Abstract
Male reproductive health has deteriorated considerably in the last few decades. Nutritional, socioeconomic, lifestyle and environmental factors (among others) have been attributed to compromising male reproductive health. In recent years, a large volume of evidence has accumulated that suggests that the trend of decreasing male fertility (in terms of sperm count, quality and other changes in male reproductive health) might be due to exposure to environmental toxicants. These environmental contaminants can mimic natural oestrogens and target testicular spermatogenesis, steroidogenesis, and the function of both Sertoli and Leydig cells. Most environmental toxicants have been shown to induce reactive oxygen species, thereby causing a state of oxidative stress in various compartments of the testes. However, the molecular mechanism(s) of action of the environmental toxicants on the testis have yet to be elucidated. This review discusses the effects of some of the more commonly used environmental contaminants on testicular function through the induction of oxidative stress and apoptosis.
Keywords: apoptosis, environmental contaminants, male reproduction, oxidative stress, reactive oxygen species, testis, spermatogenesis
Introduction
In recent years, there has been growing concern regarding the adverse effects of various environmental contaminants on male reproduction. With the advent of industrialisation, economic development and urbanisation, drastic changes have occurred in the lifestyle and surroundings of humans that have resulted in the extensive production and use of beneficial substances. As a result, many potentially hazardous chemicals have been released into the environment at an alarming rate, and their exposure to both humans and wildlife has become inevitable. These chemicals that have been released into the environment are a leading causative factor in the high incidence of various pathological conditions, including cancers.1, 2 Concurrently, there has been a declining trend in the male reproductive health of both wildlife and humans in industrialized nations.3, 4 A meta-analysis report of a 50% worldwide decline in sperm density from 1940 to 1990 raised considerable scientific and public concern regarding the imminent threat of synthetic chemicals to male reproductive health.5 Since that report, several studies have demonstrated the negative impact that synthetic chemicals have on male reproductive health.6, 7
Most environmental chemicals are hormonally active compounds that target the endocrine system and cause reproductive anomalies.8, 9, 10 Some chemicals are specifically known to perturb the testicular milieu. An increasing body of evidence suggests that environmental contaminants impair testicular functions by disturbing the pro-oxidant/antioxidant balance of testicular cells, thereby activating associated downstream pathways such as apoptosis.11 Although physiological levels of reactive oxygen species (ROS) and apoptosis are required for normal functioning of the testis, pathological levels can be deleterious. This review summarizes recent studies (including those from our laboratory) of the toxicological effects of some of the more commonly used environmental contaminants on the testis, with special emphasis on elucidating the mechanisms that act via generation of ROS and apoptosis.
The physiological role of ROS and apoptosis in spermatogenesis
The testes perform two vital, high energy-demanding functions, namely, spermatogenesis and steroidogenesis. In the testes, spermatogenesis and steroidogenesis occur within seminiferous tubules and the interstitium, respectively. These two compartments are morphologically distinct but are functionally connected.12 During spermatogenesis, a complex, interdependent population of undifferentiated germ cells multiplies and differentiates to form spermatozoa. In the seminiferous epithelium, the germ cells are sequentially organized from the base of the tubule to the lumen, signifying the various stages of development.13 The germ cells are fostered by the nursing Sertoli cells, which extend from the base to the lumen of the seminiferous tubules. The tight junctions between the Sertoli cells form an effective blood–testis barrier that regulates the flow of nutrients and growth factors that are required for the development of germ cells.14, 15 The process of spermatogenesis is hormonally regulated by a negative feedback loop that involves the hypothalamus, pituitary and testis.
Several intratesticular and extratesticular regulatory processes are involved in the regulation of normal spermatogenesis. The ROS that are generated during normal testicular function also play an important role in regulating the function of the testis. Although ROS are known to have damaging effects, controlled, low levels of ROS play a beneficial role in normal testicular function. However, the production of ROS in the testis is primarily associated with phagocytic leukocytes in the semen,16 other cell types—such as developing germ cells and spermatids—are also a ready source of ROS.17 A wealth of evidence indicates that ROS that are produced by sperm participate in the signal transduction mechanism that promotes sperm capacitation, the acrosome reaction and sperm maturation.18 However, increased levels of ROS can be detrimental to testicular function. To overcome this, the testis is equipped with very a potent antioxidant system that protects it from the damaging effects of ROS. The glutathione family of proteins, superoxide dismutase, catalase and several non-enzymatic antioxidants all help the testis by counteracting any oxidative impact.19 However, overexposure to environmental toxicants has been shown to impair the pro-oxidant/antioxidant balance in the testis and thereby hamper testicular function.20 Thus, the spermatogenic process can serve as both a source and a target of ROS.
The ROS that are produced during spermatogenesis are involved in the regulation of apoptosis within the testis.21 Testicular apoptosis occurs during differentiation of germ cells, which serves to adjust the number of germ cells in the testis.22 Testicular apoptosis occurs continuously throughout spermatogenesis, and both the intrinsic and extrinsic apoptotic pathways have been shown to play regulatory roles.23, 24 The intrinsic (or mitochondrial) pathway involves various pro-apoptotic and anti-apoptotic proteins that recruit and activate the caspase cascade to induce apoptosis. The extrinsic pathway is mediated through Fas receptor (Fas) and Fas ligand (FasL) together with caspase proteins. Sertoli cells express Fas ligand, which signals the killing of Fas-expressing germ cells, thereby limiting the number of germ cells.25 Various factors such as the withdrawal of growth factors, radiation and oxidative stress trigger apoptosis in the testis.
Environmental toxicants and spermatogenesis
Several environmental toxicants induce apoptosis in germ cells, thereby resulting in defective spermatogenesis. For example, in adult male rats, methoxychlor (MXC) induced oxidative stress by decreasing the levels of antioxidant enzymes in the testis and epididymides when administered at 50, 100 or 200 mg kg−1 body weight for 1, 4 and 7 days.26, 27 However, long-term exposure to MXC at low doses (1, 10 and 100 mg kg−1 body weight) elicited oxidative stress and depletion of the activity of antioxidant enzymes in the mitochondrial and microsomal fractions of testis.28 A single MXC dose of 50 mg kg−1 body weight transiently increased the levels of apoptotic proteins (e.g., pro- and cleaved caspase-3, cytochrome c, Fas and FasL in the peritubular germ cells), which suggests the activation of the mitochondrial and FasL-mediated death pathways upon exposure to MXC.29 A transgenerational study showed that administration of MXC to pregnant rats from gestational days 7 through 15 reduced the number of germ cells and increased the number of apoptotic germ cells in the male offspring measured at postnatal day 17.30 Perinatal and juvenile exposure to MXC (at 5, 50 or 150 mg kg−1) has been reported to reduce spermatogenic potential by decreasing the volume of the Sertoli cell nucleus and the number of Sertoli cell which suggests that MXC impairs spermatogenesis by targeting the Sertoli cell population in the testis.31
In male rats, intraperitoneal injection of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p′-DDE, a principal metabolite of DDT) at a dose of 60 or 100 mg kg−1 body weight for 10 days caused an increase in the levels of lipid peroxidation and a decrease in the activities of superoxide dismutase and glutathione peroxidase in the testis. An increase in the mRNA levels of Fas, FasL, caspase-3 and caspase-8 were also observed, which indicates that p,p′-DDE induces apoptosis through the Fas/FasL apoptotic pathway.32 Exposing Sertoli cells in vitro to p,p′-DDE (at 10, 30 or 50 µmol l−1), beta-benzene hexachloride (at 10, 30 or 50 µmol l−1) or a combination of p,p'-DDE and beta- benzene hexachloride (at 10, 30 or 50 µmol l−1 each) led to the activation of caspases-3, -8 and -9, which is indicative of apoptosis.33 Vinclozolin, a dicarboximide fungicide, was administered to mice during gestation (15–22 days) which decreased the anogenital distance, prostate weight, sperm count and induced changes in the expression of the apoptosis-related proteins p51 and p21.34 A comparative study of the anti-androgenic effects of vinclozolin and flutamide (a well-known anti-androgen drug) showed that these drugs have transgenerational effects. Vinclozolin and flutamide (at doses of 100 and 20 mg kg−1 body weight, respectively) caused an increase in spermatogenic cell apoptosis and a decrease in epididymal sperm in the testis of F1 generation rats; the F2 and F3 generations were also found to be affected in the vinclozolin-treated group but not in the flutamide-treated group, suggesting different modes of action for these compounds.35
Lindane (gamma-hexachlorocyclohexane), an organochlorine pesticide, has been shown to impair spermatogenesis in testis. Rats that were treated with gamma-hexachlorocyclohexane at critical stages of testicular development (6th–30th postnatal day) exhibited elevated levels of testicular lipid peroxidation and hydrogen peroxide synthesis, as well as reduced levels of superoxide dismutase, catalase and ascorbic acid.36 In addition, administering 5 mg of lindane daily for 30 days to male albino rats led to an induction of oxidative stress in the testis, epididymis and epididymal sperm.37, 38 Exposure to a single dose lindane (5 mg kg−1 body weight) has been shown to increase the levels of cytosolic cytochrome c along with pro-caspase-9 within 6 h of exposure. In addition, increased colocalisation of Fas and caspase-3 in peritubular germ cells was also observed in the testis, and this effect was almost fully reversed within 72 h of exposure.39 The in vitro exposure of Sertoli cells to lindane has been shown to alter gap junction intercellular communication by changing the distribution of connexin 43 and zona occludens-1.40
Several studies have reported that bisphenol A (BPA) induces oxidative stress in the testis, epididymis and sperm of various animal species.41, 42, 43 Male mice that were exposed to 480 or 960 mg kg−1 of BPA from postnatal day 35 through 49 exhibited activated mitochondrial and Fas-mediated death pathways, increased terminal deoxynucleotidyl transferase dUTP nick end labelling-positive germ cells in stage VII–VIII, and activated caspases-3, -8 and -9, Bax, Fas and FasL.44, 45 Maternal exposure to low doses of BPA caused a significant decrease in the efficiency of sperm production in male offspring46 and a significant reduction in testicular weight, daily sperm production and the efficiency of spermatogenesis (measured as daily sperm production per gram of testis) at a dose as low as 20 µg kg−1.47 A disruption of the Sertoli cell barrier and a change in the distribution of the gap junction protein connexin 43 were observed when BPA was administered to rats.48 BPA has also been reported to impair the function of gap junctions in Sertoli cells through a redistribution of occluding/zona occludin-1/focal adhesion kinase complex proteins at the blood–testis barrier and by activating the mitogen activated protein kinase pathway. Docking of BPA with gap junction protein connexin 26 revealed an interaction between BPA and the pore-lining residues of the N-terminal helix and the first transmembrane helix of connexin 26.49
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a polychlorinated dibenzo-p-dioxin, is a potent environmental toxicant. Both short- and long-term exposures to TCDD have been reported to induce oxidative stress and decrease the levels of antioxidant enzymes in the testis and epididymis of rats.50, 51, 52 TCDD targets dioxin-inducible factor-3—a nuclear factor that possesses a zinc-finger motif—to mediate reproductive toxicity.53 Gestational exposure to TCDD has been reported to impair testicular spermatogenesis and testicular development in both rhesus monkeys and mice.54, 55 Exposure of C57BL/six mice to TCDD (50 µg kg−1 body weight) caused a reduction in the mitochondrial membrane potential of epididymal spermatozoa and increased ROS levels in the spermatozoa, which was blocked by co-administration with the antioxidant N-acetylcysteine. When 3-week-old male rats were exposed for 7 days to dinbutyl phthalate, a common plasticizer, they exhibited testicular atrophy, a loss of spermatogenic cells and a high incidence of apoptosis in their spermatogenic cells, and the investigators speculated that the estrogenicity of dinbutyl phthalate could have contributed to these effects.56
Oral administration of the organic herbicide atrazine, to male rats at a dose of 120 or 200 mg kg−1 body weight for 7 and 16 days caused a decrease in the activity of antioxidant enzymes and an increase in lipid peroxidation and hydrogen peroxide synthesis, which suggests that atrazine induced a state of oxidative stress in these animals.57 Intraperitoneal administration of dinitrobenzene (at 25 mg kg−1) caused apoptosis at spermatogenesis stages VI–VIII and IX–XIII and increased DNA fragmentation within 6 h of exposure.58 Administration of 1,3-dinitrobenzene to Sprague–Dawley rats caused an upregulation of the apoptotic proteins that are involved in the mitochondrial pathway.59 Fenvalerate administration (at a dose of 15 or 60 mg kg−1 body weight) for 28 days caused an increase in the number of terminal deoxynucleotidyl transferase dUTP nick end labelling-positive germ cells, increased the levels of caspases-3 and -8 in the testis and upregulated the expression of Fas and FasL in the testis. However, fenvalerate did not activate the mitochondrial cell death pathway or caspase-9 in the testis of rats.60 Exposure to octylphenol has been shown to decrease the viability of Sertoli cells and induce apoptosis by upregulating the expression of Bax and causing the cleavage of procaspase-3,61 and administration of nonylphenol activated the endoplasmic reticulum signalling pathway and induced apoptosis in the Sertoli cells of rats.62
Most of these toxicants impair the spermatogenic process by inducting oxidative stress and apoptosis in germ cells, and some toxicants target Sertoli cells and thereby hamper spermatogenesis. Several other toxicants impair spermatogenesis and lead to infertility. The effects of a few select environmental toxicants on spermatogenesis are summarized in Table 1.
Table 1. Environmental toxicants that affect spermatogenesis.
| Toxicant | Dose and duration | Observed effects | Reference |
|---|---|---|---|
| 4-tert-octylphenol | Injection of 20 or 80 mg for 2 months | Decreased sperm count, increased head abnormalities of the sperm | 87 |
| Atrazine | Oral administration of 120 or 200 mg kg−1 body weight for 7 and 16 days | Increased oxidative stress, decreased epididymal sperm motility, viability and defoliation of germ cells | 57 |
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) | Intraperitoneal injection of 50 µg kg−1 body weight | Decreased percentage of tubules containing sperm, decreased sperm count, decreased germ cell count and Sertoli cell index and upregulation of testis-specific proteins | 88 |
| Aroclor 1254 | Intraperitoneal injection of 0.75, 1.5 or 3 mg kg−1 day-1 | Decreased weight of the testis, decreased sperm count, motility and daily sperm production, decreased levels of mitochondrial antioxidant enzymes | 89 |
| Methyl parathion | Single intraperitoneal injection of 20 mg kg−1 body weight and sperm collected on the 7th and 28th day | Decreased sperm quality, decreased DNA integrity of spermatozoa and reduced mitochondrial membrane potential | 90 |
| 1,1-dichloro-2,2 bis(p-chlorophenyl) ethylene (p,p′-DDE) | Intraperitoneal injection of 20, 60 or 100 mg kg−1 body weight for 10 days | Increased oxidative stress and increased levels of FasL, caspase-3 and caspase-8 | 32 |
| Malathion | Single intraperitoneal injection of 240 mg kg−1 body weight and killed on 1, 8, 16, 35 and 40 days | Decreased sperm count, increased incidence of teratozoospermia and depletion of seminiferous tubules | 91 |
ROS generation and apoptosis in steroidogenesis
Together with germ cells and Sertoli cells, Leydig cells also play an important role in the regulation of spermatogenesis. Leydig cells produce testosterone, which is important for the maintenance of both secondary sexual functions and spermatogenesis. It has long been known that Leydig cells originate from the mesenchymal cells that are present in the interstitium of the testis. Postnatal development of Leydig cells involves their transformation through three stages called progenitor, immature and adult Leydig cells. During the prepubertal and pubertal stages of development, there is an increase in the number of Leydig cells due to the differentiation of mesenchymal cells into Leydig cells and the mitotic division of newly formed Leydig cells.63 Although the cellular mechanisms involved in maintaining a constant population of Leydig cells are not well understood, apoptosis is thought to play an important role in the regulation of these cells.64 However, increased apoptosis can cause a decline in testosterone production that could impair fertility.
Testosterone biosynthesis occurs in the Leydig cells under the influence of luteinizing hormone (LH). Binding of LH to its receptor in a Leydig cell initiates a series of events that include an increase in intracellular cyclic adenosine monophosphate (cAMP), translocation of cholesterol into mitochondria, conversion of cholesterol to pregnenolone, translocation of pregnenolone into the smooth endoplasmic reticulum and the conversion of pregnenolone to testosterone through a cascade of reactions that are catalysed by the cytochrome P-450 family of proteins.65 The process of steroidogenesis itself can serve as a source of ROS.66 The products that are formed during normal steroidogenesis can act as pseudosubstrates and interact with P-450 enzymes, resulting in the formation of a pseudosubstrate–P-450–O2 complex, which is a source of damaging free radicals due to the inability of the pseudosubstrate to undergo hydroxylation.67
Environmental toxicants and steroidogenesis
Several endocrine-disrupting chemicals increase the production of ROS in the testis and disrupt steroidogenesis. However, very few studies have analysed toxicant-induced apoptosis in Leydig cells. Within the steroid hormone biosynthetic pathway, steroidogenic acute regulatory protein (StAR), cytochrome P-450, cytochrome P-450 17αhydroxylase/17,20 lyase/17,20 desmolase, 3β-hydroxysteroid dehydrogenase (3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD) are recognized as important targets for the actions of endocrine-disrupting chemicals. Exposing purified rat Leydig cells to the polychlorinated biphenyl Aroclor 1254 at concentrations of 10−10–10−7 mol l−1 caused a significant decline in the activities of enzymatic and non-enzymatic antioxidant enzymes, an increase in the levels of ROS and a decrease in the mRNA levels of cytochrome P-450scc, 3β-HSD and 17β-HSD.68 Exposing mice to 2,2′,4,4′,5-pentachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl 10 or 100 mg kg−1 body weight for 6 weeks increased the prevalence of apoptotic Leydig cells within the first week of exposure.69 Studies from our laboratory have demonstrated the inhibitory effect of lindane (at 5 mg kg−1 body weight) on testicular steroidogenic enzymes, 3β-HSD and 17β-HSD, which was accompanied by an induction of oxidative stress upon exposure for 30 days.37 Furthermore, a single exposure of lindane at a dose of 5 mg kg−1 body weight caused a transient decrease in testicular steroidogenesis by decreasing the levels of StAR protein, 3β-HSD and 17β-HSD after 12 and 24 h.70 When fed to rats at 7.5 or 10 mg kg−1 body weight for 15 and 30 days, the insecticide endosulfan inhibited testicular androgen biosynthesis; a significant decrease in the levels of the plasma gonadotrophin's follicle-stimulating hormone and LH, plasma and testicular testosterone and 3β-HSD and 17β-HSD was observed at both doses.71 Administration of 1 mg kg−1 body weight of endosulfan for 30 days to rats caused a decrease in the specific activities of both 3β-HSD and 17β-HSD as well as a decrease in testicular DNA and RNA levels.
MXC and its metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane exhibit weak estrogenic and anti-androgenic activities and exert their effects through oestrogen and androgen receptors, respectively. In our laboratory, we showed that a single dose of MXC at 50 mg kg−1 body weight caused oxidative stress and a transient inhibition of StAR protein and steroidogenic enzymes, 3β-HSD and 17β-HSD in the testis of rats.73 Exposing Leydig cells (progenitor, immature and adult cells) isolated from 21-, 35- and 9-day-old rats to MXC and 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane decreased the mRNA levels of P-450scc, decreased testosterone production and decreased cholesterol utilisation by Leydig cells, thereby inhibiting testosterone biosynthesis at all stages of development.74 It has also been shown that MXC and 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane inhibit the activities of 3β-HSD and 17β-HSD in both human and rat testis. The mode of action has been suggested to be by competition with the cofactors and not the substrate.75
TCDD is the most toxic dioxin congener and is known to inhibit steroidogenesis in the testis.76, 77 In vitro administration of TCDD at a concentration of 0.2 or 2 ng ml−1 has been shown to suppress hCG-induced testosterone production in purified Leydig cells. A decrease in progesterone secretion together with a decrease in P-450scc mRNA and protein levels were also observed, and it has been speculated that TCDD exerts these effects by decreasing cAMP signalling in rat Leydig cells.78 When given to mice for 6 weeks at a dose of 35 or 70 mg kg−1, permethrin, a popular synthetic pyrethroid insecticide used to control pests, impaired the mitochondrial membranes in Leydig cells and disrupted testosterone biosynthesis by decreasing the protein and mRNA levels of StAR and P-450scc. It was hypothesized that permethrin exerts this effect by diminishing the delivery of cholesterol into the mitochondria and decreasing the cellular conversion of cholesterol to pregnenolone.79 Administration of aldrin, an organochlorine insecticide, for 13 or 26 days, impaired steroidogenesis by suppressing the activities of 3β-HSD and 17β-HSD and through the release gonadotrophins from the pituitary.80 Oral exposure to atrazine at a dose of 50 of 200 mg kg−1 body weight from postnatal day 23–30 caused downregulation of the expression of the LH receptor gene, reduced cAMP levels, decreased cholesterol transport and decreased 17β-HSD activity. It has been proposed that atrazine inhibits Leydig cell steroidogenesis by inhibiting the expression of genes that are involved in steroidogenesis.81 Oral administration of linuron, a urea-based herbicide, at doses of 50 or 75 mg kg−1 body weight to pregnant rats from gestational day 13–18, caused a reduction in testosterone levels in fetal testis.82
BPA, a monomer used in the manufacturing of plastics and other products, is a ubiquitous environmental toxicant. BPA at 480 or 960 mg kg−1 day−1 has been shown to induce apoptosis of Leydig and germ cells via the upregulation of Fas, FasL and caspase-3.44 Subcutaneous administration of BPA (at 100 or 200 mg kg−1 day−1) and estradiol decreased the plasma and testicular levels of estradiol, steroidogenic enzymes and cholesterol carrier proteins in Leydig cells. A decrease in both the number of Leydig cells and the levels of estrogen receptor-α mRNA was also found following administration with BPA.83 BPA has also been shown to induce Nur77 gene expression, an orphan nuclear receptor that is involved in steroidogenesis, thereby altering steroidogenesis in testicular Leydig cells.84 Administering the industrial chemical 4-nonylphenol to rats at 250 mg day−1 for 50 days decreased testosterone levels by inhibiting P-450c17, an important enzyme in testosterone synthesis in Leydig cells.85
Triclosan is a chemical which is widely used in various antimicrobial preparations. Leydig cells that were exposed to triclosan (at 0.001, 0.01, 0.1, 1 and 10 µmol l−1) showed a significant decrease in the enzymatic activity of adenylyl cyclase, which was followed by decreased synthesis of cAMP. The transcriptional and translational activity of P-450scc, 3β-HSD, 17β-HSD and StAR were also decreased in a dose-dependent manner.86 Most toxicants impair steroidogenesis and decrease Leydig cell function by inducing ROS and/or by decreasing the levels of steroidogenic enzymes. Table 2 summarizes several key studies that reported the effects of environmental contaminants on steroidogenesis.
Table 2. Environmental toxicants that affect steroidogenesis.
| Toxicant | Dose and duration | Observed effects | Reference |
|---|---|---|---|
| Aroclor 1254 | In vitro exposure at 10−7, 10−8, 10−9, 10−10 mol l−1 for 24 h under basal and LH-stimulated conditions | Decreased LH-stimulated testosterone production, decreased activity of antioxidant enzymes and steroidogenic enzymes | 92 |
| Bisphenol A | Subcutaneous injection of 20, 100 or 200 mg kg−1 day−1 for 6 weeks | Decreased plasma levels of testosterone and LH, cholesterol carrier protein and steroidogenic enzymes and decrease numbers of Leydig cells | 83 |
| Endosulfan | Oral administration of 1 mg kg−1 body weight for 30 days | Decreased activity of steroidogenic enzymes and 3β-hydroxysteroid dehydrogenase | 72 |
| Atrazine | Oral gavage of 50, 200 mg kg−1 for 15 days or 300 mg kg−1 for 7 days | Decreased plasma and testicular testosterone levels, 3β-hydroxysteroid dehydrogenase and aryl hydrocarbon receptor expression | 93 |
| Fenvalerate | Oral administration of 60 mg kg−1 body weight from postnatal day 35 to PND 63 | Decreased testosterone biosynthesis, downregulated expression of P-450scc and 17α-hydroxylase cytochrome P-450 | 94 |
| Benzopyrene | Inhalation of 5, 75 or 100 µg benzopyrene m−3, 4 h daily for 10 days | Decreased plasma testosterone levels | 95 |
| Prochloraz | 31.3, 62.5 or 125 mg kg−1 day−1 postnatal day 23 to 42 or 51 | Decreased serum testosterone levels | 96 |
LH, luteinizing hormone.
Conclusions
Several studies have clearly demonstrated that environmental contaminants cause an imbalance in the pro-oxidant and antioxidant status of the testis. Normal testicular spermatogenesis and steroidogenesis are sources of ROS. Although physiological levels of ROS are needed for spermatogenesis, an excess of ROS resulting from environmental contaminants can have deleterious effects; a proposed mechanism of action for these toxicants is depicted in Figure 1. In addition, oxidative stress has also been associated with pathological levels of apoptosis in germ cells and Leydig cells. Future research should be directed towards studying the apoptotic effects of all toxicants that are commonly present in the environment.
Figure 1.
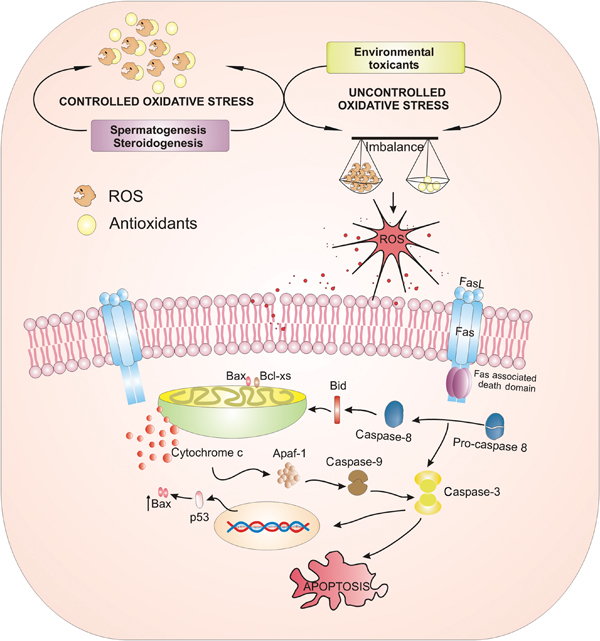
A model depicting the ROS effect of environmental contaminants on male reproduction. Spermatogenesis and steroidogenesis are sources of ROS. However, the powerful antioxidant system in the testis protects the cells from oxidative damage. Exposure to environmental contaminants causes an imbalance in pro-oxidant/antioxidant levels and thereby induces the generation of ROS. This can subsequently activate extrinsic (Fas and FasL) and intrinsic (mitochondrial) apoptotic pathways, thereby leading to apoptotic damage to the testis. ROS, reactive oxygen species.
Acknowledgments
P. P. Mathur acknowledges the receipt of financial support from the Department of Science and Technology, Government of India under the projects (1) SP/SO/β-65/99, (2) DST-FIST and (3) Indian Council of Medical Research. Shereen Cynthia D'Cruz acknowledges the Indian Council of Medical Research, New Delhi, India, for a Senior Research Fellowship. The authors also thank the staff of Bioinformatics Centre, Pondicherry University, Pondicherry, India, for providing various facilities.
The authors declare no competing financial interests.
References
- Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61:640–58. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Clapp RW, Jacobs MM, Loechler EL. Environmental and occupational causes of cancer: new evidence 2005–2007. Rev Environ Health. 2008;23:1–37. doi: 10.1515/reveh.2008.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinawat S. The environmental impact on male fertility. J Med Assoc Thai. 2000;83:880–5. [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–84. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Brown D, Sharpe RM. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environ Health Perspect. 1999;107:397–405. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104 Suppl 4:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston GP, Gooch JW, Breslin WJ, Shuey DL, Nikiforov AI, et al. Environmental estrogens and reproductive health: a discussion of the human and environmental data. Reprod Toxicol. 1997;11:465–81. doi: 10.1016/s0890-6238(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Toppari J, Keiding N, Skakkebaek NE. Do environmental estrogens contribute to the decline in male reproductive health. Clin Chem. 1995;41:1896–901. [PubMed] [Google Scholar]
- Cravedi JP, Zalko D, Savouret JF, Menuet A, Jegou B.The concept of endocrine disruption and human health Med Sci (Paris) 200723198–204.French. [DOI] [PubMed] [Google Scholar]
- Mathur PP, Saradha B, Vaithinathan S. Impact of environmental toxicants on testicular function. Immun Endoc Metab Agents Med Chem. 2008;8:79–90. [Google Scholar]
- Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl. 2004;6:259–68. [PubMed] [Google Scholar]
- Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci. 2010;365:1621–35. doi: 10.1098/rstb.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, West KM. Analysis of the relationship between reactive oxygen species production and leucocyte infiltration in fractions of human semen separated on Percoll gradients. Int J Androl. 1990;13:433–51. doi: 10.1111/j.1365-2605.1990.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10:387–99. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradha B, Mathur PP. Effect of environmental contaminants on male reproduction. Environ Toxicol Pharmacol. 2006;21:34–41. doi: 10.1016/j.etap.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Erkkila K, Pentikainen V, Wikstrom M, Parvinen M, Dunkel L. Partial oxygen pressure and mitochondrial permeability transition affect germ cell apoptosis in the human testis. J Clin Endocrinol Metab. 1999;84:4253–9. doi: 10.1210/jcem.84.11.6141. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–70. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R, Mishra DP, Shaha C. Male germ cell development: turning on the apoptotic pathways. J Reprod Immunol. 2009;83:31–5. doi: 10.1016/j.jri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Moreno RD, Lizama C, Urzua N, Vergara SP, Reyes JG. Caspase activation throughout the first wave of spermatogenesis in the rat. Cell Tissue Res. 2006;325:533–40. doi: 10.1007/s00441-006-0186-4. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–8. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide methoxychlor. Arch Toxicol. 2002;76:692–8. doi: 10.1007/s00204-002-0388-9. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Chitra KC, Mathur PP. The effect of methoxychlor on the epididymal antioxidant system of adult rats. Reprod Toxicol. 2002;16:161–72. doi: 10.1016/s0890-6238(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Effect of methoxychlor on the antioxidant system in mitochondrial and microsome-rich fractions of rat testis. Toxicology. 2002;176:67–75. doi: 10.1016/s0300-483x(02)00138-5. [DOI] [PubMed] [Google Scholar]
- Vaithinathan S, Saradha B, Mathur PP. Methoxychlor induces apoptosis via mitochondria- and FasL-mediated pathways in adult rat testis. Chem Biol Interact. 2010;185:110–8. doi: 10.1016/j.cbi.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Uzumcu M, Suzuki H, Dirks K, Phillips B, et al. Effect of transient embryonic in vivo exposure to the endocrine disruptor methoxychlor on embryonic and postnatal testis development. J Androl. 2003;24:736–45. doi: 10.1002/j.1939-4640.2003.tb02736.x. [DOI] [PubMed] [Google Scholar]
- Johnson L, Staub C, Silge RL, Harris MW, Chapin RE. The pesticide methoxychlor given orally during the perinatal/juvenile period, reduced the spermatogenic potential of males as adults by reducing their Sertoli cell number. Reprod Nutr Dev. 2002;42:573–80. doi: 10.1051/rnd:2002043. [DOI] [PubMed] [Google Scholar]
- Shi YQ, Wang YP, Song Y, Li HW, Liu CJ, et al. p,p′-DDE induces testicular apoptosis in prepubertal rats via the Fas/FasL pathway. Toxicol Lett. 2010;193:79–85. doi: 10.1016/j.toxlet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Liang XM, Hu YF, Yu HG, Yang KD.Effects of p,p′-DDE and beta-BHC on the apoptosis of Sertoli cells in vitro Zhonghua Yu Fang Yi Xue Za Zhi 200842648–52.Chinese. [PubMed] [Google Scholar]
- Elzeinova F, Novakova V, Buckiova D, Kubatova A, Peknicova J. Effect of low dose of vinclozolin on reproductive tract development and sperm parameters in CD1 outbred mice. Reprod Toxicol. 2008;26:231–8. doi: 10.1016/j.reprotox.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Anway MD, Rekow SS, Skinner MK. Comparative anti-androgenic actions of vinclozolin and flutamide on transgenerational adult onset disease and spermatogenesis. Reprod Toxicol. 2008;26:100–6. doi: 10.1016/j.reprotox.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta L, Roy A, Chainy GB. Changes in rat testicular antioxidant defence profile as a function of age and its impairment by hexachlorocyclohexane during critical stages of maturation. Andrologia. 1999;31:83–90. [PubMed] [Google Scholar]
- Sujatha R, Chitra KC, Latchoumycandane C, Mathur PP. Effect of lindane on testicular antioxidant system and steroidogenic enzymes in adult rats. Asian J Androl. 2001;3:135–8. [PubMed] [Google Scholar]
- Chitra KC, Sujatha R, Latchoumycandane C, Mathur PP. Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian J Androl. 2001;3:205–8. [PubMed] [Google Scholar]
- Saradha B, Vaithinathan S, Mathur PP. Lindane induces testicular apoptosis in adult Wistar rats through the involvement of Fas–FasL and mitochondria-dependent pathways. Toxicology. 2009;255:131–9. doi: 10.1016/j.tox.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Defamie N, Mograbi B, Roger C, Cronier L, Malassine A, et al. Disruption of gap junctional intercellular communication by lindane is associated with aberrant localization of connexin43 and zonula occludens-1 in 42GPA9 Sertoli cells. Carcinogenesis. 2001;22:1537–42. doi: 10.1093/carcin/22.9.1537. [DOI] [PubMed] [Google Scholar]
- Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology. 2003;185:119–27. doi: 10.1016/s0300-483x(02)00597-8. [DOI] [PubMed] [Google Scholar]
- Kabuto H, Hasuike S, Minagawa N, Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003;93:31–5. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- Chitra KC, Rao KR, Mathur PP. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: a histological and biochemical study. Asian J Androl. 2003;5:203–8. [PubMed] [Google Scholar]
- Li YJ, Song TB, Cai YY, Zhou JS, Song X, et al. Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci. 2009;108:427–36. doi: 10.1093/toxsci/kfp024. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao XF, Ji YL, Wang H, Liu P, et al. Mitochondrial signaling pathway is also involved in bisphenol A induced germ cell apoptosis in testes. Toxicol Lett. 2010;199:129–35. doi: 10.1016/j.toxlet.2010.08.014. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–60. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, et al. Bisphenol-A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–190. [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Disruption of the blood–testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood–testis barrier dynamics. Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Wong EW, Lie PP, Li MW, Su L, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:1–12. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumycandane C, Chitra KC, Mathur PP. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the antioxidant system in mitochondrial and microsomal fractions of rat testis. Toxicology. 2002;171:127–35. doi: 10.1016/s0300-483x(01)00563-7. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Chitra KC, Mathur PP. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress in the epididymis and epididymal sperm of adult rats. Arch Toxicol. 2003;77:280–4. doi: 10.1007/s00204-003-0439-x. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Effects of vitamin E on reactive oxygen species-mediated 2,3,7,8-tetrachlorodi-benzo-p-dioxin toxicity in rat testis. J Appl Toxicol. 2002;22:345–51. doi: 10.1002/jat.866. [DOI] [PubMed] [Google Scholar]
- Ohbayashi T, Oikawa K, Iwata R, Kameta A, Evine K, et al. Dioxin induces a novel nuclear factor, DIF-3, that is implicated in spermatogenesis. FEBS Lett. 2001;508:341–4. doi: 10.1016/s0014-5793(01)03039-3. [DOI] [PubMed] [Google Scholar]
- Arima A, Kato H, Ooshima Y, Tateishi T, Inoue A, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces a reduction in epididymal and ejaculated sperm number in rhesus monkeys. Reprod Toxicol. 2009;28:495–502. doi: 10.1016/j.reprotox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Jin MH, Ko HK, Hong CH, Han SW. In utero exposure to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin affects the development of reproductive system in mouse. Yonsei Med J. 2008;49:843–50. doi: 10.3349/ymj.2008.49.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MT, Nagarkatti M, Nagarkatti PS. Aryl hydrocarbon receptor-dependent induction of loss of mitochondrial membrane potential in epididydimal spermatozoa by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Lett. 2005;157:99–107. doi: 10.1016/j.toxlet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Abarikwu SO, Adesiyan AC, Oyeloja TO, Oyeyemi MO, Farombi EO. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch Environ Contam Toxicol. 2010;58:874–82. doi: 10.1007/s00244-009-9371-2. [DOI] [PubMed] [Google Scholar]
- Strandgaard C, Miller MG. Germ cell apoptosis in rat testis after administration of 1,3-dinitrobenzene. Reprod Toxicol. 1998;12:97–103. doi: 10.1016/s0890-6238(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Muguruma M, Yamazaki M, Okamura M, Moto M, Kashida Y, et al. Molecular mechanism on the testicular toxicity of 1,3-dinitrobenzene in Sprague–Dawley rats: preliminary study. Arch Toxicol. 2005;79:729–36. doi: 10.1007/s00204-005-0006-8. [DOI] [PubMed] [Google Scholar]
- Zhao XF, Wang Q, Ji YL, Wang H, Liu P, et al. Fenvalerate induces germ cell apoptosis in mouse testes through the Fas/FasL signaling pathway Arch Toxicole-pub ahead of print 30 January 2011; doi: 10.1007/s00204-011-0654-9. [DOI] [PubMed]
- Qian J, Bian Q, Cui L, Chen J, Song L, et al. Octylphenol induces apoptosis in cultured rat Sertoli cells. Toxicol Lett. 2006;166:178–86. doi: 10.1016/j.toxlet.2006.06.646. [DOI] [PubMed] [Google Scholar]
- Gong Y, Wu J, Huang Y, Shen S, Han X. Nonylphenol induces apoptosis in rat testicular Sertoli cells via endoplasmic reticulum stress. Toxicol Lett. 2009;186:84–95. doi: 10.1016/j.toxlet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HB, Teunissen van Manen KR, Haupt RL. Differentiation of adult Leydig cells in the neonatal rat testis is arrested by hypothyroidism. Biol Reprod. 1998;59:351–7. doi: 10.1095/biolreprod59.2.351. [DOI] [PubMed] [Google Scholar]
- Yuan SH, Xu SF.Leydig cell apoptosis and its regulation Zhonghua Nan Ke Xue 20039218–20, 25.Chinese. [PubMed] [Google Scholar]
- Haider SG. Leydig cell steroidogenesis: unmasking the functional importance of mitochondria. Endocrinology. 2007;148:2581–2. doi: 10.1210/en.2007-0330. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–96. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- Quinn PG, Payne AH. Steroid product-induced, oxygen-mediated damage of microsomal cytochrome P-450 enzymes in Leydig cell cultures. Relationship to desensitization. J Biol Chem. 1985;260:2092–9. [PubMed] [Google Scholar]
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol. 2008;25:447–54. doi: 10.1016/j.reprotox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Oskam IC, Ropstad E, Smith AJ, Skaare JU, Tverdal A, et al. Effects of PCB99 and PCB153 exposure on spermatogenesis in young adult C57BL6 mice. Reprod Toxicol. 2004;19:169–80. doi: 10.1016/j.reprotox.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Saradha B, Vaithinathan S, Mathur PP. Single exposure to low dose of lindane causes transient decrease in testicular steroidogenesis in adult male Wistar rats. Toxicology. 2008;244:190–7. doi: 10.1016/j.tox.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Singh SK, Pandey RS. Effect of suβ-chronic endosulfan exposures on plasma gonadotrophins, testosterone, testicular testosterone and enzymes of androgen biosynthesis in rat. Indian J Exp Biol. 1990;28:953–6. [PubMed] [Google Scholar]
- Chitra KC, Latchoumycandane C, Mathur PP. Chronic effect of endosulfan on the testicular functions of rat. Asian J Androl. 1999;1:203–6. [PubMed] [Google Scholar]
- Vaithinathan S, Saradha B, Mathur PP. Transient inhibitory effect of methoxychlor on testicular steroidogenesis in rat: an in vivo study. Arch Toxicol. 2008;82:833–9. doi: 10.1007/s00204-008-0301-2. [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Ge RS, Klinefelter GR, Gunsalus GL, Hardy MP. A metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, reduces testosterone biosynthesis in rat Leydig cells through suppression of steady-state messenger ribonucleic acid levels of the cholesterol side-chain cleavage enzyme. Biol Reprod. 2000;62:571–8. doi: 10.1095/biolreprod62.3.571. [DOI] [PubMed] [Google Scholar]
- Hu GX, Zhao B, Chu Y, Li XH, Akingbemi BT, et al. Effects of methoxychlor and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane on 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase-3 activities in human and rat testes. Int J Androl. 2011;34:138–44. doi: 10.1111/j.1365-2605.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- Fukuzawa NH, Ohsako S, Wu Q, Sakaue M, Fujii-Kuriyama Y, et al. Testicular cytochrome P450scc and LHR as possible targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the mouse. Mol Cell Endocrinol. 2004;221:87–96. doi: 10.1016/j.mce.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Johnson L, Dickerson R, Safe SH, Nyberg CL, Lewis RP, et al. Reduced Leydig cell volume and function in adult rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin without a significant effect on spermatogenesis. Toxicology. 1992;76:103–18. doi: 10.1016/0300-483x(92)90158-b. [DOI] [PubMed] [Google Scholar]
- Lai KP, Wong MH, Wong CK. Inhibition of CYP450scc expression in dioxin-exposed rat Leydig cells. J Endocrinol. 2005;185:519–27. doi: 10.1677/joe.1.06054. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Ito Y, Yamanoshita O, Yanagiba Y, Kobayashi M, et al. Permethrin may disrupt testosterone biosynthesis via mitochondrial membrane damage of Leydig cells in adult male mouse. Endocrinology. 2007;148:3941–9. doi: 10.1210/en.2006-1497. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Ray A, Bagchi P, Deb C. Suppression of testicular steroidogenesis in rats by the organochlorine insecticide Aldrin. Environ Pollut. 1988;51:87–94. doi: 10.1016/0269-7491(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Pogrmic K, Fa S, Dakic V, Kaisarevic S, Kovacevic R. Atrazine oral exposure of peripubertal male rats downregulates steroidogenesis gene expression in Leydig cells. Toxicol Sci. 2009;111:189–97. doi: 10.1093/toxsci/kfp135. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright CR, Furr JR, Howdeshell KL, Earl Gray L., Jr The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. Toxicol Lett. 2009;186:73–7. doi: 10.1016/j.toxlet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett. 2010;194:16–25. doi: 10.1016/j.toxlet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Song KH, Lee K, Choi HS. Endocrine disrupter bisphenol a induces orphan nuclear receptor Nur77 gene expression and steroidogenesis in mouse testicular Leydig cells. Endocrinology. 2002;143:2208–15. doi: 10.1210/endo.143.6.8847. [DOI] [PubMed] [Google Scholar]
- Gong Y, Han XD. Effect of nonylphenol on steroidogenesis of rat Leydig cells. J Environ Sci Health B. 2006;41:705–15. doi: 10.1080/03601230600701866. [DOI] [PubMed] [Google Scholar]
- Kumar V, Balomajumder C, Roy P. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology. 2008;250:124–31. doi: 10.1016/j.tox.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Boockfor FR, Blake CA. Chronic administration of 4-tert-octylphenol to adult male rats causes shrinkage of the testes and male accessory sex organs, disrupts spermatogenesis, and increases the incidence of sperm deformities. Biol Reprod. 1997;57:267–77. doi: 10.1095/biolreprod57.2.267. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim IW, Hwang SY, Shin BJ, Kim SK. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on testicular spermatogenesis-related panels and serum sex hormone levels in rats. BJU Int. 2008;101:250–5. doi: 10.1111/j.1464-410X.2007.07202.x. [DOI] [PubMed] [Google Scholar]
- Aly HA, Domenech O, Abdel-Naim AB. Aroclor 1254 impairs spermatogenesis and induces oxidative stress in rat testicular mitochondria. Food Chem Toxicol. 2009;47:1733–8. doi: 10.1016/j.fct.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Pina-Guzman B, Sanchez-Gutierrez M, Marchetti F, Hernandez-Ochoa I, Solis-Heredia MJ, et al. Methyl-parathion decreases sperm function and fertilization capacity after targeting spermatocytes and maturing spermatozoa. Toxicol Appl Pharmacol. 2009;238:141–9. doi: 10.1016/j.taap.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Bustos-Obregon E, Gonzalez-Hormazabal P. Effect of a single dose of malathion on spermatogenesis in mice. Asian J Androl. 2003;5:105–7. [PubMed] [Google Scholar]
- Murugesan P, Balaganesh M, Balasubramanian K, Arunakaran J. Effects of polychlorinated biphenyl (Aroclor 1254) on steroidogenesis and antioxidant system in cultured adult rat Leydig cells. J Endocrinol. 2007;192:325–38. doi: 10.1677/joe.1.06874. [DOI] [PubMed] [Google Scholar]
- Victor-Costa AB, Bandeira SM, Oliveira AG, Mahecha GA, Oliveira CA. Changes in testicular morphology and steroidogenesis in adult rats exposed to Atrazine. Reprod Toxicol. 2010;29:323–31. doi: 10.1016/j.reprotox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang H, Wang Q, Zhao XF, Liu P, et al. Pubertal and early adult exposure to fenvalerate disrupts steroidogenesis and spermatogenesis in mice at adulthood. J Appl Toxicol. 2010;30:369–77. doi: 10.1002/jat.1507. [DOI] [PubMed] [Google Scholar]
- Inyang F, Ramesh A, Kopsombut P, Niaz MS, Hood DB, et al. Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod Toxicol. 2003;17:527–37. doi: 10.1016/s0890-6238(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Blystone CR, Furr J, Lambright CS, Howdeshell KL, Ryan BC, et al. Prochloraz inhibits testosterone production at dosages below those that affect androgen-dependent organ weights or the onset of puberty in the male Sprague Dawley rat. Toxicol Sci. 2007;97:65–74. doi: 10.1093/toxsci/kfm004. [DOI] [PubMed] [Google Scholar]


