Abstract
Background
Vitamin D is a modulator of the immune system. There is some limited evidence that it also increases local blood flow in response to stress.
Material/Methods
In the present study, we examined 20 age matched subjects; 10 whom were from India and 10 Caucasians from the United States. Subjects were administered 4000 IU of Vitamin D3 for 3 weeks at breakfast. The function of the endothelial cells was evaluated in 2 ways; first, the response to 4 minutes of vascular occlusion was measured with a laser Doppler flow meter and second, the blood flow response to local heat at 42°C for 6 minutes.
Results
The results of the experiments showed that, as reported previously, the endothelial function in people from India was less than their Caucasian counterparts. The blood flow response to heat was reduced after 3 weeks administration of vitamin D in both groups and the response to vascular occlusion in the Caucasian group. But there was only a 20% reduction in the blood flow response to heat in the Caucasian group and a 50% reduction in the group from India.
Conclusions
Thus acute doses of vitamin D may increase vascular tone and reduce blood flow to tissue during stressors. Dosages administered for a longer duration may have beneficial effects on endothelial function but this was not examined here.
Keywords: vitamin D, inflammation, endothelial cells, South Asian Indians
Background
Endothelial dysfunction is a common cause of morbidity and mortality in the elderly, and in people with Diabetes [1–3]. The endothelial dysfunction is commonly caused by oxidative stress [2]. This type of stress, while normally associated with daily activities such as exercise [4], can, if strenuous enough, damage the vascular endothelial cells lining blood vessels [5]. The most common causes of increased oxidative stress are high fat diets [1,6], ageing [7], diabetes [8–11] and smoking [12]. The resulting damage to the endothelial cell impairs the ability of blood vessels to vasodilate. Free radicals damage only the vasodilator pathway of blood vessels leaving the vasoconstrictor pathway intact so that it predominates in the control of blood flow [1,2,13]. This results in a shift in the balance in the blood vessels to constriction at rest and even during stressors such as local heat [2,3,14,14]. With high oxidative stress, blood flow is impaired in response to local heating of the skin.
One potential solution is the use of antioxidants in the diet [15,16]. It has been shown that a healthy diet rich in greens and colored vegetables, provides antioxidants to ward off damage from free radicals [15,16]. In contrast, even a single high fat meal can increase free radicals and damage blood vessel function [1,6]. Unfortunately, most diets do not contain enough of these natural sources of free radical scavengers to stop the damage that occurs from a westernized diet and lifestyle [15].
In the past 40 years an important vitamin that has been investigated is Vitamin D [17]. This vitamin is actually a hormone that regulates the immune system [18,19]. Recent evidence shows that it is involved, not just in bone and mineral metabolism, but it is associated with reducing the incidence of many types of cancers and autoimmune diseases such as multiple scleroses [18,19]. The shift in society from an agrarian to an urban society is associated with low levels of vitamin D in the blood [18,19]. In fact, most people in the United States are extremely substandard in vitamin D in their blood [20–24].
The push in internal medicine to increase vitamin D to doses of over 2000 IU daily has been associated with research on the effects of vitamin D on multiple organ systems [20–24]. One pathway altered by vitamin D is a direct effect on Phosphotidyl inositol 3 kinase (PI3K) in endothelial cells [25]. This compound (PI3K) causes an increase in glucose transport in the cell [26]. It is of no surprise then that vitamin D has been shown in some studies to cause a reduction in blood glucose, especially in people with diabetes [27]. But PI3K has another role. It also causes the activation of endothelial nitric oxide synthetase, an enzyme that catalyzes the production of the blood vessel vasodilator nitric oxide (NO) from l-arginine [2,14]. It is of no surprise then, that the brachial artery response to vascular occlusion (flow mediated vasodilation) is increased in people who are on vitamin D supplements [17,20,23,28,29].
But micro vascular function and vitamin D have not been investigated. The response to stressors such as heat or the micro vascular response (tissue) to vascular occlusion has not been investigated. Typically, flow mediated dilation refers to the brachial artery size [30]. Its increase in size is mediated by shear receptors and is a reaction to an increase in peripheral blood flow [31]. Shear receptors mediate their response through a prostaglandin pathway and this response may not reflect what happens at the level of the micro vascular beds [32]. It can be predicted that stressors such as the application of local heat should show improved blood flow response to vitamin D administration since the main mechanism of the vasodilator response is nitric oxide. But the response to heat has not been investigated. This was the purpose of the present investigation.
Material and Methods
Twenty subjects participated in the experiments. Ten subjects were in each group. The demographics of the Caucasian and subjects from India are listed in Tables 1 and 2 respectively. Subjects were not taking alpha blockers, beta blockers, alpha agonists or antagonists, or any other medication that would affect peripheral blood flow. They were not taking calcium channel blockers or any pain medications. All subjects were naïve for vitamin D at least for a month prior to the beginning of this study. No subjects were smokers. All methods and procedures were approved by the Institutional Review Board of Loma Linda University. All subjects signed a statement of informed consent.
Table 1.
Caucasian subjects.
| Age (years) | Weight Kg | Height Cm | BMI | |
|---|---|---|---|---|
| Mean | 25.3 | 59.2 | 165.7 | 21.5 |
| Standard deviation | 3.3 | 9.7 | 5.9 | 2.9 |
Table 2.
Subjects from India.
| Age (years) | Weight Kg | Height Cm | BMI | |
|---|---|---|---|---|
| Mean | 24.9 | 62.9 | 164.9 | 23.1 |
| Standard deviation | 1.2 | 8.8 | 4.0 | 3.0 |
Measurement of skin temperature
Skin temperature was measured with a thermistor (SKT RX 202A) manufactured by BioPac systems (BioPac Inc., Goleta, CA). The thermistor output was sensed by an SKT 100 thermistor amplifier (BioPac Inc., Goleta, CA). The output, which was a voltage between 0 and 10 volts, was sampled with an analog to digital converter at a frequency of a 1,000 samples per second with a resolution of 24 bits with a BioPac MP150 analog to digital converter. The converted data was then stored on a desktop computer using Acknowledge 3.9.1 software for future analysis. Data analysis was done over a 5 second period for mean temperature.
Measurement of skin blood flow
Skin blood flow will be measured with either a Moor Laser Doppler Imager (LDF) or Moor optical fiber flow meter (VMS LDF2)(Moor LTD. Oxford England). The imager used a red laser beam (632.8 nm) at a power of 2.5 mw to measure skin blood flow using the Doppler Effect. The laser, in this case, was used in single point mode. After warming the laser for 20 minutes prior to use, the laser was focused on one area of the skin and, by comparing the reflected to the source light, the change of the frequency of the light and absorption of the light was used to calculate the red cell velocity and the red cell content in that area of the skin. The Moor Laser Doppler Imager measured blood flow through most of the dermal layer of the skin but did not penetrate the entire dermal layer. Blood flow was then calculated in a unit called Flux based on the red cell concentration and red cell velocity with a stated accuracy of ±10%. It was used for the occlusion studies. For the heat studies, a single point fiber optic laser Doppler flow meter was used (VMS LDF2). This Moor instrument unit sampled a smaller area of the skin but was coupled to a heated probe as described below so that the assembly temperature was controlled. This flow meter provides less flow output due to the smaller surface area of the probe.
Control of skin temperature
Skin temperature will be controlled by a Moor temperature controller (Moor VMS-heat, Moor LTD. Oxford England). This is a closed loop electric warmer where temperature is controlled to 0.1°C.
Vitamins
The vitamins used in the study will be 4000 IU of D3, taken daily (Kirkland).
Measurement of endothelial function
Endothelial function was measured by the blood flow response to occlusion and heat.
Occlusion
The blood flow to the arm was occluded for 4 minutes by placing a pneumatic occlusion cuff on the upper arm above the elbow and inflating the cuff for 4 minutes. After the pressure was released, forearm blood flow was measured for 2 minutes to assess the reactivity of the blood vessels to occlusion and anoxia.
Measurement of the response to heat
The response of the skin to heat was measured by applying a heated probe to the skin for 6 minutes. The thermode was set at a temperature of 42 degrees centigrade. This warmed the skin and blood flow was then recorded.
Procedures
The study design was a pre post non randomized quasi experimental study. All subjects were administered vitamin D for 3 weeks in this longitudinal study. Before and after this period of time, the entire group had their response to heat and occlusion measured.
Data analysis
Data analysis consisted of means and standard deviations and related and unrelated T tests. Mixed Factorial ANOVA was used to compare within and between groups. The level of significance was p<0.05.
Results
Caucasian subjects
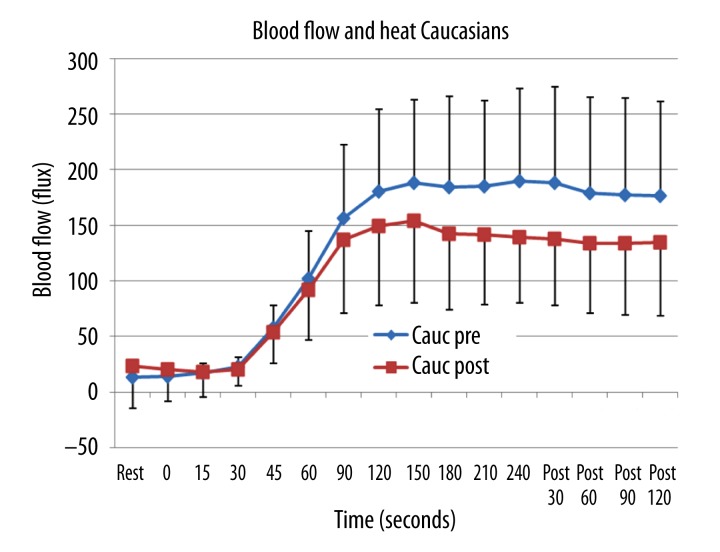
The results of the experiments are shown in Figures 1–4. The blood flow response to a locally applied heat source is shown in Figure 1 for the young Caucasian subjects. Before ingesting vitamin D, after heat was applied, there was a 30 second period before blood flow started to increase. After this, flow increased rapidly for the first 2 minutes and then plateaued. The final blood flow measured during heat application averaged 189.1±83.1 flux. After heat was turned off, flow remained elevated for at least 2 minutes post as shown in this Figure. Three weeks after administration of vitamin D, the rate of increase in blood flow in the first 1.5 minutes was almost the same and there was no significant statistical difference in the blood flow comparing their magnitude before and after vitamin D administration. But after that point, blood flow was significantly less in the subjects after administration of vitamin D (Figure 1). For the last 2.5 minutes and 2 minute recovery period the difference between the group was significant (ANOVA p=0.001) or a 21% reduction in the sustained blood flow response to heat. The area under the curve in Figure 1 gives the total blood flow response due to heat. For the Caucasian subjects pre vitamin D, the average was 1814 flux while post the total excess blood flow was 1120 flux, this difference being significant (p<0.001).
Figure 1.
Illustrated here is the skin blood flow as the mean of 10 subjects plus or minus the standard deviation measured at rest and during 6 minutes of passive heating of the skin. Data is shown before (pre) and 3 weeks after (post) administration of vitamin D in 10 Caucasian subjects. The x axis shows the resting blood flow and blood flow during 240 seconds of heat exposure and for 120 seconds after heat exposure.
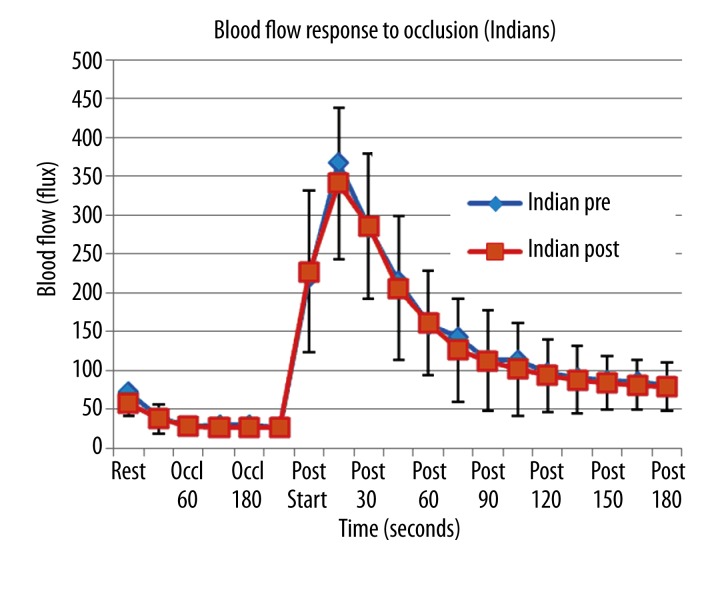
Figure 4.
Illustrated here is the skin blood flow as the mean of 10 subjects plus or minus the standard deviation measured at rest and during and after 4 minutes of vascular occlusion. Data is shown before (pre) and 3 weeks after (post) administration of vitamin D in 10 Indian subjects. The x axis shows the resting blood flow, blood flow during occlusion and for 180 seconds post occlusion.
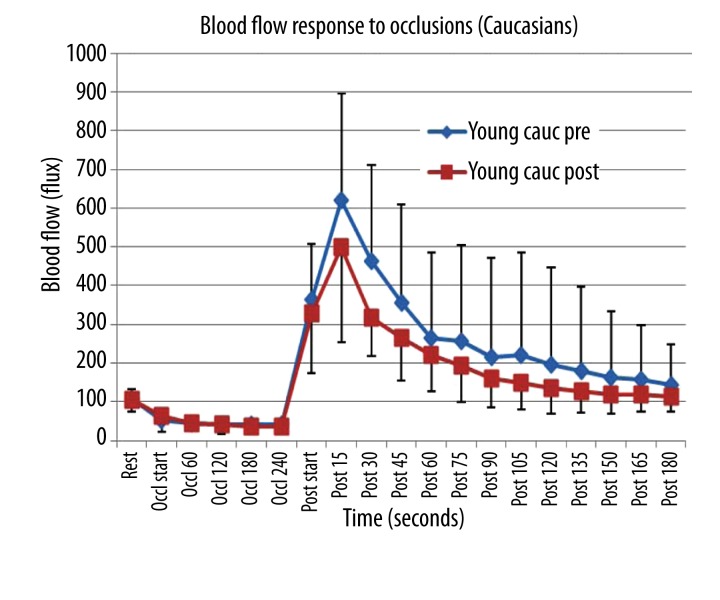
The response to occlusion showed a similar pattern. The blood flow was higher in this series than the heat series since a different laser flow meter was used. To measure the response to heat, a single fiber optic probe was used as cited in methods. For occlusion, a laser Doppler imager was used that sampled a much larger area of the skin. As shown in Figure 2, for the Caucasian subjects, blood flow after 4 minutes of occlusion increased by almost 10 fold. Pre vitamin D, the blood flow increased to 618.9±238 flux and then exponentially returned toward the baseline blood flow. Subtracting resting blood flow from the blood flows recorded for 3 minutes post occlusion, the total increase in blood flow due to the occlusion averaged 2201 flux units. Post vitamin D, the same pattern was seen but the peak blood flow was less, averaging 498 flux. The total excess blood flow above rest post occlusion was 1370.3 flux. Comparing blood flow pre and post vitamin D, there was a significant difference for the first minute after occlusion was released and for the area under the post occlusion curve (p<0.001).
Figure 2.
Illustrated here is the skin blood flow as the mean of 10 subjects plus or minus the standard deviation measured at rest and during and after 4 minutes of vascular occlusion. Data is shown before (pre) and 3 weeks after (post) administration of vitamin D in 10 Caucasian subjects. The x axis shows the resting blood flow, blood flow during occlusion and for 180 seconds post occlusion.
Indian subjects
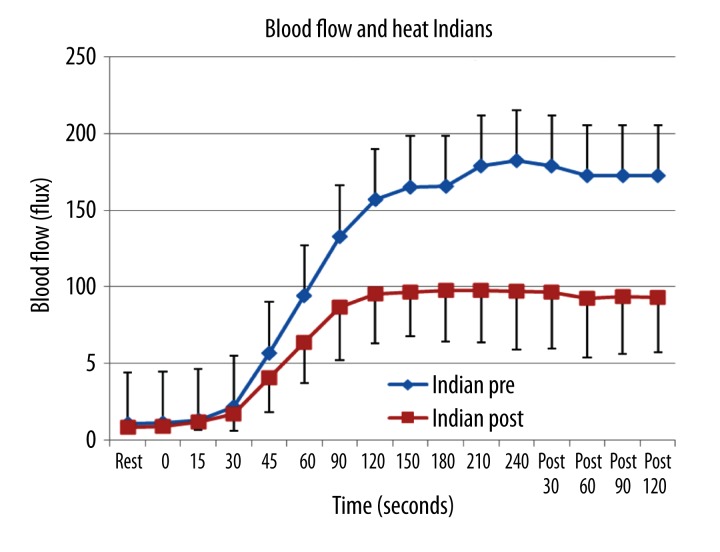
The results for the subjects from India are shown in Figures 3 and 4. For the response to heat, Figure 3, Pre vitamin D there was a similar pattern as that seen to the young Caucasian subjects. The only difference was that the blood flow at rest and throughout heat exposure was significantly less than that of the Caucasian subjects (p<.05). This is especially true post vitamin D. Here the blood flow was significantly less 60 seconds after exposure to the heat but not at rest or before 60 seconds comparing the Indian subjects before and after vitamin D. After 60 seconds, the blood flow was reduced by almost 50% comparing data before and after vitamin D administration in these subjects. The total excess blood flow in the Indian group pre vitamin D after heat exposure was 1709 flux, decreased to 889 flux post vitamin D, a significant loss in total blood flow (p<0.001).
Figure 3.
Illustrated here is the skin blood flow as the mean of 10 subjects plus or minus the standard deviation measured at rest and during 6 minutes of passive heating of the skin. Data is shown before (pre) and 3 weeks after (post) administration of vitamin Din 10 subjects from India. The x axis shows the resting blood flow and blood flow during 240 seconds of heat exposure and for 120 seconds after heat exposure.
The resting blood flow was significantly less after vitamin D (p<0.05). For the response to occlusion, there was no difference in the blood flow response throughout occlusion, however, the blood flow response was significantly less at each time point as well as the area under the curve compared to the Caucasian group (p<0.05).
Discussion
Numerous studies have been conducted on vitamin D, a secosteroid hormone with genomic and nongenomic signaling, and its potential cardiovascular benefits [33]. Some studies show that vitamin D upregulates the immune system [34], may down regulate the renin-angiotensin system [35], increases the release of vascular endothelial growth factor [36], and helps regulate calcium homeostasis [37]. Some studies show an increase in endothelial function after administration of vitamin D while others do not [33,38]. Endothelial function is commonly measured by brachial artery flow mediated dilation [33,39], (a measure of macro vascular and not micro vascular endothelial function). Even though numerous studies use brachial artery flow mediated dilation to assess endothelial function, these studies show very different results on the cardiovascular benefits of vitamin D administration. Studies, while largely on people with pathologies or lab animals, show, in some cases, no effect while others show macro vascular improvement [40] and a reduction in renin and angiotensin 2 in the body, thereby lowering blood pressure [33]. Some studies show a decrease in parathormone but others show an increase in arterial stiffness after vitamin D administration [41,42]. The confusion comes from the fact that studies of vitamin D are usually based on correlations between vitamin D in the blood and the incidence of disease [33]. This does not mean that vitamin D in itself will reduce vascular disease but is a factor observed to be inversely correlated with diabetes and cardiovascular disease [33]. Since most studies have been conducted for 3 months or more in people with hypertension, heart disease or diabetes, the potential complications of inflammation and drugs such as the statins on vitamin D function are unknown and may negate the effects of vitamin D administered to the subjects [33]. Inflammation, for example, reduces vitamin D in the body [43]. Few studies have looked at vitamin D administration in healthy young people and very few have looked at micro vascular effects of vitamin D. For example, in a study of blood flow in the fingertip (micro vascular function) vitamin D administration increased brachial artery flow mediated dilation but not fingertip blood flow [28]. But brachial artery dilation is mediated by shear receptors which activate through microvilli and a prostaglandin mediated process including the release of nitric oxide [44–46]. The local response to occlusion is not mediated by prostaglandins or nitric oxide [47]. The micro vascular response to heat is mediated by other mechanisms. Thus the present investigation adds another piece to this puzzle by examining other pathways in young asymptomatic people.
The blood flow response to local heat was investigated because it is largely a nitric oxide mediated pathway. Inhibition of nitric oxide synthetase is common in disease states such as diabetes and even ageing, causing a reduced blood flow response to local heat. Local heating induces a biphasic skin blood flow (SBF) response. With heat application, the initial phase is a rapid increase in skin blood flow [48] and is mediated by sensory nerve neurotransmitters, such as calcitonin gene related peptide (CGRP) and substance P (SP) [49]. The second phase is a slow and prolonged increase in SBF, which is mediated by nitric oxide (NO) [49–54]. In the present series of experiments, vitamin D did not alter the initial response to heat but did alter the prolonged response to heat. One possibility is that an acute dose of vitamin D has altered the nitric oxide pathway by reducing the production of nitric oxide. Nitric oxide is released through voltage gated calcium channels that are sensitive to temperature (TRPV4 channels). There are vitamin D receptors on TRPV6 calcium channels that alter calcium movement into endothelial cells. It is possible that calcium may have been reduced in these temperature channels and hence nitric oxide production was also reduced. This would account for the lower slow phase blood flow response in Caucasian and Indians both. The fact that the response to heat is less in Indians and more greatly diminished in response to vitamin D dosage (acute) may be due to the difference in their endothelial function due to the presence of what has been termed a “thrifty genotype” [6,55,56]. This genotype is very sensitive to free radicals and can cause a major reduction in the response to heat or vascular occlusion in response to even a single high fat meal [56,57]. Asian populations have been shown to have less blood flow response to heat than that seen in Caucasians [6,58].
Treatment with vitamin D in asymptomatic controls has been shown to reduce free radicals in the blood as measured by TBARS [59]. But in this study, brachial artery mediated dilation increased. However the study, while using similar dosages of vitamin D as in the present investigation, was conducted over 3 months. After 3 months, insulin resistance decreased and brachial artery flow mediated dilation increased. Two factors are noteworthy, first, the study was 3 months duration and second, the mechanism for arterial blood flow mediated dilation is different than that seen in the micro vascular bed in response to anoxia after occlusion or the second phase response to heat.
But vitamin D is also a major regulator of calcium metabolism [60]. Some animal studies show that acute administration of vitamin D can increase arterial stiffness. This is due to increased calcium influx into vascular smooth muscle. This may be a mechanism in action here in the blood flow response to heat.
But the response to occlusion is caused by an anoxia mediated mechanism and unrelated to nitric oxide or prostaglandin production. The mechanism is poorly understood. The micro vascular response to occlusion seen here showed a small reduction in blood flow in the Caucasian group but none in the group from India. Some studies show an improvement in the response to occlusion while others do not. Mostly these studies on flow mediate dilation require months of administration of vitamin D. For both legs of this study, a 3 week period would normally not increase vitamin D substantially in the blood in most studies because vitamin D is absorbed in the fat in the body. But the subjects in this study were all young and lean subjects and vitamin D should have increased even after 3 weeks. It was not possible to measure vitamin D here and therefore this is a limitation of the study. In many studies of asymptomatic people there were no cardiovascular benefits of vitamin D as assessed by flow mediated dilation in the brachial artery.
Conclusions
This study examined the peripheral micro circulation so it was very different than previous studies. More investigation is needed.
Footnotes
Source of support: Departmental sources
References
- 1.Yim J, Petrofsky J, Berk L, et al. Differences in endothelial function between Korean-Asians and Caucasians. Med Sci Monit. 2012;18(6):CR337–43. doi: 10.12659/MSM.882902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrofsky J, Berk L, Al-Nakhli H. The influence of autonomic dysfunction associated with aging and type 2 diabetes on daily life activities. Exp Diabetes Res. 2012;2012:657103. doi: 10.1155/2012/657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrofsky JS, Alshahmmari F, Lee H, et al. Reduced endothelial function in the skin in Southeast Asians compared to Caucasians. Med Sci Monit. 2012;18(1):CR1–8. doi: 10.12659/MSM.882185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadley AJ, Veldhuijzen van Zanten JJ, Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: the vascular health triad. Age (Dordr) 2013;35(3):705–18. doi: 10.1007/s11357-012-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira DM, Lima RM, El-Bacha RS. Brain rust: Recent discoveries on the role of oxidative stress in neurodegenerative diseases. Nutr Neurosci. 2012;15(3):94–102. doi: 10.1179/1476830511Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 6.Bui C, Petrofsky J, Berk L, et al. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41:490–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes SC, Little JP, Candow DG. Exercise and nutritional interventions for improving aging muscle health. Endocrine. 2012;42(1):29–38. doi: 10.1007/s12020-012-9676-1. [DOI] [PubMed] [Google Scholar]
- 8.Hegde SV, Adhikari P, Kotian S, et al. Response to Comment on: Hegde et al. Effect of 3-Month Yoga on Oxidative Stress in Type 2 Diabetes With or Without Complications: A Controlled Clinical Trial. Diabetes Care, 2011;34: 2208–10. Diabetes Care. 2012;35:e43. doi: 10.2337/dc10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegde SV, Adhikari P, Kotian S, et al. Effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled clinical trial. Diabetes Care. 2011;34:2208–10. doi: 10.2337/dc10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde KR, Varma SD. Combination of glycemic and oxidative stress in lens: implications in augmentation of cataract formation in diabetes. Free Radic Res. 2005;39:513–17. doi: 10.1080/10715760400013755. [DOI] [PubMed] [Google Scholar]
- 11.Hegde HR. Diabetes mellitus, acquired immune deficiency syndrome and tuberculosis. Med Hypotheses. 2005;64:1065. doi: 10.1016/j.mehy.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Vande Loock K, Ciardelli R, Decordier I, et al. Preterm newborns show slower repair of oxidative damage and paternal smoking associated DNA damage. Mutagenesis. 2012;27(5):573–80. doi: 10.1093/mutage/ges022. [DOI] [PubMed] [Google Scholar]
- 13.Petrofsky JS. The effect of type-2-diabetes-related vascular endothelial dysfunction on skin physiology and activities of daily living. J Diabetes Sci Technol. 2011;5:657–67. doi: 10.1177/193229681100500319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrofsky J, Alshahmmari F, Yim JE, et al. The interrealtionship between locally applied heat, ageing and skin blood flow on heat transfer into and from the skin. J Med Eng Technol. 2011;35:262–74. doi: 10.3109/03091902.2011.580039. [DOI] [PubMed] [Google Scholar]
- 15.Landberg R, Naidoo N, van Dam RM. Diet and endothelial function: from individual components to dietary patterns. Curr Opin Lipidol. 2012;23(2):147–55. doi: 10.1097/MOL.0b013e328351123a. [DOI] [PubMed] [Google Scholar]
- 16.Hansen L, Skeie G, Landberg R, et al. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. Int J Cancer. 2012;131:469–78. doi: 10.1002/ijc.26381. [DOI] [PubMed] [Google Scholar]
- 17.Caprio M, Mammi C, Rosano GM. Vitamin D: a novel player in endothelial function and dysfunction. Arch Med Sci. 2012;8:4–5. doi: 10.5114/aoms.2012.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–72. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 19.Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging. 2007;24:1017–29. doi: 10.2165/00002512-200724120-00005. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 24.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–16. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang HJ, Cao JP, Yu JK, et al. Calbindin-D28K expression induced by glial cell line-derived neurotrophic factor in substantia nigra neurons dependent on PI3K/Akt/NF-kappaB signaling pathway. Eur J Pharmacol. 2008;595:7–12. doi: 10.1016/j.ejphar.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 26.Xu TJ, Liu Y, Yuan B. Effect of insulin in combination with selenium on Irs/PI3K-mediated GLUT4 expression in cardiac muscle of diabetic rats. Eur Rev Med Pharmacol Sci. 2011;15:1452–60. [PubMed] [Google Scholar]
- 27.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin d and diabetes. Rheum Dis Clin North Am. 2012;38(1):179–206. doi: 10.1016/j.rdc.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Duprez D, de Buyzere M, de Backer T, Clement D. Relationship between vitamin D3 and the peripheral circulation in moderate arterial primary hypertension. Blood Press. 1994;3:389–93. doi: 10.3109/08037059409102292. [DOI] [PubMed] [Google Scholar]
- 29.Zawada ET, Jr, TerWee JA, McClung DE. Systemic and renal vascular responses to dietary calcium and vitamin D. Hypertension. 1986;8:975–82. doi: 10.1161/01.hyp.8.11.975. [DOI] [PubMed] [Google Scholar]
- 30.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.09.047. pii: S0167-5273(12)01153-9. [DOI] [PubMed] [Google Scholar]
- 31.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–19. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 32.Merkus D, Sorop O, Houweling B, et al. NO and prostanoids blunt endothelin-mediated coronary vasoconstrictor influence in exercising swine. Am J Physiol Heart Circ Physiol. 2006;291:H2075–81. doi: 10.1152/ajpheart.01109.2005. [DOI] [PubMed] [Google Scholar]
- 33.Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int. 2013;24(8):2167–80. doi: 10.1007/s00198-013-2281-1. [DOI] [PubMed] [Google Scholar]
- 34.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–99S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grundmann M, Haidar M, Placzko S, et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303:C954–62. doi: 10.1152/ajpcell.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 38.Sokol SI, Srinivas V, Crandall JP, et al. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17:394–404. doi: 10.1177/1358863X12466709. [DOI] [PubMed] [Google Scholar]
- 39.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 40.Sugden JA, Davies JI, Witham MD, et al. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–25. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer M, Begerow B, Minne HW, et al. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes. 2001;109(2):87–92. doi: 10.1055/s-2001-14831. [DOI] [PubMed] [Google Scholar]
- 42.Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–37. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 43.Oplander C, Volkmar CM, Paunel-Gorgulu A, et al. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circ Res. 2009;105:1031–40. doi: 10.1161/CIRCRESAHA.109.207019. [DOI] [PubMed] [Google Scholar]
- 44.Jones H, Lewis NC, Green DJ, et al. alpha1-Adrenoreceptor activity does not explain lower morning endothelial-dependent, flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1437–42. doi: 10.1152/ajpregu.00042.2011. [DOI] [PubMed] [Google Scholar]
- 45.Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–35. [PubMed] [Google Scholar]
- 46.Lavallee M, Takamura M, Parent R, Thorin E. Crosstalk between endothelin and nitric oxide in the control of vascular tone. Heart Fail Rev. 2001;6:265–76. doi: 10.1023/a:1011448007222. [DOI] [PubMed] [Google Scholar]
- 47.Lopez MG, Silva BM, Joyner MJ, Casey DP. Roles of nitric oxide and prostaglandins in the hyperemic response to a maximal metabolic stimulus: redundancy prevails. Eur J Appl Physiol. 2013;113(6):1449–56. doi: 10.1007/s00421-012-2570-y. [DOI] [PubMed] [Google Scholar]
- 48.Charkoudian N, Eisenach JH, Atkinson JL, et al. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol. 2002;92:685–90. doi: 10.1152/japplphysiol.00758.2001. [DOI] [PubMed] [Google Scholar]
- 49.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 50.Kellogg DL, Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–90. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 51.Minson JB, Llewellyn-Smith IJ, Arnolda LF. Neuropeptide Y mRNA expression in interneurons in rat spinal cord. Auton Neurosci. 2001;93:14–20. doi: 10.1016/S1566-0702(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 52.Petrofsky J, Bains G, Prowse M, et al. Dry heat, moist heat and body fat: are heating modalities really effective in people who are overweight? J Med Eng Technol. 2009;33:361–69. doi: 10.1080/03091900802355508. [DOI] [PubMed] [Google Scholar]
- 53.Petrofsky JS, Goraksh N, Alshammari F, et al. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrofsky JS, Laymon M. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33:337–48. doi: 10.1080/03091900802069547. [DOI] [PubMed] [Google Scholar]
- 55.Newsome BB, McClellan WM, Allison JJ, et al. Racial differences in the competing risks of mortality and ESRD after acute myocardial infarction. Am J Kidney Dis. 2008;52:251–61. doi: 10.1053/j.ajkd.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Andersen LL, Jay K, Andersen CH, et al. Acute effects of massage or active exercise in relieving muscle soreness: Randomized controlled trial. J Strength Cond Res. 2013 doi: 10.1519/JSC.0b013e3182908610. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Vrontou S, Wong AM, Rau KK, et al. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature. 2013;493:669–73. doi: 10.1038/nature11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mata-Greenwood E, Chen DB. Racial differences in nitric oxide-dependent vasorelaxation. Reprod Sci. 2008;15:9–25. doi: 10.1177/1933719107312160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howatson G, Hoad M, Goodall S, et al. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr. 2012;8(9):20. doi: 10.1186/1550-2783-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabini A, Piazzini DB, Tancredi G, et al. Deep heating therapy via microwave diathermy relieves pain and improves physical function in patients with knee osteoarthritis: a double-blind randomized clinical trial. Eur J Phys Rehabil Med. 2012;48(4):549–59. [PubMed] [Google Scholar]