Abstract
Erectile dysfunction (ED) is an important worldwide health issue that has a significant negative impact on the quality of life and life satisfaction of both the affected individual and his partner. Here we review the prevalence of ED in Asia, associated factors that may influence sexual attitudes and sexual behaviours, and randomized clinical trials (RCTs) of phosphodiesterase-5 (PDE-5) inhibitors to evaluate the clinical efficacy and safety of PDE-5 inhibitors in Asian men. We searched for English-language articles in MEDLINE and PubMed from January 2000 to September 2010. Our results showed that the overall reported prevalence rate of ED in Asia ranged widely, from 2% to 88%. This finding indicates that ED is a common and major health problem in this region. However, sociocultural and economic factors in Asia prevent people from seeking and obtaining appropriate medical care. We found reports on five kinds of PDE-5 inhibitors for the management of ED: sildenafil, vardenafil, tadalafil, udenafil and mirodenafil. The results of RCTs showed that these five PDE-5 inhibitors are more effective than placebo in improving erectile function in Asian men with ED and that these drugs have similar efficacy and safety profiles.
Keywords: Asia, erectile dysfunction, phosphodiesterase inhibitors, prevalence
Introduction
Erectile dysfunction (ED) has been defined as the inability to achieve or maintain an erection sufficiently rigid for achieving satisfying sexual intercourse.1 ED is an important worldwide health issue that affects nearly half of men over the age of 40 years, and it has a significant negative impact on the quality of life and life satisfaction of the affected individual as well as his partner.2, 3 One of the most frequently cited epidemiological surveys reporting prevalence data for ED is the Massachusetts Male Aging Study.2 The Massachusetts Male Aging Study—the first longitudinal, community-based, randomized, wide-scale epidemiological study of ED—included 1709 men between the ages of 40 and 70 years who completed a self-reported ED questionnaire. Over that age range, the probabilities of minimal, moderate and complete ED were 16.5%, 17.5% and 4.9%, respectively, at age of 40 years, and 18%, 34% and 15% at age of 70 years, showing that the prevalence of ED increases with age.
Another pivotal epidemiological study of sexual dysfunction was the Global Study of Sexual Attitudes and Behaviors (GSSAB), which included 27 500 men and women aged 40–80 years in 29 countries.4 This study showed that physical, social/emotional and relationship factors all have a significant impact on the prevalence of ED.
The incidence of ED is higher in men with diabetes mellitus, hypertension, hypercholesterolemia, atherosclerosis or cardiovascular disease. All of these diseases and conditions are known to be associated with endothelial dysfunction.5, 6 Men who reported having at least one type of vascular disease were more likely to experience erectile difficulties.4 In the Global Better Sex Survey, Asian men and women agreed that sex was an important part of their lives and partner relationships; however, many men may not recognize that ED is a treatable medical condition.7
Here we review the prevalence rate of ED in Asia, associated factors that influence sexual attitudes and sexual behaviour, help-seeking patterns, and the clinical efficacy and safety of phosphodiesterase-5 (PDE-5) inhibitors in Asian men.
Prevalence rate of ED in Asia
The prevalence of ED in Asian populations was recently analyzed by Cheng et al.8 They performed computer-based searches through MEDLINE, PubMed, PsycINFO and other general Internet search engines for articles documenting the prevalence of ED in Asian countries between 1986 and 2006. Among the 219 relevant articles initially identified, 34 articles were retrieved, 18 of which were analyzed as general population studies. The overall reported prevalence rate of ED in Asia ranged widely, from 2% to 81.8%.8 It was 11% in Indonesia, 19.5%–28.3% in China, 8%–50% in Hong Kong, 13%–81.1% in Japan, 22.4%–59% in Malaysia, 33%–65% in the Philippines, 2%–53% in Singapore, 18%–36.6% in South Korea, 9%–17.7% in Taiwan (China) and 29%–65% in Thailand. The prevalence of ED increased with age. The pooled random-effects age-specific prevalence rates were 15.1%, 29.6%, 40.6%, 54.3% and 70.0% for the age groups 20–29, 30–39, 40–49, 50–59 and 60–69 years, respectively. The overall, age-standardized prevalence was 36.8%. This meta-analysis showed that the prevalence rates of ED differed between countries and that there was also a wide range of overall ED prevalence in a country. The limitation of this analysis was that the tools used to evaluate ED prevalence were not the same across studies. In addition, even though the International Index of Erectile Function (IIEF) was the most frequently used assessment tool, for some of the studies a validated IIEF was not available in the local language.8
We searched for epidemiological studies through MEDLINE and PubMed for the period from 2001 to 2010. We reviewed 20 general population studies of ED in Asian regions (Table 1).9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Among them, there were three important epidemiological studies of ED in Asian populations: the GSSAB subgroup study in Asian countries,12 the Asian Men's Attitudes to Life Events and Sexuality study11 and the Asian Survey of Aging Males.15 The GSSAB subgroup study compared sexual behaviours, the prevalence of sexual dysfunction and help-seeking patterns in nine Asian countries. In that study, the prevalence of ED was the highest in the Philippines (33%), followed by Thailand (29%), Malaysia (28%), Korea (18%), Japan (13%), Taiwan (China) (9%), Hong Kong (China) (8%) and Singapore (2%) (Table 1). The Men's Attitudes to Life Events and Sexuality Phase I study consisted of 10 934 men aged 20–75 years from Mainland China, Japan, Korea, Malaysia and Taiwan (China). In that study, the self-reported prevalence of ED was the highest in Japan (14%) and the lowest in Malaysia (3%).11 The Asian Survey of Aging Males survey was conducted in 1155 men aged 50–80 years from Hong Kong (China), Singapore, Malaysia, Philippines and Thailand. ED was reported by 63% of the participants, via the sexual function using Danish prostate symptom score questionnaire. The prevalence of ED was the highest in the Philippines (65%) and Thailand (65%), followed by Malaysia (59%), Singapore (53%) and Hong Kong (China) (50%). For these three studies, as shown in Table 1, the overall reported prevalence rates of ED in Asia ranged widely, from 2% to 88%. This high prevalence of ED indicates that ED is a common health problem in the Asian region.
Table 1. Prevalence of ED in Asian countries9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28.
| Region | Study | Study size (n) | Age range (year) | Range of overall ED prevalence (%) | Assessment tools |
|---|---|---|---|---|---|
| Mainland China | Bai et al.9 | 2226 | 20–86 | 28.4a | Self-report |
| Lau et al.10 | 298 | 20–39 | 19.5 | Self-report | |
| Tan et al.11 | 225 | 20–75 | 6 | Self-report | |
| Nicolosi et al.12 | 250 | 40–80 | 20 | Self-report | |
| Hong Kong (China) | Wong et al.13 | 1566 | 65–92 | 88 | IIEF |
| Ng et al.14 | 1506 | 26–70 | 36.7 | Self-report | |
| Li et al.15 | 201 | 50–80 | 50 | DAN-PSS-Sex, IIEF | |
| Nicolosi et al.12 | 250 | 40–80 | 8 | Self-report | |
| Indonesia | Nicolosi et al.12 | 250 | 40–80 | 11 | Self-report |
| Japan | Marumo et al.16 | 1517 | 23–79 | 1.0–25.9b | IIEF |
| Sasayama et al.17 | 6112 | 30–80 | 32 | Self-report | |
| Nicolosi et al.18 | 600 | 40–70 | 34a | Self-report | |
| Tan et al.11 | 228 | 20–75 | 14 | Self-report | |
| Nicolosi et al.12 | 750 | 40–80 | 13 | Self-report | |
| Korea | Ahn et al.19 | 1570 | 40–79 | 45.8 | Self-report, IIEF |
| Cho et al.20 | 3501 | >20 | 36.5a | IIEF | |
| Moreira et al.21 | 600 | 40–80 | 31.9 | Self-report | |
| Tan et al.11 | 225 | 20–75 | 8 | Self-report | |
| Nicolosi et al.12 | 600 | 40–80 | 18 | Self-report | |
| Malaysia | Khoo et al.22 | 351 | >50 | 70.1 | IIEF |
| Quek et al.23 | 430 | >20 | 41.6 | IIEF | |
| Li et al.15 | 250 | 50–80 | 59 | DAN-PSS-Sex, IIEF | |
| Tan et al.11 | 380 | 20–75 | 3 | Self-report | |
| Nicolosi et al.12 | 250 | 40–80 | 28 | Self-report | |
| Philippines | Li et al.15 | 250 | 50–80 | 65 | DAN-PSS-Sex, IIEF |
| Nicolosi et al.12 | 250 | 40–80 | 33 | Self-report | |
| Singapore | Li et al.15 | 204 | 50–80 | 53 | DAN-PSS-Sex, IIEF |
| Tan et al.24 | 729 | >30 | 51.3 | IIEF | |
| Nicolosi et al.12 | 250 | 40–80 | 2 | Self-report | |
| Taiwan (China) | Hwang et al.25 | 1060 | >30 | 27 | IIEF, EHS, QEQ |
| Wu et al.26 | 990 | >40 | 26 | Self-report, IIEF | |
| Chen et al.27 | 1002 | >40 | 17.7 | Self-report | |
| Tan et al.11 | 228 | 20–75 | 4 | Self-report | |
| Nicolosi et al.12 | 250 | 40–80 | 9 | Self-report | |
| Thailand | Permpongkosol et al.28 | 2269 | 40–70 | 42.1 | Self-report |
| Li et al.15 | 250 | 50–80 | 65 | DAN-PSS-Sex, IIEF | |
| Nicolosi et al.12 | 250 | 40–80 | 29 | Self-report |
Abbreviations: DAN-PSS-Sex, sexual function using Danish prostate symptom score; ED, erection dysfunction; EHS, erection hardness score; IIEF, International Index of Erectile Function; QEQ, quality of erection questionnaire.
Age adjusted.
Prevalence of ED according to age.
Most of the other epidemiological studies in Asian regions were based on populations within a country. The majority were reported from East Asia and Southeast Asia because epidemiological data from other Asian countries are not well represented in the international database search engines. The sample sizes, age groups and assessment tools for ED were different in each study. The prevalence rate of ED varies according to the definitions and methodologies used in ED research. Therefore, future studies should be considered to collect age-specific, ED severity-specific prevalence data and to adopt an appropriate ED assessment tool with a validated cutoff value.
Sexual attitudes and sexual behaviour in Asian men
The GSSAB study showed that most men and women with sexual dysfunction had not consulted a doctor about these problems.12 This lack of communication between physician and patient is not unique to Asia; the global study showed that only 9% of men and women had been asked about their sexual health by a doctor.29 Sexual attitudes can be influenced by cultural differences. Asian men are known to be conservative towards sex and less sexually active than Western men.12 More specifically, the pattern of treatment-seeking behaviour differs between East and Southeast Asian regions because of cultural and religious influences. In East Asia, a Confucian cultural background predominates, whereas in Southeast Asia, people may have a Buddhist background (Thailand), a Muslim background (Malaysia and Indonesia) or a Catholic background (the Philippines). As one study reported, in Korea and other East Asian countries most patients took no action, whereas in Southeast Asia help was often sought from a partner, a family member or another source of social support12 (Figure 1). Another substantial barrier to seeing a physician is the cost. Limited accessibility of medical care for financial reasons was most common in Mainland China, Indonesia and Thailand and least common in Hong Kong (China), Singapore and Japan.12 These sociocultural and economic factors appear to prevent people from seeking and obtaining medical care. The findings imply that public awareness of ED is needed in order to encourage men to consult a physician.
Figure 1.
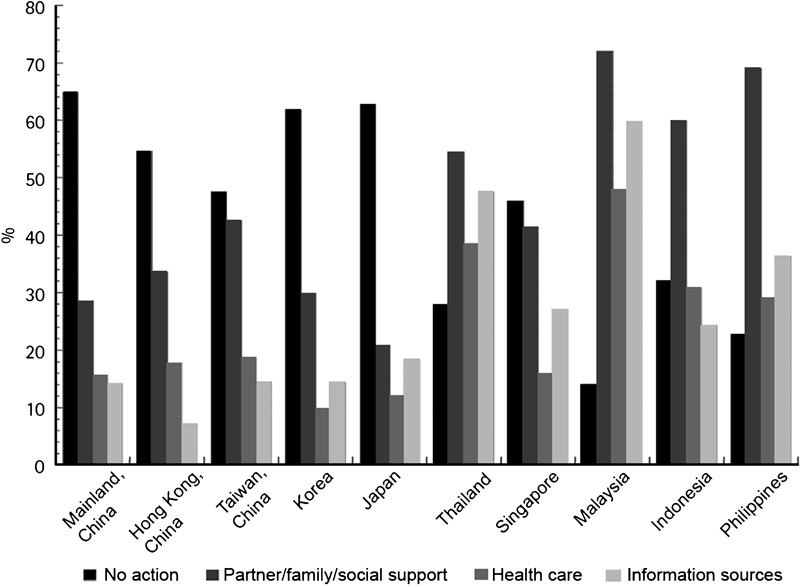
Help-seeking behaviour of men and women with sexual dysfunction in Asia as determined by the GSSAB, 2001–2002.12 Adapted with permission. GSSAB, Global Study of Sexual Attitudes and Behaviors.
Medical treatment of ED in Asia
The first-line therapeutic option for the treatment of ED is PDE-5 inhibitors. Five types of PDE-5 inhibitors have been introduced: sildenafil, tadalafil, vardenafil, udenafil and mirodenafil. A systematic review and meta-analysis of data on oral PDE-5 inhibitors was recently reported.30 In the 130 randomized clinical trials (RCTs) analyzed, 72 were for sildenafil, 27 were for vardenafil, 28 were for tadalafil, two were for mirodenafil and one was for udenafil. Four RCTs directly compared PDE-5 inhibitors. This study concluded that oral PDE-5 inhibitors improve erectile functioning and have similar efficacy and safety profiles.
In the current review, after a search of MEDLINE and PubMed for articles published from January 2000 to September 2010, we selected 14 Asian RCTs of PDE-5 inhibitors (Table 2). These included four RCTs of sildenafil,31, 32, 33, 34 two RCTs of vardenafil,35, 36 four RCTs of tadalafil,37, 38, 39, 40 two RCTs of udenafil41, 42 and two RCTs of mirodenafil.43, 44 The efficacy and safety of two novel PDE-5 inhibitors, mirodenafil and udenafil, were evaluated only in Korean men. In this review, we did not include clinical trials of PDE-5 inhibitors in men with specific comorbid diseases, such as diabetes, hypertension and other neurovascular diseases.
Table 2. Studies evaluating PDE-5-I administration for ED in Asian men31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44.
| Study | Drug | Study design | Patients | Outcome measures | Efficacy | Adverse event |
|---|---|---|---|---|---|---|
| Tan et al.31 | Sildenafil versus placebo (50 mg increased to 100 mg or decreased to 25 mg) for 12 weeks | Multinational, multi-institutional randomized controlled trial | 254 men;aged 26–78 years;ED duration >6 months | IIEF, patients' event logs of sexual activity, and a global efficacy question | Higher EF with sildenafil than with placebo, IIEF Q3 (4.22 versus 2.59) and Q4 (4.15 versus 2.41) (P<0.0001), | 22.8% of sildenafil versus 10.2% of placebo; flushing, dizziness and headache |
| significantly improved IIEF-EF and GEAQ in sildenafil group (P<0.0001) | ||||||
| Kongkanand et al.32 | Sildenafil versus placebo (50 mg increased to 100 mg or decreased to 25 mg) for 12 weeks | Multi-institutional randomized controlled trial in Thailand | 125 men;aged 26–77 years;ED duration >6 months | IIEF, patients' event logs of sexual activity, and a global efficacy question | Higher EF with sildenafil than with placebo, successful attempts at sexual intercourse (66.16% versus 33.05%) | 30.2% of sildenafil versus 11.3% of placebo; flushing, dizziness and headache |
| Chen et al.33 | Sildenafil versus placebo (50 mg increased to 100 mg or decreased to 25 mg) for 12 weeks | Multi-institutional randomized controlled trial in Taiwan | 236 men;aged 26–80 years;ED duration >6 months | IIEF, patients' event logs of sexual activity, and a global efficacy question | Higher EF with sildenafil than with placebo, IIEF Q3 (4.17 versus 2.98) and Q4 (4.14 versus 2.88) (P<0.0001), significantly improved IIEF-EF and GEAQ in sildenafil group (P<0.0001) | 43.7% of sildenafil versus 18.8% of placebo; flushing, dizziness and headache |
| Choi et al.34 | Sildenafil versus placebo (50 mg increased to 100 mg or decreased to 25 mg) for 8 weeks | Multi-institutional randomized controlled trial in Korea | 133 men;aged 28–78 years;ED duration >6 months | IIEF, patients' event logs of sexual activity, and a global efficacy question | Higher EF with sildenafil than with placebo, IIEF Q3 (4.19 versus 2.67) and Q4 (3.74 versus 2.07) (P<0.0001), significantly improved IIEF-EF and GEAQ in sildenafil group (P<0.0001) | 56.1% of sildenafil versus 20.9% of placebo; flushing, headache and abnormalities in colour vision |
| Tan et al.35 | Vardenafil (10 mg) versus placebo for 12 weeks | Multinational, multi-institutional randomized controlled trial | 358 men;aged ≥20 years;ED duration >6 months | IIEF-EF domain score, success rate of vaginal penetration (SEP2), penile rigidity (SEP3), GAQ | Increased IIEF-EF scores in vardenafil compared with placebo (22.4 versus 14.3, P<0.001), improved SEP2 (82.2 versus 43.6, P<0.001), SEP3 (66.1 versus 24.0, P<0.001), positive GAQ responses (81.8% versus 24.3%) | 25.4% of the vardenafil group, the majority mild and transient headache, flushing, nasal congestion and dizziness |
| Chen et al.36 | Vardenafil (10 mg) versus placebo for 12 weeks | Multinational, multi-institutional randomized controlled trial | 306 men;aged ≥20 years;ED duration >6 months | IIEF-EF domain score, success rate of vaginal penetration (SEP2), penile rigidity (SEP3), GAQ | Greater increase in IIEF-EF score in vardenafil group compared with placebo (24.2 versus 15.9), higher SEP2 (88% versus 58%), higher SEP3 (69% versus 23%), higher positive GAQ responses (85% versus 33%) | 54.2% of vardenafil versus 43.0% of placebo; mild intensity flushing, rhinitis and headache |
| Yip et al.37 | Tadalafil (20 mg) versus placebo for 12 weeks | Multinational, multi-institutional randomized controlled trial | 242 men;aged >18 years;ED duration >3 months | IIEF,SEP diary,GAQ | Significantly improved EF in tadalafil compared with placebo (P<0.001), greater SEP3 (70.9% versus 33.5%), greater GAQ (86.2% versus 30.1%) | Headache (11.3%), back pain (7.5%), dizziness (3.8%) and dyspepsia (3.1%) |
| Guo et al.38 | Tadalafil (10 or 20 mg) versus placebo for 12 weeks | Multinational, multi-institutional randomized controlled trial | 367 men;aged >18 years;(>21 in Singapore)ED duration >3 month | IIEF,SEP diary,GAQ | Tadalafil 10 and 20 mg improve IIEF (8.1 and 8.7, versus placebo 2.4, P<0.001), SEP3 (62% and 70%, placebo 32%, P<0.001), GAQ (81% and 86% versus 44%, P<0.001) | Headache, back pain, dyspepsia and dizziness |
| Chen et al.39 | Tadalafil (10 or 20 mg) versus placebo for 12 weeks | Multi-institutional randomized controlled trial in Taiwan | 196 men;aged >21 years;ED duration >3 months | IIEF,SEP diary,GAQ | Tadalafil improved EF compared with placebo (P<0.005), SEP Q3: 70.0%, 10 mg; 78.0%, 20 mg versus 42.8% placebo, GAQ: 92.3% and 84.6% versus 54.5% placebo | Back pain, dyspepsia and myalgia |
| Guo et al.40 | Tadalafil (10 or 20 mg) versus placebo for 12 weeks | Integrated analysis of five double-blind, placebo-controlled trials | 1046 men;aged >18 years;ED duration >3 months | IIEF,SEP diary,GAQ | Tadalafil 10 or 20 mg improved IIEF-EF (P<0.001), better SEP Q3 (64.1%, 70.5% versus placebo 33.4%, P<0.001), improved GAQ (85.5%, 85.4% versus placebo 43.5%, P<0.001) | 1.3% of tadalafil versus 1.1% of placebo;headache and back pain |
| Paick et al.41 | Udenafil (100 or 200 mg) versus placebo for 12 weeks | Multi-institutional randomized controlled trial in Korea | 167 men;aged 19–70 years;ED duration >6 months | IIEF, SEP diary, GAQ | Significantly improved IIEF-EF, SEP Q2 and SEP Q3 (P<0.0001), GAQ (placebo, 25.9% udenafil 100 mg, 81.5% udenafil 200 mg, 88.5%, P<0.0001) | Flushing, headache and nasal congestion |
| Park et al.42 | Udenafil (100 mg) versus placebo for 4 weeks | Multi-institutional randomized controlled trial in Korea | 103 men;aged 19–70 years;ED duration >6 months | IIEF-EF domain score,SEP2, SEP3 | Enhanced SEP Q3 (placebo, 28.3% versus udenafil, 54.7% P<0.0001), significant change from base line in IIEF-EF (placebo, 0.58; udenafil, 4.40; P<0.0001) | 4% of placebo, 11.3% of udenafil;flushing, headache and toothache |
| Paick et al.43 | Mirodenafil (SK3530; 50, 100 or 150 mg) versus placebo for 8 weeks | Multi-institutional randomized controlled trial in Korea | 119 men;aged 19–70 years;ED duration >6 months | IIEF, SEP diary, GAQ | All primary and secondary efficacy end points significantly improved (P<0.05) 42.3% achieved normal erectile function after treatment | Flushing, headache, dizziness and eye redness (10.9%, 7.6%, 2.5% and 2.5%, respectively) |
| Paick et al.44 | Mirodenafil (50 or 100 mg) versus placebo for 12 weeks | Multi-institutional randomized controlled trial in Korea | 223 men;aged 19–70 years;ED duration >6 months | IIEF, SEP diary, GAQ, LSC | Mirodenafil 50 and 100 mg, greater increase in IIEF Q3, Q4 (P<0.0001), improved IIEF-EF, SEP2, SEP3, GAQ and LSC | Mild intensity flushing, headache, nausea and eye redness |
Abbreviations: ED, erectile dysfunction; EF, erectile function; IIEF, International Index of Erectile Function; GAQ, global assessment question; GEAP, global efficacy assessment question; LSC, life satisfaction checklist; PDE-5, phosphodiesterase-5; SEP, sexual encounter profile.
Efficacy and safety of sildenafil
Of the five PDE-5 inhibitors, sildenafil was the first to be launched in Asia. Three multinational or multi-institutional studies, collectively called the Asian Sildenafil Efficacy and Safety Study (ASSESS), have evaluated the efficacy, safety and tolerability of oral sildenafil in Asian men with ED with broad-spectrum aetiology.31, 32, 33 The ASSESS-1 trial was carried out at eight centres in Malaysia, the Philippines and Singapore.31 ASSESS-2 was performed in four centres in Thailand32 and ASSESS-3 was conducted in Taiwan (China).33 In these three studies, a total of 616 Asian men were randomly assigned to 12 weeks of sildenafil or placebo taken as needed 1 h before anticipated sexual activity. Initially, the sildenafil or matching placebo dose was 50 mg but could be increased to 100 mg or decreased to 25 mg depending on efficacy or intolerance, respectively. The primary efficacy variables related to achievement and maintenance of erections sufficient for sexual intercourse and the secondary efficacy variables, such as IIEF-EF domains, the percentage of successful intercourse attempts and the global assessment of erections, were all statistically significantly improved by sildenafil as compared with placebo. In one trial, only the sexual-desire domain showed no significant difference.32 These results demonstrate that sildenafil is effective in restoring sexual function in Asian men with ED of various aetiologies. The other RCT of sildenafil, the ASSESS-K study, was a flexible-dose escalation study performed at six centres in Korea.34 ASSESS-K was similar to the other ASSESS studies except for a shorter trial period (8 weeks). In all four studies, common adverse events with sildenafil were flushing, dizziness and headache, most of which were mild.
Efficacy and safety of vardenafil
Two prospective, randomized, double-blind, placebo-controlled, fixed-dose, parallel-group studies have been conducted to assess the safety and efficacy of vardenafil 10 mg for the treatment of ED in Asian regions.35, 36 The first such study was performed in six countries or regions: Malaysia, Singapore, Thailand, the Philippines, Hong Kong (China) and Indonesia.35 In this study, 358 men with ED were randomly assigned to receive vardenafil 10 mg or placebo for 12 weeks. The primary efficacy variables were the IIEF-EF and the sexual encounter profile (SEP) questions related to penetration (SEP2) and intercourse completion (SEP3). Secondary efficacy variables were the global assessment question (GAQ) regarding erection improvement.
Treatment with vardenafil significantly improved the IIEF-EF domain scores as compared with placebo (22.4 versus 14.3; P<0.001) and also improved SEP2 (vardenafil 82.2 versus placebo 43.6; P<0.001) and SEP3 (vardenafil 66.1 versus placebo 24.0; P<0.001). Positive GAQ responses were reported by 81.8% of the participants in the vardenafil group and 24.3% of those in the placebo group. The most frequent adverse events were headache, flushing, nasal congestion and dizziness.
The other vardenafil study was conducted in 306 men with ED in Taiwan, South Korea and Japan.36 Treatment with vardenafil produced significant improvement in the main efficacy measures of IIEF-EF score and SEP2 and SEP3 as well as the GAQ. The efficacy and safety of vardenafil in East Asian men were similar to those reported in previous studies of Western populations.45, 46
Efficacy and safety of tadalafil
Tadalafil is considered an effective, well-tolerated therapy for Asian men with ED of broad-spectrum severity and aetiology.37, 38, 39, 40, 47 Yip et al.37 assessed the efficacy and safety of tadalafil as compared with a placebo in a multicentre, randomized, double-blind, parallel-group, placebo-controlled study conducted at 17 centres across East and Southeast Asia: Hong Kong (China) (one site, 38 patients), Indonesia (two sites, 21 patients), Malaysia (three sites, 32 patients), the Philippines (four sites, 40 patients), Singapore (three sites, 30 patients) and Taiwan (China) (four sites, 81 patients). In this study, men older than 18 years with mild to severe ED of various aetiologies were randomly assigned to receive a placebo or 20 mg tadalafil taken as needed. Tadalafil significantly improved erectile function as compared with placebo (P<0.001). At the end point, the tadalafil group reported successful intercourse attempts (SEP3: 70.9% compared with 33.5% in the placebo) and improved erections (GAQ: 86.2% compared with 30.1%). The most common treatment-emergent adverse events were headache, back pain, dizziness and dyspepsia.
Tadalafil was also shown to be an effective and well-tolerated treatment for Southeast Asian men with ED.38, 39 In these studies, the study design was a randomized, double-blind, placebo-controlled study of men with mild to severe ED of various aetiologies who were randomly assigned to receive placebo, tadalafil 10 mg or tadalafil 20 mg taken as needed without restrictions on food intake and timing of sexual activity for 12 weeks. Tadalafil RCTs were conducted in 10 centres across Mainland China, Singapore and the Philippines38 (n=367) and eight centres in Taiwan (China)39 (n=196). Compared with placebo, tadalafil significantly improved erectile dysfunction on all efficacy outcomes (P<0.001). The most common adverse events reported by patients were headache, back pain, dyspepsia and dizziness.
Guo et al.40 conducted an integrated analysis of five Asian tadalafil clinical trials involving 1046 patients and determined the efficacy and safety of tadalafil in diverse clinical populations with comorbid medical conditions. In this study, patients receiving 10 mg or 20 mg tadalafil, as compared with patients receiving placebo, showed significant improvement from baseline to the end point in the IIEF-EF domain score in all clinical subpopulations (P<0.001). The 10 mg and 20 mg tadalafil groups showed mean success rates of 64.1% and 70.5%, respectively, for SEP3, as compared with 33.4% in the placebo group (P<0.001). Also, 85.5% and 85.4%, respectively, reported improved erections on the end point GAQ versus 43.5% in the placebo group (P<0.001). Tadalafil was well tolerated across all groups, and the most frequently reported adverse events were headache and back pain.
Efficacy and safety of udenafil
Udenafil (Zydena®, Dong-A Phamaceutical, Seoul, Korea) is a newly developed, potent, selective PDE-5 inhibitor that can also inhibit cyclic guanosine monophosphate hydrolysis.48 Its pharmacokinetic profile includes unique clinical properties of both a relatively rapid onset and a long duration of action.49 In addition, the isoenzyme selectivity profile of udenafil is similar to that of sildenafil. On the other hand, unlike tadalafil, it does not inhibit PDE-11. In a multicentre, double-blind, placebo-controlled, fixed-dose, parallel-group phase III trial, 167 Korean patients with ED of diverse origin and severity were randomly assigned to take placebo or udenafil at fixed doses of 100 mg or 200 mg as needed for 12 weeks.41 The efficacy of udenafil was assessed by IIEF-EF domain scores, SEP2, SEP3 and GAQ. The patients treated with udenafil showed a significantly greater change from baseline in the IIEF-EF domain score as compared with those given placebo (placebo, 0.20; 100 mg udenafil, 7.52; and 200 mg udenafil, 9.93) (P<0.0001). Udenafil significantly enhanced SEP2 and SEP3 as compared with placebo (P<0.0001). The GAQ also increased significantly in the udenafil group as compared with the placebo group (GAQ: 100 mg udenafil, 81.5% 200 mg udenafil, 88.5% and placebo, 25.9%) (P<0.0001). These results indicate that udenafil is an effective and well-tolerated therapy for ED of broad-spectrum aetiology and severity. The most common treatment-related adverse events were facial flushing and headache.
Another RCT evaluated the efficacy of udenafil in treating ED for up to 12 h after dosing.42 This study was performed to evaluate the duration of udenafil efficacy for sexual intercourse. Udenafil significantly enhanced the SEP3 (placebo 28.3% versus udenafil 54.7% P<0.0001) and IIEF-EF domain from baseline, whereas there was no significant difference in the SEP2 between the two groups. These data suggest that udenafil may be a reliable treatment option in patients who have relatively spontaneous and unscheduled sexual intercourse.
Efficacy and safety of mirodenafil
Mirodenafil (SK3530) is a newly developed oral PDE-5 inhibitor for the treatment of ED.43 Preclinical studies revealed that SK3530 has selectivity comparable with that of conventional PDE-5 inhibitors.43, 50 Paick et al.43 evaluated the efficacy and safety of mirodenafil in a total of 119 patients randomized at 10 centres in Korea. The patients received either mirodenafil (50, 100 or 150 mg) or placebo for an 8-week period. The efficacy of mirodenafil was assessed using the IIEF, SEP and GAQ. At the end of the study, all primary and secondary efficacy end points were statistically significantly improved by mirodenafil as compared with placebo (P<0.05). The most common adverse events were flushing, headache, dizziness and redness of the eyes (10.9%, 7.6%, 2.5% and 2.5%, respectively), and most were mild.
A consecutive clinical study of mirodenafil was conducted with 223 subjects who were randomly assigned to placebo or mirodenafil at fixed doses of 50 or 100 mg for 12 weeks.44 The mirodenafil 50 and 100 mg groups showed a significantly greater increase in the scores for IIEF question 3 regarding the availability of penile penetration to partner (P=0.0001 and P<0.0001, respectively) and question 4 (both P<0.0001) at the end point as compared with the placebo group. Mirodenafil in both doses significantly improved the IIEF, SEP2 and SEP3 scores and the GAQ as compared with placebo. The most frequent adverse events were facial flushing, headache, nausea and redness of the eyes.
Conclusion
The prevalence rate of ED in Asia ranged widely, from 2% to 88%. This finding indicates that ED is a common and major health problem in this region. However, sociocultural and economic factors in Asia prevent people from seeking and obtaining appropriate medical care. Five kinds of PDE-5 inhibitors for the treatment of ED have been studied in Asian countries: sildenafil, vardenafil, tadalafil, udenafil and mirodenafil. The results of RCTs have shown that these five PDE-5 inhibitors are more effective than placebo in improving erectile function in Asian men with ED. The adverse events reported by patients tended to be mild or moderate in severity, and the rate of discontinuation caused by adverse events was similar to those observed in Western countries. Further well-designed, long-term PDE-5 inhibitor trials are needed to evaluate their efficacy and safety in the presence of comorbid conditions or specific causes of ED.
The authors declare no competing financial interests.
References
- NIH Consensus Conference Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Nehra A, Kulaksizoglu H. Global perspectives and controversies in the epidemiology of male erectile dysfunction. Curr Opin Urol. 2002;12:493–6. doi: 10.1097/00042307-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, et al. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- Maas R, Schwedhelm E, Albsmeier J, Boger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002;7:213–25. doi: 10.1191/1358863x02vm429ra. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- Tan HM, Marumo K, Yang DY, Hwang TI, Ong ML. Sex among Asian men and women: the Global Better Sex Survey in Asia. Int J Urol. 2009;16:507–14. doi: 10.1111/j.1442-2042.2009.02283.x. [DOI] [PubMed] [Google Scholar]
- Cheng JY, Ng EM, Chen RY, Ko JS. Prevalence of erectile dysfunction in Asian populations: a meta-analysis. Int J Impot Res. 2007;19:229–44. doi: 10.1038/sj.ijir.3901517. [DOI] [PubMed] [Google Scholar]
- Bai Q, Xu QQ, Jiang H, Zhang WL, Wang XH, et al. Prevalence and risk factors of erectile dysfunction in three cities of China: a community-based study. Asian J Androl. 2004;6:343–8. [PubMed] [Google Scholar]
- Lau JT, Wang Q, Cheng Y, Yang X. Prevalence and risk factors of sexual dysfunction among younger married men in a rural area in China. Urology. 2005;66:616–22. doi: 10.1016/j.urology.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Tan HM, Low WY, Ng CJ, Chen KK, Sugita M, et al. Prevalence and correlates of erectile dysfunction (ED) and treatment seeking for ED in Asian men: the Asian Men's Attitudes to Life Events and Sexuality (MALES) study. J Sex Med. 2007;4:1582–92. doi: 10.1111/j.1743-6109.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- Nicolosi A, Glasser DB, Kim SC, Marumo K, Laumann EO. Sexual behaviour and dysfunction and help-seeking patterns in adults aged 40–80 years in the urban population of Asian countries. BJU Int. 2005;95:609–14. doi: 10.1111/j.1464-410X.2005.05348.x. [DOI] [PubMed] [Google Scholar]
- Wong SY, Leung JC, Woo J. Sexual activity, erectile dysfunction and their correlates among 1,566 older Chinese men in Southern China. J Sex Med. 2009;6:74–80. doi: 10.1111/j.1743-6109.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- Ng EM, Cheng JY. Prevalence and biopsychosocial correlates of erectile dysfunction in Hong Kong: a population-based study. Urology. 2007;70:131–6. doi: 10.1016/j.urology.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Li MK, Garcia LA, Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int. 2005;96:1339–54. doi: 10.1111/j.1464-410X.2005.05831.x. [DOI] [PubMed] [Google Scholar]
- Marumo K, Nakashima J, Murai M. Age-related prevalence of erectile dysfunction in Japan: assessment by the International Index of Erectile Function. Int J Urol. 2001;8:53–9. doi: 10.1046/j.1442-2042.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Sasayama S, Ishii N, Ishikura F, Kamijima G, Ogawa S, et al. Men's health study: current status of erectile dysfunction of 6,112 ambulatory patients at general practitioners offices in Japan J Cardiol 20034257–65.Japanese. [PubMed] [Google Scholar]
- Nicolosi A, Moreira ED, Jr, Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61:201–6. doi: 10.1016/s0090-4295(02)02102-7. [DOI] [PubMed] [Google Scholar]
- Ahn TY, Park JK, Lee SW, Hong JH, Park NC, et al. Prevalence and risk factors for erectile dysfunction in Korean men: results of an epidemiological study. J Sex Med. 2007;4:1269–76. doi: 10.1111/j.1743-6109.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- Cho BL, Kim YS, Choi YS, Hong MH, Seo HG, et al. Prevalence and risk factors for erectile dysfunction in primary care: results of a Korean study. Int J Impot Res. 2003;15:323–8. doi: 10.1038/sj.ijir.3901022. [DOI] [PubMed] [Google Scholar]
- Moreira ED, Jr, Kim SC, Glasser D, Gingell C. Sexual activity, prevalence of sexual problems, and associated help-seeking patterns in men and women aged 40–80 years in Korea: data from the Global Study of Sexual Attitudes and Behaviors (GSSAB) J Sex Med. 2006;3:201–11. doi: 10.1111/j.1743-6109.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Khoo EM, Tan HM, Low WY. Erectile dysfunction and comorbidities in aging men: an urban cross-sectional study in Malaysia. J Sex Med. 2008;5:2925–34. doi: 10.1111/j.1743-6109.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- Quek KF, Sallam AA, Ng CH, Chua CB. Prevalence of sexual problems and its association with social, psychological and physical factors among men in a Malaysian population: a cross-sectional study. J Sex Med. 2008;5:70–6. doi: 10.1111/j.1743-6109.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Tan JK, Hong CY, Png DJ, Liew LC, Wong ML. Erectile dysfunction in Singapore: prevalence and its associated factors—a population-based study. Singapore Med J. 2003;44:20–6. [PubMed] [Google Scholar]
- Hwang TI, Tsai TF, Lin YC, Chiang HS, Chang LS. A survey of erectile dysfunction in Taiwan: use of the Erection Hardness Score and Quality of Erection Questionnaire. J Sex Med. 2010;7:2817–24. doi: 10.1111/j.1743-6109.2010.01837.x. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Hsieh JT, Lin JS, Hwang TI, Jiann BP, et al. Comparison of prevalence between self-reported erectile dysfunction and erectile dysfunction as defined by five-item International Index of Erectile Function in Taiwanese men older than 40 years. Urology. 2007;69:743–7. doi: 10.1016/j.urology.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Chen KK, Chiang HS, Jiann BP, Lin JS, Liu WJ, et al. Prevalence of erectile dysfunction and impacts on sexual activity and self-reported intercourse satisfaction in men older than 40 years in Taiwan. Int J Impot Res. 2004;16:249–55. doi: 10.1038/sj.ijir.3901218. [DOI] [PubMed] [Google Scholar]
- Permpongkosol S, Kongkakand A, Ratana-Olarn K, Tantiwong A, Tantiwongse K. Increased prevalence of erectile dysfunction (ED): results of the second epidemiological study on sexual activity and prevalence of ED in Thai males. Aging Male. 2008;11:128–33. doi: 10.1080/13685530802278128. [DOI] [PubMed] [Google Scholar]
- Moreira ED, Jr, Brock G, Glasser DB, Nicolosi A, Laumann EO, et al. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6–16. doi: 10.1111/j.1742-1241.2005.00382.x. [DOI] [PubMed] [Google Scholar]
- Tsertsvadze A, Fink HA, Yazdi F, MacDonald R, Bella AJ, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med. 2009;151:650–61. doi: 10.7326/0003-4819-151-9-200911030-00150. [DOI] [PubMed] [Google Scholar]
- Tan HM, Moh CL, Mendoza JB, Gana T, Albano GJ, et al. Asian sildenafil efficacy and safety study (ASSESS-1): a double-blind, placebo-controlled, flexible-dose study of oral sildenafil in Malaysian, Singaporean, and Filipino men with erectile dysfunction. The Assess-1 Study Group. Urology. 2000;56:635–40. doi: 10.1016/s0090-4295(00)00688-9. [DOI] [PubMed] [Google Scholar]
- Kongkanand A, Ratana-Olarn K, Ruangdilokrat S, Tantiwong A. The efficacy and safety of oral sildenafil in Thai men with erectile dysfunction: a randomized, double-blind, placebo controlled, flexible-dose study. J Med Assoc Thai. 2003;86:195–205. [PubMed] [Google Scholar]
- Chen KK, Hsieh JT, Huang ST, Jiaan DB, Lin JS, et al. ASSESS-3: a randomised, double-blind, flexible-dose clinical trial of the efficacy and safety of oral sildenafil in the treatment of men with erectile dysfunction in Taiwan. Int J Impot Res. 2001;13:221–9. doi: 10.1038/sj.ijir.3900685. [DOI] [PubMed] [Google Scholar]
- Choi HK, Ahn TY, Kim JJ, Kim SC, Paick JS, et al. A double-blind, randomised- placebo, controlled, parallel group, multicentre, flexible-dose escalation study to assess the efficacy and safety of sildenafil administered as required to male outpatients with erectile dysfunction in Korea. Int J Impot Res. 2003;15:80–6. doi: 10.1038/sj.ijir.3900944. [DOI] [PubMed] [Google Scholar]
- Tan HM, Chin CM, Chua CB, Gatchalian E, Kongkanand A, et al. Efficacy and tolerability of vardenafil in Asian men with erectile dysfunction. Asian J Androl. 2008;10:495–502. doi: 10.1111/j.1745-7262.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- Chen KK, Paick JS, Ishii N. The efficacy and safety of vardenafil in East Asian men with erectile dysfunction. J Sex Med. 2007;4:753–61. doi: 10.1111/j.1743-6109.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- Yip WC, Chiang HS, Mendoza JB, Tan HM, Li MK, et al. Efficacy and safety of on demand tadalafil in the treatment of East and Southeast Asian men with erectile dysfunction: a randomized double-blind, parallel, placebo-controlled clinical study. Asian J Androl. 2006;8:685–92. doi: 10.1111/j.1745-7262.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- Guo YL, Zhu JC, Pan TM, Ding Q, Wang YX, et al. Efficacy and safety of on-demand tadalafil for the treatment of erectile dysfunction in South-East Asian men. Int J Urol. 2006;13:721–7. doi: 10.1111/j.1442-2042.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- Chen KK, Jiann BP, Lin JS, Lee SS, Huang ST, et al. Efficacy and safety of on-demand oral tadalafil in the treatment of men with erectile dysfunction in Taiwan: a randomized, double-blind, parallel, placebo-controlled clinical study. J Sex Med. 2004;1:201–8. doi: 10.1111/j.1743-6109.2004.04029.x. [DOI] [PubMed] [Google Scholar]
- Guo YL, Viswanathan VP, Chiang HS, Choi HK, Yip AW, et al. Efficacy and safety of tadalafil taken as needed for the treatment of erectile dysfunction in Asian men: results of an integrated analysis. Asian J Androl. 2009;11:423–33. doi: 10.1038/aja.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paick JS, Kim SW, Yang DY, Kim JJ, Lee SW, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008;5:946–53. doi: 10.1111/j.1743-6109.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- Park HJ, Park JK, Park K, Min K, Park NC. Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sex Med. 2010;7:2209–16. doi: 10.1111/j.1743-6109.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- Paick JS, Choi HK, Kim SC, Ahn TY, Kim JJ, et al. Efficacy and safety of oral SK3530 for the treatment of erectile dysfunction in Korean men: a multicenter, randomized, double-blind, placebo-controlled, fixed dose, parallel group clinical trial. Asian J Androl. 2008;10:791–8. doi: 10.1111/j.1745-7262.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- Paick JS, Ahn TY, Choi HK, Chung WS, Kim JJ, et al. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med. 2008;5:2672–80. doi: 10.1111/j.1743-6109.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- Porst H, Rosen R, Padma-Nathan H, Goldstein I, Giuliano F, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int J Impot Res. 2001;13:192–9. doi: 10.1038/sj.ijir.3900713. [DOI] [PubMed] [Google Scholar]
- Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, et al. Sustained efficacy and tolerability of vardenafil, a highly potent selective phosphodiesterase type 5 inhibitor, in men with erectile dysfunction: results of a randomized, double-blind, 26-week placebo-controlled pivotal trial. Urology. 2003;61:8–14. doi: 10.1016/s0090-4295(03)00115-8. [DOI] [PubMed] [Google Scholar]
- Choi HK, Kim JJ, Kim SC, Suh J, Park YK, et al. A randomised, double-blind, parallel, placebo-controlled study of the efficacy and safety of tadalafil administered on-demand to men with rectile dysfunction in Korea. Korean J Urol. 2006;47:852–58. [Google Scholar]
- Doh H, Shin CY, Son M, Ko JI, Yoo M, et al. Mechanism of erectogenic effect of the selective phosphodiesterase type 5 inhibitor, DA-8159. Arch Pharm Res. 2002;25:873–8. doi: 10.1007/BF02977007. [DOI] [PubMed] [Google Scholar]
- Salem EA, Kendirci M, Hellstrom WJ. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs. 2006;7:661–9. [PubMed] [Google Scholar]
- Lee J, Yoo HH, Rhim KJ, Sohn DR, Kim DH. Metabolism and excretion of 5-ethyl-2-{5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-7-propyl-3,5 dihydropyrrolo [3,2-d]-pyrimidin-4-one (SK3530) in rats. Rapid Commun Mass Spectrom. 2007;21:1139–49. doi: 10.1002/rcm.2943. [DOI] [PubMed] [Google Scholar]


