and low-density lipoprotein were observed. These findings are in agreement with previous studies. Dockery et al.25 reported elevated total cholesterol and high-density lipoprotein 3 months after the beginning of ADT. In another short-term study, increased total cholesterol, triglycerides and high-density lipoprotein were observed.24 Braga-Basaria et al.26 performed a retrospective study and found higher total cholesterol and low-density lipoprotein levels in patients receiving ADT. Although the outcomes of these studies are slightly different, there may be a link between ADT and hyperlipidemia. Hyperlipidemia and insulin resistance, as we discussed above, are also risk factors for cardiovascular diseases.27 It is possible that ADT could increase cardiovascular risk because of its adverse effect on the risk factors of cardiovascular diseases. There may also be a relation between ADT and cardiovascular risk.28
It is reported that metabolic syndrome is seen in over half of men undergoing long-term ADT.29 In our study, the incidence rates of metabolic syndrome in the three groups were comparable at baseline. After 12 months of treatment, 17 out of 46 patients in the ADT group met the criteria for metabolic syndrome compared with 6 out of 37 patients in the non-ADT group and 9 out of 50 subjects in the control group. The incidence rate of metabolic syndrome in the ADT group was higher than in the other two groups (P=0.040). We suppose that the increased incidence rate may be to some extent treatment-related, because ADT in our study could lead to increased fasting blood glucose and waist circumference, which are both components of metabolic syndrome. A close relationship between hypogonadism and central obesity, which is one of the criteria of metabolic syndrome, has been established.30, 31 From baseline to 1 year, ADT may increase fat mass by 9.4%–11.0%,32, 33 which consists of subcutaneous fat rather than visceral fat. Nevertheless, metabolic syndrome caused by ADT is to some extent distinct from the classic metabolic syndrome. In all three definitions of metabolic syndrome—by the National Cholesterol Education Program34, the World Health Organization35 and the International Diabetes Federation15—one of the criteria is decreased high-density lipoprotein. However, high-density lipoprotein was higher in men undergoing ADT in our study. Similar results were observed in other studies.24, 25 Moreover, a low level of adiponectin and elevated inflammatory markers characterize classic metabolic syndrome. Conversely, it is reported that ADT increases the serum adiponectin level and does not change the level of C-reactive protein.36, 37
Our study has several strengths. First, this was a prospective study in which all subjects had been followed for at least 12 months. Second, all patients in the ADT group underwent surgical castration, while most studies reported on medical castration. When medical castration is performed, the serum testosterone level increases, rather than decreases, during the following week, which is called flare-up phenomenon.38 It takes 3–4 weeks to achieve a castration level.38 Surgical castration, still considered the gold standard of ADT, is the quickest way to reach a castration level, usually within 12 h. Bilateral orchiectomy, which is a simple procedure, is still preferred in some circumstances to avoid flare-up phenomenon, especially when treating metastatic diseases. Third, we recruited two different groups to compare with the ADT group. The recruitment of the non-ADT group provided us the opportunity to rule out the influence of prostate cancer itself. By comparing the ADT group with the control group, we could remove the influence of aging. Forth, this study provides data of metabolic changes in Asian men undergoing ADT. There are also some limitations in this study. First, although our sample size was comparable with those of former studies performed in this field, it was still relatively small. Second, this was not a randomized study. It may be possible that men in the ADT group had more advanced disease in the first place. Third, because the metabolic syndrome caused by ADT might be different from classic metabolic syndrome, more metabolic parameters, including adiponectin, C-reactive protein, glycosylated haemoglobin, waist-to-hip ratio and lean body mass, should be tested.
In conclusion, ADT is a cornerstone of treatment for metastatic, recurrent and locally advanced prostate cancer, although it is a treatment with some complications. Fortunately, increasing attention is being paid to the side effects of ADT, and the Food and Drug Administration has ordered manufacturers of gonadotropin-releasing hormone agonists to add warnings related to the increased risk of cardiovascular diseases and diabetes. Results of the current study demonstrate that ADT may lead to hyperglycemia, hyperlipidemia and an increased risk of metabolic syndrome. This study might aid patients and physicians in making crucial decisions when they are evaluated for ADT. Future research is needed to confirm the findings using a larger sample size, so as to further demonstrate the relationship between ADT and its complications and to develop strategies to prevent treatment-related mortality.
Author contributions
JJB participated in the design and coordination of the study and helped to draft and revise the manuscript. CZ contributed to data acquisition, performed the statistical analysis and drafted the manuscript. LHZ participated in the design and coordination of the study. PL made substantial contributions to data acquisition. JJS and JWL made contributions to data acquisition and helped with data interpretation. DML and YRH revised the manuscript critically and gave final approval of the version to be published. ZL conceived of the study and participated in its design. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by the Key Project of Science and Technology Commission of Shanghai Municipality (No.09DJ1400400).
The authors indicate no financial support or financial conflict of interests.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Ye DW, Li CL. Epidemiological trends of prostate cancer: retrospect and prospect. Chin Oncol. 2007;17:177–80. [Google Scholar]
- Ries LA, Eisner MP, Kosary CL, Hankey BF, Miller BA, et al. editors. SEER Cancer Statistics Review, 1975–2001 BethesdaMD; National Cancer Institute; 2004. Available at: http://seer.cancer.gov/csr/1975_2001/ [Google Scholar]
- Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. 2001;92:2211–9. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer. II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–23. [Google Scholar]
- Barry MJ, Delorenzo MA, Walker-Corkery ES, Lucas FL, Wennberg DC. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: a population-based cohort study. BJU Int. 2006;98:973–8. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- Payne H. Management of locally advanced prostate cancer. Asian J Androl. 2009;11:81–7. doi: 10.1038/aja.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbarn H, Boccon-Gibod L, Carroll PR, Montorsi F, Schulman C, et al. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol. 2009;55:62–75. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt A, Garcia JA. Complications of androgen deprivation therapy in prostate cancer. Curr Opin Urol. 2009;19:322–6. doi: 10.1097/MOU.0b013e32832a082c. [DOI] [PubMed] [Google Scholar]
- Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- Wadhwa VK, Weston R, Parr NJ. A large proportion of patients with prostate cancer undergoing androgen deprivation therapy continue to die from non-cancer causes in the PSA era. Br J Med Surg Urol. 2009;2:191–6. [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Tadros NN, Garzotto M. Androgen deprivation therapy for prostate cancer: not so simple. Asian J Androl. 2011;13:187–8. doi: 10.1038/aja.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst. 1993;85:979–87. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]
- Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts Male Aging Study. Diabetes Care. 2000;23:490–4. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- Simon D, Charles MA, Nahoul K, Orssaud G. Kremski et al Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab. 1997;82:682–5. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–08. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who received androgen deprivation therapy. Cancer. 2006;106:581–8. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci. 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- Braga-Basaria M, Muller DC, Carducci MA, Dobs AS, Basaria S. Lipoprotein profile in men with prostate cancer undergoing androgen deprivation therapy. Int J Impot Res. 2006;18:494–8. doi: 10.1038/sj.ijir.3901471. [DOI] [PubMed] [Google Scholar]
- Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci. 2010;23:51–61. [PubMed] [Google Scholar]
- Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–40. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–83. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Impot Res. 2009;21:1–8. doi: 10.1038/ijir.2008.41. [DOI] [PubMed] [Google Scholar]
- Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13:353–63. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–5. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Smith MR, Lee H, Fallon MA, Nathan DM. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–22. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Lee H, McGovern F, Fallon MA, Goode M, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–94. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limonta P, Montagnani MM, Moretti RM. LHRH analogues as anticancer agents: pituitary and extrapituitary sites of action. Expert Opin Investig Drugs. 2001;10:709–20. doi: 10.1517/13543784.10.4.709. [DOI] [PubMed] [Google Scholar]


