Summary
Throughout Southeast Asia there is a strikingly high incidence of cholangiocarcinoma (CCA - hepatic cancer of the bile duct epithelium), particularly in people from rural settings in Laos and Northeast Thailand who are infected with the liver fluke, Opisthorchis viverrini, one of only three carcinogenic eukaryotic pathogens. More ubiquitous carcinogenic microbes, such as Helicobacter pylori, induce cancer in less than 1% of infected people, while as many as one-sixth of people with opisthorchiasis will develop CCA. The mechanisms by which O. viverrini causes cancer are multi-factorial, involving mechanical irritation from the activities and movements of the flukes, immunopathology, dietary nitrosamines and the secretion of parasite proteins that promote a tumourigenic environment. Genomic and proteomic studies of the liver fluke secretome have accelerated the discovery of parasite proteins with known/potential roles in pathogenesis and tumourigenesis, establishing a framework towards understanding, and ultimately preventing, the morbidity and mortality attributed to this highly carcinogenic parasite.
Carcinogenic helminths
More than 340 species of helminths infect humans, of which at least 25 cause serious and occasionally fatal outcomes 1. Currently, only three of these helminths, Schistosoma haematobium (blood fluke), Clonorchis sinensis (Chinese liver fluke) and Opisthorchis viverrini (Thai liver fluke), have been definitively linked to cancer (Table 1) 2. It has been suggested that over a lifetime almost one in six of O. viverrini infected individuals will contract cholangiocarcinoma (CCA), or cancer of the bile ducts 3, 4. CCA is a highly aggressive cancer with a very poor prognosis 5, 6. Despite this, flukes contribute only minimally (0.12%) to world cancer rates. One major reason is simply the limited distribution of these parasites. As with many parasitic helminths, liver flukes tend to be restricted geographically due to their reliance on specific intermediate hosts (freshwater snails and fish) for propagation and transmission (Figures 1 and 2). This limits endemic areas to tens of millions, rather than the billions of individuals inhabiting regions rife with viral infections 7. Moreover, with O. viverrini cultural habits play a critical role in transmission and subsequent restriction of endemic regions (see below). Another contributing factor to the small proportion of fluke-derived cancers is the difficulty in identifying (unambiguously) correlations between infections with multicellular organisms and cancer, particularly in developing countries, and as a result, current estimates are conservative 4. It has been asserted that the incidence of O. viverrini-derived cancer may be six times greater than otherwise estimated 4. Other studies suggest that O. viverrini infection may initiate as many as 89% of the 28,000 annual liver cancer cases in Thailand, making the infection the fourth largest cause of death in Thailand behind AIDS, stroke and traffic accidents 8, 9.
Table 1.
| Pathogen Type | Pathogen | Malignancies | Non-Malignant Disease | Percent of annual cancers |
|---|---|---|---|---|
| Human papilloma virus: 13 carcinogenic strains (HPV) | cervix, vulva, vagina, penis, anus, oral cavity, oropharynx and tonsil | Warts, condyloma | 5.2 | |
|
|
||||
| Viruses | Hepatitis B or C viruses (HBV and HCV) | Liver: Hepatocellular carcinoma (HCC), non-HLP (Hepatitis C only) | Hepatitis, cirrhosis | 4.9 |
|
|
||||
| Epstein Barr virus (EBV) | Nasopharyngeal, extranodal NK/T-cell LP (nasal type), HLP, Burkitt’s LP, immune-suppression-related non-HLP | Infectious mononucleosis | 1 | |
|
|
||||
| Human immunodeficiency virus, type 1 (HIV-1) | Kaposi’s sarcoma, non-HLP, HLP, cervix, anus, conjunctiva | AIDS | 0.9 | |
|
|
||||
| Human T-cell lymphotrophic virus type 1 (HTLV-1) | Adult T-cell leukaemia and LP | 0.03 | ||
|
| ||||
| Bacteria | H. pylori | Non-cardia gastric carcinoma, MALT gastric LP | Gastritis, peptic ulcers | 5.5 |
|
| ||||
| Helminths | S. haematobium (blood fluke) | Urinary bladder cancer | Schistosomosis | 0.1 |
|
|
||||
| O. viverrini and C. sinensis (liver flukes) | Liver: Cholangiocarcinoma (CCA) | Opisthorchiasis and Clonorchiasis | 0.02 | |
| Total | 17.80% | |||
HLP: Hodgkin’s lymphoma, LP: Lymphoma, MALT: Low-grade B-cell mucosa-associated lymphoid tissue
Figure 1. Images depicting cultural practices that result in liver fluke infection.
A. Local creeks in North-East Thailand that harbour fish rife with O. viverrini metacercariae. B. Thai Farmer net fishing. C. Infected fish catch. D. Fermented fish that gives consumers the carcinogenic combination of O. viverrini and nitrosamines. E. The fermented fish dish, pla-ra.
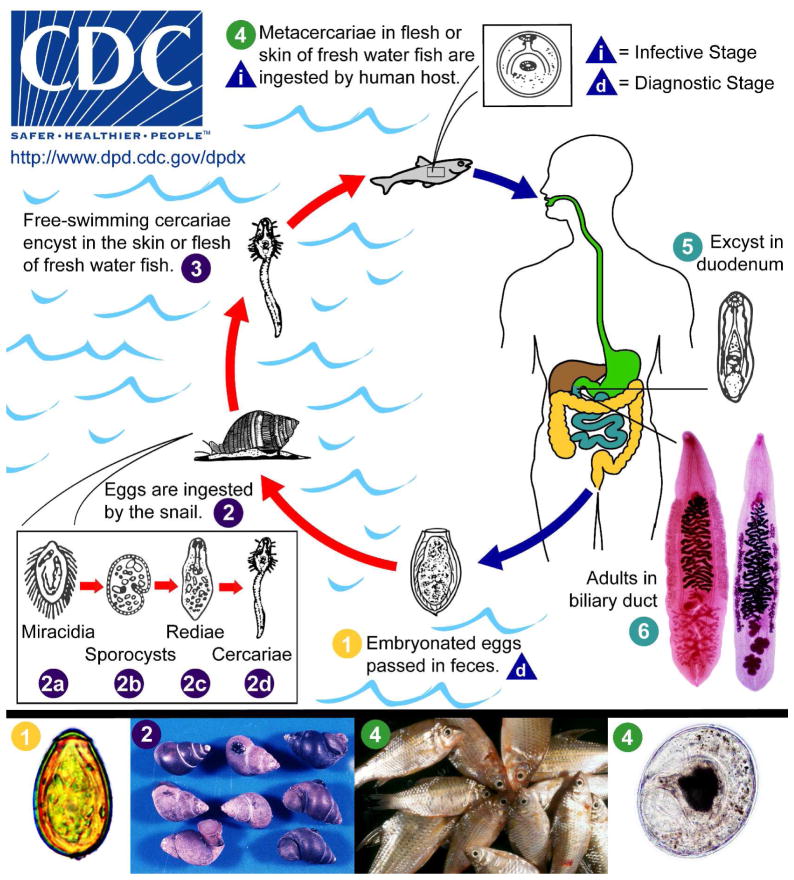
Figure 2. Life Cycles of O. viverrini and C. sinensis.
Embryonated eggs are discharged in the biliary ducts and in the stools (stage 1). Eggs are ingested by a suitable snail intermediate host (2); there are more than 100 species of snails that can serve as intermediate hosts. Each egg releases a miracidium (2a), which morphs through several developmental stages (sporocyst [2b], redia [2c], to become a cercaria [2d]). Cercariae are released from the snail and after a short period as free-swimming larvae in fresh water, they penetrate the flesh of freshwater fishes, such as Cyclocheilichthys armatus or Puntius leiacanthus, where they encyst as metacercariae (3). Humans are infected through ingestion of undercooked, salted, pickled, or smoked freshwater fishes (4). After ingestion, the metacercariae excyst in the duodenum (5) and ascend the biliary tract through the Ampulla of Vater. Maturation to adulthood (right, O. viverrini; left, C. sinensis) takes approximately one month (6). The adult flukes reside in the small and medium-sized bile ducts. In addition to humans, carnivorous animals can serve as reservoir hosts 90.
While O. viverrini has been recognised as a group I carcinogen since 1994, its close relative C. sinensis has only recently been classified within group I 2. The CCA rates in Korea, a major region of C. sinensis endemicity, ranges from 7.6–12 ASR (age standardised ratio); while high compared to the rest of the world (<5 ASR) these values are low compared to areas in North-East Thailand where O. viverrini is endemic, with a CCA rate of 58.1 ASR 10, 11. The reasons for the substantially lower ASR in Korea are unclear. Additional data might also elevate the status of the lesser researched Siberian liver fluke, Opisthorchis felineus, to group I carcinogen status, where O. viverrini and C. sinensis are currently ranked 2, 12.
While investigations have been limited, 12 reports over the last 20 years describe potential links between other parasitic worms and carcinogenesis – some of these studies describe soluble parasite extracts that cause DNA damage and mitogenesis, such as the liver fluke of livestock Fasciola hepatica, Taenia spp. tapeworms and various nematodes [reviewed in 13]. Behind O. viverrini however, the most widely accepted carcinogenic helminth is the blood fluke S. haematobium. Of the human schistosomes, only S. haematobium has been unequivocally linked to cancer and is a group I carcinogen 12. Adult worms reside in the vasculature of the urinary bladder where the female fluke lays eggs which penetrate the bladder wall to be passed in the urine. Some eggs are trapped in the tissue by granulomatous immune reactions, resulting in chronic fibrosis over time. In Egypt, where S. haematobium infection is common, bladder cancer is the most prevalent of all cancers in males compared with the fifth to seventh most common cancer in countries where schistosomiasis is not endemic [reviewed in 14]. Squamous cell carcinoma (SCC) is the most frequently encountered subtype of bladder cancer associated with S. haematobium, while transitional cell carcinoma is the dominant form in non-endemic areas. A role for nitrosamines (urinary) in S. haematobium has been proposed to predispose infected individuals to SCC 15. As with opisthorchiasis associated CCA (see later), there is a proposed role for S. haematobium soluble proteins in the development of SCC. S. haematobium adult worm extract caused CHO cells to undergo increased proliferation and decreased apoptosis, accompanied by down-regulation of tumour suppressor p27 and up-regulation of the anti-apoptotic B-cell lymphoma -2 protein (Bcl-2). Moreover, cells exposed to parasite proteins displayed increased migration and invasion capabilities 16, and formed sarcomas when injected into male nude mice 17. For a comprehensive recent review on the role of S. haematobium in carcinogenesis, see 14.
Liver flukes, with a specific focus on Opisthorchis viverrini
To date 13 species of liver flukes have been recovered from humans, but only four are commonly encountered – F. hepatica, C. sinensis, O. viverrini and O. felineus. Worldwide infections with liver flukes are estimated at over 45 million, predominantly across Asia and Eastern Europe [reviewed in 18]. The most pathogenic of these flukes is O. viverrini, which infects 10 million individuals throughout Thailand, Laos, Cambodia and Vietnam 7. C. sinensis infects over 35 million individuals across a far wider area than O. viverrini, encompassing Korea, mainland China, Taiwan, Vietnam, probably Thailand and formerly Japan 18, 19. F. hepatica is estimated to infect 2.4 million people around the world, and O. felineus, primarily a parasite of cats, infects only 1.2 million people in Eastern Europe and the Russian Federation 20–23.
O. viverrini has three hosts, two fresh water intermediate hosts (a pulmonate snail and a cyprinoid fish) and a primary mammalian host, usually humans, but can also be fish-eating mammals such as domesticated cats and dogs (Figure 2). Still, these alternate primary hosts are largely insignificant as carriers in endemic areas, as low prevalence is observed, and their egg output is considered a minor contributor to the maintenance of the life cycle relative to that of humans 24. Unlike the many soil transmitted helminths, which often directly infect the mammalian host through the skin, O. viverrini is food-borne and is infective when a viable metacercaria is ingested. Hence, regions where they are endemic are not only limited by availability of intermediate hosts, but also to areas where people routinely consume uncooked and under-cooked fish. These constraints reflect the striking regional distribution between the four major geographical regions within Thailand, with only the North-East and Northern regions suffering from appreciable levels of infection (19% and 15.7% prevalence, respectively). It should be noted that North-East Thailand has the highest prevalence of infection with O. viverrini but it also has extensive mass anthelmintic drug administration programs; before these were widely implemented the region had an infection rate of 34.6% 8, 25.
After ingestion by the mammalian definitive host, the metacercariae excyst in the duodenum with the help of the host’s digestive enzymes. They then migrate up the common bile duct to the intrahepatic bile duct branches where they mature into dorso-ventrally flattened, leaf shaped adult worms of approximately 7 × 1.5 mm in size. It takes about one month from ingestion of metacercariae to the detection of eggs in the human faeces. The exact life span of adult worms is unknown, but is thought to be at least 10 years and possibly 25–30 years 26. Some enzyme-immunoassays have been developed for diagnosis of past and present infections but suffer from lack of specificity and so currently observation of eggs in the faeces is the best way to determine current infection status 7, 8, 21, 27, 28.
Liver cancer and Opisthorchis viverrini
Primary liver malignancies
The majority of primary malignant liver cancers are of two main histological types distinguished by their cellular origin. First, hepatocellular carcinoma (HCC) is derived from hepatocytes and is the most common form throughout the world 2. There are several important risk factors for HCC, with 75–80% of cases attributable to hepatitis B and C infection 29, 30. Second, CCA is derived from cholangiocytes, which form the epithelial lining of the bile ducts. CCA is by far the least common of the two cancers, except for regions within East Asia where opisthorchiasis and/or clonorchiasis are endemic. These regions show a 15-fold increase in the liver cancer incidence compared to the incidence of this cancer in the developed world (71.9 vs <5 ASR) and 50–100 times the CCA incidence (58.1 vs <1 ASR) 11. As previously mentioned, C. sinensis causes significant CCA in Korea, but at rates more than 5 times lower (58.1 vs 7.6–12 ASR) than those seen in North-East Thailand 2.
Both CCA and HCC have a low survival rate with a yearly fatality ratio of approximately 1.0, which indicates for the most part that patients do not survive one year past diagnosis 8. Even with treatment, the five year relative survival rates during the 1990s in the United States and Europe were only 8.3% and 6.5% respectively 31, 32. In developing countries, prognosis with liver cancer usually is poor.
Mechanisms of Opisthorchis viverrini-initiated CCA
Tumours are rare in infections with carcinogenic viruses and bacteria
The viral modification of cell growth resulting in cancer has been extensively studied and was vital in establishing many current concepts of cancer biology. Research on viruses has shown that the malignant phenotype, in most cases, is the end result of a series of genetic and epigenetic changes rather than a single event. The likelihood of these aberrant alterations synchronising in a single cell, even upon exposure to a carcinogen, is low due to the complex, multi-faceted nature of the phenomenon. One common example illustrating this point is H. pylori. Half the human population is infected with this gram positive bacterium but less than 1% will proceed to develop the associated gastric cancer and/or Gastric Mucosa–Associated Lymphoid Tissue (MALT) Lymphoma 33. By contrast, and notwithstanding controversy on the exact proportion of cancers attributable to O. viverrini, it is suspected over a lifetime of infection that as many as one in six infected people will develop CCA 3, 4.
Proving an infectious agent is carcinogenic is a challenge, but exposing all or even some of the mechanisms involved is an extremely complicated process, especially where parasitic worms are concerned. Infected populations usually suffer from multiple parasitic infections and other medical conditions and risk factors that confound the analyses. Additionally, there have recently been cases of C. sinensis infections in central Thailand villages, detected by PCR-based methods 19. As the diagnosis of infection is by detection of eggs in faeces and both C. sinensis and O. viverrini eggs are morphologically very similar, it is unknown how widespread Clonorchis may be throughout Thailand. This distinction between parasites may be crucial for ongoing studies involved in widespread screening for Opisthorchis infection and liver abnormalities or cancer 4.
Proposed mechanisms contributing to liver fluke-induced carcinogenesis
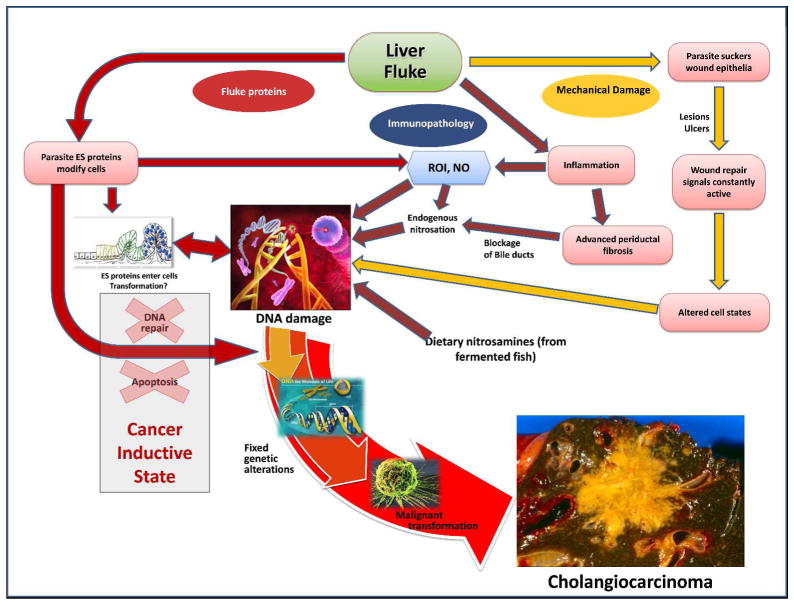
Three main mechanisms are proposed to contribute to CCA through chronic infection with O. viverrini: (a) mechanical damage to the biliary epithelium caused by feeding parasites; (b) immunopathology due to the inflammatory effect of the infection; (c) toxic effects of parasite secreted molecules (Figure 3). The interplay of these three mechanisms aligns with current knowledge of malignancies, suggesting formation and progression relies on many interrelated factors creating a microenvironment that is conducive for malignant transformation 34.
Figure 3. Hypothesized pathways of pathogenesis of opisthorchiasis-associated cholangiocarcinoma.
The liver fluke Opisthorchis viverrini induces damage to the bile duct tissue via at least three distinct pathways: (1) mechanical damage to biliary epithelia caused by feeding parasites (yellow arrows); (2) inflammation-induced immunopathology, particularly due to reactive oxygen intermediates (ROI) and nitric oxide (NO) (blue arrows); (3) direct effects of fluke secreted proteins on biliary epithelia including cell proliferation induced by parasite-derived growth factors (red arrows). These pathways converge to result in oxidative DNA damage and excessive, chronic cell proliferation. Damaged DNA/genes after successive replications become fixed, leading to malignant transformation of the transformed cholangiocytes. Adapted from 94.
Mechanical damage induces a wound response
The body’s response to wounding
Wound repair has long been implicated in tumourigenesis with striking similarities between tumour stroma and the wounded tissues 35. This carcinogenic potential of wound repair was first demonstrated in the 1980s. An adult chicken injected with the carcinogenic respiratory syncytial virus developed a tumour solely at the infection site even though the virus spread completely throughout the body 36. After this initial malignancy, any subsequent wounding of an infected bird was sufficient to elicit new carcinogenic growth at the injury site. These wound healing responses disrupt the cellular microenvironment by inducing production of enzymes, including matrix metallo- and other proteases, that degrade the extracellular matrix, interfere with epithelial cell-cell adhesion and induce cell division to create a cellular covering of the wound 34, 37. In a normal situation, after the wound has been repaired, a series of secreted factors act to halt the process.
How does this apply to liver flukes?
Undoubtedly liver flukes cause mechanical injury to the bile ducts, easily visualised as tissue damage from the oral and ventral suckers hooking into the bile duct epithelium to secure the parasite in place (Figure 4) 38. If the parasite is removed the damage is repaired and the regenerative growth of the wound response halts. But in a fluke infection, persistent damage of the epithelium resulting from feeding and migratory activities hinders complete wound repair and recovery from the injury. The constant cell division associated with this mechanically driven tissue injury, in the presence of exogenous co-factors, such as dietary nitrosamines (see later), is thought to result in DNA damage and subsequent oncogenesis, as indicated in Figure 3. These carcinogenic properties of fluke-induced wounding have been illustrated in hamsters, where the bile ducts were surgically ligated, simulating damage seen in fluke infections, followed by co-administration of sub-carcinogenic doses (20 mg/kg) of nitrosamines. The hamsters showed significant biliary lesion development whereas the controls, which received biliary ligation or nitrosamines alone, did not 39. Therefore, invoking the wound repair response likely triggers cell proliferation, which in the presence of co-factors, significantly contributes to cancer development 40.
Figure 4. Opisthorchis in a liver section.
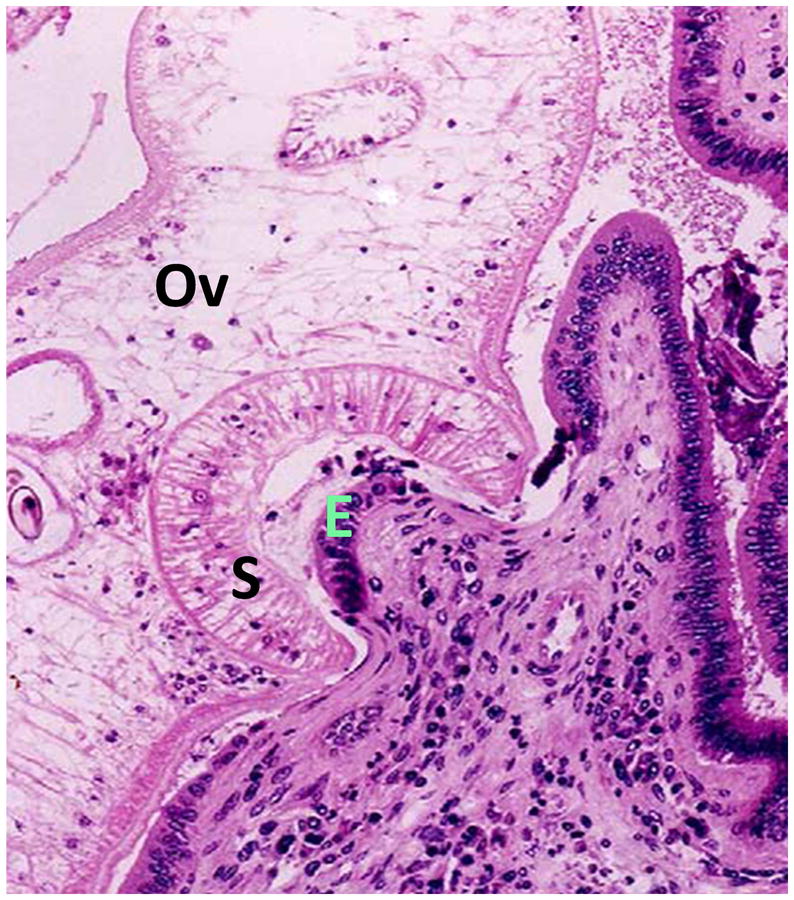
The ventral sucker (S) of the liver fluke (Ov) is attached to the bile duct epithelium (E) 41. Note the infiltration of eosinophils into the site of inflammation.
Immunopathological changes
An intense infiltration of inflammatory cells in infected livers has been associated with Opisthorchis antigens in the bile duct epithelium, observed by immunohistochemical staining of liver sections from experimentally-infected hamsters 41. This intense inflammatory response can result in malignancy in a number of ways - directly through production of oxidative radicals (discussed further below) or indirectly by influencing DNA replication which creates a phenotype that is predisposed to mutations 42. In other pre-cancerous conditions, such as ulcerative colitis, chronic inflammation has been shown to produce a ‘mutator’ phenotype, inducing conditions conducive to microsatellite instability and erroneous DNA repair 43.
Early inflammatory events predominantly comprise the recruitment of neutrophils, eosinophils and macrophages, whereas later stage infections show the major infiltrating cells are mononuclear subtypes including lymphocytes, macrophages, plasma cells and mast cells 44. In addition to the fluke invoking a localised response, the excretory/secretory (ES) molecules released by the parasite induce inflammation in the first order bile ducts which are too small for flukes to reside in 41. Despite these findings, it is still unknown whether these released antigens are entrapped by the biliary epithelium via a receptor mediated or a general endocytotic process. Notably, a host genetic component may be involved, as not all infected hamsters exhibit parasite antigens on biliary epithelial surfaces 41, 44.
In humans infected with O. viverrini, inflammatory changes to the liver can be detected early during O. viverrini infection before chronic disease sequelae are apparent. In a recent case-control study, we determined the presence of advanced periductal fibrosis (APF) in asymptomatic, O. viverrini-infected individuals and then measured cytokine responses to O. viverrini ES proteins. In persons with APF, levels of the pro-inflammatory cytokine interleukin-6 (IL-6) to O. viverrini ES products were 8-fold higher than levels in O. viverrini infected individuals without APF 45. Moreover, elevated IL-6 was associated with increased risk of APF by 63% in a model adjusted for sex and age. O. viverrini-infected individuals with APF showed other hepatobiliary abnormalities, including reduced gall bladder contractility. Taken together, these data strongly implicate a role for parasite-specific IL-6 in the pathogenesis of APF in opisthorchiasis.
Chronic immune responses can lead to cancer
Activated phacogytes produce bursts of nitric oxide (NO) and oxygen radicals which normally destroy many pathogens 46. This immune defence mechanism, however, has potentially inimical side-effects - NO is mutagenic and results in the release of endogenous carcinogenic nitrosamines, one of the key initiators of CCA (Figure 3) 47. Unfortunately, for the host, when the invading pathogen is unaffected by these inflammatory responses, such as with O. viverrini, a prolonged reaction and associated NO accumulation develops 48–50. Fluke infections in humans results in elevated plasma and urinary nitrite levels, even in the absence of elevated levels of dietary nitrosamines and their precursors 50, 51. This rise in nitrites indicates high endogenous nitrosamine production, as nitrites are the excretory metabolites of nitrosamines. This consequence of fluke infection is reversible upon removal of the parasite, which is readily accomplished by treatment with the anthelmintic drug, praziquantel 52. Follow-up investigations proved that DNA damage from nitrite production is present at the sites of fluke-induced carcinogenesis 53. Further evidence implicating nitrosamines in the pathogenesis of opisthorchiasis comes from studies linking urinary nitrite levels, infection intensity and an increased risk of CCA 3. It is unclear if the increased nitrite levels are the result of elevated exogenous nitrites from fermented foods, such as fish sauce, or differences in host endogenous nitrite production.
Another source of prolonged inflammation is not directly from the flukes but the eggs they produce. Eggs released from the fluke become entrapped as a consequence of bile duct blockage or embedded within the periductal tissue through ulceration at the site of parasite attachment 39. Significantly, this egg related inflammation of surrounding tissue can operate long after the adult flukes have been expelled by chemotherapy with praziquantel. This carcinogenic potential of O. viverrini eggs has not been studied in depth but we suggest that it may significantly contribute to carcinogenesis as seen with the other major carcinogenic fluke, S. haematobium 54.
Exogenous nitrosamines: critical for carcinogenesis?
Nitrosamines are common in the fermented foods that are a dietary staple in North East Thailand 28. When ingested in large quantities, such as in fermented fish, nitrosamines are carcinogenic 55. Short term infection (under 7 months) of hamsters with O. viverrini alone does not commonly lead to cancerous lesions, however, substantial numbers of lesions are induced with the addition of normally sub-carcinogenic doses of nitrosamines to the diet of infected hamsters 39, 56. It should be noted that longer infections (20 months) have shown that O. viverrini alone is sufficient for CCA induction 57. While the exact role of nitrosamines in human fluke infections is unclear, regular consumption of fermented fish containing metacercariae poses an added risk for CCA.
Mitogenic properties of fluke ES products
To survive long periods in hostile environs, parasitic helminths excrete and secrete a range of soluble proteins and other mediators that perform many roles at the host-parasite interface, including digestion of nutrients, tissue invasion and regulating the host immune system. This interaction has long been thought to modify host cellular homeostasis and contribute to malignant transformations 58, 59. Secretions of other infectious agents have supported this notion: H. pylori causes cell proliferation through protein transfer, and schistosome secretions and tapeworm soluble products induce hyperplasia 60–63. A routine observation in fluke infections is altered cell states, one of the gateways to genomic instability that can be induced by a variety of growth factors 64. Lesions of epithelial dysplasia and metaplasia including goblet cell metaplasia and adenomatous hyperplasia are commonly seen 65. Fluke associated CCA cases frequently demonstrate an intestinal goblet cell phenotype which differs from non-infection related CCA 66. O. viverrini ES products are mitogenic and taken up by host biliary cells (discussed below), and in vitro application of ES products to mammalian cell lines induces morphological changes 67. Subsequent in vivo investigations confirmed that CCA development in the hamster model of opisthorchiasis is more rapid when hamsters are administered with ES products prior to O. viverrini infection (B. Sripa, unpublished), suggesting that immunological memory might further accelerate tumourigenesis.
O. viverrini ES products induce cell proliferation
Using a non-contact in vitro co-culture technique, O. viverrini ES products were shown to induce proliferation of non-cancerous fibroblasts, a keystone of cancerous transformation 67. This insightful experiment involved co-culturing of O. viverrini adult worms with NIH-3T3 mouse fibroblasts physically separated by an 8 μm membrane, thereby removing mechanical (and immunological) damage from the interpretation of the outcome of the experiment. Three-fold growth of cells compared to control cells cultured in the absence of parasite proteins was observed, confirming that ES products of O. viverrini contain growth factors or other molecules that result indirectly in cell growth (and potentially contribute to the development of CCA). Excessive cell growth resulted from stimulation of cells at the G1/S transition in the cell cycle and was induced by over expression of cyclin D1 and phosphorylation of retinoblastoma protein 67.
The effect on signalling of mouse fibroblasts after exposure to O. viverrini ES products was explored using complimentary DNA microarrays. More than 200 genes underwent significantly elevated expression upon addition of ES products 68, two of which had known roles in cell proliferation - eps 8, or epidermal growth factor (EGF) receptor pathway substrate, and tgf-β 1i4, or transforming growth factor-β-1 (TGF-β)-induced transcript 4. The TGF-β super family generally act as tumour suppressors by blocking cell cycle progression at the G1 stage, but TGF-β is more often linked to later stages of cancer progression 69. EGF is also a large gene family with links to proliferation, and errant activation of EGF signalling pathways can result in cancer 70.
The transcriptome and secreted proteins of O. viverrini
Recently, the transcriptomes of O. viverrini and C. sinensis were sequenced using high throughput sequencing. Approximately 50,000 sequences were assembled for each species, defining molecules that are essential for the development, reproduction and survival of these flukes 71, 72, including a number of secreted growth factors which might act on host cells 73. This extensive dataset provided the framework for characterisation of the secretome using proteomic approaches 74. To identify parasite proteins critical for liver fluke survival and the aetiology of CCA, various proteomic approaches were employed to characterise 300 O. viverrini secreted and surface membrane proteins. The ES products included a complex mixture of proteins that have been associated with cancers, including proteases, protease inhibitors, orthologues of mammalian growth factors and anti-apoptotic proteins. Of particular interest was the identification of Ov-GRN-1, a protein with sequence similarity to the mammalian growth factor, granulin.
Granulin: a parasite growth factor that causes proliferation of host cells
Ov-GRN-1 is the only helminth-derived growth factor reported to date that causes proliferation of mammalian cells 75. Ov-GRN-1 is secreted by adult flukes where it binds to and is internalised by biliary epithelial cells of O. viverrini-infected hamsters (Figure 5). Recombinant Ov-GRN-1 caused mouse fibroblasts and human CCA cell lines to proliferate via the mitogen-activated protein kinase (MAPK) pathway, and anti-Ov-GRN-1 antibodies blocked the mitogenic activity of fluke ES products 75, attributing the mitogenic properties of ES products exclusively to the one protein.
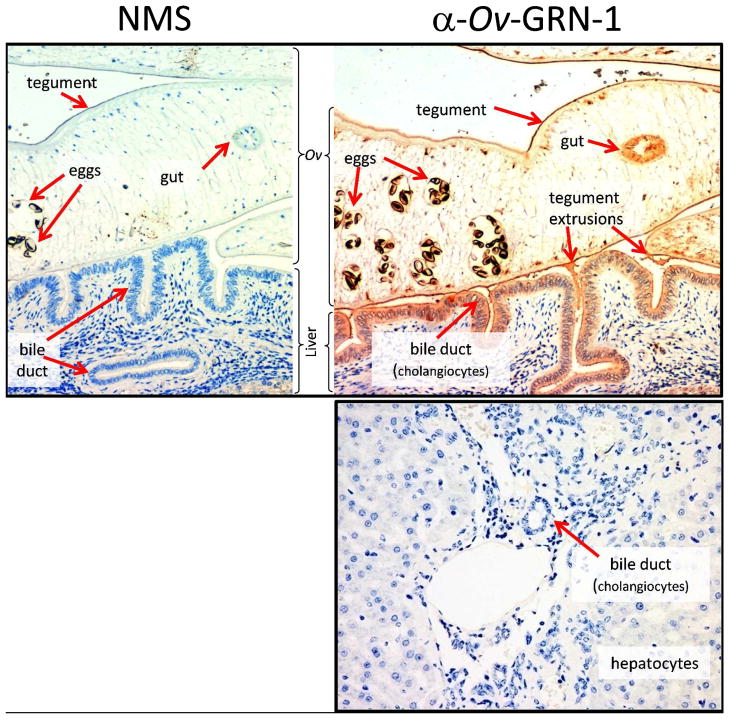
Figure 5. Immunolocalisation of Ov-GRN-1 in histological sections of adult O. viverrini in the bile ducts of experimentally infected hamsters.
The left panel was probed with IgG purified from control normal mouse serum (NMS); the right panel was probed with anti-Ov-GRN-1 IgG. The bottom panel shows a liver section from an uninfected hamster that was probed with anti-Ov-GRN-1 IgG. All three sections were stained with peroxidase staining revealed as a brown/rust colored deposit and Mayer’s Haematoxylin counterstained the nuclei in blue. Red arrows highlight the regions within the O. viverrini parasite and bile duct tissue that stained positive for Ov-GRN-1 75.
GRN protein family members occur in diverse species including bacteria, plants and animals. Human pro-GRN (PGRN) is associated with many aggressive cancers. It is over-expressed in human liver 76, 77, renal 78, breast 79–81, bladder 82 and brain 83 tumours. It may promote cancer progression by stimulating angiogenesis, suppressing anoikis (a form of apoptosis), promotion of tumour invasion and anchorage independence, all of which support tumour expansion in the unfavourable interstitial environment 82, 84, 85. Preventing the activity of GRN in a range of tumour types, either through gene silencing or antibody neutralization, reduces or entirely inhibits tumour progression 86. Transfection of fibroblasts with GRN induces serum independent proliferation but does not transform them into neoplastic cells, suggesting that the protein is probably not oncogenic by itself, but over-expression of PGRN in the SW-13 non-malignant adrenal carcinoma cell line made it highly tumourigenic 87. Indeed, PGRN is a therapeutic target for liver cancer, particularly HCC. An anti-PGRN monoclonal antibody inhibited tumour growth in vivo in nude mice transplanted with human HCC 77. The anti-PGRN antibody also inhibited growth of hepatoma cells but had little effect on normal liver cells, and inhibited the growth and proliferation of established tumours via the p44/42 MAPK and Akt signalling pathways. These findings demonstrate that GRN is an important factor in the initiation of liver cancer and the migration of cancerous cells. Recent high throughput DNA sequencing has uncovered a multidomain GRN protein from F. hepatica and a single domain GRN protein from C. sinensis homologous to Ov-GRN-1 72.
Host-parasite interplay and granulin
Why O. viverrini secretes such a potent growth factor that acts on host cells is unclear. One potential role for fluke GRN involves wound repair. Mammalian inflammatory cells secrete peptides derived from PGRN 88, and PGRN mRNA is highly induced in dermal fibroblasts and epithelial cells following transcutaneous puncture wounds 89. Furthermore, human recombinant PGRN increased the accumulation of inflammatory cells, blood vessels and fibroblasts at puncture sites, implying a direct role as a wound-healing growth factor 89. O. viverrini adult worms grasp the bile duct wall with their suckers and feed on the biliary cells, often severely damaging the epithelium. Additional inflammation occurs as a result of the local immune response to resident worms [reviewed in 90]. Ov-GRN-1 might therefore play a role in wound repair at and around the feeding site to minimize the pathology that the parasite causes to the host. Another potential role for Ov-GRN-1 is in the “farming” of host cells for nutritional purposes. By promoting growth of cells at the feeding site, the parasite is ensured of a steady supply of nutrients. Blood-feeding leeches secrete a GRN that inhibits thrombin activity 91, and Ov-GRN-1 might also perform a similar function to interfere with clot formation while feeding.
Future directions
Progression from opisthorchiasis to CCA is multi-factorial. As emphasized herein, we hypothesize that one of the major predisposing factors towards carcinogenesis is chronic exposure of host tissues to parasite metabolites. To this end, we have begun to catalogue putative carcinogenic molecules from the ES products and surface tegument of O. viverrini, taking bioinformatic approaches based on Gene Ontology 71, 72, proteomes 74 and exposure of host cells to parasite products that have been purified using a range of chromatographic techniques (M. Smout and A. Loukas, unpublished). Advances in biotechnology are opening up new fields of research in both parasite and cancer biology alike. Such advances will not only provide answers but will also result in new questions to be addressed. Studies of gene expression changes induced in cells exposed to defined O. viverrini proteins will shed light on the molecular mechanisms of disease progression. We and others 92 are using proteomics to search for biomarkers of hepatobiliary damage and/or CCA in cell lines and animal models but ultimately in infected patients. Better diagnostic methods are required to identify individuals at-risk of developing cancer in endemic settings, such that preventative chemotherapy can be specifically targeted at these individuals rather than the currently implemented intermittent mass drug administration programs. Future work on the molecular basis of human-Opisthorchis interactions should focus on potential targets for the development of anti-cancer therapies, tailored specifically to fluke-induced CCA. Such approaches will also reveal parasite proteins that might be suitable targets for the development of new drugs as well as recombinant vaccines against opisthorchiasis.
Despite the deployment of mass drug administration programs throughout Thailand, opisthorchiasis is still a major public health problem, and the prevalence of the infection in some areas is increasing 8, 93. Any advance in elucidating the complex associations of Opisthorchis or Clonorchis and CCA could assist in reducing both parasite burdens and the incidence of CCA, the most prevalent and fatal of the liver cancers in north-east Thailand 8. Additionally, the novelty of carcinogenic metazoans may lead to fundamental insights into carcinogenesis in general.
Acknowledgments
Support from award number UO1AI065871 from the National Institute of Allergy and Infectious Disease and the National Health and Medical Research Council of Australia (NHMRC) is gratefully acknowledged. MJS was supported by a Dora Lush postgraduate scholarship and AL by a Senior Research Fellowship from NHMRC. (The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH).
References
- 1.Crompton DW. J Parasitol. 1999;85:397–403. [PubMed] [Google Scholar]
- 2.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. Lancet Oncol. 2009;10:321–322. [Google Scholar]
- 3.Haswell-Elkins MR, Mairiang E, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, Sithithaworn P, Elkins DB. Int J Cancer. 1994;59:505–509. doi: 10.1002/ijc.2910590412. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 5.Green A, Uttaravichien T, Bhudhisawasdi V, Chartbanchachai W, Elkins DB, Marieng EO, Pairqjkul C, Dhiensiri T, Kanteekaew N, Haswell-Elkins MR. Trop Geogr Med. 1991;43:193–198. [PubMed] [Google Scholar]
- 6.Kurathong S, Lerdverasirikul P, Wongpaitoon V, Pramoolsinsap C, Kanjanapitak A, Varavithya W, Phuapradit P, Bunyaratvej S, Upatham ES, Brockelman WY. Gastroenterology. 1985;89:151–156. doi: 10.1016/0016-5085(85)90755-3. [DOI] [PubMed] [Google Scholar]
- 7.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 8.Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vatanasapt V, Sriamporn S, Vatanasapt P. Jpn J Clin Oncol. 2002;32(Suppl):S82–91. doi: 10.1093/jjco/hye134. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F, Ferlay J, Pisani P. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 11.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DBE. Cancer incidence in five continents. VIII. International Agency for Research on Cancer; Lyon: 2002. [Google Scholar]
- 12.IARC. IARC Monogr Eval Carcinog Risks Hum. 1994;61:121–175. [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer DA, Fried B. Adv Parasitol. 2007;65:239–296. doi: 10.1016/S0065-308X(07)65004-0. [DOI] [PubMed] [Google Scholar]
- 14.Fried B, Reddy A, Mayer D. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Tricker AR, Mostafa MH, Spiegelhalder B, Preussmann R. Carcinogenesis. 1989;10:547–552. doi: 10.1093/carcin/10.3.547. [DOI] [PubMed] [Google Scholar]
- 16.Botelho M, Ferreira AC, Oliveira MJ, Domingues A, Machado JC, da Costa JM. Int J Parasitol. 2009 doi: 10.1016/j.ijpara.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Botelho M, Oliveira P, Gomes J, Gartner F, Lopes C, da Costa JM, Machado JC. Int J Exp Pathol. 2009;90:448–453. doi: 10.1111/j.1365-2613.2009.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keiser J, Utzinger J. Clin Microbiol Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traub RJ, Macaranas J, Mungthin M, Leelayoova S, Cribb T, Murrell KD, Thompson RC. PLoS Negl Trop Dis. 2009;3:e367. doi: 10.1371/journal.pntd.0000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. World Health Organ Tech Rep Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- 21.Kaewkes S. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 23.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Cancer Sci. 2010;101:579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harinasuta C, Harinasuta T. Arzneimittelforschung. 1984;34:1164–1167. [PubMed] [Google Scholar]
- 25.Sithithaworn P, Haswell-Elkins M. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Upatham ES, Viyanant V. Acta Trop. 2003;88:171–176. doi: 10.1016/j.actatropica.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Jongsuksuntigul P, Imsomboon T. Southeast Asian J Trop Med Public Health. 1998;29:327–332. [PubMed] [Google Scholar]
- 28.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, Todoroki T, Jedpiyawongse A, Kittiwatanachot P, Sripa B, Deerasamee S, Miwa M. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 29.Tabor E. AIDS Res Hum Retroviruses. 1992;8:793–796. [PubMed] [Google Scholar]
- 30.Bosch FX, Ribes J, Diaz M, Cleries R. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 32.Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, Allemani C, Ciccolallo L, Santaquilani M, Berrino F. Ann Oncol. 2003;14(Suppl 5):v128–149. doi: 10.1093/annonc/mdg756. [DOI] [PubMed] [Google Scholar]
- 33.Kuper H, Adami HO, Trichopoulos D. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 34.Bissell MJ, Radisky D. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dvorak HF. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 36.Rous P. JAMA. 1983;250:1445–1449. [PubMed] [Google Scholar]
- 37.Sripa J, Laha T, To J, Brindley PJ, Sripa B, Kaewkes S, Dalton JP, Robinson MW. Cell Microbiol. 2010;12:781–795. doi: 10.1111/j.1462-5822.2010.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kullavanijaya P, Tangkijvanich P, Poovorawan Y. Southeast Asian J Trop Med Public Health. 1999;30:96–105. [PubMed] [Google Scholar]
- 39.Thamavit W, Pairojkul C, Tiwawech D, Itoh M, Shirai T, Ito N. Carcinogenesis. 1993;14:2415–2417. doi: 10.1093/carcin/14.11.2415. [DOI] [PubMed] [Google Scholar]
- 40.Kenny PA, Bissell MJ. Int J Cancer. 2003;107:688–695. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sripa B, Kaewkes S. Int J Parasitol. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesan RN, Bielas JH, Loeb LA. DNA Repair (Amst) 2006;5:294–302. doi: 10.1016/j.dnarep.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Loeb LA, Loeb KR, Anderson JP. Proc Natl Acad Sci U S A. 2003;100:776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sripa B, Kaewkes S. Parasite Immunol. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 45.Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, Tessana S, Loukas A, Brindley PJ, Bethony JM. Hepatology. 2009;50:1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James SL, Hibbs JB., Jr Parasitol Today. 1990;6:303–305. doi: 10.1016/0169-4758(90)90261-2. [DOI] [PubMed] [Google Scholar]
- 47.Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Gastroenterology. 2001;120:190–199. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- 48.Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Carcinogenesis. 2004;25:1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 49.Satarug S, Haswell-Elkins MR, Sithithaworn P, Bartsch H, Ohshima H, Tsuda M, Mairiang P, Mairiang E, Yongvanit P, Esumi H, Elkins DB. Carcinogenesis. 1998;19:485–491. doi: 10.1093/carcin/19.3.485. [DOI] [PubMed] [Google Scholar]
- 50.Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB. Mutat Res. 1994;305:241–252. doi: 10.1016/0027-5107(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 51.Satarug S, Haswell-Elkins MR, Tsuda M, Mairiang P, Sithithaworn P, Mairiang E, Esumi H, Sukprasert S, Yongvanit P, Elkins DB. Carcinogenesis. 1996;17:1075–1081. doi: 10.1093/carcin/17.5.1075. [DOI] [PubMed] [Google Scholar]
- 52.Ohshima H, Bandaletova TY, Brouet I, Bartsch H, Kirby G, Ogunbiyi F, Vatanasapt V, Pipitgool V. Carcinogenesis. 1994;15:271–275. doi: 10.1093/carcin/15.2.271. [DOI] [PubMed] [Google Scholar]
- 53.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 54.IARC. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 55.Griciute L. Bull Cancer. 1978;65:53–58. [PubMed] [Google Scholar]
- 56.Srivatanakul P, Ohshima H, Khlat M, Parkin M, Sukaryodhin S, Brouet I, Bartsch H. Int J Cancer. 1991;48:821–825. doi: 10.1002/ijc.2910480606. [DOI] [PubMed] [Google Scholar]
- 57.Songserm N, Prasongwattana J, Sithithaworn P, Sripa B, Pipitkool V. Asian Pac J Cancer Prev. 2009;10:299–302. [PubMed] [Google Scholar]
- 58.Pairojkul C, Shirai T, Hirohashi S, Thamavit W, Bhudhisawat W, Uttaravicien T, Itoh M, Ito N. Princess Takamatsu Symp. 1991;22:77–86. [PubMed] [Google Scholar]
- 59.Vatanasapt V, Sriamporn S, Kamsaard S, Suwanrungruang K, Pengsaa P, Charoensiri DJ, Chaiyakum J, Pesee M. IARC Sci Publ. 1998:123–134. [PubMed] [Google Scholar]
- 60.Almeida CA, Goes AM. Parasitol Int. 2000;48:255–264. doi: 10.1016/s1383-5769(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 61.Konno K, Oku Y, Nonaka N, Kamiya M. Parasitol Res. 1999;85:431–436. doi: 10.1007/s004360050573. [DOI] [PubMed] [Google Scholar]
- 62.Lax AJ, Thomas W. Trends Microbiol. 2002;10:293–299. doi: 10.1016/s0966-842x(02)02360-0. [DOI] [PubMed] [Google Scholar]
- 63.Hofman P, Waidner B, Hofman V, Bereswill S, Brest P, Kist M. Helicobacter. 2004;9(Suppl 1):15–22. doi: 10.1111/j.1083-4389.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz L, Balosso J, Baillet F, Brun B, Amman JP, Sasco AJ. Med Hypotheses. 2002;58:340–346. doi: 10.1054/mehy.2001.1539. [DOI] [PubMed] [Google Scholar]
- 65.Moore MA, Thamavit W, Hiasa Y, Ito N. Carcinogenesis. 1988;9:1185–1189. doi: 10.1093/carcin/9.7.1185. [DOI] [PubMed] [Google Scholar]
- 66.Hughes NR, Pairojkul C, Royce SG, Clouston A, Bhathal PS. J Clin Pathol. 2006;59:1073–1078. doi: 10.1136/jcp.2005.033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thuwajit C, Thuwajit P, Kaewkes S, Sripa B, Uchida K, Miwa M, Wongkham S. Parasitology. 2004;129:455–464. doi: 10.1017/s0031182004005815. [DOI] [PubMed] [Google Scholar]
- 68.Thuwajit C, Thuwajit P, Uchida K, Daorueang D, Kaewkes S, Wongkham S, Miwa M. World J Gastroenterol. 2006;12:3585–3592. doi: 10.3748/wjg.v12.i22.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narayan S, Thangasamy T, Balusu R. Front Biosci. 2005;10:1135–1145. doi: 10.2741/1606. [DOI] [PubMed] [Google Scholar]
- 70.Anagnostopoulos K, Tentes I, Kortsaris AH. J BUON. 2008;13:17–22. [PubMed] [Google Scholar]
- 71.Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, Gasser RB, Brindley PJ, Loukas A. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young ND, Campbell BE, Hall RS, Jex AR, Cantacessi C, Laha T, Sohn WM, Sripa B, Loukas A, Brindley PJ, Gasser RB. PLoS Negl Trop Dis. 2010;4:e719. doi: 10.1371/journal.pntd.0000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young ND, Jex AR, Cantacessi C, Campbell BE, Laha T, Sohn WM, Sripa B, Loukas A, Brindley PJ, Gasser RB. Biotechnol Adv. 2010 doi: 10.1016/j.biotechadv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Mulvenna J, Sripa B, Brindley PJ, Gorman J, Jones MK, Colgrave ML, Jones A, Nawaratna S, Laha T, Suttiprapa S, Smout MJ, Loukas A. Proteomics. 2009;10:1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, Brindley PJ, Loukas A. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheung ST, Wong SY, Leung KL, Chen X, So S, Ng IO, Fan ST. Clin Cancer Res. 2004;10:7629–7636. doi: 10.1158/1078-0432.CCR-04-0960. [DOI] [PubMed] [Google Scholar]
- 77.Ho JC, Ip YC, Cheung ST, Lee YT, Chan KF, Wong SY, Fan ST. Hepatology. 2008;47:1524–1532. doi: 10.1002/hep.22191. [DOI] [PubMed] [Google Scholar]
- 78.Donald CD, Laddu A, Chandham P, Lim SD, Cohen C, Amin M, Gerton GL, Marshall FF, Petros JA. Anticancer Res. 2001;21:3739–3742. [PubMed] [Google Scholar]
- 79.Lu R, Serrero G. Biochem Biophys Res Commun. 1999;256:204–207. doi: 10.1006/bbrc.1999.0253. [DOI] [PubMed] [Google Scholar]
- 80.Lu R, Serrero G. Proc Natl Acad Sci U S A. 2001;98:142–147. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tangkeangsirisin W, Serrero G. Carcinogenesis. 2004;25:1587–1592. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 82.Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- 83.Liau LM, Lallone RL, Seitz RS, Buznikov A, Gregg JP, Kornblum HI, Nelson SF, Bronstein JM. Cancer Res. 2000;60:1353–1360. [PubMed] [Google Scholar]
- 84.Ong CH, Bateman A. Histol Histopathol. 2003;18:1275–1288. doi: 10.14670/HH-18.1275. [DOI] [PubMed] [Google Scholar]
- 85.Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 86.Jones MB, Houwink AP, Freeman BK, Greenwood TM, Lafky JM, Lingle WL, Berchuck A, Maxwell GL, Podratz KC, Maihle NJ. J Soc Gynecol Investig. 2006;13:304–311. doi: 10.1016/j.jsgi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 87.He Z, Bateman A. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]
- 88.Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Biochem Biophys Res Commun. 1990;173:1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 89.He Z, Ong CH, Halper J, Bateman A. Nat Med. 2003;9:225–229. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 90.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong SJ, Kang KW. Protein Expr Purif. 1999;16:340–346. doi: 10.1006/prep.1999.1077. [DOI] [PubMed] [Google Scholar]
- 92.Khoontawad J, Wongkham C, Hiraku Y, Yongvanit P, Prakobwong S, Boonmars T, Pinlaor P, Pinlaor S. Parasite Immunol. 2010;32:314–323. doi: 10.1111/j.1365-3024.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- 93.Andrews RH, Sithithaworn P, Petney TN. Trends Parasitol. 2008 doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sripa B, Pairojkul C. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]