Abstract
Compounds with dual action on cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) may be a treatment option for erectile dysfunction, as they not only promote penile erection but also prevent the upregulation of phosphodiesterase-5. In this study, we examined the possible relaxant effect and mechanism of 17-nor-subincanadine E (SEC, 0.2–200 µmol l−1), a plant-derived alkaloid, in rabbit corpus cavernosum (RbCC) strips that had been precontracted by exposure to phenylephrine (10 µmol l−1) or a high concentration of K+ (60 mmol l−1) in vitro. In addition to SEC's effect on cAMP and cGMP levels, electrical field stimulation (EFS) in phenylephrine-precontracted RbCC and calcium chloride (1–100 mmol l−1) evoked responses in depolarized RbCC were analysed. SEC relaxed the phenylephrine-precontracted RbCCs in a concentration-dependent manner. Atropine, guanethidine and N-ω-nitro-ℓ-arginine methyl ester (L-NAME) did not have any effect on the relaxation of RBCCs. When 1H-1, 2, 4oxadiazole[4,3-a] quinoxalin-1-one (ODQ) was added, it effectively blocked the relaxant response of SEC. Although SEC enhanced the maximal relaxation produced by sodium nitroprusside (SNP) and forskolin in phenylephrine-precontracted cavernosal smooth muscle, it caused a decrease in the maximal contractile response induced by calcium chloride in depolarized RbCCs. The relaxant effect of SEC was paralleled by an increase in the tissue levels of the cyclic nucleotides cAMP and cGMP. We conclude that SEC promotes the relaxation of RbCC, possibly favouring cAMP and cGMP accumulation and calcium blockade. This novel mechanism could be useful for patients who do not benefit from phosphodiesterase inhibitors and for those with endothelial and nitrergic dysfunction, such as patients with diabetes, hypertension and dyslipidaemias.
Keywords: 17-nor-subincanadine E, cyclic nucleotides, electrical field stimulation, nitric oxide, rabbit corpus cavernosum, smooth muscle relaxation
Introduction
The erect penis has always been a symbol of power, virility and fertility. Erectile dysfunction (ED) is the consistent inability to achieve and maintain an erection sufficient for satisfactory sexual activity. This condition is a complex neurovascular disorder that affects 52% of men aged 40–70 years and causes considerable distress, unhappiness and relationship problems for these men.1, 2 Penile erection occurs as a function of trabecular smooth muscle relaxation and a subsequent increase in blood flow to lacunar spaces, which results in engorgement of the penis and restriction of venous drainage. Basically, the process is mediated by nitric oxide (NO) release from cavernosal nerve varicosities and endothelial cells, and this release activates intracellular guanylate cyclase to produce cGMP.3 Recently, the selective phosphodiesterase 5 (PDE5) inhibitors sildenafil, tadalafil and vardenafil were shown to enhance the proerectile effect of NO by decreasing cGMP catabolism rates, thereby reinforcing the physiological signals that facilitate smooth muscle relaxation and erection;4 however, their use is contraindicated with concomitant nitrate administration or alpha-adrenergic blockers. Furthermore, endothelial and nitrergic dysfunction, which are both progressive, are common in patients with ED associated with cardiovascular risk factors, such as those with diabetes and hypertension. Decreased synthesis and activity of NO associated with endothelial and nitrergic dysfunction may account for the observed tolerance and inefficacy (as high as 56%) of PDE5 inhibitors in these patients.5 Increasing awareness of the adverse side effects with commonly prescribed medications on sexual function provides a rationale for developing new treatment strategies, and studies point out that compounds with dual action on cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) may be a treatment option for ED, as they not only promote penile erection but also prevent the upregulation of PDE5.6, 7 Therefore, alternative forms of medical treatment remain clinically interesting, including the use of plant extracts, which many patients prefer as a treatment option.8
Intense activity exists in the area of testing semipurified plant extracts for ED. Some plant-derived alkaloids, such as apomorphine, berberine, neferine and yohimbine, have been effective in alleviating impotence and ED.9, 10, 11, 12, 13 Aspidosperma ulei (Markgr.) (Apocynaceae), which grows largely in the Amazon region of Brazil and elsewhere in South America, is a plant rich in indole alkaloids14 and has an ethnomedicinal history as a traditional remedy for the treatment of ED. In the search for novel drugs effective against ED, our previous studies evaluated semi-purified extracts of Aspidosperma ulei, in vitro as well as in vivo, and the results obtained were encouraging.15, 16 In a continuation study, we examined the effect of 17-nor-subincanadine E (SEC), a pure indole alkaloid isolated from the stem bark of Aspidosperma ulei, on penile erection-related behavioural responses in mice, and we found that SEC has a proerectile activity in vivo simulating yohimbine, a known indole alkaloid used to treat ED.17 The present study aimed at assessing the possible relaxant effect of SEC on isolated rabbit corpus cavernosum (RbCC) strips and elucidating the underlying mechanism.
Materials and methods
Plant material and isolation of SEC
Aspidosperma ulei (Markgr.) stem bark was collected from the Garapa area of Acarape, Ceará, Brazil, after its identification, and a specimen of the plant material has been deposited in Herbarium Prisco Bezerra (# 30823) of Federal University of Ceará, Fortaleza, Brazil, for future reference. The air-dried plant material (4.0 kg) was extracted with EtOH at room temperature. The corresponding extract (432.32 g, 10.81%) was designated AUE. AUE (20.7 g) was suspended in 200 ml of 2 mol l−1 HCl and was mixed by shaking for 30 min. The acid suspension was filtered under vacuum, and the resultant solution was partitioned with CH2Cl2 (3×200 ml), yielding 776.9 mg of a residue, designated AUEA, after work-up. The aqueous layer was alkalinized to pH 9 with NH4OH and extracted with CH2Cl2 (3×200 ml), yielding 781.8 mg of a residue designated AUEB. AUEA (350 mg) was preabsorbed on 500 mg of silica and was subjected to silica gel (2.5 g) chromatography by elution with a mixture of CH2Cl2–CH3OH (98∶2–94∶6), affording three subfractions. Among these, fraction 2 was identified as a new alkaloid named SEC (Figure 1), by using spectroscopy analysis (mass spectrometry, infrared and nuclear magnetic resonance), including modern 2D NMR techniques and by comparison to data in the literature.18 The characteristics of fraction 2 are as follows: 83.1 mg; melting point 229.1–230.4 °C; [α]25D=−36.7 ° (c 0.33, CH3OH); IR: 3410, 1618, 1375, 1247 and 748 cm−1. Spectral details are provided as Supplemental information. The isolated compound was 95%–98% pure, with a molecular weight of 496.02.
Figure 1.
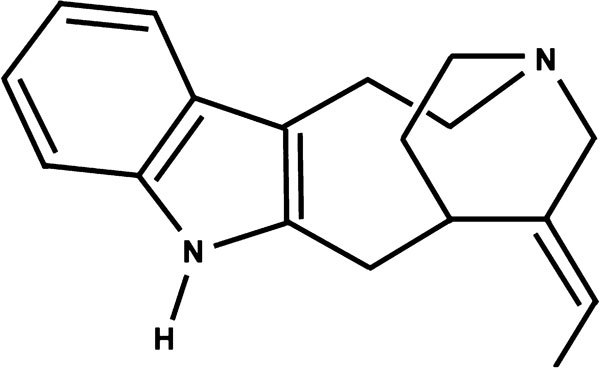
Chemical structure of 17-nor-subincanadine E.
Chemicals and reagents
Phenylephrine (PE), phentolamine hydrochloride, guanethidine atropine, N-ω-nitro-L-arginine methyl ester (L-NAME), 1H-1, 2, 4oxadiazole[4,3-a] quinoxalin-1-one (ODQ), acetylcholine chloride, carbamylcholine chloride, sodium nitroprusside (SNP), glibenclamide, apamin, forskolin, indomethacin, lidocaine, guanethidine, scopolamine and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Chemicals Co. (São Paulo, Brazil). Pentobarbitone sodium was purchased from Hypnol-Cristália (São Paulo, Brazil) and iberiotoxin from Tocris Bioscience (Ellisville, MO, USA). Drug solutions were freshly prepared on the day of the experiment. Indomethacin (Sigma, Poole, UK) was dissolved in 5% NaHCO3, and all other chemicals were dissolved and diluted in isotonic saline. First, SEC was dissolved in 3% (v/v) DMSO in saline to make a 60 mmol l−1 stock solution. For studies, the final concentration of DMSO did not exceed 0.1% in nutrient solution. A similar volume and DMSO concentration (maximal concentration used in tissue baths was 0.03%) were used as a vehicle control in all experimental protocols. Male New Zealand rabbits were obtained from Zootecnia College cuniculture, Federal University of Ceará. The study protocols were approved by the Institutional Ethics Committee of the Federal University of Ceará in accordance with the guidelines of National Institutes of Health on the use and care of animals for experimentation.
Preliminary studies on the relaxant effect of SEC on different smooth muscles
SEC concentration–response curves (0.2–600 µmol l−1) were constructed using RbCC strips, aortic rings (endothelium-intact and -denuded), tracheal rings and the duodenum. Isolated RbCCs and aortic rings were precontracted with PE (10 µmol l−1). Tracheal rings (five rings) were mounted as described by Castillo and de Beer19 and were precontracted with carbamylcholine (10 µmol l−1), and duodenum was mounted vertically and precontracted with a high-potassium solution (K+, 60 mmol l−1). To obtain endothelial-denuded preparations, a thoracic aortic segment was rapidly removed and cut into rings (5 mm), and the vessel's lumen was gently rubbed. The procedure was considered successful when no relaxation was observed after a 1 µmol l−1 acetylcholine challenge.20 These tissues were suspended on steel hooks, under 1 g of resting tension, in 5 ml organ baths containing Krebs–Henseleit solution gassed with 95% O2/5% CO2. The tension was recorded by force-displacement transducers (F-60; Narco Biosystems, Houston, TX, USA) coupled to a four-channel polygraph (Narco BioSystems). The tissues were allowed to equilibrate for 60 min, washing them every 15 min before starting any pharmacological observation. After an equilibration period of 1 h, tissues were precontracted, and the contraction tonic component was used as an internal standard control. After washing, an SEC concentration–response curve was performed in precontracted tissues, and the effect was expressed as the percentage of standard contraction. SEC effects were studied using two paired segments mounted on different baths and cumulatively increasing the SEC concentration in one bath while adding vehicle, isovolumetrically, to the other. The differences between the control baseline and test segment were used to express the relaxation induced by SEC. The relaxation (negative deflection) was measured and expressed as the percentage of maximal tonic contraction (positive deflection) induced by constrictor agents.
SEC relaxant effect on rabbit corpus cavernosa smooth muscle
Male New Zealand white rabbits (6-month-old; 2.5–3.0 kg) were used. Study protocols were approved by the Institutional Ethics Committee of the Federal University of Ceará in accordance with the guidelines of National Institute of Health on the use and care of animals for experimentation. For experiments, animals were anesthetized with pentobarbital sodium (Hypnol, 35–40 mg kg−1, i.v.) and were killed by exsanguination. The penis was removed at the level of corporal body attachment to the ischium and was immersed in cold Krebs solution (pH 7.4). The corporal tissues were carefully dissected free from the tunica albuginea, and strips were prepared and mounted under 1 g of resting tension in 5 ml organ baths filled with warmed (37 °C) and oxygenated (95% O2 in 5% CO2) in Krebs solution. Following a 60-min equilibration period, tension was induced by adding PE (10 µmol l−1). At the contraction plateau, relaxation responses to cumulative concentrations (0.2–600 µmol l−1) of SEC were registered on a desk model polygraph (DMP-4B; Narco Bio-Systems) using a model FT-60 (Narco Bio-System) force-displacement transducer.
In separate experiments, when a stable contraction to PE (10 µmol l−1) was attained, SEC (0.2–200 µmol l−1) was added to the organ bath in the presence of vehicle or one of the following pharmacological blockers: 10 µmol l−1 atropine, 100 µmol l−1 L-NAME or 100 µmol l−1 ODQ. SEC concentration–response curve was also obtained in 10 µmol l−1 phenylephrine-precontracted strips with 100 µmol l−1 glibenclamide or the combination of 1 µmol l−1 apamin plus 100 nmol l−1 iberiotoxin. Cavernosal strips were pre-incubated with the above pharmacological agents in the bath chamber for 30 min before the addition of SEC, and the curves were performed in the presence of these compounds. In another set of experiments, RbCCs were pre-treated for 30 min with 10 µmol l−1 guanethidine and 10 µmol l−1 phentolamine, and thereafter, an SEC concentration–response curve was performed in strips tonically precontracted with 60 mmol l−1 K+. When a stable contraction to PE (10 µmol l−1) was reached, concentration–response curves for sodium nitrate (pH 2.0; 1–3000 nmol l−1) and forskolin (10−9–10−5 mol l−1) were constructed. The tissues were incubated with SEC (20 and 60 µmol l−1) for 15 min and then precontracted with 10 µmol l−1 phenylephrine; in the contraction plateau, the concentration–response curves for sodium nitrate and forskolin were determined (usually about 60 min) , where SEC was still present.
Effect of SEC on electrical field stimulation (EFS)-induced cavernosal muscle relaxation
EFS was accomplished by means of two platinum ring electrodes and a stimulus isolation unit in series with an S48 stimulator (Astro-Med Industrial Park, West Warwick, RI, USA). After an equilibration period, the strips were contracted with phenylephrine (10 µmol l−1). When the phenylephrine-induced contractile responses had stabilized, the tissue was subjected to the first EFS-induced relaxation at 20 V (0.5 ms pulse duration), using sequential frequencies of 0.5, 1, 2, 4, 8 and 16 Hz delivered as 20 s trains. Fifty minutes after addition of SEC (2, 6 or 20 µmol l−1) to the bath at basal tonus, tissues were precontracted, and the second frequency–response curve was performed. For this experiment, we added 10 µmol l−1 indomethacin, 5 µmol l−1 scopolamine and 5 µmol l−1 guanethidine to obtain clear nitrergic responses. Furthermore, we verified the neurogenic nature of the responses by checking whether they could be blocked by lidocaine (1 mmol l−1), a neuronal voltage-dependent sodium channel blocker.
Effect of SEC on calcium chloride-induced contractions in depolarized RbCC
Concentration–response curves obtained by increasing concentrations of calcium chloride (CaCl2; 1–100 mmol l−1) were performed in RbCCs previously depolarized with Krebs–Henseleit with 60 mmol l−1 K+ and containing zero nominal calcium. The whole procedure was repeated in the presence of SEC (7, 15 or 30 µmol l−1) or vehicle in separate experiments.
Effect of SEC on phenylephrine-induced cavernosal contractions in the presence of SNP or forskolin and tissue levels of cAMP and cGMP
The cavernosal strips were contracted with PE (10 µmol l−1). After the contractile response plateaued, SEC (15 or 30 µmol l−1), SNP (1 µmol l−1) or forskolin (1 µmol l−1) was added to the tissue during tonic contraction. When drugs caused a maximal change in tension, the tissues were immediately frozen in liquid nitrogen. The frozen tissues were homogenized with a microhomogenizer in 1 ml 6% trichloroacetic acid containing 1 mmol l−1 EDTA. After centrifugation (3000g, 15 min, 4 °C), the supernatant was extracted with water-saturated diethyl ether, and aliquots of the aqueous phase were dried under nitrogen atmosphere and then reconstituted in the respective buffer kit (cAMP or cGMP) for analysis. Cyclic nucleotide solution levels were measured by the acetylation method with commercially available cAMP and cGMP immunoassay kits (Cayman Chemical Company, Ann Arbor, MI, USA). Protein content was determined using the Bradford method.21 The cyclic nucleotide levels were expressed in pmol mg−1 protein.
Statistical analysis
The effect of SEC at each concentration was calculated as a percentage decrease from the maximal contractile responses of agonists. The concentration producing 50% of the maximal effect (EC50) and the maximal effect were calculated for each agent. The differences between groups were verified using a one-way analysis of variance (ANOVA) followed by a Tukey–Kramer post hoc test with 5% significance. The data were analysed using SIGMA STAT software (Sigma Chemical Co., St Louis, MO, USA).
Results
SEC evoked concentration-dependent relaxation in all tissues tested (i.e., the corpora cavernosa, endothelium-intact and endothelium-denuded aortic rings, tracheal chains and the duodenum). Nevertheless, SEC was relatively selective for corpora cavernosa tissue, with an EC50 approximately fourfold smaller than the calculated EC50 for relaxation in aortic rings and approximately 10-fold smaller than the EC50 for relaxation induced in tracheal rings and duodenum (Table 1). The EC50 did not differ between endothelium-denuded and endothelium-intact aortic rings (Table 1).
Table 1. EC50 (µmol l−1) values with 95% confidence intervals of SEC-induced relaxation in rabbit corpora cavernosa (RbCC), aortic rings, tracheal chains and duodenum.
| Experimental condition | Tissue | EC50 (µmol l−1) |
|---|---|---|
| Precontracted with phenylephrine (10 µmol l−1) | Corpus cavernosum | 13.6 (9.8–19.0)* |
| Precontracted with phenylephrine (10 µmol l−1) | Aortic rings E+ | 52.4 (32.2–164.8) |
| Precontracted with phenylephrine (10 µmol l−1) | Aortic rings E− | 59.6 (24.6–144.6) |
| Precontracted with carbamylcholine (10 µmol l−1) | Tracheal rings | 120.4 (66.0–219.6) |
| Spontaneous contractility | Duodenum | 153.6 (66.2–356.4) |
Abbreviations: E+, endothelium-intact; E−, endothelium-denuded.
*P<0.05, compared with other four groups, analysis of variance (ANOVA) followed by Tukey–Kramer test.
The relaxant effect of SEC on RbCC strips was apparent after 2 µmol l−1 (11.3%) and was statistically significant at 6 µmol l−1 (28.3%); 20 µmol l−1 attained approximately equal to 60% level and, a more complete relaxation was apparent at higher concentrations (Figure 2a). The SEC concentration–response curves obtained in phenylephrine-precontracted RbCC were unaffected by pretreatment with selective blockers of ATP-dependent potassium channels (KATP), glibenclamide or calcium-dependent potassium channels (Kca), the combination of apamin and iberiotoxin (Figure 2b). Similarly, the incubation of RbCC strips with 10 µmol l−1 atropine, a concentration that could completely block 1 µmol l−1 acetylcholine-induced relaxation, did not affect the SEC concentration–response curve (Figure 3a).
Figure 2.
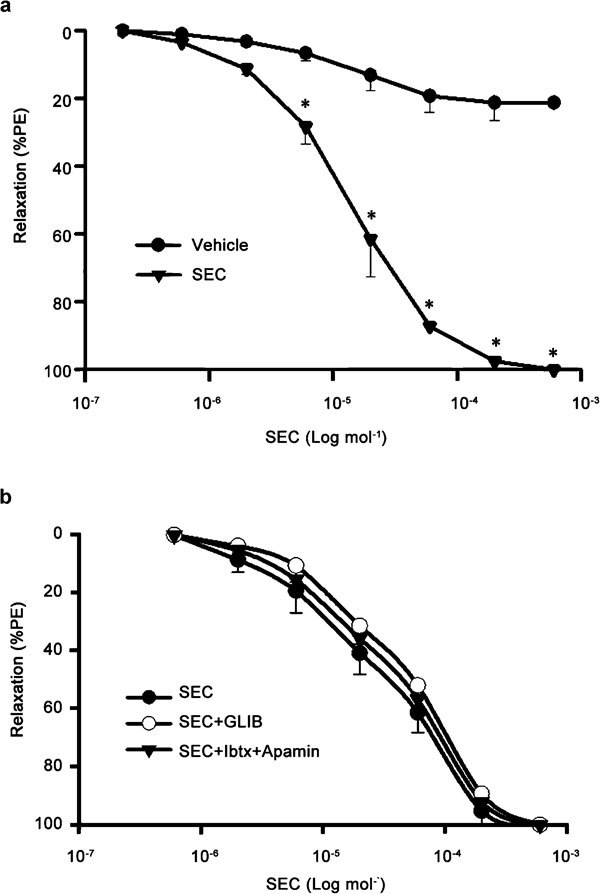
Concentration–response curves for 17-nor-subincanadine E (SEC, 0.2–600 µmol l−1) in rabbit corpora cavernosa (RbCC) precontracted with phenylephrine (PE, 10 µmol l−1) alone (a) or in the presence of glibenclamide (GLIB, 10 µmol l−1) or the combination of 0.1 µmol l−1 iberiotoxin (Ibtx) plus 0.1 µmol l-1 apamin (b). Data are presented as mean±s.e.m. of the relaxation expressed as percentage of phenylephrine-induced contraction. *P<0.05 vs. control (vehicle), one-way analysis of variance (ANOVA) followed by Tukey–Kramer test.
Figure 3.
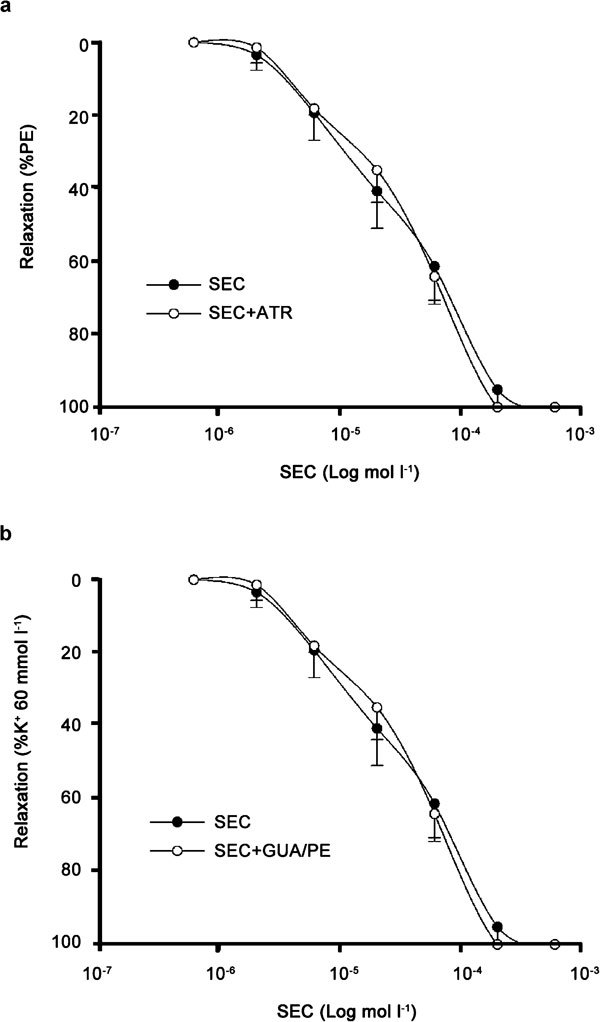
Concentration–response curves for 7-nor-subincanadine E (SEC, 0.2–600 µmol l−1) in rabbit corpora cavernosa precontracted (a) with phenylephrine (PE, 10 µmol l-1), alone or in the presence of atropine (ATR, 10 µmol l−1), and (b) with a high-concentration potassium solution (K+, 60 mmol l−1), alone or in the presence of the combination of guanethidine (GUA, 10 µmol l−1) and phenylephrine (10 µmol l−1). Data are presented as mean±s.e.m. (n=5) of the relaxation expressed as percentage of phenylephrine or high potassium concentration-induced contraction.
In addition, the association of guanethidine, which is known to deplete catecholamine from nerve endings, and phentolamine, an alpha-adrenergic blocker, failed to alter the SEC concentration–response curve in K+ (60 mmol l−1) precontracted RbCC strips (Figure 3b). Similarly, a 30-min incubation period with L-NAME, a NO synthase inhibitor, did not have a significant influence on the concentration-dependent relaxation induced by SEC (Figure 4a and b). However, the incubation of RbCC strips with ODQ, a selective, potent, non-competitive inhibitor of NO-stimulated sGC, shifted the SEC concentration–response curve to the right (Figure 4c). SEC (20, 60 and 200 µmol l−1) evoked 61.5%, 91.1% and 100% relaxation in the absence of ODQ, respectively, and 19.8%, 37.7% and 79.1% in the presence of ODQ (Figure 4c), respectively. The calculated EC50 for SEC-induced relaxation in the presence of ODQ was 114.4 µmol l−1 (range: 85.8–152.8 µmol l−1), which corresponds to approximately an eightfold increase when compared with the response obtained in the absence of ODQ (EC50=13.6 µmol l−1 (range: 9.6–19.0 µmol l−1)).
Figure 4.
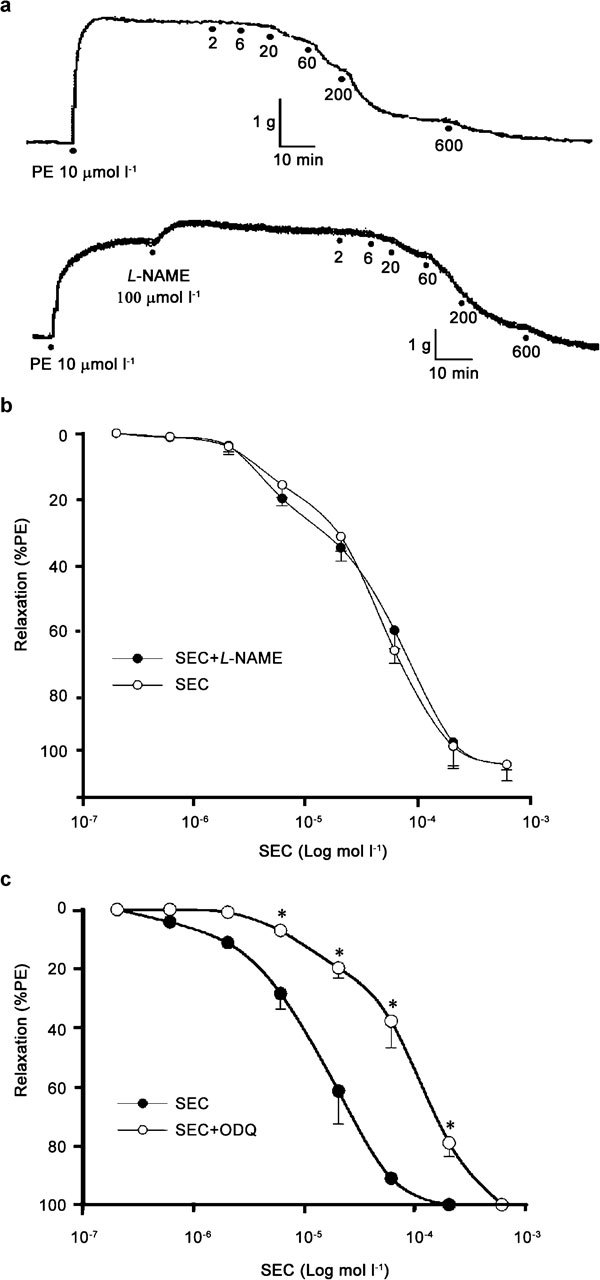
(a) Physiographic recordings representative of the effect of 7-nor-subincanadine E (SEC, 2–600 µmol l−1) on rabbit corpora cavernosa (RbCC) in the absence (upper) and presence (lower) of N-ω-nitro-L-arginine methyl ester (L-NAME, 100 µmol l−1). (b) Concentration–response curve for SEC (2–600 µmol l−1) in RbCC, alone or in the presence of L-NAME (100 µmol l−1. (c) Concentration–response curve for SEC (2-600 µmol l−1) on RbCC, alone or in the presence of 1H-1, 2, 4oxadiazole[4,3-a] quinoxalin-1-one (ODQ, 30 µmol l−1). Data are presented as mean±s.e.m. (n=5) of the relaxation expressed as percentage of phenylephrine (PE)-induced contraction. *P<0.05 vs. control (SEC alone), (ANOVA) followed by Tukey–Kramer test.
The maximal relaxant response to sodium nitrate (NaNO2, 3 µmol l−1) was 61% in control phenylephrine-precontracted RbCC strips and 77% and 93% in tissues incubated for 15 min with 20 and 60 µmol l−1 SEC, respectively (Figure 5a). The concentration–response curves for both sodium nitrate and forskolin were shifted upwards and to the left in tissues treated with SEC (Figure 5b). The EC50 for sodium nitrate-induced relaxation was 297.9 nmol l−1 (range: 206.1–430.7 nmol l−1) in control RbCC strips and 145.5 nmol l−1 (range: 89.3–236.8 nmol l−1) and 91.8 nmol l−1 (range: 57.2–147.3 nmol l−1) in tissues pre-treated with SEC 20 and 60 µmol l−1, respectively. Similarly, the maximal relaxant response evoked by 1 µmol l−1 forskolin was 62% in control RbCC strips and 84.7% and 96% in tissues treated with 20 and 60 µmol l−1 SEC, respectively (Figure 5b). The calculated EC50 for relaxation evoked by forskolin varied from 550 nmol l−1 (range: 425–709 nmol l−1) to 187.6 nmol l−1 (range: 143.7–244.9 nmol l−1) and 58.9 nmol l−1 (range: 39.9–86.8 nmol l−1) in control RbCC strips.
Figure 5.
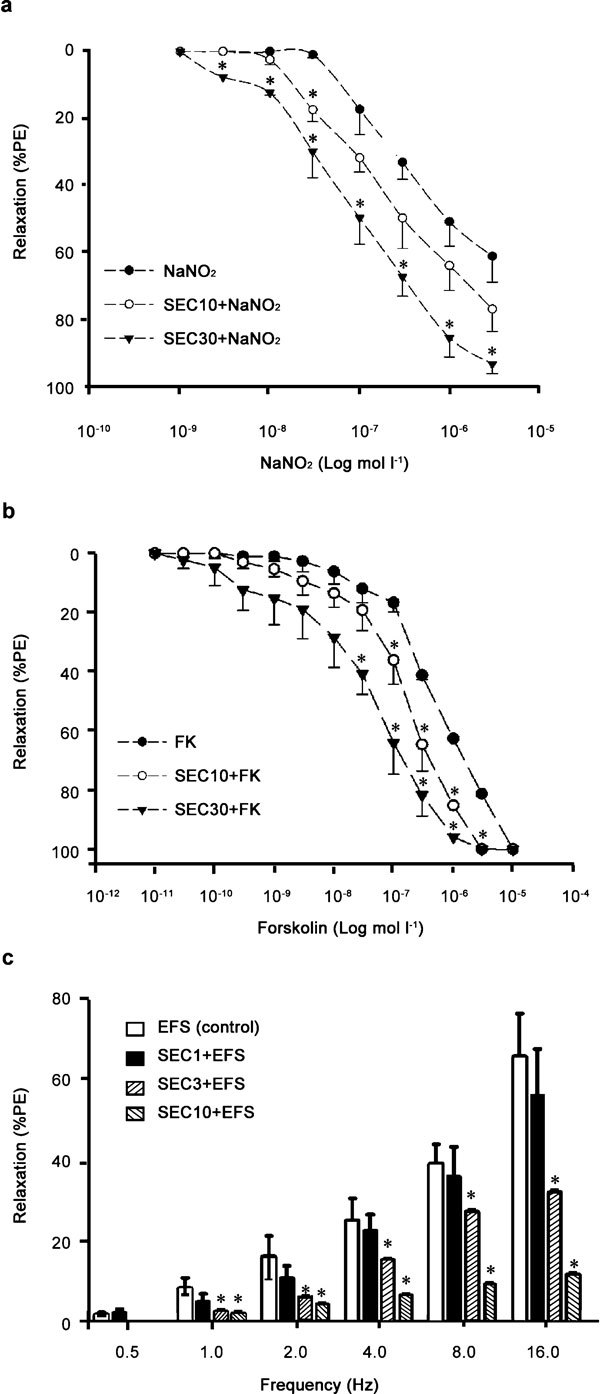
(a) Concentration–response curve for sodium nitrate (NaNO2) at pH 2.0 (1–3000 nmol l−1) and (b) concentration–response curve for forskolin (FK, 10−11–10−5 mol l−1) on rabbit corpora cavernosa (RbCC) strips precontracted with phenylephrine (PE, 10 µmol l−1) in control situations or after incubation with 7-nor-subincanadine E (SEC) (SEC 10 and SEC 30 in graph represent SEC 20 and 60 µmol l−1, respectively). (c) Neurogenic relaxation obtained after transmural electrical field stimulation (30 V, 1.0-ms pulse duration; 0.5–16 Hz) before or after incubation with SEC (SEC 1, SEC 3 and SEC 10 in graph represent SEC 2, 6 and 20 µmol l−1, respectively) in RbCC precontracted with PE (10 µmol l−1), using a nutrient solution that contained 10 µmol l−1 indomethacin, 5 µmol l−1 scopolamine and 5 µmol l−1 guanethidine. Data are expressed as mean±s.e.m. (n=5) of the relaxation expressed as percentage of phenylephrine-induced contraction. *P<0.05 vs. control, analysis of variance (ANOVA) followed by Tukey–Kramer test.
The relaxation evoked by transmural EFS at different frequencies was inhibited in a concentration-dependent fashion by a 20-min incubation period with SEC (2, 6 and 20 µmol l−1; Figure 5c). The maximal relaxation observed at 16 Hz was 65% in control RbCC strips and was reduced to 55.1%, 31.2% and 10.8% in tissues pre-treated with 2, 6 and 20 µmol l−1 SEC, respectively (Figure 5c). SEC also impaired the cumulative concentration-response curves for calcium chloride on isolated RbCC strips mounted in organ baths containing calcium-free depolarizing solution (Figure 6a). The maximal contraction induced by this procedure was blocked by SEC (28.1%, 34.3% and 51.3% at 7, 15 and 30 µmol l−1 concentrations, respectively; Figure 5a). Finally, the incubation of RbCC strips with 15 µmol l−1 SEC induced 45.8%±3.2% relaxation, but did not change the levels (pmol mg−1 of protein) of cAMP (SEC, 134.5±9.8 vs. DMSO, 117.3±7.9; P>0.05; n=4 in triplicate) or cGMP (SEC, 8.5±1.0 vs. DMSO, 8.3±2.9; P>0.05; n=4 in triplicate). On the other hand, 30 µmol l−1 SEC induced relaxation (68.7%±4.8%), which was associated to an increased cAMP (SEC, 331.9±26.1 vs. 117.3±7.0; P<0.05; n=4 in triplicate) and cGMP (SEC, 21.5±4.2 vs. 8.3±2.9; P<0.05; n=4 in triplicate) levels (Figure 6b and c).
Figure 6.
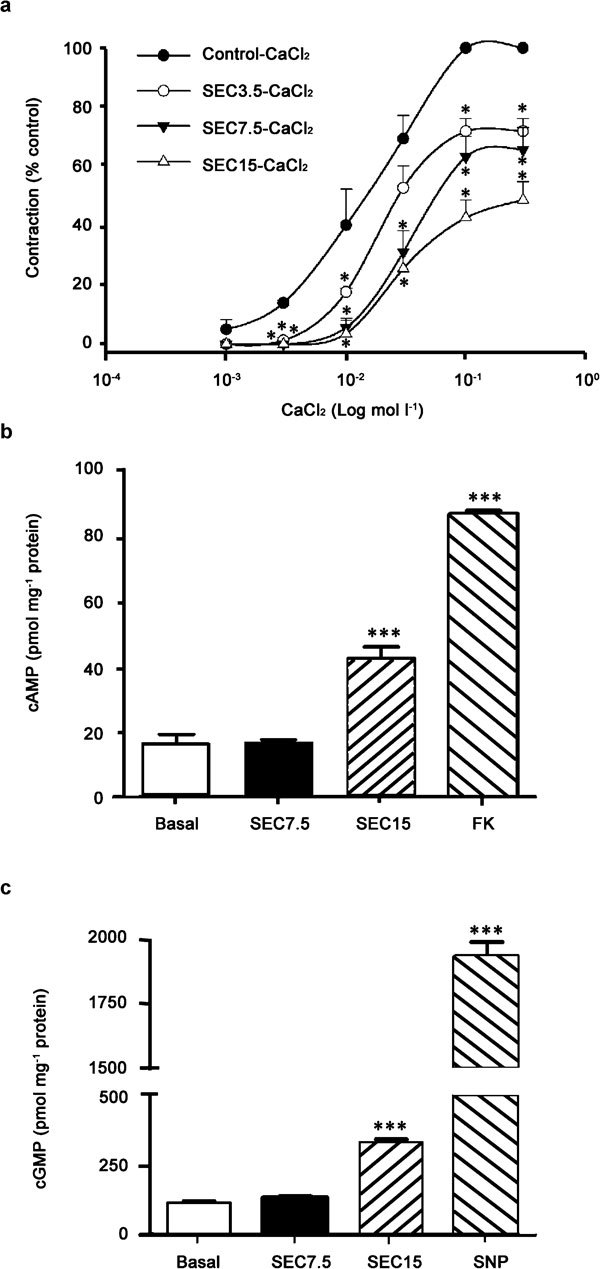
(a) Concentration–response curves for calcium chloride (CaCl2, 1–300 mmol l−1) on rabbit corpora cavernosa (RbCC) strips. The curves were obtained by cumulatively increasing CaCl2 in the organ bath where RbCC strips were kept in a ‘calcium-free' medium enriched with K+ (60 mmol l−1). The curves were obtained in the absence or presence of 7-nor-subincanadine E (SEC) (SEC 3.5, SEC 7.5 and SEC 15 in graph represent 7, 15 and 30 µmol l−1, respectively). Data are presented as mean±s.e.m. (n=5) and were expressed as percentages of relaxation in relation to maximal contractile response (mN) in control tissues (i.e., not incubated with SEC). Data are expressed as mean±s.e.m. (n=4). *P<0.05 vs. control. (b, c) Cyclic nucleotides (cGMP and cAMP) levels in homogenates of RbCC strips were incubated with SEC (15 and 30 µmol l−1) or positive controls (FK for cAMP and SNP for cGMP). The strips were mounted in organ baths and incubated with specific drugs, and after the relaxant effect plateau, they were rapidly frozen in liquid nitrogen and stored at −80 °C until assays were performed. The measurement was performed by using commercially available kits for immunoassays (Cayman, Ann Arbour, MI, USA). Data are expressed as mean±s.e.m. (n=4 in triplicates). ***P<0.001, vs. control (basal level), analysis of variance (ANOVA) followed by Tukey–Kramer test. cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; SNP, sodium nitroprusside.
Discussion
RbCC has been a common experimental model for assessing the activity of compounds on erectile function, and cavernosum relaxation has been considered a positive result for the test substance.22, 23, 24, 25 We used this in vitro system to characterize the relaxant effect of the plant alkaloid SEC and to study the underlying mechanism. SEC showed a concentration-dependent relaxant effect on phenylephrine-precontracted RbCC muscle, with an EC50 of 13.6 µmol l−1, which is four times lower than the EC50 of 52.4 µmol l−1, which was observed with aortic smooth muscle. Statistical differences were not found between maximal relaxation induced by SEC on endothelium-intact or endothelium-denuded aortic rings or in the calculated EC50, and this finding suggests that the endothelium is unlikely to have a primary role in vasorelaxation. This mechanism of action is different from another indole alkaloid, yohimbine, whose relaxant effect is endothelium-dependent.23 Therefore, we assumed that endothelium-derived hyperpolarizing factor, NO and prostanoids (PGE1, PGE2 and PGI2), are probably not involved. The relaxation induced in aortic rings seems to be myogenic in regard to its pharmacological mechanisms.
SEC-induced relaxation was not dependent on KATP or calcium-activated potassium channel activation, because it is insensitive to glibenclamide and iberiotoxin–apamin combinations. In addition, the relaxation induced by SEC occurred by a non-cholinergic, non-noradrenergic mechanism, because it is insensitive to atropine and guanethidine–phentolamine combinations. In the present study, the effective blockage of SEC-induced relaxation by ODQ, but not L-NAME, may suggest that part of the SEC effect depends on a downstream effect in the NO–sGC–cGMP pathway.
This study has shown that SEC relaxes phenylephrine or high potassium-precontracted tissues with the same potency, raising the possibility for calcium antagonistic activity that does not occur in L-type voltage-dependent calcium channels. The main possibility considered was that SEC can affect neuronal (N-type) calcium channels. In this context, it was suggested that within the corpus cavernosum, neuronal release or synthesis of NO partly depends on intracellular calcium bioavailability.26 To elucidate the mechanism of SEC-mediated relaxation, its effects on EFS and tissue levels of cAMP and cGMP in phenylephrine-precontracted RbCC were analysed. It was found that nerve-mediated relaxation is inhibited by SEC at 6 and 10 µmol l−1 in a concentration-related manner. The inhibitory effect may be related to calcium antagonism by SEC in nitrergic nerve endings leading to decreased activation of neuronal NOS (a calcium-dependent enzyme). In this regard, an earlier study pointed out that blockade of N-type, but not L-type, voltage-dependent calcium channels inhibits the transmural electrical field-evoked relaxation.27 Furthermore, a related fraction of alkaloids from this plant was shown to inhibit calcium uptake in rat cortical synaptosomes (unpublished observations). On the other hand, a classical NOS inhibitor would not decrease corpora cavernosa smooth muscle tonus in a concentration-related fashion as with SEC. Indeed, NOS inhibition and sGC inhibition would increase smooth muscle tonus. In our control experiments, NOS inhibition or sGC inhibition only evoked sustained increase in tonus (data not shown). In this regard, it has been shown that the increase in cGMP in nerve varicosities has a negative effect on electrical field-induced relaxation by an unknown molecular mechanism.28 The possibility that specific inhibition of neuronal nitric oxide synthase by phosphorylation has a role on SEC effect was not ruled out and needs to be addressed in a future study.
SEC at 15 µmol l−1 effectively relaxes (45%) phenylephrine-precontracted corpus cavernosum tissues without producing an observable change in tissue levels of cyclic nucleotides, but at 30 µmol l−1, its relaxant effect (65%) was associated with significant increases of cAMP and cGMP, which may be a consequence of enhanced biosynthesis. Interestingly, increases in both cGMP and cAMP are related to penile erection and there seems to have a cross-talk signalling between these nucleotides in the regulation of penile smooth muscle tone.29, 30 For instance, increased levels of cGMP decreased the rate of cAMP hydrolysis. In this context, a previous study has shown that sildenafil, a clinically useful drug for ED, increases both cAMP and cGMP in isolated human corpus cavernosum.31 However, further experimentation is needed to better understand the communication between different phosphodiesterases and to determine SEC activity against a full range of phosphodiestrases isoenzymes, its proerectile effect in vivo, and whether it would enhance intracavernous pressure.
In conclusion, our data demonstrated for the first time that the plant alkaloid SEC from Aspidosperma ulei stem bark exerts a potent relaxant effect on rabbit corpus cavernosum smooth muscle in vitro by a mechanism that is partly independent of the classical nitric oxide pathway and may bypass nitrergic and endothelial dysfunctions associated with several cardiovascular risk factors that contribute to ED, such as diabetes, hypertension and dyslipidaemia.
Author contributions
NRFN, MCF and VSR participated in the study design and data interpretation, and helped draft the manuscript. FAS helped coordinate and perform the statistical analysis. ODB and ARC contributed substantially to the acquisition of experimental data. KMAC and CFS carried out the immunoassays. ERS and DEU were involved in realizing chemical studies on SEC utilized for the study. All authors read the manuscript and approved the final version.
Acknowledgments
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Proc. No. 472717/2003-0) and Fundação Cearense de Pesquisa e Cultura (FUNCAP, Proc. No. 30/2002).
The authors declare no competing financial interests.
Supplementary Information
References
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinmann KP, et al. Incidence of erectile dysfunction in men 40–69 years old. Longitudinal Results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- Maggi M, Filippi S, Leda F, Magini A, Forti G. Erectile dysfunction: from biochemical pharmacology to advences in medical therapy. Eur J Endocrinol. 2000;143:143–54. doi: 10.1530/eje.0.1430143. [DOI] [PubMed] [Google Scholar]
- Seftel AD. Challenges in oral therapy for erectile dysfunction. J Androl. 2002;23:729–36. [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–83. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Guirguis WR. Oral treatment of erectile-dysfunction: from herbal remedies to designer drugs. J Sex Marital Ther. 1998;24:69–73. doi: 10.1080/00926239808404920. [DOI] [PubMed] [Google Scholar]
- El-Thaher TS, Khatib S, Saleem M, Shnoudeh A, Badwan AA. A novel compound JPM8: in vivo penile activity promotion in rats, effect on the relaxation and cGMP/cAMP accumulation in isolated rabbit corpora cavernosa. Int J Impot Res. 2002;14:453–61. doi: 10.1038/sj.ijir.3900908. [DOI] [PubMed] [Google Scholar]
- Mas M. Molecular mechanisms of penile erection. Arch Esp Urol. 2010;63:589–98. [PubMed] [Google Scholar]
- Zaher TF. Papaverine plus PGE, versus PGE1 alone for intracorporeal injection therapy. Intl Urol Nephrol. 1998;30:193–6. doi: 10.1007/BF02550576. [DOI] [PubMed] [Google Scholar]
- Deutsch HF, Evenson MA, Drescher P, Sparwasser C, Madsen PO. Isolation and biological activity of aspidospermine and quebrachamine from Aspidosperma tree source. J Pharmaceut Biomed Anal. 1994;12:1283–7. doi: 10.1016/0731-7085(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Chen J, Chen CF. Relaxation of corpus cavernosum and raised intracavernous pressure by berberine in rabbit. Br J Pharmacol. 1998;125:1677–84. doi: 10.1038/sj.bjp.0702249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmore WW. Available and future treatments for erectile dysfunction. Clin Cornerstone. 2005;7:37–45. doi: 10.1016/s1098-3597(05)80047-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshida M, Andersson KE, Hedlund P. Effects in vitro and in vivo by apomorphine in the rat corpus cavernosum. Br J Pharmacol. 2005;146:259–67. doi: 10.1038/sj.bjp.0706317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu JH, Wang T, Xiao HJ, Yin CP, et al. Effects of plant extract neferine on cyclic adenosine monophosphate and cyclic guanosine monophosphate levels in rabbit corpus cavernosum in vitro. . Asian J Androl. 2008;10:307–12. doi: 10.1111/j.1745-7262.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Pereira MM, Jacome RL, Alcântara AF, Alves RB, Raslan DS. Alcalóides indólicos isolados de espécies do gênero Aspidosperma (Apocynaceae) Quim Nova. 2007;30:970–83. [Google Scholar]
- Campos AR, Lima Júnior RCP, Uchoa DEA, Silveira ER, Santos FA, et al. Pro-erectile effects of an alkaloidical rich fraction from Aspidosperma ulei root bark in mice. J Ethnopharmacol. 2006;104:240–4. doi: 10.1016/j.jep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Campos AR, Otacílio BD, Jr, Ucho DEA, Silveira ER, Rao VS. 15R-16,17-Seco subincanadine E, a proerectile indole alkaloid isolated from Aspidosperma ulei stem Bark. Evid Based Complement Altern Med. 2007;4 Suppl:56–8. [Google Scholar]
- Campos AR, Cunha KM, Santos FA, Silveira ER, Uchoa DE, et al. Relaxant effects of an alkaloid-rich fraction from Aspidosperma ulei root bark on isolated rabbit corpus cavernosum. Int J Impot Res. 2008;20:255–63. doi: 10.1038/sj.ijir.3901624. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Sekiguchi M, Shimamoto S, Shigemori H, Ishiyama H, et al. Subincanadines A–C, novel quaternary indole alkaloids from Aspidosperma subincanum. . J Org Chem. 2002;67:6449–55. doi: 10.1021/jo025854b. [DOI] [PubMed] [Google Scholar]
- Castillo JC, de Beer EJ. The tracheal chain-l. A preparation for the study of antispasmodics with particular reference to bronchodilator drugs. J Pharmacol Exp Ther. 1947;90:104–9. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature (London) 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Lin RJ, Wu BN, Shen KP, Lo YT, Huang CH, et al. KMUP-1 relaxes rabbit corpus cavernosum smooth muscle in vitro and in vivo: involvement of cyclic GMP and Kþ channels. Br J Pharmacol. 2002;135:1159–66. doi: 10.1038/sj.bjp.0704554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi S, Luconi M, Granchi S, Natali A, Tozzi P, et al. Endothelium-dependency of yohimbine-induced corpus cavernosum relaxation. Int J Impot Res. 2002;14:295–307. doi: 10.1038/sj.ijir.3900890. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Nishimura J, Hirano K, Naito S, Kanaide H. Modulation of Ca2+ sensitivity regulates contractility of rabbit corpus cavernosum smooth muscle. J Urol. 2003;169:2412–6. doi: 10.1097/01.ju.0000065808.45445.a1. [DOI] [PubMed] [Google Scholar]
- Sharabi FM, Daabees TT, El-Metwally MA, Senbel AM. Comparative effects of sildenafil, phentolamine, yohimbine and L-arginine on the rabbit corpus cavernosum. Fund Clin Pharmacol. 2004;18:187–94. doi: 10.1111/j.1472-8206.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Broderick G, Wein AJ, Levin RM. Effect of alteration in the extracellular potassium and calcium on field-stimulated relaxation of the rabbit corpus cavernosum. Gen Pharmacol. 1996;27:375–8. doi: 10.1016/0306-3623(95)02029-2. [DOI] [PubMed] [Google Scholar]
- Okamura T, Fujioka H, Ayajiki K. Effects of calcium antagonists on the nitrergic nerve function in canine corpus cavernosum. Jpn J Pharmacol. 2001;87:208–13. doi: 10.1254/jjp.87.208. [DOI] [PubMed] [Google Scholar]
- Hallén K, Wiklund NP, Gustafsson LE. Inhibitors of phosphodiesterase 5 (PDE 5) inhibit the nerve-induced release of nitric oxide from the rabbit corpus cavernosum. Br J Pharmacol. 2007;150:353–60. doi: 10.1038/sj.bjp.0706991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Resn. 2007;100:1569–78. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- Uckert S, Hedlund P, Waldkirch E, Sohn M, Jonas U, et al. Interactions between cGMP- and cAMP-pathways are involved in the regulation of penile smooth muscle tone. World J Urol. 2004;22:261–6. doi: 10.1007/s00345-003-0394-4. [DOI] [PubMed] [Google Scholar]
- Stief CG, Uckert S, Becker AJ, Harringer W, Truss MC, et al. Effects of sildenafil on cAMP and cGMP levels in isolated human cavernous and cardiac tissue. Urology. 2000;55:146–50. doi: 10.1016/s0090-4295(99)00371-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


