The XX/XY sex chromosomal system of mammals, including human, challenges the chromosome pairing mechanism during male meiosis. Pairing and subsequent separation of homologous chromosomes generates haploid cells from diploid cells during the meiotic divisions. One of the basic requirements for recognition between homologous chromosomes is DNA sequence identity. Since the X and Y chromosome share little homology, their quest for each other is difficult, and has special characteristics. During the lengthy meiotic prophase, all autosomal chromosomes synapse, by forming a special protein structure called the synaptonemal complex, which connects the chromosomal axes. In contrast, the X and Y chromosome synapse only in the short homologous pseudoautosomal regions, and form the so-called XY body.1 In this specialized chromatin area, transcription is shut down by a mechanism named meiotic sex chromosome inactivation (MSCI) (reviewed by Refs. 2 and 3). The sex chromosomes remain silent throughout the rest of meiotic prophase, and only few sex-linked genes are (re)activated in postmeiotic spermatids.2 Since long, scientists have wondered how MSCI is achieved, and what its biological significance is.
Recently, two papers4, 5 significantly increased our understanding of how and why MSCI works.
So far, the only molecule that was known to be absolutely essential for initiation of MSCI was γH2AX.6 This phosphorylated form of histone H2AX marks the XY body from its formation until diplotene in mouse.7 In mice lacking H2AX, no XY body is formed, the sex chromosomes are not silenced and meiosis arrests at pachytene.6 The phosphorylation of H2AX at the XY body is mediated by the checkpoint kinase ATR that can be visualized along the unsynapsed axes of X and Y from late zygotene onwards.8 In their recent paper, Ichijima et al.4 reveal that the phosphorylation of H2AX occurs in two phases; first, it is restricted to the chromosomal axes, then the area extends, and H2AX also becomes phosphorylated in the surrounding chromatin loops. This second phase, when γH2AX spreads, depends on a protein named mediator of DNA damage checkpoint 1 (MDC1), and this function is required for transcriptional silencing of the sex chromosomes (Figure 1). MDC1 is a known binding partner of γH2AX,9, 10 and both proteins, as well as ATR, are well known for their role in the DNA damage response pathway in somatic cells. This pathway plays a pivotal role in sensing the presence of single-stranded DNA at stalled replication fork to allow repair as well as activation of cell cycle checkpoints. Interestingly, the authors also analyzed the role of MDC1 in somatic cells. In analogy to their observations in meiotic cells, they observed reduced amplification of ATR-dependent γH2AX signals after replicative stress in the absence of MDC1, compared to control cells. They also showed that RNA polymerase II is excluded from chromatin at replication-stalled sites that are marked by ATR- and MDC1-dependent γH2AX accumulation, thereby confirming and extending the observations described by Shanbag et al.,11 who discovered that DNA double-strand breaks (DSBs) elicit transcriptional silencing in cis. These data reveal an analogy between somatic and meiotic cells, with respect to the link between DNA repair and DNA damage response protein accumulation (at respectively DNA damage sites and unsynapsed axes), and transcriptional silencing. It should be noted that 200–400 enzymatically generated DSBs are induced at the onset of meiotic prophase, to mediate homology recognition and meiotic recombination. Meiotic DSBs are also induced in the non-homologous regions of X and Y, and DSB repair molecules are present on the unsynapsed X chromosomal axis.12 Together with the observation that meiotic silencing is impaired when repair of meiotic DSBs is hampered,13 this indicates that MSCI is also mechanistically related to DSB repair and the DNA damage response pathway in somatic cells.
Figure 1.
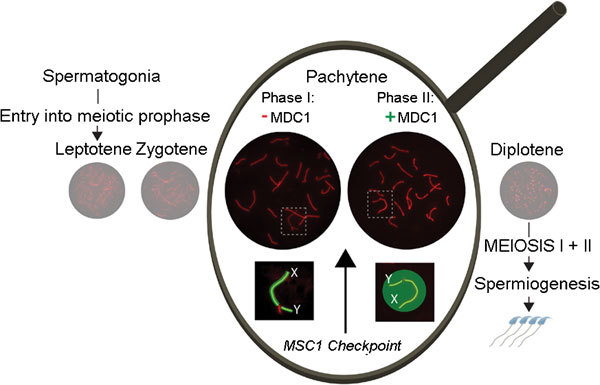
Zoom in on sex chromosomes during pachytene. Schematic representation of spermatogenesis, showing the different substages of meiotic prophase (leptotene, zygotene, pachytene and diplotene). During meiotic prophase, progression of chromosome pairing and synapsis can be followed by immunofluorescent staining of SYCP3 (red), a component of the axial/lateral elements of the synaptonemal complex. In the enlarged area, the recent findings of Ichijima et al.4 and Royo et al.5 are summarized: mediator of DNA damage checkpoint 1 (MDC1) mediates spreading of γH2AX (green), phosphorylated by ATR (yellow) from the unsynapsed chromosomal axes (phase I) into the surrounding chromatin (phase II). Spreading of γH2AX and ATR is required to achieve meiotic sex chromosome inactivation (MSCI) and MSCI failure induces apoptosis (MSCI checkpoint) of pachytene spermatocytes, caused by misexpression of one or more sex-linked genes.
In yeast, a pachytene checkpoint operates to block development when meiotic recombination or synapsis has failed. In mouse, there also is a very strong pachytene arrest during male meiosis whenever synapsis and DSB repair are affected.2 In addition, MSCI fails in such situations.13 A recent series of experiments by Royo et al.5 has shown that pachytene arrest in male mice can be induced by inappropriate expression of sex-linked genes, and they identified a single Y-linked gene, named Zfy, whose expression is sufficient to induce apoptosis of pachytene spermatocytes. Thus, in the Mdc1 knockout mice analyzed by Ichijima et al.,4 it is to be expected that the failure to achieve MSCI also explains the strict pachytene arrest that is observed in these mice (Figure 1). Whether the presence of autosomal asynapsed chromatin and/or persistent DNA damage can also trigger activation of a pachytene checkpoint is not clear. In mouse oocytes with an impaired meiotic recombination machinery, checkpoint activation is not very efficient, leading to the formation of many aneuploid oocytes and early embryo loss upon fertilization of such oocytes.14 Thus, although the presence of heteromorphic sex chromosomes could be viewed as a problem for the male, it seems to have evolved into a blessing in disguise by providing a very efficient means to avoid the generation of aberrant sperm. In this context, it is also relevant to mention that the same gene (Zfy2) that needs to be repressed by MSCI to prevent an apoptotic response during pachytene, is somehow required to mediate apoptotic removal of cells with misaligned sex chromosomes during the first meiotic metaphase.15 This provides another male advantage to the fidelity of meiosis.
Meiosis is a tricky business; no other cell generates hundreds of DSBs on purpose, and their complete repair is required to generate gametes with an intact genome. As a back-up, unrepaired damage present in postmeiotic cells may be repaired during spermiogenesis or in the zygote,16 but such repair would be associated with an increased risk of generating mutations. Human spermatocytes also contain a silenced XY body, and meiotic arrest is a frequent cause of infertility. To be able to asses whether meiotic defects could be associated with an increased risk to transmit mutations or chromosome aberrations via intacytoplasmatic sperm injection, it will be important to determine the quality of the pachytene checkpoint/arrest in human males. In a case report of a patient with an impairment of meiotic DSB repair, cell degeneration was reported to occur both during meiotic prophase and during meiotic divisions.17 X-autosome or autosome-to-autosome translocations that might be associated with impaired MSCI are usually associated with infertility, but sperm cells for intacytoplasmatic sperm injection could be obtained in at least one case, leading to transmission of the translocation.18 Solari and Rey Valzacchi19 have analyzed spermatocytes in an XYY human male. Since YY synapsis is observed in almost half of these cells, it would be expected that this would lead to a failure to induce MSCI, which should be associated with cell death during pachytene, as was observed in mouse XYY cells. However, Solari and Rey Valzacchi19 observe more prominent loss of XYY germ cells during metaphase I, indicating that the pachytene arrest that should be elicited by defective MSCI may be weaker in man compared to mouse. This is in accordance with data from microarray analyses of testicular gene expression in man and chimpanzee, that indicate that human MSCI may be less efficient or complete.20 Future in-depth analyses of meiotic progression in human spermatocytes from infertile patients should reveal whether MSCI in man also acts to safeguard the male germline against transmitting a damaged or incomplete genome.
References
- Monesi V. Differential rate of ribonucleic acid synthesis in the autosomes and sex chromosomes during male meiosis in the mouse. Chromosoma. 1965;17:11–21. doi: 10.1007/BF00285153. [DOI] [PubMed] [Google Scholar]
- Turner JM. Meiotic sex chromosome inactivation. Development. 2007;134:1823–31. doi: 10.1242/dev.000018. [DOI] [PubMed] [Google Scholar]
- Inagaki A, Schoenmakers S, Baarends WM. DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics. 2010;5:255–66. doi: 10.4161/epi.5.4.11518. [DOI] [PubMed] [Google Scholar]
- Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Camerini-Otero RD, et al. MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev. 2011;25:959–71. doi: 10.1101/gad.2030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, et al. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol. 2010;20:2117–23. doi: 10.1016/j.cub.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, et al. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell. 2003;4:497–508. doi: 10.1016/s1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–6. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Turner JM, Aprelikova O, Xu X, Wang R, Kim S, et al. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14:2135–42. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Du LL, Redon C, Russell P. Histone H2A phosphorylation controls Crb2 recruitment at DNA breaks, maintains checkpoint arrest, and influences DNA repair in fission yeast. Mol Cell Biol. 2004;24:6215–30. doi: 10.1128/MCB.24.14.6215-6230.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Melander F, Stucki M, Falck J, Bekker-Jensen S, et al. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 2004;23:2674–83. doi: 10.1038/sj.emboj.7600269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell. 2010;141:970–81. doi: 10.1016/j.cell.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Chen DJ, Shen Z, Kolas N, Tarsounas M, et al. Rad51 immunocytology in rat and mouse spermatocytes and oocytes. Chromosoma. 1997;106:207–15. doi: 10.1007/s004120050241. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Bourc'his D, de Rooij DG, Bestor TH, Turner JM, et al. Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol. 2008;182:263–76. doi: 10.1083/jcb.200710195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu JG, Hoja MR, Wilbertz J, Nordqvist K, et al. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science. 2002;296:1115–8. doi: 10.1126/science.1070594. [DOI] [PubMed] [Google Scholar]
- Vernet N, Mahadevaiah SK, Ojarikre OA, Longepied G, Prosser HM, et al. The y-encoded gene zfy2 acts to remove cells with unpaired chromosomes at the first meiotic metaphase in male mice. Curr Biol. 2011;21:787–93. doi: 10.1016/j.cub.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer P, Ramos L, de Vries M, Gochhait S. Memoirs of an insult: sperm as a possible source of transgenerational epimutations and genetic instability. Mol Human Reprod. 2010;16:48–56. doi: 10.1093/molehr/gap098. [DOI] [PubMed] [Google Scholar]
- Sciurano RB, Rahn MI, Pigozzi MI, Olmedo SB, Solari AJ. An azoospermic man with a double-strand DNA break-processing deficiency in the spermatocyte nuclei: case report. Hum Reprod. 2006;21:1194–203. doi: 10.1093/humrep/dei479. [DOI] [PubMed] [Google Scholar]
- Ma S, Yuen BH, Penaherrera M, Koehn D, Ness L, et al. ICSI and the transmission of X-autosomal translocation: a three-generation evaluation of X;20 translocation: case report. Hum Reprod. 2003;18:1377–82. doi: 10.1093/humrep/deg247. [DOI] [PubMed] [Google Scholar]
- Solari AJ, Rey Valzacchi G. The prevalence of a YY synaptonemal complex over XY synapsis in an XYY man with exclusive XYY spermatocytes. Chromosome Res. 1997;5:467–74. doi: 10.1023/a:1018469030537. [DOI] [PubMed] [Google Scholar]
- Mulugeta Achame E, Baarends WM, Gribnau J, Grootegoed JA. Evaluating the relationship between spermatogenic silencing of the X chromosome and evolution of the Y chromosome in chimpanzee and human. PloS One. 2010;5:e15598. doi: 10.1371/journal.pone.0015598. [DOI] [PMC free article] [PubMed] [Google Scholar]


