Abstract
In this study, we investigated the effects of a combination of Ginkgo biloba extracts (GBE) and phosphodiesterase type 5 (PDE-5) inhibitors on the muscular tone of the corpus cavernosum and potassium channel activity of corporal smooth muscle cells. Strips of corpus cavernosum from male New Zealand white rabbits were mounted in organ baths for isometric tension studies. After contraction with 1×10−5 mol l−1 norepinephrine, GBE (0.01–1 mg ml−1) and mirodenafil (0.01–100 nmol l−1) were added together into the organ bath. In electrophysiological studies, whole-cell currents were recorded by the conventional patch-clamp technique in cultured smooth muscle cells of the human corpus cavernosum. The corpus cavernosum was relaxed in response to GBE in a dose-dependent manner (from 0.64%±8.35% at 0.01 mg ml−1 to 52.28%±11.42% at 1 mg ml−1). After pre-treatment with 0.03 mg ml−1 of GBE, the relaxant effects of mirodenafil were increased at all concentrations. After tetraethylammonium (TEA) (1 mmol l−1) administration, the increased effects were inhibited (P<0.01). Extracellular administration of GBE increased the whole-cell K+ outward currents in a dose-dependent fashion. The increase of the outward current was inhibited by 1 mmol l−1 TEA. These results suggest that GBE could increase the relaxant potency of mirodenafil even at a minimally effective dose. The K+ flow through potassium channels might be one of the mechanisms involved in this synergistic relaxation.
Keywords: calcium-activated potassium channels, erectile dysfunction, Ginkgo biloba, phosphodiesterase inhibitors, smooth muscles
Introduction
Ginkgo biloba extract (GBE) is derived from dried Ginkgo biloba leaves and contains 24% ginkgo flavonol glycosides and 6% terpene lactones such as ginkgolides A, B, C, J and bilobalide. Clinically, it is one of the most commonly prescribed botanical drugs. Vasorelaxative effects are the main function of GBE, and there are many clinical therapeutic applications associated with chronic vascular insufficiency, such as peripheral arterial obstructive disease, cognitive dysfunction,1 ageing damage2 and Alzheimer's disease.3
Traditionally, GBE is believed to have positive effects on sexual function in men; therefore, it is widely used as a potential enhancer of penile erection in Korea. Penile erection is a haemodynamic event, which is coordinated with smooth muscle relaxation of the corpus cavernosum. Reduced penile vascular resistance induced by corporal smooth muscle relaxation is the most important step in penile erection.4 Although the efficacy of GBE for erectile dysfunction (ED) has been infrequently studied, recent work by Paick and Lee5 demonstrated relaxant effects of GBE on corpus cavernosal tissues in studies using organ bath models and suggested its potential use for ED in a clinical setting.
ED is a common disorder in men.6, 7 The incidence of ED has increased due to the modern lifestyle associated with limited physical activity and high-calorie intake.8 ED is prevalent in patients with cardiovascular comorbidities, diabetes and elevated fasting glucose levels, and is reportedly associated with future coronary risk.9 Although oral forms of phosphodiesterase type 5 (PDE-5) inhibitors have been prescribed as a first-line treatment for ED, some men discontinue PDE-5 inhibitor usage because of the lack of effectiveness. Even after usage instructions are optimized as either an as-needed or a daily protocol, the success rate per attempts at intercourse has been reported to be less than 50%.10, 11 Although other invasive options such as intracavernousal injections or penile prostheses are available, they are less acceptable for most men due to their invasiveness and inconvenience.12 Therefore, many men diagnosed with ED could benefit from additional treatment options with non-invasive approaches.
Considering the possible efficacy of GBE for relaxing corpus cavernosal smooth muscle, concurrent administration with a PDE-5 inhibitor might potentiate the effects of the PDE-5 inhibitor. In this study, we evaluated the potential benefit of the combination of GBE and mirodenafil, a PDE-5 inhibitor, on the relaxation of the corpus cavernosum in an ex vitro tissue model. In addition, we performed electrophysiological studies to investigate the mechanism of action of GBE on the potassium channel in human corporal smooth muscle cells (SMCs).
Materials and methods
Tissue relaxation measurements
This experiment was reviewed and approved by the Institutional Animal Care and Use Committee of Samsung Biomedical Research Institute. The Samsung Biomedical Research Institute is an International Association for Assessment and Accreditation of Laboratory Animal Care accredited facility and abides by the Institute of Laboratory Animal Resources guidelines. Penile erectile tissue was obtained from male rabbits (22–26 weeks old; 3–4 kg body weight). The rabbits were killed by transvenous embolisation. The entire penis was surgically removed and rapidly placed in a modified Krebs solution (NaCl 114.0, NaHCO3 25.0, CaCl2 2.5, KCl 4.7, MgSO 1.2, KH2PO4 1.2, glucose 11.7 and ascorbic acid 1.0; all concentrations in mmol l−1) under conditions of 95% O2 and 5% CO2.
The corpus cavernosum was carefully dissected by removing surrounding adipose and muscular tissues and the tunica albuginea under a microscope. The corpus cavernosum was prepared as two longitudinal strips (0.6–0.8 cm) and mounted under 1 g of resting tension in a 5-ml jacketed organ bath containing modified Krebs solution (composition as described above) bubbled with 95% O2 and 5% CO2, and maintained at 37 °C. An equilibration period of 1 h was used for all tissues in this study. During this period, the tissues were washed with fresh Krebs solution, and the baseline tension was readjusted to 1 g. Tissue responses were measured using isometric strain gauges connected to a polygraph and an electronic data acquisition system run on a personal computer. Corpus cavernosal tissue was precontracted with 1×10−5 mol l−1 of norepinephrine (NE). After a stable plateau of contractions was obtained, the relaxant compounds were added.
First, to determine the dose of GBE to use in combination with mirodenafil, GBE was cumulatively added from 0.01 to 1 mg ml−1, and the relaxation was measured. Second, mirodenafil was cumulatively added from 0.01 to 100 nmol l−1, and the relaxation was measured. After pre-treatment with 0.03 mg ml−1 of GBE, mirodenafil was cumulatively added. Finally, the whole organ bath procedure was repeated after administration of tetraethylammonium (TEA), a selective blocker of large-conductance calcium-activated potassium channels (BKCa channels).
Explant cell cultures and electrophysiological recordings
This experiment was approved by the Institutional Review Board (IRB) of Samsung Medical Center (Seoul, Korea). All participants granted written informed consent. Human erectile tissue was obtained from the corpus cavernosum of patients undergoing surgery for implantation of penile prostheses or penectomy for penile cancer. Homogeneous explant cell cultures of human corporal SMCs were prepared as previously described.13 SMCs were cultured with Dulbecco's medium (Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum, 1% penicillin/streptomycin and 2 mmol l−1 L-glutamine at 37 °C in a humidified incubator with 5% CO2 atmosphere. These cultures were morphologically homogeneous, and furthermore, we did not observe cobblestone morphologies characteristic of endothelial cells or the flattened and spread-out shapes characteristic of fibroblasts. Cellular homogeneity was further verified by the presence of smooth muscle specific α-actin immunoreactivity. All experiments were performed within four culture passages, and during this time, changes were not observed in any experimentally relevant pharmacological and molecular properties, including cyclic adenosine monophosphate (cAMP) formation, calcium mobilisation and expression or function of the gap junction protein connexin43. For the electrophysiological studies, the confluent cells were trypsinized (0.05% (w/v) trypsin and 5 mmol l−1 EDTA) and replated onto glass coverslips in 35-mm culture dishes. The experiments were conducted within 3 h of the cells attaching to the glass coverslips.
The conventional whole-cell patch-clamp method was used to measure the membrane currents under voltage clamp conditions. The cells on glass coverslips were placed into a small chamber (0.6 ml) on the stage of an inverted microscope (TMD Diaphot; Nikon, Tokyo, Japan). Membrane currents in the SMCs were recorded using a patch-clamp amplifier (Axopatch-lD; Axon Instruments, Foster City, CA, USA). The patch electrodes were made from borosilicate glass capillary tubing (World Precision Instruments, Sarasota, FL, USA) and had resistances of 2.5–5 MΩ. Series resistance (about 6–10 MΩ) and capacitative currents were not compensated for because the cell size and measured currents were relatively small. The membrane capacitance was determined from the current amplitude elicited in response to hyperpolarizing voltage ramp pulses from a holding potential of 0 to −5 mV (duration 25 ms at 0.2 V s−1); there was no interference due to any time-dependent ionic currents throughout the duration of this experiment. The average cell capacitance was 35.3±2.6 pF (n=44). pCLAMP software v.9.2 and Digidata-1322A (both from Axon Instruments Inc.) were used for data acquisition and the application of command pulses. Membrane currents were measured during ramp and filtered at 5 kHz (−3 dB frequency). Current signals were filtered at 5 kHz, digitized and analysed on a personal computer using the pCLAMP software (version 9.2; Axon Instruments) and Origin v.7.0 (Microcal Software Inc., Northampton, MA, USA). The standard bath solution contained the following components (mmol l−1): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaC12, 5 glucose and 10 HEPES, pH 7.4 with NaOH. The pipette solution contained the following (in m mol l−1): 140 KCl, 10 HEPES, 2 K2ATP, 3.3 CaCl2, 2 MgCl2, 5 ethyleneglycol-bis-[2-aminoethyl ether]-N,N′-tetraacetic acid and 1 GTP, and the pH was adjusted to 7.2 with KOH.
Drugs and chemicals
All drugs and chemicals were purchased from Sigma (St Louis, MO, USA), with the exception of mirodenafil and GBE. Mirodenafil (a PDE-5 inhibitor) and GBE were provided by SK Chemical (Seoul, Korea). GBE was prepared in dimethylsulphoxide and freshly diluted in bath solution immediately before use. The final concentration of dimethylsulphoxide did not exceed 0.1%. All other drugs were prepared in distilled water.
Data analysis
The relaxant effects of drugs on corpus cavernosal tissues were expressed as the per cent inhibition of the contractions that were induced by phenylephrine before adding relaxant drugs. To compare the relaxation effect, the Wilcoxon rank-sum test was performed. Descriptive statistics were used for electrophysiological recordings. SPSS 17.0K software (SPSS Inc., Chicago, IL, USA) was used, and statistical significance was accepted at P<0.05.
Results
Relaxant effect of GBE on corporal tissue
The corpus cavernosum was relaxed in response to GBE in a dose-dependent manner. The cumulative relaxation effects of GBE were 0.64%±8.35% at 0.01 mg ml−1, 8.79%±9.26% at 0.03 mg ml−1, 20.52%±9.26% at 0.1 mg ml−1, 32.72%±8.53% at 0.3 mg ml−1 and 52.28%±11.42% at 1 mg ml−1. The relaxation potency was not significant at the dose of 0.01 mg ml−1 (P=0.786); however, GBE did begin to demonstrate significant relaxant effects on the corpus cavernosum at 0.03 mg ml−1 (P=0.017; Figure 1). Based on these data, we decided to use 0.03 mg ml−1 of GBE in consecutive experiments measuring the additional relaxant effects of GBE on the corpus cavernosum with mirodenafil.
Figure 1.
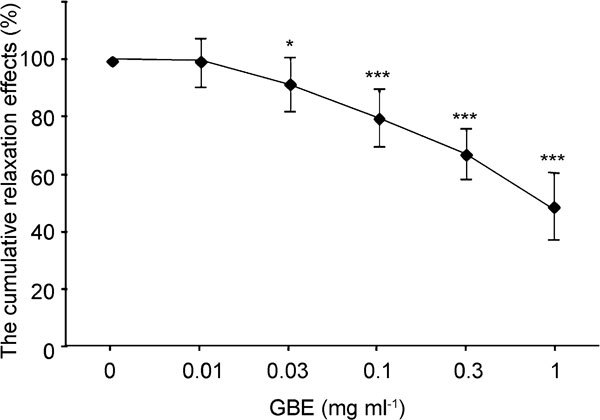
Relaxation effects of Ginkgo biloba extracts (GBE) on 10−5 mol l−1 norepinephrine (NE)-induced contractions of rabbit cavernosal strips (n=12). *P<0.05, ***P<0.0001, compared with the control.
Relaxant effect of mirodenafil on corporal tissue
The corpus cavernosum was relaxed in response to mirodenafil in a dose-dependent manner. The cumulative relaxation effects of mirodenafil were 10.53%±7.94% at 0.1 nmol l−1, 18.95%±8.50% at 1 nmol l−1, 27.36%±11.28% at 10 nmol l−1 and 46.33%±12.12% at 100 nmol l−1. When pre-treated with 0.03 mg ml−1 of GBE, relaxation effects of mirodenafil were enhanced. The per cent relaxations of mirodenafil combined with GBE were 12.21±6.05% at 0.1 nmol l−1, 20.53±5.89% at 1 nmol l−1, 33.51%±13.53% at 10 nmol l−1 and 60.39%±13.82% at 100 nmol l−1 (n=8; Figure 2).
Figure 2.
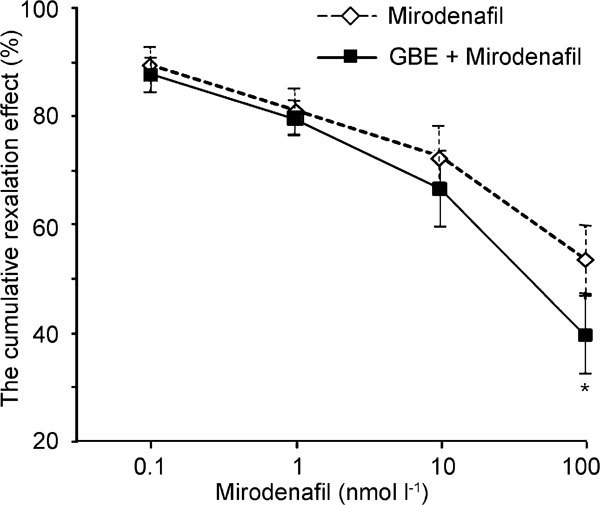
Relaxation effects of mirodenafil on 10−5 mol l−1 norepinephrine (NE)-induced contractions of rabbit cavernosal strips in the absence (round circle) or presence (square) of 0.03 mg ml−1 Ginkgo biloba extracts (GBE) (n=8). *P<0.05, compared with the control.
After administration of the TEA, the per cent relaxation by the mirodenafil and GBE was measured. Following precontraction by NE (1×10−5 mol l−1), 1 mmol l−1 of TEA was administered prior to adding GBE to the organ bath. The relaxation effects of different doses of mirodenafil and 0.3 mg ml−1 of GBE after administration of TEA (1 mmol l−1) were 7.71%±2.73% at 0.1 nmol l−1, 11.20%±3.47% at 1 nmol l−1, 15.99%±5.39% at 10 nmol l−1 and 22.20%±7.78% at 100 nmol l−1. The increasing relaxation effects caused by mirodenafil and GBE were inhibited by TEA administration (n=8, P<0.01; Figure 3).
Figure 3.
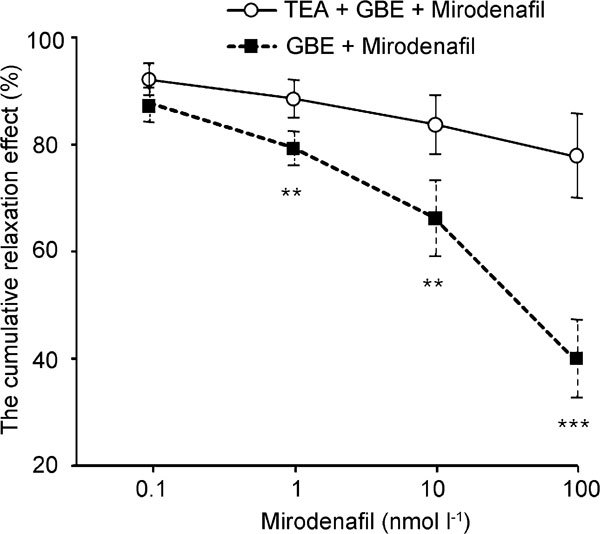
Relaxation effects of mirodenafil on 10−5 mol l−1 norepinephrine (NE)-induced contractions of rabbit cavernosal strips with pretreatment of 0.03 mg ml−1 Ginkgo biloba extracts (GBE) in the absence (round circle) or presence (square) of 1 mmol l−1 tetraethylammonium (TEA) (n=8). **P<0.01, ***P<0.0001, compared with the control.
Activation of BKCa whole-cell currents by GBE
The effects of GBE on potassium currents in human corporal SMCs were investigated using the patch-clamp recording technique. The membrane currents were elicited by a 500-ms depolarizing pulse from −100 to 60 mV. The extracellular administration of GBE increased the whole cell K+ outward currents (Figure 4). The response magnitude was dose-dependent at +60 mV (control: 8.7±1.8 pA/pF, n=18; 0.01 mg GBE: 16.2±3.1 pA/pF, n=7; 0.03 mg GBE: 44.5±9.0 pA/pF, n=7; 0.1 mg GBE: 49.9±9.0 pA/pF, n=8; 0.3 mg: GBE 52.9±5.0 pA/pF, n=12). The increase of outward current was inhibited by 1 mmol l−1 of TEA, a selective blocker of BKCa channels at this concentration (Figure 4b). A summary of the stimulatory effects of GBE followed by administration of TEA is presented in Figure 4c.
Figure 4.

Effect of Ginkgo biloba extracts (GBE) on the BKCa channel whole-cell currents in human corporal smooth muscle cells. (a) Current–voltage relationships obtained using a 500-ms ramp pulse from −100 to +60 mV. Membrane currents were recorded before and 5 min after administration of GBE. (b) GBE-stimulated currents were inhibited by 1 mmol l−1 tetraethylammonium (TEA), a BKCa channel selective inhibitor. (c) Summary of the means±s.e. of peak whole-cell currents at +60 mV (n=12).
Discussion
PDE-5 inhibitors are commonly prescribed as first-line treatment for ED; however, a significant proportion of men do not respond to PDE-5 inhibitor treatment.14 The mechanism of action of PDE-5 inhibitors is through the inhibition of intracellular cyclic guanosine monophosphate (cGMP) degradation, but the underlying mechanism associated with non-responsiveness is unclear. Nevertheless, there are a considerable number of men who are PDE-5 non-responders and seek additional non-invasive treatment options.12 The combination of a PDE-5 inhibitor with other drugs might be an alternative option, if efficacy and safety can be demonstrated. If a drug combination could improve the potency of the PDE-5 inhibitor, this population of men may respond to the PDE-5 inhibitor. Furthermore, some PDE-5 inhibitor responders could potentially reduce their PDE-5 inhibitor dose with the combination treatment.
Some studies have shown that severe vascular lesions and atrophy of SMCs were observed in sildenafil non-responders; therefore, drugs that maintain the structure and function of the penile vasculature, by preventing endothelial and SMC dysfunction and damage, may improve the response to PDE-5 inhibitors.12 Clinically, GBE has been commonly prescribed to protect from vascular insufficiencies and peripheral vascular diseases.15 The main action of GBE is suggested to be the relaxation of vascular smooth muscles and the enhancement of endothelial functions; there are many clinical therapeutic applications of GBE for conditions associated with chronic vascular insufficiency.1, 2, 3 In the current experiment, GBE induced relaxation of the corpus cavernosum in a dose-dependent manner. The relaxant potency of GBE itself was demonstrated to be more than 50% at a dose of 1 mg ml−1 and about 9% at a dose of 0.03 mg ml−1. There are several potential mechanisms by which GBE plays a role in the relaxation of corporal smooth muscles. As Ginkgo biloba dimeric flavonoids were shown to inhibit cAMP-specific PDE activity in a rat aorta model and have also been shown to inhibit human cGMP-specific PDE-5, inhibition of PDE is one possible mechanism that explains the relaxation activity of the corpus cavernosum by GBE.16, 17 GBE maintains ATP content by protecting mitochondrial respiration and preserving oxidative phosphorylation, and it also exerts arterial and venous vasoregulator effects involving the release of endothelial factors.18 In addition, GBE regulates the ionic balance in damaged cells and exerts specific and potent platelet-activating factor antagonist activity.19
The endothelium-dependent vasorelaxation of GBE has previously been studied with regard to biochemical and pharmacological characteristics.20 Various mechanisms including NO release,21 activation of the K+ channel,22 and prostacyclin release23 have been suggested to be involved in the smooth muscle relaxation by GBE. In the corporal smooth muscle, K+ channels perform a key role in determining smooth muscle tone. In human corporal smooth muscle cells that express ATP-sensitive potassium channels, the BKCa channel is thought to be the most important for modulating muscle tone.24, 25 Due to the large conductance and high density of expression, BKCa channels help set and maintain the resting membrane potential of human corporal smooth muscle.26 In an electrophysiological study using porcine aorta, it was demonstrated that the application of GBE to the intracellular surface of excised inside-out patches activated K+ channels in a concentration-dependent manner in the concentration range of 1–100 µg ml−1.23 The authors suggested that GBE increased nitric oxide synthase (NOS) activity in a manner dependent upon the activity of BKCa channels and that GBE may regulate NO release by changing the cell membrane potential in vascular endothelial cells. However, there are controversies for the effects of GBE on the BKCa channel of smooth muscles. In a previous study, the relaxation effects of GBE were assessed after a 30-min pre-treatment with TEA. Authors demonstrated that TEA did not reduce the GBE-induced vasorelaxation in a rat aorta model at any concentration of GBE (0.03–3 mg ml−1).27 Our data, however, show that GBE increased the probability of opening the BKCa channel in the cell-attached patch, consistent with the increased whole-cell current in the human corpus cavernosal cells. The increased K+ flow caused by GBE was effectively inhibited by TEA. Additionally, TEA showed significant inhibitory effects on the relaxation potency of GBE combined with mirodenafil in the organ bath model. Based on these results, the activation of potassium channels, mainly BKCa channels, appears to have an important role in the GBE-induced relaxation of the corpus cavernosum.
GBE is usually prescribed for daily use, whereas PDE-5 inhibitors are prescribed for use as necessary. In the present study, we added mirodenafil after pre-administration of GBE to duplicate the clinical situation. To our knowledge, there have been no other studies evaluating whether GBE could show additional relaxation effects on the corpus cavernosum when combined with PDE-5 inhibitors. In our current experiments, GBE increased the relaxant potency of PDE-5 inhibitors on the corpus cavernosum even with the minimally significant dose (Figure 1). Additional relaxation effects were demonstrated to increase in a dose-dependent manner, but the statistics were significant only at the maximal dose (Figure 2). Because the average maximum plasma concentration (Cmax) of mirodenafil was shown to be 354.9 ng ml−1 (587.0 nM),28 experimental doses of mirodenafil used in this study (0.1–100 nM) seem to be relevant to actual therapeutic ranges. These results could provide evidence to support additional in vivo studies and clinical trials using the combination therapy of GBE and PDE-5 inhibitors to treat erectile dysfunction, especially in PDE-5 inhibitor non-responders.
Conclusion
GBE induced the relaxation of the corpus cavernosum in a dose-dependent manner and also effectively increased the relaxant potency of mirodenafil in rabbit corpus cavernosal tissues even at the minimally effective dose. The K+ flow through potassium channels, mainly by BKCa channels, might be one mechanism involved in the synergistic relaxation observed. Our results may be used to support further in vivo experiments or clinical investigations on the combination therapy of GBE with PDE-5 inhibitors for the treatment of ED.
Author contributions
JJK and DHH conceived of the study, and participated in its design and drafted the manuscript. SHL and THK carried out tissue relaxation measurements by organ bath and helped to draft the manuscript. MRC, KJC and SCK participated in the design of the study and performed explant cell cultures and electrophysiological studies, and performed the statistical analysis. JHJ and JKP conceived of the study, and participated in the design of the study and involved in revising the manuscript critically for important intellectual content. SWL conceived of the study, and participated in its design and coordination and helped to draft the manuscript and involved in revising the manuscript critically. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Korea (A090583).
The authors have nothing to disclose.
References
- Kennedy DO, Scholey AB, Wesnes KA. The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacology (Berl) 2000;151:416–23. doi: 10.1007/s002130000501. [DOI] [PubMed] [Google Scholar]
- Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, et al. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–78. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327–32. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- Han DH, Chae MR, So I, Park JK, Lee SW. The effects of dopamine receptor agonists on BKCa channels and signal transduction mechanism in corpus cavernosal smooth muscle cells. Int J Impot Res. 2008;20:53–9. doi: 10.1038/sj.ijir.3901623. [DOI] [PubMed] [Google Scholar]
- Paick JS, Lee JH. An experimental study of the effect of ginkgo biloba extract on the human and rabbit corpus cavernosum tissue. J Urol. 1996;156:1876–80. [PubMed] [Google Scholar]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Kubin M, Wagner G, Fugl-Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res. 2003;15:63–71. doi: 10.1038/sj.ijir.3900949. [DOI] [PubMed] [Google Scholar]
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–8. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- Grover SA, Lowensteyn I, Kaouache M, Marchand S, Coupal L, et al. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–9. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- Atiemo HO, Szostak MJ, Sklar GN. Salvage of sildenafil failures referred from primary care physicians. J Urol. 2003;170:2356–8. doi: 10.1097/01.ju.0000096221.67967.ae. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K, Moysidis K, Bekos A, Tsimtsiou Z, Ioannidis E, et al. Treatment strategy for “non-responders” to tadalafil and vardenafil: a real-life study. Eur Urol. 2006;50:126–32; discussion 132–3. doi: 10.1016/j.eururo.2006.02.060. [DOI] [PubMed] [Google Scholar]
- Gholamine B, Shafiei M, Motevallian M, Mahmoudian M. Effects of pioglitazone on erectile dysfunction in sildenafil poor-responders: a randomized, controlled study. J Pharm Pharm Sci. 2008;11:22–31. doi: 10.18433/j3tg6h. [DOI] [PubMed] [Google Scholar]
- Wespes E, Rammal A, Garbar C. Sildenafil non-responders: haemodynamic and morphometric studies. Eur Urol. 2005;48:136–9; discussion 139. doi: 10.1016/j.eururo.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Souverein PC, Egberts AC, Meuleman EJ, Urquhart J, Leufkens HG. Incidence and determinants of sildenafil (dis)continuation: the Dutch cohort of sildenafil users. Int J Impot Res. 2002;14:259–65. doi: 10.1038/sj.ijir.3900883. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Tanaka N, Umegaki K, Takenaka H, Mizuno H, et al. Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001;69:2327–36. doi: 10.1016/s0024-3205(01)01303-0. [DOI] [PubMed] [Google Scholar]
- Dell'Agli M, Galli GV, Bosisio E. Inhibition of cGMP-phosphodiesterase-5 by biflavones of Ginkgo biloba. . Planta Med. 2006;72:468–70. doi: 10.1055/s-2005-916236. [DOI] [PubMed] [Google Scholar]
- Saponara R, Bosisio E. Inhibition of cAMP-phosphodiesterase by biflavones of Ginkgo biloba in rat adipose tissue. J Nat Prod. 1998;61:1386–7. doi: 10.1021/np970569m. [DOI] [PubMed] [Google Scholar]
- Clostre F.Ginkgo biloba extract (EGb 761). State of knowledge in the dawn of the year 2000 Ann Pharm Fr 199957Suppl 11S8–88.French. [PubMed] [Google Scholar]
- Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- Delaflotte S, Auguet M, DeFeudis FV, Baranes J, Clostre F, et al. Endothelium-dependent relaxations of rabbit isolated aorta produced by carbachol and by Ginkgo biloba extract. Biomed Biochim Acta. 1984;43:S212–6. [PubMed] [Google Scholar]
- Chen X, Salwinski S, Lee TJ. Extracts of Ginkgo biloba and ginsenosides exert cerebral vasorelaxation via a nitric oxide pathway. Clin Exp Pharmacol Physiol. 1997;24:958–9. doi: 10.1111/j.1440-1681.1997.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Nakaya Y, Niwa Y, Chen X. KCa channel-opening activity of Ginkgo Biloba extracts and ginsenosides in cultured endothelial cells. Clin Exp Pharmacol Physiol. 2001;28:441–5. doi: 10.1046/j.1440-1681.2001.03456.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS.Effects of Ginkgo biloba extract on cerebral metabolic processesIn: Agnoli A, Rapin JR, Scapagnini V, Weitbrecht WV, editors. Effects of Ginkgo Biloba Extract on Organic Cerebral Impairment London; John Libbery; 1985pp5–14. [Google Scholar]
- Christ GJ, Spray DC, Brink PR. Characterization of K currents in cultured human corporal smooth muscle cells. J Androl. 1993;14:319–28. [PubMed] [Google Scholar]
- Fan SF, Brink PR, Melman A, Christ GJ. An analysis of the Maxi-K+ (KCa) channel in cultured human corporal smooth muscle cells. J Urol. 1995;153:818–25. [PubMed] [Google Scholar]
- Archer SL. Potassium channels and erectile dysfunction. Vascul Pharmacol. 2002;38:61–71. doi: 10.1016/s1537-1891(02)00127-1. [DOI] [PubMed] [Google Scholar]
- Nishida S, Satoh H. Mechanisms for the vasodilations induced by Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Life Sci. 2003;72:2659–67. doi: 10.1016/s0024-3205(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Shin BS, Hu SK, Kim J, Oh JG, Youn WN, et al. Development of LC/MS/MS assay for the determination of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5-dihydropyrrolo[3,2-d]pyrimidin-4-one (SK3530) in human plasma: application to a clinical pharmacokinetic study. J Pharm Biomed Anal. 2007;45:176–84. doi: 10.1016/j.jpba.2007.06.021. [DOI] [PubMed] [Google Scholar]


