Abstract
The interleukin-7 receptor α chain (IL-7Rα) gene was identified as a top non–major histocompatibility complex–linked risk locus for multiple sclerosis (MS). Recently, we showed that a T helper 1 (TH1)–driven, but not a TH17-driven, form of MS exhibited a good clinical response to interferon-β (IFN-β) therapy. We now demonstrate that high serum levels of IL-7, particularly when paired with low levels of IL-17F, predict responsiveness to IFN-β and hence a TH1-driven subtype of MS. We also show that although IL-7 signaling is neither necessary nor sufficient for the induction or expansion of TH17 cells, IL-7 can greatly enhance both human and mouse TH1 cell differentiation. IL-7 alone is sufficient to induce human TH1 differentiation in the absence of IL-12 or other cytokines. Furthermore, targeting IL-7/IL-7Rα is beneficial in experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. Mice treated with IL-7Rα–blocking antibodies before or after onset of paralysis exhibited reduced clinical signs of EAE, with reduction in peripheral naïve and activated T cells, whereas central memory T, regulatory T, B, and natural killer cell populations were largely spared. IL-7Rα antibody treatment markedly reduced lymphocyte infiltration into the central nervous system in mice with EAE. Thus, a serum profile of high IL-7 may signify a TH1-driven form of MS and may predict outcome in MS patients undergoing IFN-β therapy. Blockade of IL-7 and the IL-7Rα pathway may have therapeutic potential in MS and other autoimmune diseases.
INTRODUCTION
Multiple sclerosis (MS), a chronic recurring autoimmune disease of the central nervous system (CNS), is characterized by inflammation, demyelination, and axonal injury (1, 2). Disease onset usually occurs in young adults, and it is more common in females (3). Recently, several independent genome-wide association studies have identified a single-nucleotide polymorphism (SNP) in the interleukin-7 receptor α (IL-7Rα) gene that may be associated with susceptibility to MS (4–6). The SNP involved influences alternative splicing of exon 6, which in turn may have potential consequences for the function of the receptor (6). Lundmark et al. showed that both IL-7R and IL-7 mRNA levels were higher in the cerebrospinal fluid of patients with MS than in non-inflammatory neurological diseases (4), suggesting that IL-7/IL-7Rα may be involved in the pathogenesis of MS. The precise roles of IL-7/IL-7Rα in the pathogenesis of MS remain unclear.
IL-7 is a member of the γc cytokine receptor superfamily that includes IL-2, IL-4, IL-9, IL-15, and IL-21 (7–9). IL-7 binds to its receptor, which is composed of IL-7Rα and γ-chains (10, 11). Alternatively IL-7Rα can heterodimerize with the unique thymic stromal lymphopoietin receptor (TSLPR) to form a distinct multicomponent receptor for another cytokine TSLP (12, 13). IL-7/IL-7R signaling is crucial for proliferation and survival of T lymphocytes in humans and in animal models (14–19); in humans, IL-7Rα deficiency results in the absence of T cells, but B cell counts remain normal (16). On the other hand, mice that lack IL-7Rα are essentially devoid of T and B cells (17), suggesting that the role of IL-7/IL-7R signaling in T cell, but not B cell, development is shared between humans and mice.
Given that the Il7r gene may be associated with susceptibility to MS (4, 5, 20), here we investigate whether serum levels of IL-7 can stratify outcome in MS patients undergoing interferon-β (IFN-β) therapy and dissect the role of IL-7/IL-7R in the pathogenesis of experimental autoimmune encephalomyelitis (EAE) in mice. We found that high levels of serum IL-7 predict clinical responsiveness in MS patients undergoing IFN-β therapy. When high IL-7 levels are paired with low IL-17F levels in serum, the prediction is stronger. IL-7 alone or in combination with IL-12 can promote human and mouse T helper 1 (TH1) cell differentiation. These results are consistent with the notion that IL-7 drives a TH1 form of MS, which was previously shown to respond better to IFN-β therapy than the TH17 form of MS (21). In addition, we show that IL-7Rα–blocking antibodies given to EAE mice before or after onset of paralysis reduced clinical signs of EAE without affecting regulatory T (Treg), B, or natural killer (NK) cells. Therefore, blockade of IL-7 or IL-7Rα may be a potential therapeutic strategy for treating MS.
RESULTS
High Serum Levels of IL-7 Predict MS Patient Responsiveness to IFN- β Therapy
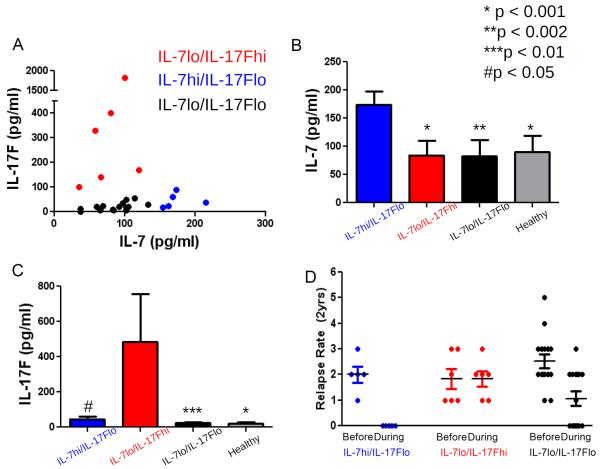
The clinical response to IFN-β therapy is strongly influenced by TH1 and TH17 cells (21). For example, EAE disease induced in mice with pathogenic TH1 T cells is prevented when IFN-β treatment is given before symptom onset, and reversed when IFN-β is given after mice are paralyzed (21). In contrast, when EAE is induced with T cells cultured under cytokine conditions that induce the TH17 pathway, the degree of paralysis is exacerbated clinically and inflammation in the CNS is increased after administration of IFN-β (21). These differential effects of IFN-β in mice were studied in patients with relapsing remitting multiple sclerosis (RRMS) and in normal human controls. Using a multiplex cytokine platform, we found that serum levels of IL-17F are elevated in patients who do not respond to IFN-β (nonresponders) before treatment. Nonresponders relapsed in the 2 years after treatment, whereas responders were relapse-free for 2 years after the therapy (21). Further mining of this same data set from 26 patients with RRMS revealed (Fig. 1) that serum levels of IL-7 were inversely correlated with IL-17F and that the RRMS patients could be stratified into three groups: IL-7hi/IL-17Flo, IL-7lo/IL-17Fhi, and IL-7lo/IL-17Flo (Fig. 1A). IL-7 concentrations were increased in the serum of IL-7hi/IL-17Flo (174.3 ± 23.6 pg/ml) compared to the other RRMS groups (IL-7lo/IL-17Fhi: 84.5 ± 25.1 pg/ml, P < 0.001; IL-7lo/IL-17Flo: 82.3 ± 29.2 pg/ml, P < 0.002) and the healthy control group (90.2 ± 28.8 pg/ml, P < 0.001) (Fig. 1B). IL-17F concentrations were significantly elevated in the IL-7lo/IL-17Fhi group (571.4 ± 23.6 pg/ml) compared to the other RRMS groups (IL-7hi/IL-17Flo: 46.3 ± 29.7 pg/ml, P < 0.05; IL-7lo/IL-17Flo: 25.6 ± 24.9 pg/ml, P < 0.01) (Fig. 1C). Moreover, high levels of IL-7 predicted responsiveness to IFN-β such that patients with IL-7 levels greater than 150 pg/ml were relapse-free in the 2 years after IFN-β therapy was instituted (Fig. 1D). The percentage of relapse-free patients with IL-7 above 150 pg/ml was 100% (n = 5/5) compared to 33% (n = 7/21) of relapse-free patients with IL-7 less than 150 pg/ml (P < 0.012, Fisher's exact test). The IL-7lo/IL-17Flo group also had both responders and nonresponders to IFN-β, but comparing levels of IL-7 or IL-17F within this group did not discriminate responsiveness to therapy (fig. S1).
Fig. 1. High serum IL-7 is a strong predictor of clinical response to IFN-β.
(A) A Luminex 200 cytometer was used to measure serum concentrations of IL-7 and IL-17F in 26 patients with RRMS before the start of IFN-β treatment. (B and C) Concentrations of (B) IL-7 and (C) IL-17F in the stratified groups of RRMS patients and healthy controls. Results are represented as means ± SEM, and statistics were performed with Student's t test (*P < 0.001; **P < 0.002; ***P < 0.01; #P < 0.05). (D) Number of relapses over 2 years before and during IFN-β therapy. IL-7 concentrations in patients with RRMS can be stratified into high versus low with a multiplex cytokine platform (Affymetrix). High levels of IL-7 predicted responsiveness to IFN-β therapy; responsiveness is defined as relapse-free in the 2 years after therapy with IFN-β.
To further substantiate the association between IL-7 and response to IFN-β, we analyzed the additional 15 patients who were neither IL-7lo/IL-17Fhi nor IL-7hi/IL-17Flo and asked whether there was a relationship between levels of IL-7 and response across the entire patient population. When we performed this analysis on the entire data set of 14 nonresponders and 12 responders (21), serum levels of IL-7 had a significant P value for discriminating responders from nonresponders (P < 0.028, t test) (table S1). The area under the receiver operating characteristic (ROC) curve for IL-7 was 0.708 [for details of ROC methodology, see (22)]. Notably, emerging from such refined analysis, IL-7 was the second most discriminatory analyte behind platelet-derived growth factor type BB (PDGF-BB) (ROC area = 0.83). The combination of baseline PDGF-BB levels with knowledge of treatment group allowed perfect separation of responders from nonresponders in this data set of 26. We tested several multivariate modeling methods including logistic regression, lasso, random forest, boosting, support vector machine, and others, but none gave significant improvements over PDGF-BB alone. The top 10 analytes with this analysis are shown in table S1. Given a larger sample size, we would expect additional analytes, including IL-7 and IL-17F, to have even higher ROC values and to add to the predictive value of PDGF-BB. Large-scale confirmatory trials, appropriately powered, are now under way to assess how these analytes, taken one by one or in groups, will predict outcome. Constellations of various analytes may give the best discrimination.
Role of IL-7 human TH cell differentiation
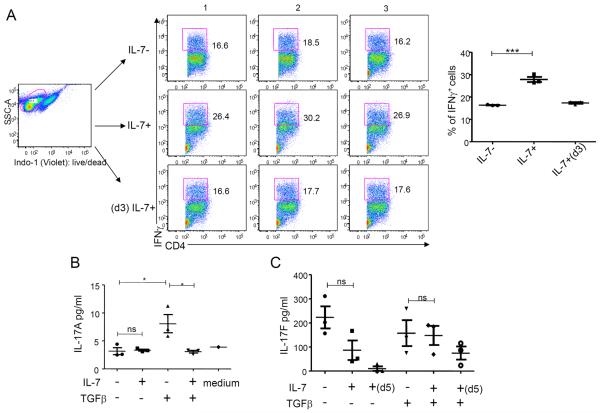
Next, we sought to examine the effects of IL-7 during differentiation and expansion of human TH1 and TH17 cells. We activated naïve TH cells (CD4+CD45RA+CD45RO−CD25− T cells) with antibodies to CD3 and CD28 under cytokine conditions that favored their differentiation into TH1 (IL-12) or TH17 (IL-1 and IL-23) cells in the presence or absence of IL-7. We found that IL-7 significantly induced IFN-γ–producing cells indicative of TH1 cells in the presence of IL-12 (Fig. 2A). The increase in IFN-γ–producing cells was not observed when IL-7 was added later at day 3. Thus, IL-7 can further promote TH1 differentiation from naïve T cells when added together with IL-12 during the early phase of the culture favoring TH1 cell development. Under cytokine conditions favoring TH17 cell development in the absence of transforming growth factor–β (TGFβ), IL-7 had no significant effect on IL-17A production (Fig. 2, B and C). In contrast, IL-7 significantly decreased IL-17A production in the presence of TGFβ (Fig. 2B) and IL-7 treatment also exhibited a trend in reducing IL-17F in the absence of TGFβ (Fig. 2B). The proportion of IFN-γ–expressing cells and IFN-γ production indicative of TH1 cells was significantly increased in the presence of IL-7 even under TH17-polarizing conditions. This effect was also seen in the presence of TGFβ (fig. S2, A and B). Therefore, IL-7 can partially reverse the TH17-polarizing conditions in favor of TH1 differentiation.
Fig. 2. IL-7 promotes TH1 cell differentiation from human naïve CD4+ T cells.
(A) Intracellular staining of IFN-γ was measured in cord blood–sorted naïve CD4+ T cells activated with hIL-12 in the presence or absence of hIL-7 for 6 days or with hIL-12 and hIL-7 added at day 3 (d3). The percent of IFN-γ+ cells are represented as mean ± SEM. ***P < 0.001. (B) Cytometric bead assay measured IL-17A production by naïve cord blood CD4+ T cells. These cells were activated with antibodies to CD3 and CD28 and a combination of IL-1 and IL-23, with or without TGFβ and with or without IL-7 as indicated. Data were analyzed on day 5 (d5) after activation. (C) IL-17F production was measured by ELISA assay in naïve cord blood CD4+ T cells activated with antibodies to CD2, CD3, and CD28 and a combination of IL-1 and IL-23, with or without TGFβ and with or without IL-7. Data were analyzed on day 13. (D) Intracellular staining of IFN-γ in cord blood–sorted naïve CD4+ T cells activated with hIL-12 or hIL-7 in the presence or absence of anti–IL-7Rα antibody (Ab). Data are representative of at least four independent donors. Error bars represent mean ± SEM, and statistics were performed with Student's t test (*P < 0.05; **P < 0.01; ***P < 0.001). n.s., not significant.
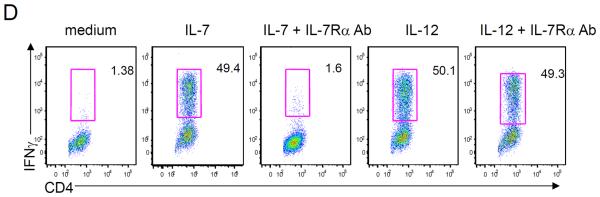
To further assess the effects of IL-7 on TH1 cell differentiation, we performed the experiment in the absence of IL-12. IL-7 alone induces the production of IFN-γ in CD4 T cells to levels that are comparable to stimulation with IL-12 (Fig. 2D), and blocking IL-7Rα with an antibody abolished IL-7–mediated, but not IL-12–mediated, induction of IFN-γ (Fig. 2D). IL-7 increases IFN-γ+ cells and T-bet+ cells in CD4 T cells in a dose-dependent fashion (fig. S2, C and D), further confirming the identity of TH1 cell development. In addition, IL-7 alone also significantly increased IL-2, IL-6, and tumor necrosis factor–α (TNFα) similar to the effects of IL-12 (fig. S2E); however, IL-7 also increased IL-4 and IL-10 production (fig. S2E). Under TH17-polarizing conditions, IL-7 also significantly increased TNF, IL-6, and IL-10 production in the absence of TGFβ, whereas IL-7 significantly increased IL-2 production in the presence or absence of TGFβ (fig. S2F). Together, these results indicated that IL-7, either alone or in combination with IL-12, can promote human TH1, but not TH17, cell differentiation from naïve human T cells.
Role of IL-7 in mouse TH cell differentiation and EAE
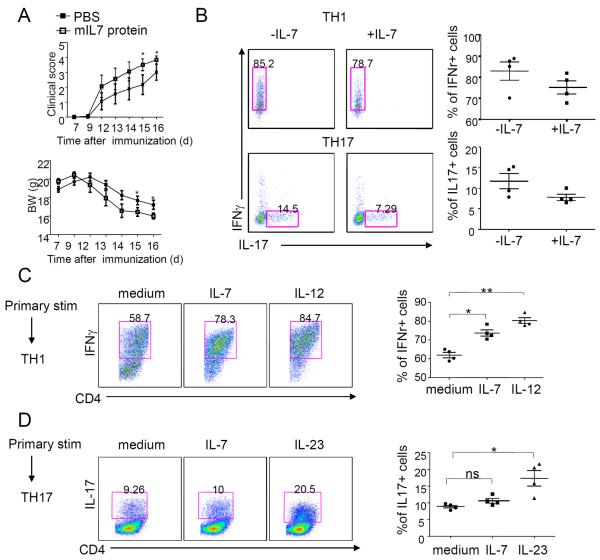
Next, we studied the effect of IL-7 in mice with EAE and on pathogenic T cell differentiation. Wild-type B6 mice immunized with myelin oligodendrocyte glycoprotein (MOG) to induce EAE and treated with mouse IL-7 (mIL-7) starting at day 7 after immunization showed significantly elevated disease activity (Fig. 3A, upper panel, *P < 0.05 on days 15 and 16) accompanied by more severe body weight loss (Fig. 3A, bottom panel, *P < 0.05 on days 15 and 16) compared to control mice, suggesting that increased IL-7 exacerbates disease severity.
Fig. 3. IL-7 is required for TH1 cell expansion from 2D2 naïve CD4+ T cells.
(A) Clinical scores (upper) and body weight (BW) (bottom) are shown for MOG-immunized wild-type B6 mice treated with IL-7 (1 μg) or vehicle control every 3 days starting from day 7 before symptom onset. (B to D) Frequency of CD4+ T lymphocytes expressing IFN-γ and IL-17. Sorted naïve CD4+ T cells from naïve 2D2 mice were activated in the presence of MOG(35–55) and irradiated antigen-presenting cells plus the respective cytokines for TH1 (upper) or TH17 (bottom) polarization. (B) During the differentiation period, cells were cultured in the presence or absence of IL-7 for 3 days. (C to D) In a separate experiment, after 3 days of culture in the presence of TH1- or TH17-polarizing conditions, cells were rested for 48 hours followed by restimulation with MOG(35–55) in the presence of different cytokines (for example, IL-12, IL-7, IL-23, or medium only as indicated above each individual FACS plot) during the expansion period. CD4+ T cells were analyzed for IFN-γ and IL-17 production by flow cytometry. The mean percentage ± SEM (n = 2 experiments) of cytokine-positive cells is given. Each experiment comprised four mice per group. Statistics were generated with Student's t test. *P < 0.05; **P < 0.01.
Then, we investigated whether IL-7/IL-7R signaling had effects on mouse TH1 or TH17 cell differentiation. In the presence of IL-12 (for mouse TH1 polarization) or in the presence of TGFβ plus IL-6 (for mouse TH17 polarization), IL-7 did not promote either TH1 or TH17 differentiation (Fig. 3B). Rather, a slight but statistically insignificant decrease in both IFN-γ– and IL-17–producing T cells was observed in the presence of IL-7. Next, we addressed the effect of IL-7 on the expansion of TH1 or TH17 cells. CD4+ naïve T cells from naïve 2D2 mice were first cultured under TH1- or TH17-polarizing conditions, and then were allowed to rest for 48 hours; these cells were then restimulated with MOG in the presence of individual cytokines to assess their effect on TH expansion. Under cytokine conditions that promoted the TH1 pathway, IL-12 significantly expanded TH1 cells (P < 0.01), as expected, and so did IL-7 (P < 0.05) (Fig. 3C). Under cytokine conditions that promoted the TH17 pathway, IL-23 significantly expanded previously differentiated TH17 cells, whereas IL-7 did not (Fig. 3D).
To further assess the role of IL-7 in the expansion of TH1 or TH17 cells, we cultured CD4+ T cells from MOG-immunized 2D2 mice under conditions that promote polarized differentiation along either the TH1 or the TH17 pathways. Again, under cytokine conditions that promoted the TH17 pathway, IL-23 and IL-6 expanded TH17 cells but IL-7 did not (fig. S3, A and B). IL-7Rα–blocking antibodies alone or IL-7 plus IL-7Rα antibody, when added to T cells cultured under cytokine conditions that promote the TH17 pathway, also did not have any effect on expansion of TH17 cells. Likewise, IL-12 expanded TH1 cells, whereas IL-7 did not (fig. S3, A and B). These findings have been confirmed in an independent experiment (fig. S3D and table S2) using different sources of IL-7 (R&D Systems and BD Biosciences). We observed that IL-7 was not able to expand TH17 cells under any of these defined conditions in the laboratory at either Pfizer or Stanford (fig. S3D and table S2).
Previously, Liu et al. showed that IL-7 promotes the expansion but not the differentiation of mouse TH17 cells and that IL-7 does not affect TH1 cells (23). To reconcile the difference between our study's findings and the results of the Liu et al. study (23), we also performed experiments to culture purified CD4+ T cells from MOG-immunized 2D2 mice with IL-7 in a protocol identical to that described by Liu et al. (23). However, we did not observe TH17 cell expansion by IL-7 under these conditions (fig. S3C).
Inhibition of EAE with antagonists of IL-7Rα
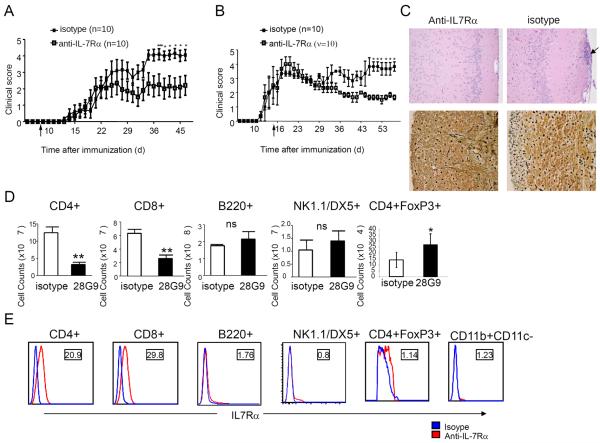
To assess the potential roles of IL-7Rα in MS disease models, we tested whether an antagonist IL-7Rα antibody, 28G9, is efficacious in MOG-immunized mice with EAE. EAE mice were given IL-7Rα antibody, isotype control, or phosphate-buffered saline (PBS) weekly at day 7 (that is, before disease onset). The IL-7Rα antibody treatment was effective in reducing disease activity: clinical score of 4.15 ± 0.30 (mean ± SEM) in immunoglobulin G (IgG) isotype control-treated (n = 10) versus 2.05 ± 0.61 in 28G9-treated group (n = 10) on day 44 (P < 0.05) (Fig. 4A); clinical score of 3.25 ± 0.60 in IgG isotype control-treated (n = 10) versus 0.55 ± 0.35 in 28G9-treated group (n = 10) on day 24 (P < 0.001) (fig. S4A). To investigate IL-7Rα antibody efficacy in treating established disease, we treated EAE animals with IL-7Rα antibody starting at day 14 (that is, after the onset of paralysis, with a mean clinical score of grade 2). Compared to the control group that was treated with isotype-matched, nonspecific IgG (isotype control), treatment with IL-7Rα antibody significantly reduced EAE severity (Fig. 4B): clinical score of 3.83 ± 0.60 in IgG isotype control–treated (n = 10) versus 1.67 ± 0.17 in 28G9-treated group (n = 10) on day 46 (P < 0.05), suggesting that an IL-7Rα antibody can effectively ameliorate established, ongoing EAE after the onset of paralysis. We also observed a similar beneficial effect of anti–IL-7Rα given either before or after symptom onset in proteolipid protein–induced EAE in the SJL mouse model, demonstrating that the efficacy of IL-7Rα antibody treatment was not dependent on the mouse strain (fig. S4, A and B). In accordance with the reduction of clinical score of EAE in treated mice, histological analysis showed that anti–IL-7Rα treatment decreased cellular inflammation and axon loss in the CNS (Fig. 4C).
Fig. 4. Anti–IL-7Rα antibody is efficacious in EAE before or after symptom onset.
(A and B) Clinical scores of EAE mice treated with IL-7Rα–specific antibody (1 mg/kg, anti–IL-7Rα) or isotype control once a week starting from day 7 before symptom onset (A) or with anti–IL-7Rα antibody (10 mg/kg) from day 14 after symptom onset twice weekly after immunization (B) as indicated by an arrow. *P < 0.05; ***P < 0.001 (n = 10 mice per treatment group). Error bars represent mean ± SEM. Statistics were performed with two-way analysis of variance (ANOVA). (C) Histology of cerebrum (top) and spinal cord section (bottom) from treated or isotype control at day 21 after immunization was examined for the degree of inflammation by H&E (top) and for axon loss by Bielschowsky stain (bottom). Inflammatory cells are indicated by an arrow. (D) Absolute cell counts of CD4+, CD8+, B220+, NK1.1/DX5+, and CD4+FoxP3+ cells from spleen of treated or isotype control mice at day 21 after immunization. *P < 0.05. Statistics were generated with Student's t test. (E) Profile of IL-7Rα surface expression in B6 mice. IL-7Rα (red) or isotype control (blue). The numbers in the square represent relative intensity: mean fluorescence intensity (MFI) of IL-7Rα stains/MFI of isotype stains.
To gain insight into the mechanisms by which anti–IL-7Rα antibody acts to ameliorate EAE in mice, we stained for T, B, and NK cells and found a significant reduction in the composition of CD4+ and CD8+ cells in bone marrow, spleen, blood, and lymph nodes (Fig. 4D and fig. S4, C and D). However, B and NK cell populations were not significantly reduced in the peripheral lymphoid organs (Fig. 4D and fig. S4, C to E), but we did observe a 33% decrease in B cell number in the bone marrow (fig. S4C). In agreement with the phenotype analysis, CD4+ and CD8+ T cells expressed higher levels of IL-7Rα, whereas B and NK cells did not express IL-7Rα at comparable levels (Fig. 4E). Because IL-7Rα antibody treatment resulted in T cell lymphopenia, we examined the effects this treatment had on Treg cells. MOG-specific Treg cells in lymph nodes from antibody-treated animals were not reduced compared to control, untreated animals; instead, we found a significant increase in the absolute number of Treg cells as characterized by CD4+FoxP3+ expression (Fig. 4D, last panel, and fig. S5, A and B). These data are consistent with the observation that Treg cells express low levels of IL-7Rα expression compared to effector CD4+ or CD8+ T cells (Fig. 4E), and thus, Treg cells may be preferentially spared by the anti–IL-7Rα treatment.
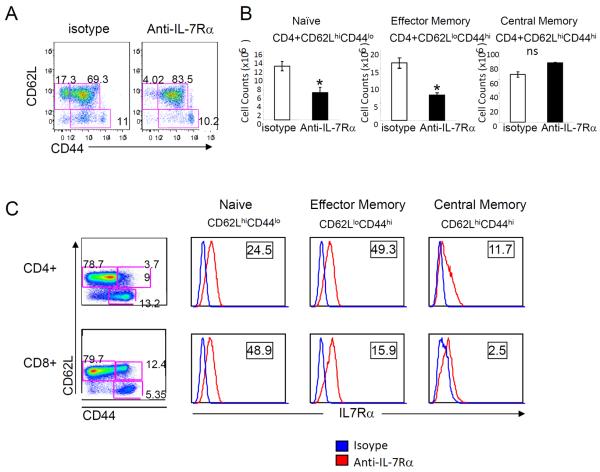
IL-7R signaling is critical for naïve T cell survival and for memory T cell homeostatic proliferation (15, 24, 25). The effect of the anti–IL-7Rα antibody in the regulation of peripheral T cells was analyzed using activation markers CD44 and CD62L. Compared to isotype-treated animals, IL-7Rα antibody–treated mice had depleted naïve T cell and effector memory T cell populations. On the other hand, central memory T cell populations were minimally affected (Fig. 5, A and B). Consistent with this phenotypic analysis, naïve and effector memory T cells expressed a high level of IL-7Rα, whereas the frequency of IL-7Rαhigh central memory T cells was low (Fig. 5C).
Fig. 5. IL-7Rα–specific antibody reduced naïve and effector memory T cells.
(A) Representative expression of CD44 and CD62L in splenic T cells from antibody or isotype-treated EAE at day 22 after immunization. These are representative results of three independent experiments. (B) Number of splenic naïve (CD44loCD62Lhi), effector memory (CD44hiCD62Llo), and central memory (CD44hiCD62Lhi) T cells from antibody and isotype control mice (n = 4). *P < 0.05; **P < 0.01. (C) IL-7Rα surface expression was examined in CD4 and CD8 naïve, effector memory, and central memory T cells. IL-7Rα (red) or isotype control (blue). The numbers in the square represent relative intensity: MFI of IL-7Rα stains/MFI of isotype stains.
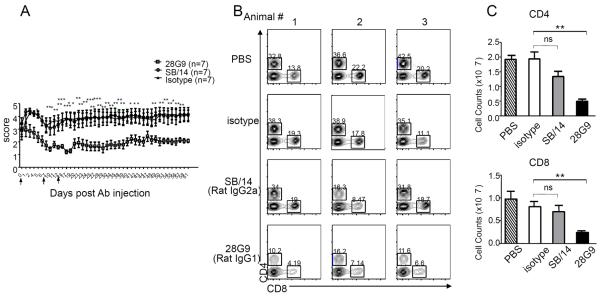
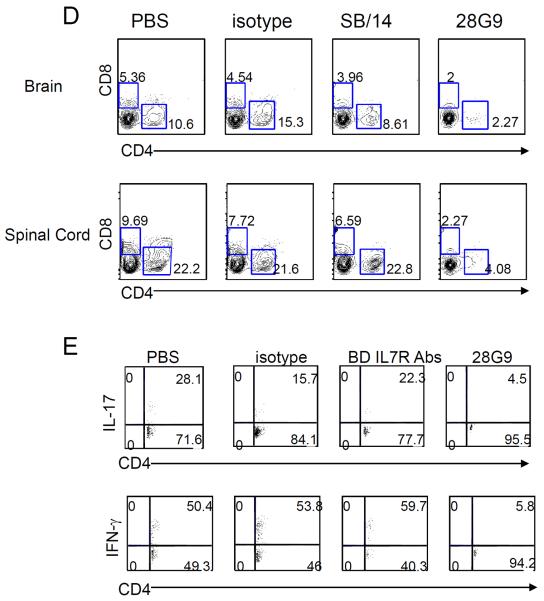
Finally, we compared the biological effects of 28G9 antibody used in all of our studies versus those of the SB/14 antibody (BD Biosciences) used in the study by Liu et al. (23). We found that, after symptom onset with clinical paralysis, three injections of 28G9 antibody significantly reduced EAE symptoms in mice compared to isotype antibody–treated mice (Fig. 6A) with a clinical score of 4.00 ± 0.39 in IgG isotype control–treated (n = 7) versus 1.64 ± 0.34 in 28G9-treated group (n = 7) on day 27 (P < 0.001). In contrast, SB/14 antibody treatment did not (Fig. 6A), with a clinical score of 4.00 ± 0.39 in IgG isotype control–treated (n = 7) versus 3.93 ± 0.64 in SB/14-treated group (n = 7) on day 27 (not significant). As shown above, 28G9 antibody treatment in mice significantly reduced CD4+ and CD8+ T cells (Fig. 6, B and C). In animals treated with SB/14 antibody, their T cells in the periphery (Fig. 6, B and C) and their CD4+ and CD8+ T cell infiltrates (including TH1 and TH17 cells) in the CNS were not significantly different compared to control-treated EAE animals (Fig. 6, D and E). In contrast, mice treated with 28G9 showed significant reductions in the CD4+ and CD8+ T cell infiltrates including TH1 and TH17 cells in the CNS (Fig. 6, D and E). To understand the differences between SB/14 and 28G9 antibodies observed in vivo, we checked whether these two antibodies are comparable in their potency for blocking IL-7–mediated signaling and whether their IgG Fc immune effector function may be different. Both 28G9 and SB/14 antibodies showed similar degrees of blockade of IL-7–mediated signal transducer and activator of transcription 5 (STAT5) phosphorylation in a dose-dependent manner (fig. S6). 28G9 (rat IgG1) binds more strongly to mouse Fcγ receptors than SB/14 (rat IgG2a) (table S3). Thus, mouse Fcγ receptor binding by 28G9-rIgG1 may contribute to the efficacy of 28G9 in ameliorating disease severity after the onset of paralytic disease in EAE mice.
Fig. 6. Anti–IL-7Rα antibody 28G9 showed stronger therapeutic efficacy than SB/14.
(A) Mice that reached clinical scores 2 to 3 were started with 28G9, SB/14, or isotype control once a week for 3 weeks. Dosing was stopped after three injections, and mice were monitored for 7 weeks after injection. Error bars represent mean ± SEM. Statistics were performed with two-way ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001, n = 7 mice per group). (B and C) CD4+ and CD8+ T cells from mice treated with SB/14 antibody, 28G9 antibody, PBS, or isotype control were analyzed at day 21 after immunization. (B) Percent of CD4+ and CD8+ T cells in the spleen from mice treated with IL-7Rα–specific antibody, 28G9, SB/14, or control. (C) Total CD4+ and CD8+ T cell counts. (D and E) Effects of both IL-7Rα–specific antibodies, SB/14 and 28G9, in CNS. (D) Percentage of CD4+ and CD8+ T cells in the brain (upper) and spinal cord (bottom) from EAE mice treated with SB/14 antibody, 28G9 antibody, PBS, or isotype control analyzed at day 21 after immunization. (E) Percentage of IL-17–producing (upper) and IFN-γ–producing (bottom) CD4+ T cells in the brains of EAE mice treated with IL-7Rα–specific antibody or control. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In this report, we show that serum levels of IL-7 predict clinical response to IFN-β therapy in patients with RRMS. We demonstrate that in human T cell cultures, IL-7, either alone or in combination with IL-12, can promote differentiation of naïve T cells into TH1 cells. These data are consistent with the notion that high IL-7 levels may signify the TH1-driven form of MS. Congruent with these human data, we also find that IL-7 promotes the expansion of mouse TH1 cells. In contrast to the study by Liu et al. (23), we find that there is no effect on either human or mouse TH17 cell differentiation or expansion. In EAE, recombinant IL-7 protein exacerbates the disease, whereas the IL-7Rα–blocking antibody 28G9 given either before or after disease onset effectively reduces the severity of symptoms. The prophylactic and therapeutic efficacy of IL-7Rα–blocking antibody is best correlated with and explained by the partial depletion of naïve (CD62LhiCD44lo) and effector memory (CD62Llo44hi) CD4 and CD8 T cells. Reduction of effector T cells may directly dampen ongoing inflammation. Reduction of naïve T cells would further restrain the activation and recruitment of nascent autoantigen-reactive T cells from the naïve pool (26).
In RRMS, polymorphisms in the IL-7Rα gene confer increased risk of disease. This genetic association may have a mechanistic basis that perhaps can be explained by the findings in this study. The IL-7 pathway promotes the differentiation of pathogenic TH1 cells as well as sustaining the naïve pool from which autoreactive T cells are activated and recruited to cause disease. Conversely, IL-7Rα antibody treatment targets effector and naïve T cells, but not B and NK cell populations, which is consistent with the T cell lymphopenia observed in both humans and mice that are deficient in IL-7Rα (14, 16, 17). The specific T cell populations implicated in our proposed mechanism here are supported by two recent publications. First, Walline et al. demonstrated that the attenuation of EAE in IL-7R−/− mice was associated with a decrease in the generation of effector TH1 and TH17 cells (27), and not just TH17 cells as suggested by Liu et al. (23). Second, Hartgring et al. reported that an IL-7R–specific antibody inhibits arthritis symptoms and that the beneficial effect is associated with reduced numbers of total, naïve, and effector memory CD4+ and CD8+ T cells (28). In the Hartgring et al. study, IL-7Rα blockade did not significantly affect B cells, macrophages, and dendritic cell subsets (28).
Our results on the therapeutic efficacy of IL-7Rα–blocking antibody in EAE mice are largely consistent with the study by Liu et al. (23). Our detailed mechanistic studies, however, have yielded results that are quite different from their findings. They showed that IL-7 expands TH17 cells, and IL-7R antibody treatment preferentially targets TH17 cells. We consistently observed that IL-7, either alone or in combination with IL-12, promoted human TH1 but not TH17 cell differentiation (Fig. 2 and fig. S2). One of the major differences between the Liu study and ours is the starting population of human cells used for the generation of TH1 and TH17 cells in vitro. In Liu et al.'s study, human memory T cell subsets (CD45RA−CD45RO+) were generated from MS patients and then were expanded in vitro with IL-7 (23). Given that these memory T cells have already been primed and differentiated in vivo, this population of cells is likely to be composed of multiple T cell subsets at different stages of differentiation. Such a population thus may not be ideally suited to study the differentiation and expansion of specific TH subsets. In contrast, we cultured fluorescence-activated cell sorting (FACS)–sorted CD4+ naïve T cells from human cord blood in fresh XVIVO-20 serum-free medium under TH1-or TH17-polarizing conditions as described by Manel et al. (29).
The conflicting observations on IL-7 in TH17 expansion in the mouse system, however, were not likely due to differences in the experimental protocols, because we diligently followed their methods (23). Nevertheless, we found that IL-7 had no effect on mouse TH17 cell expansion or differentiation in experiments independently repeated at two sites (Pfizer and Stanford) (Fig. 3, fig. S3, C and D, and table S2). Because cells from immunized 2D2 mice have already been primed and potentially differentiated in vivo, using cells from these animals may not be optimal to study in vitro differentiation. To overcome this issue, we purified T cells from naïve 2D2 mice for in vitro differentiation and we still did not find any evidence that IL-7 could promote mouse TH17 expansion or differentiation (Fig. 3, B and D). One may speculate that a formal possibility for this discrepancy might be that the microflora harbored in the mice used in our studies in California, in two different laboratories, and those in Liu et al.'s study in China are different. For example, Ivanov et al. demonstrated that two mouse colonies (Taconic versus Jackson, both in the United States) have different gut microbes that differentially regulate the TH17/Treg balance in the lamina propria (30–33). Nonetheless, it is important to emphasize that we observed significant TH17 expansion in the presence of IL-23 or IL-6 using CD4+ T cells derived from naïve 2D2 or MOG-immunized 2D2 mice (Fig. 3D and fig. S3C) to a level comparable to that of published data (34). Therefore, regardless of the status of gut microflora, the mice used in our studies at Pfizer and at Stanford are permissive to the differentiation and growth of mouse TH17 cells.
Overall, we found that IL-7 promotes TH1 cell differentiation and expansion in human and mouse cell cultures, respectively (Figs. 2 and 3). Most strikingly, we have found that IL-7, independent of IL-12, promotes human TH1 cell differentiation. These results have an unexpected but potentially very important clinical correlate. We previously reported that IL-17F is a marker for nonresponsiveness to IFN-β treatment in RRMS patients (21). Moreover, we showed that IFN-β provides benefit in TH1-polarized EAE but exacerbated symptoms in TH17-polarized EAE (21). We now show here that high levels of serum IL-7 are associated with low levels of IL-17F and that elevated levels of IL-7 correlate with full responsiveness to IFN-β in RRMS. Full responsiveness is defined as no relapses in the 2 years after therapy was instituted. Our finding of a role for IL-7 in directing and promoting the generation of human TH1 cells, therefore, is entirely consistent with the elevated IL-7 levels seen in patients with the IFN-β–responsive form of MS. Together with our previous report, the current data suggest that high serum concentrations of IL-7 in RRMS patients signify a TH1 form of MS, whereas high serum concentrations of IL-17F portend a TH17 form of MS. The former patient population had no relapses within 2 years in response to IFN-β therapy, whereas the latter population tended to have less favorable outcomes.
The relationship between polymorphisms in IL-7Rα and the response to IFN-β is relevant for both understanding susceptibility to MS and predicting responsiveness to IFN-β. In a recent study of MS patients, for instance, Hoe and colleagues found that the IL-7Rα proximal promoter contains response elements to IFN-β (35). They also report that IL-7Rα mRNA is up-regulated in peripheral blood mononuclear cells (PBMCs) in response to IFN-β in vitro using PBMCs from carriers of haplotypes 1 and 2, but not 4, of the IL-7Rα locus (35). Furthermore, they found that in the PBMCs, IL-7Rα cell surface protein (CD127) is lower in haplotype 4 carriers than in noncarriers after incubation with IFN-β, with haplotype 1 conferring increased risk for MS, haplotype 2 conferring protection against MS, and haplotype 4 carrying intermediate risk (35). Thus, whereas the effects of different IL-7Rα haplotypes on gene expression and disease risk are complex, the human genetic architecture of the IL-7Rα locus does suggest a potential relationship between the IL-7Rα pathway and the cellular response to IFN-β (35).
In conclusion, our data suggest that IL-7 plays a crucial role in the generation of pathogenic TH1 cells and that high serum levels of IL-7 precede and predict a TH1 form of MS. It is possible that serum IL-7 levels may be useful for predicting clinical benefit from IFN-β treatment in RRMS patients. It is conceivable that patients with high levels of serum IL-7 might directly benefit from potential new therapies targeting either IL-7 or its receptor. Indeed, blockade of IL-7Rα with the antibody 28G9 effectively ameliorated established ongoing EAE after the onset of paralysis. These results not only help to clarify our understanding of a genetic risk factor associated with MS but also suggest new predictive, mechanism-based measurements for MS patient stratification. These measurements may include a constellation of markers including PDGF-BB, IL-7, and IL-17F. They also highlight the IL-7/IL-7Rα pathway as a potential new therapeutic target for treating autoimmune diseases including those
MATERIALS and METHODS
EAE induction and treatment
We induced EAE by complete Freund's adjuvant–MOG(35–55) peptide (Stanford Protein and Nucleic Acid Biotechnology Facility) immunization and monitored mice daily for clinical signs. The clinical grading scale is described by Axtell et al. (21). Typical EAE symptoms were monitored daily with a standard clinical score range: 1, loss of tail tone; 2, incomplete hindlimb paralysis; 3, complete hindlimb paralysis; 4, forelimb paralysis; 5, moribund/dead. We obtained MOG-specific T cell receptor (TCR)–transgenic B6 mice (2D2) through a licensing agreement with Brigham and Women's Hospital. The various treatments included recombinant mIL-7 (rmIL-7) (made in-house, 1 μg per mouse every 3 days) or IL-7 purchased from R&D Systems (AB-407-NA). Antibodies to IL-7Rα (SB/14; BD Biosciences) or 28G9 made in-house or respective isotype controls. The doses were given as indicated.
We fixed and sectioned the brainstem, cerebrum, and spinal cords for histology [Luxol fast blue, Bielschowsky stain, and hematoxylin and eosin (H&E)]. We isolated infiltrating cells from the brainstem and cerebrum or spinal cords from three to four perfused mice per group. CNS homogenates were incubated with collagenase (Sigma) and deoxyribonuclease (DNase) (Sigma) for 1 hour at 37°C and cells were purified with a Percoll gradient.
Clinical classification and detection of cytokines in serum of MS patients
We identified 26 patients with RRMS receiving IFN-β treatment for at least 12 months as responders or nonresponders to IFN-β therapy. Two neurologists, blinded to the laboratory data, classified the patients as responder or nonresponder on the number of relapses and steroid interventions in the 2 years before initiation of treatment compared to the 2 years after starting treatment. We obtained serum samples the day before starting IFN-β therapy. The study received approval from the Medical Ethics Board of the VU University Medical Center. All subjects signed written informed consent.
To determine the levels of serum analytes, we used a Human 50-plex Procarta Cytokine Assay Kit (Affymetrix) and ran the samples on a Luminex 200 cytometer in accordance with the manufacturer's instructions. Standards and each sample were analyzed in duplicate. Data analysis was performed with BeadView software package by Millipore.
Human TH cell differentiation
Human mononuclear cells were prepared from the buffy coats of samples from healthy adult donors (Stanford Blood Center) or from cord blood purchased from AllCells on Ficoll-Paque gradients. CD4+ T cells were isolated by magnetic bead depletion of CD19+, CD14+, CD56+, CD16+, CD36+, CD123+, CD8+, TCR-γ+, and TCR-δ+, and glycophorin A+ cells (Miltenyi Biotec). Naïve CD4+ T cells were further purified as CD4+CD45RA+CD45RO−CD25− by cell sorting on a FACSAria (BD). Cells (5 × 105) were seeded in fresh XVIVO-20 serum-free medium (Lonza) in 96-well U-bottomed plates. For polarization conditions, cells were cultured in the presence of anti-CD3/CD28 activation beads and IL-2 (10 U/ml) in nonpolarizing conditions (no cytokines), TH1-polarizing conditions [human IL-12 (hIL-12) (10 ng/ml) and anti–IL-4 (1 μg/ml)], or TH17-polarizing conditions [IL-1β (10 ng/ml), IL-23 (10 ng/ml), anti–IL-4 (1 μg/ml), and anti–IFN-γ (1 μg/ml)] in the presence or absence of TGFβ (10 ng/ml) as described (29). The medium was replaced on day 3, and cells were split in the presence of IL-2. For long-term experiments, cells were split as needed. In some cases, naïve CD4+CD45RA+CD45RO−CD25− T cells were seeded for 5 days at a density of 5 × 105 cells per well in Yssel's medium containing 1% human AB serum (Gemini Bio-Products) along with beads coated with antibodies to CD2, CD3, and CD28 (one bead per 10 cells; Miltenyi) in TH17-polarizing conditions [hIL-1 (10 ng/ml) and hIL-23 (50 ng/ml)]. Cells were split and cultured for 6 additional days with the various cytokines and IL-2 (100 U/ml; R&D Systems). For day 5 cultures, cytokines were directly assayed from cell supernatants. For day 11 cultures, 1 × 106 cells/ml were stimulated with T cell activation beads with IL-2, and after 48 hours, cytokines were assayed as indicated.
Mouse TH cell differentiation
Purified CD4+ naïve T cells (CD44loCD62LhiCD25−) were stimulated for 3 days with MOG(35–55) peptides, irradiated allophycocyanin (APC), or anti-CD3/CD28 activation beads in the presence of TH1-polarizing conditions [rmIL-12 (10 ng/ml) (419-ML) and IL-4–specific antibody (10 μg/ml, BVD-1D11; BD Biosciences)] or TH17-polarizing conditions [recombinant human TGFβ (3 ng/ml) and rmIL-6 (20 ng/ml) (406-ML) (all R&D Systems)] plus antibodies to IFN-γ (XMG1.2) and IL-4 (10 μg/ml, BD Biosciences) in the presence or absence of rmIL-7 (10 ng/ml) as indicated.
Flow cytometry
The following surface antibodies were purchased from BD Biosciences: CD4 (GK1.5), CD8, B220 (RA3-6B2), CD44 (IM7), CD62L (MEL-14), CD25 (PC61), DX5 (CD49), and CD127 (SB/199). For intracellular cytokine FACS, we stimulated cells with phorbol 12-myristate 13-acetate (Sigma-Aldrich) and ionomycin (Sigma-Aldrich) and monensin (BD Biosciences) for 4 hours, stained them for CD4 and CD8 (BD Biosciences), fixed and permeabilized them with Cytofix/Cytoperm (BD Biosciences), and then stained them for IFN-γ, IL-17, or FoxP3 (FJK-165, eBiosciences) according to the manufacturer's protocol. MOG(38–49)/IAb tetramer and control tetramer (CLIP/IAb) were constructed and supplied by the National Institutes of Health Tetramer Core Facility. Background staining was assessed with nonreactive, isotype-matched control monoclonal antibodies (mAbs) (Caltag Laboratories). For tetramer staining, lymphocytes were stained for 3 hours at 37°C as described (36). We also performed enzyme-linked immunosorbent assay (ELISA) for IL-17 (R&D Systems) and IFN-γ (BD Biosciences) on culture supernatants. All the experiments included live/dead fixable stain kits from Invitrogen to exclude dead cells.
Generation of mAb 28G9 to IL-7Rα
Briefly, an 8-week-old female Sprague-Dawley rat was immunized with 50 μg of rmIL-7Rα/CD127/Fc chimera (747-MR, R&D Systems). The antigen was prepared for immunization by mixing 50 μg of antigen in 100 μl of PBS with 100 μl of Sigma Adjuvant System (S6322). After repeated injection of antigens, the spleen cells were prepared as a single-cell suspension at day 13 and fused with mouse myeloma cells following a standard fusion protocol. The fused cells were resuspended in medium containing 18% fetal bovine serum (FBS), 2 mM l-glutamine, penicillin/streptomycin, HAT (hypoxanthine, aminopterin, and thymidine) (Sigma H0262), and 10% hybridoma fusion and cloning supplement (HFCS) (11 363 735 001, Sigma), and then plated out in 96-well plates at 200 μl per well. At day 7 after fusion, 150 μl of the medium was aspirated from each well, and the wells were refed with 200 μl of fresh medium. At days 11 to 13, supernatant from each well was tested for antibody to IL-7Rα and human Fc with ELISA screening.
In vitro phospho-STAT5 functional assay
BALB/c or B6 spleens were homogenized in PBS and 5 mM EDTA, filtered through 70-μm pore size mesh, pelleted, and resuspended at 5 × 106 cells/ml in room temperature to 37°C RPMI 1640 containing 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine. Cells were maintained at 37°C in 5% CO2 in conical tubes (to prevent monocyte/macrophage adherence) for 1 to 2 hours before stimulation. IL-7 and antibodies to IL-7Rα were typically added for 15 min at the indicated concentrations. Formaldehyde was added directly to the culture medium to a final concentration of 1.6%, and cells were fixed for 15 min at room temperature. Methanol was then added directly and samples were stored at 4°C for 30 min to 1 hour. Cell suspensions in methanol were washed twice with staining medium and then resuspended in staining medium at 107 cells/ml for intracellular phosphoprotein staining as described (37, 38). Typically, surface mixture, CD11b-fluorescein isothiocyanate (FITC), TCR-PE (phycoerythrin), B220-Cy5.5PerCP, and phospho-STAT5–Alexa 647 antibodies were added simultaneously for 20 to 30 min and then washed with 15 to 20 volumes of staining medium. Cells were resuspended in staining medium and kept at 4°C before analysis on an LSR instrument (BD Biosciences) equipped with a 633 helium-neon laser to allow for Alexa 647 excitation and detection. Data were analyzed in FlowJo.
Supplementary Material
Acknowledgments
We thank M. Gilbert for flow cytometry, M. Chin and A. Rajpal for discussing IL-7 expression and purification approaches, D. Malashock and Y. Abdiche for biosensor assay, S. LaPorte for general discussion on cytokine biology, G. Vergara and T. Radcliff for vivarium support, and A. Gholston for help with figures.
Funding: This study was funded in part by NIH grants R01NS 55997 (to L.S.) and 1K99NS075099-01 (to R.A.) and a National Multiple Sclerosis Society fellowship FG 1817-A-1 (to R.A.).
Footnotes
Author contributions: L.F.-L. and R.A. designed and performed the research, analyzed the data, and wrote the paper; G.H.T. performed the research and analyzed the data; K.L. performed the research; J.D., J.Y., and M.R. contributed vital new reagents; B.H. and W.E. performed the histopathology study and evaluated the slides; M.G.W. and J.S. performed statistical analysis of patient samples; B.A.d.J., J.K., and C.H.P. collected all patient samples; L.S. and J.C.L. designed the research, analyzed the data, and wrote the paper.
Competing interests: L.F.-L., G.H.T., K.L., J.D., J.Y., M.R., B.H., W.E., and J.C.L. are full-time employees of Pfizer Inc. Pfizer Inc. filed a patent application, with L.F.-L., J.C.L., and W. Zhai as co-inventors: U.S. Application Serial No. 13/033,491, entitled “Antagonist Anti-IL-7 Receptor Antibodies and Methods.” Neither R.A. nor L.S. has consulting relationships with Pfizer Inc. or its Rinat unit. The work at Stanford was not sponsored by Rinat or Pfizer Inc. L.S. has a consulting relationship with GlaxoSmithKline. Stanford University has filed a patent application, with R.A. and L.S. as co-inventors: U.S. Application Serial No. 13/026,181 for “Markers for Determination of Patient Responsiveness.” The other authors declare that they have no competing interests.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain. 2003;126:770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 4.Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallström E, Khademi M, Oturai A, Ryder LP, Saarela J, Harbo HF, Celius EG, Salter H, Olsson T, Hillert J. Variation in interleukin 7 receptor α chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 5.International Multiple Sclerosis Genetics Consortium. Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 6.Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, Lincoln R, McCauley JL, Sawcer SJ, Compston DA, Dubois B, Hauser SL, Garcia-Blanco MA, Pericak-Vance MA, Haines JL. Multiple Sclerosis Genetics Group, Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 7.Leonard WJ, Shores EW, Love PE. Role of the common cytokine receptor γ chain in cytokine signaling and lymphoid development. Immunol. Rev. 1995;148:97–114. doi: 10.1111/j.1600-065x.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 8.Habib T, Senadheera S, Weinberg K, Kaushansky K. The common γ chain (γc) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–8731. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- 9.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: The common γ-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Palmer MJ, Mahajan VS, Trajman LC, Irvine DJ, Lauffenburger DA, Chen J. Interleukin-7 receptor signaling network: An integrated systems perspective. Cell. Mol. Immunol. 2008;5:79–89. doi: 10.1038/cmi.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard WJ. TSLP: Finally in the limelight. Nat. Immunol. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- 14.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene–deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 16.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat. Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 17.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry TJ, Mackall CL. The many faces of IL-7: From lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 19.Bielekova B, Muraro PA, Golestaneh L, Pascal J, McFarland HF, Martin R. Preferential expansion of autoreactive T lymphocytes from the memory T-cell pool by IL-7. J. Neuroimmunol. 1999;100:115–123. doi: 10.1016/s0165-5728(99)00200-3. [DOI] [PubMed] [Google Scholar]
- 20.Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, Lincoln R, McCauley JL, Sawcer SJ, Compston DA, Dubois B, Hauser SL, Garcia-Blanco MA, Pericak-Vance MA, Haines JL. Multiple Sclerosis Genetics Group, Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 21.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: Visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Leung S, Wang C, Tan Z, Wang J, Guo TB, Fang L, Zhao Y, Wan B, Qin X, Lu L, Li R, Pan H, Song M, Liu A, Hong J, Lu H, Zhang JZ. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat. Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 24.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Walline CC, Kanakasabai S, Bright JJ. IL-7Rα confers susceptibility to experimental autoimmune encephalomyelitis. Genes Immun. 2011;12:1–14. doi: 10.1038/gene.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, van Roon JA. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis Rheum. 2010;62:2716–2725. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
- 29.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol. 2010;3:209–212. doi: 10.1038/mi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov II, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 2011;14:106–114. doi: 10.1016/j.mib.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Hoe E, McKay F, Schibeci S, Heard R, Stewart G, Booth D. Interleukin 7 receptor α chain haplotypes vary in their influence on multiple sclerosis susceptibility and response to interferon β. J. Interferon Cytokine Res. 2010;30:291–298. doi: 10.1089/jir.2009.0060. [DOI] [PubMed] [Google Scholar]
- 36.Falta MT, Fontenot AP, Rosloniec EF, Crawford F, Roark CL, Bill J, Marrack P, Kappler J, Kotzin BL. Class II major histocompatibility complex–peptide tetramer staining in relation to functional avidity and T cell receptor diversity in the mouse CD4+ T cell response to a rheumatoid arthritis–associated antigen. Arthritis Rheum. 2005;52:1885–1896. doi: 10.1002/art.21098. [DOI] [PubMed] [Google Scholar]
- 37.Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J. Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- 38.Krutzik PO, Clutter MR, Nolan GP. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J. Immunol. 2005;175:2357–2365. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.