Abstract
Genome sequences for Schistosoma japonicum and Schistosoma mansoni are now available. The schistosome genome encodes ~13,000 protein encoding genes for which the function of only a minority is understood. There is a valuable role for transgenesis in functional genomic investigations of these new schistosome gene sequences. In gain-of-function approaches, transgenesis can lead to integration of transgenes into the schistosome genome which can facilitate insertional mutagenesis screens. By contrast, transgene driven, vector-based RNA interference (RNAi) offers powerful loss-of-function manipulations. Our laboratory has focused on development of tools to facilitate schistosome transgenesis. We have investigated the utility of retroviruses and transposons to transduce schistosomes. Vesicular stomatitis virus glycoprotein (VSVG) pseudotyped murine leukemia virus (MLV) can transduce developmental stages of S. mansoni including eggs. We have also observed that the piggyBac transposon is transpositionally active in schistosomes. Approaches with both VSVG-MLV and piggyBac have resulted in somatic transgenesis and have lead to integration of active reporter transgenes into schistosome chromosomes. These findings provided the first reports of integration of reporter transgenes into schistosome chromosomes. Experience with these systems is reviewed herewith, along with findings with transgene mediated RNAi and germ line transgenesis, in addition to pioneering and earlier reports of gene manipulation for schistosomes.
Keywords: transgenesis, transposon, piggyBac, retrovirus, integration, vector based RNAi
The integral role transgenesis plays in the study of various models and biomedically significant species is well documented. Unquestionably the vast majority of scientific information available in regard to pathogens has been generated through transgenesis studies of model species (e.g. Ding et al. 2005). The technologies have subsequently been transferred to other systems (Langridge et al. 2009). Schistosomes along with other helminths are the causative agents of neglected tropical diseases; there is a paucity of genetic manipulation techniques available for investigation of their physiology. Nonetheless, the scope of functional genomics studies in schistosomes has advanced in recent years mainly due to the availability of draft genome sequences for two of the medically important schistosome species, namely Schistosoma mansoni and Schistosoma japonicum (Berriman et al. 2009, Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium 2009). Progress made on this front includes the use of integration competent vectors, i.e. transposons and retroviruses for the delivery of reporter transgenes into the schistosome genome (Morales et al. 2007, Kines et al. 2008). Additionally, both conventional and vector based RNAi studies have been successfully employed for gene expression studies in schistosomes, this could potentially identify future drug targets in experimental chemotherapeutic studies. However, despite these advances the attainment of a stable heritable system for transgenesis has not been brought to fruition.
Pioneering reports - functional genomics for schistosomes
Unlike some protozoan parasites for which techniques for genetic manipulation are well advanced (e.g. Balu et al. 2005), schistosomes and other helminths lag substantially in terms of tools for genetic manipulation. The transfer of exogenously derived nucleic acids into a target organism or cells referred to as transgenesis is a relatively recent accomplishment in the field of schistosome molecular biology. Functional genomics approaches would enable manipulation of the genome of these worms, including forward and reverse genetics. However, the fundamental nature of parasitic helminths, such as the blood flukes - intricate developmental cycles, large size, multicellular tissues and complex organization - along with the absence of immortalized cell lines and our inability to rear the entire life cycle in vitro has hindered development of tractable transgenesis tools and models (Brindley & Pearce 2007).
Nevertheless, the first reports of successful transgenesis in schistosomes utilized biolistic particle delivery (gene gun) to introduce plasmids encoding firefly luciferase or jellyfish green fluorescent protein reporters (Davis et al. 1999, Wippersteg et al. 2002). These reports were able to demonstrate the utility of particle bombardment for foreign gene introduction into adult (Davis et al. 1999, Wippersteg et al. 2002) and sporocyst (Wippersteg et al. 2002) stages. Following these successes, Correnti and Pearce (2004) introduced a luciferase encoding RNA into schistosomules using square wave electroporation for the delivery of the transgene. In a time course experiment they observed that transfection efficiency was highest in schistosomules that had been cultured for 18 h prior to electroporation (Correnti & Pearce 2004). Moreover, the findings indicated that the electroporation was a less damaging method than biolistics with regard to transfer of nucleic acids into cultured schistosomes. Both of these gain-of-function approaches have shown success in the transfer of mRNAs and plasmid based reporter genes to several developmental stages of S. mansoni and S. japonicum. These and other pioneering studies into genetic manipulation of schistosomes have been reviewed (Grevelding 2006, Brindley & Pearce 2007, Kalinna & Brindley 2007, Mann et al. 2008).
Loss-of-function procedures involving RNA interference (RNAi) targeting schistosome genes in several developmental stages have been reported (e. g. Skelly et al. 2003, Correnti et al. 2005, Dinguirard & Yoshino 2006, Osman et al. 2006) and reviewed in Brindley and Pearce (2007), Geldhof et al. (2007) and Kalinna and Brindley (2007). RNAi was first demonstrated in schistosomes by targeting the hemoglobinolytic protease cathepsin B in schistosomules of S. mansoni soaked with double stranded RNA (dsRNA) (Skelly et al. 2003). Correnti et al. (2005) extended the investigation by introducing dsRNA in schistosomules by square wave electroporation. These important advances have demonstrated transgene delivery and activity of functional promoters. However, they are unlikely to lead to integration of transgenes into schistosome chromosomes, without which the transgene effect would likely only be transitory.
Accessing the germ line
In order to advance our ability to develop novel interventions for schistosomiasis, there is likely to be a valuable role for stable heritable transgenic lines of schistosomes. Establishment of lines of transgenic schistosomes will require integration of transgenes into the chromosomes of the schistosome germ line. In order to achieve germ line transgenesis in any organism several obstacles have to be overcome. The vectors containing the constructs have to be able to achieve stable integration into the genome. The transgenes not only have to integrate into the genome, they have to specifically integrate into the germ cells. The fundamental nature of the schistosome makes this challenging. Blood flukes are large, complex, multicellular organisms composed of both somatic and germinal tissues further lowering the chances of transforming the germ line. In addition, once integrated, the transgenic schistosome has to be viable to continue the develop- mental cycle producing subsequent generations of transgenic progeny (Heyers et al. 2003). Using transfer by particle bombardment, earlier investigations into germ line transgenesis in schistosomes circumvented the latter impediment by introducing the transgene in the miracidia (Heyers et al. 2003) and allowing the transformed miracidia to infect the intermediate host snail via the natural route thereby obviating the need for additional manipulation of the transformed organisms, e.g. microinjection of sporocysts into snails. Furthermore, these workers demonstrated the possibility of penetrating the germ cells through biolistic approaches. Though their studies and others similar investigations provided suitable methods in introducing constructs in the germ cells, strategies for integration into the germ cells still require further development.
The germ cell cycle in schistosomes
The conventional meaning of “germ line” connotes the fusion of gametes from the male and female gonads (testis and ova, respectively) through the process of fertilization resulting in the formation of a zygote that contains half the DNA of each of the two parents, culminating in the formation of the embryo from which individuals develop, this is, the hallmark of sexual reproduction. Thus the germ line of an organism contains the germ cells that contain heritable genetic material that may be passed on to offspring. Theoretically, if exogenous genetic material was introduced into the germ line, the transgene would constitute part of the heritable genetic material and would be present in subsequent generations since germ line cells, unlike somatic cells, are not constrained by the Hayflick limit (Shay & Wright 2000), and are therefore immortal because they can divide indefinitely. Therefore the ratio of germ cells to somatic cells is highest during gastrulation and progressively decreases after onset of organogenesis due to cellular differentiation.
Schistosomes have a complex developmental cycle that encompasses both an asexual and a sexual phase in an intermediate and a definitive host, respectively. The asexual cycle is characterized by a series of germ cell rich developmental stages that result in the progression of the organism from the egg, to the miracidium, to the mother/ primary sporocyst, to the daughter/secondary sporocyst and the cercariae (Yoshino & Laursen 1995). Although the intramolluscan development of S. mansoni is asexual in nature, studies into the method of schistosome reproduction in the snail host have demonstrated that larval development commences when daughter sporocysts arise from the multiplication of germ cell derived germinal cells in the mother sporocysts (Cort & Olivier 1943). This process is then followed sequentially by the formation of cercariae which arise from the germ cells in the daughter sporocysts. It should be further noted that during progression through both the asexual and sexual life cycle the germ cells are the only continuous cell line in the schistosome life cycle (Grevelding 2006). And in general, these germ cells as they progress through the life stages form a cell lineage that is ultimately dedicated to the production of future gametes.
Towards lines of transgenic schistosomes
Ideally, target stages for the introduction of transgenes into the genome of S. mansoni would be either the immature/ newly laid egg (where it contains only the single-celled zygote prior to embryogenesis) or the early larval stages (egg derived miracidia or miracidia derived sporocysts). According to (Jurberg et al. 2009), S. mansoni eggs remain undeveloped when laid and contain the single cell zygote that has not undergone any cleavage. In addition, according to (Freitas et al. 2007), development of an immature egg into a mature egg containing a miracidium proceeds in vitro outside the female worm and takes about five days at 37°C. Therefore, to increase the probability of transforming the germ line introduction of transgenes in the egg stage when the ratio of germ cells to somatic cells is highest during the developmental cycle would be optimal. Miracidia and sporocysts are also attractive stages to target the germ line because at these stages of development the schistosome exhibits a comparatively high germ to somatic cell ratio. The miracidia, on average has ~20 germinal cells that are located at the posterior of the larval fluke (Pan 1980). After penetrating the snail host, the miracidium discards its ciliated epidermal plates, forms a new tegument and transforms into a mother sporocyst (Olivier & Mao 1949, Pan 1996). The mother sporocyst asexually produces 200–400 germ balls from the 20 germinal cells of the miracidium (Pan 1980). Each of these germ balls develops into daughter sporocysts that in turn produce thousands of cercariae. The miracidia and sporocyst stage are also suitable for in vitro cultivation (Grevelding 2006).
Transposable elements
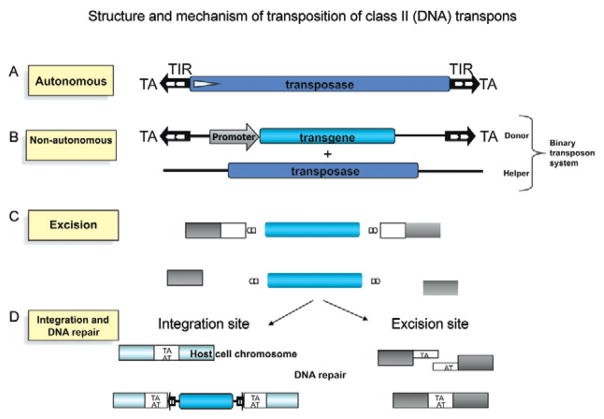
Transposons are mobile genetic elements that colonize the genomes of a variety of organisms. Discovered by Barbara McClintock through her studies of mutations in maize (McClintock 1950), transposons move within and between genomes via a process referred to as transposition. Transposons are classified into two classes based on their mechanism of transposition. piggyBac, a class II DNA transposon has been used to mediate somatic transgenesis of S. mansoni (Morales et al. 2007). DNA transposons move within the genome via a “cut and paste” mechanism mediated by a single or double stranded DNA intermediate and facilitated by a self encoded transposase enzyme. In addition to encoding transposase enzyme, autonomous DNA transposons are characterized by the presence of terminal inverted repeats (TIRs) (Feschotte & Pritham 2007). Transposition is initiated when the transposase recognizes the TIRs and excises the transposon. Depending on the particular transposon and the transposase it encodes, the enzyme either arbitrarily or sequence specifically binds to a target site. The transposase then makes a staggered cut producing sticky ends, excises the transposon and ligates it into the target site. A DNA polymerase fills in the resulting gaps from the sticky ends and DNA ligase closes the sugar-phosphate backbone resulting in target site duplication. DNA transposons can be classified into three groups: those that excise as double stranded DNA and reinsert elsewhere in the genome, i.e., the classic cut and paste transposons (Fig. 1) (Mann et al. 2008), helitrons that may use a mechanism similar to rolling circle replication (Kapitonov & Jurka 2001) and mavericks/politrons, for which the mechanism of transposition is not yet well characterized but may replicate using a self encoded DNA polymerase (Kapitonov & Jurka 2006, Pritham et al. 2007). [Notably, in nature, parasitism appears to provide a bridge for dispersal of transposons among phylogenetically unrelated host species genomes (Gilbert et al. 2010).]
Fig. 1.
Fig. 1A: schematic representation of the structure and mechanism of transposition of transposons. The terminal inverted repeats (TIR) (black arrows) contain binding sites for the transposase (white arrows). The element contains a single gene encoding the transposase. The NH2-terminal part of the transposase contains a DNA binding domain, followed by a nuclear localization signal. The COOH-terminal part of the protein is responsible for catalysis, including the DNA cleavage and rejoining reactions (not shown); B: schematic of the donor cassette in which the transposase open reading frame has been replaced with a reporter transgene driven by an endogenous promoter; C: cut and paste mechanism of transposition. The transposase initiates the excision of the transposon donor cassette with staggered cuts and reintegrates it at the target integration site; D: the single-stranded gaps at the integration site as well as the double-strand DNA breaks in the donor DNA are repaired by the host DNA repair machinery. After repair, the target is regenerated at the integration site in the host cell chromosome and at the site of excision from the donor plasmid [adapted from Handler (2002), Miskey et al. (2005), Morales et al. (2007) and Mann et al. (2008)].
piggyBac
piggyBac, a lepidopteran class II DNA transposon originally isolated from the genome of the cabbage looper moth Trichoplusia ni (Fraser et al. 1983), is a short inverted terminal repeat element that is 2.5 kilo-bases (kb) in length with 13 bp inverted terminal repeat sequences and a 2.1 kb open reading frame (Cary et al. 1989, Elick et al. 1996). Being a class II transposon, this element moves via cut and paste transposition mediated by a self encoded transposase enzyme. Unlike other transposons, piggyBac demonstrates precise excision upon transposition (Cary et al. 1989, Fraser et al. 1996) as well as an affinity for TTAA target sites (Fraser et al. 1996). This element has been harnessed for somatic transgenesis in a variety of model and pathogenic organisms including Plasmodium falciparum (Balu et al. 2005), the planarian Girardia tigrina (González-Estévez et al. 2003), S. mansoni (Morales et al. 2007) and mammalian cell lines, including human cell lines (Wilson et al. 2007). piggyBac is not known to be active in prokaryotic cells (Elick et al. 1996). In addition, piggyBac has facilitated stable heritable (germ line) transgenesis in various insects including the Mediterranean fruit fly Ceratitis capitata (Handler et al. 1998), the silkworm Bombyx mori L (Tamura et al. 2000), the pink bollworm Ectinophora gossypiella (Peloquin et al. 2000), the oriental fruit fly Bactrocera dorsalis and the malaria mosquito Anopheles stephensi (Nolan et al. 2002). With the ability of piggyBac to jump within phylogenetically diverse taxa and the successful somatic transgenesis of S. mansoni (Fig. 2), this system has the potential to achieve the vertical transmission of transgenes within the S. mansoni genome in both the sexual and asexual phases of the lifecycle.
Fig. 2.
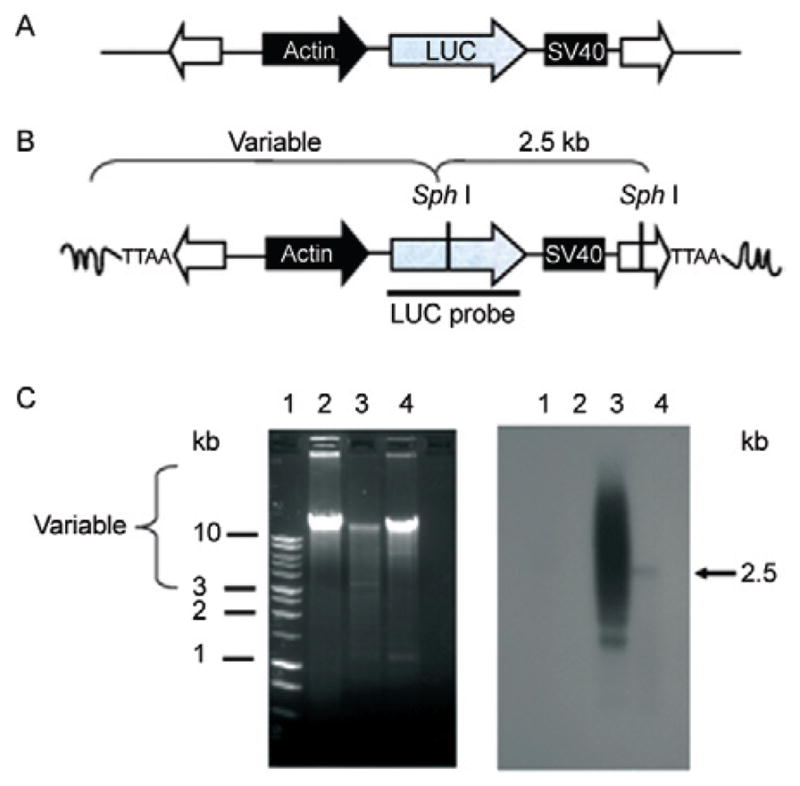
southern hybridization analysis of piggyBac transposon integration into schistosome chromosomes. A: representation of the (BssS 1)-linearized pXL-BacII-SmAct-Luc transposon construct. The transposon cassette included the firefly luciferase reporter (gray arrow) followed by the SV40 polyadenylation site (black box), driven by the schistosome actin gene promoter (black arrow) and flanked by the piggyBac terminal inverted repeats (white arrows); B: the structure of a piggyBac-mediated integration site, depicting variable length fragments expected following Sph I digestion of gDNA from piggyBac-transformed schistosomules. The luciferase probe (LUC) is indicated by a black bar; C: ethidium-stained gel of gDNA from Schistosoma mansoni digested with Sph I (left), southern hybridization of Sph I digested DNA (gDNA) to the labeled LUC probe (right), size standards in kilobases (kb) (Lane 1), gDNA from control schistosomes (Lane 2), gDNA from schistosomules after electroporation with donor piggyBac plasmid plus in vitro-transcribed transposase mRNA (Lane 3), gDNA from schistosomules seven days after electroporation with donor piggyBac plasmid alone (Lane 4). [From Morales et al. (2007), with permission].
Investigations with human and mouse cell lines further demonstrate the versatility of piggyBac as a vector for the delivery of transgenes (Ding et al. 2005, Wilson et al. 2007). piggyBac, unlike some other transposons (e.g. the P element of Drosophila) is active in numerous species from diverse phyla (Handler 2002, Sumitani et al. 2003). In addition, it has a high cargo capacity, endowing the capacity to efficiently transpose transgenes ranging in size from 9.1–14.3 kb without a reduction in transposition frequency (Ding et al. 2005, Wilson et al. 2007). By contrast, sleeping beauty loses transposition efficiency with larger transgenes (Karsi et al. 2001, Geurts et al. 2003). piggyBac also appears to be unaffected by overproduction inhibition a phenomenon whereby an extremely high ratio of transposase (helper) to the donor construct results in a decrease in transposition efficiency and frequency (Geurts et al. 2003, Zayed et al. 2004, Wilson et al. 2005). Overproduction inhibition has been observed in members of the Tc1/mariner family of transposons, including sleeping beauty (Lampe et al. 1998).
Mos-1 mariner
Mos-1 mariner is a member of Tc1/ mariner superfamily. It is a small (1.3 kb) transposon that is flanked by short inverted repeats sequence of 28 bp. It was isolated originally from the fruit fly Drosophila mauritania (Jacobson et al. 1986, Medhora et al. 1991) and also been found in closely related species, including Drosophila simulans and Drosophila sechellia (Capy et al. 1991). It exhibits widespread natural distribution - with orthologues known from nematodes, arthropods, fish, dogs and humans (Plasterk et al. 1999). The Mos-1 mariner transposon gene encodes a single gene, transposase, which is necessary for the transposition process (Lampe et al. 1996). Transposition involves excision at the donor site followed by insertion at the integration target site. Mos-1 is capable of transposition in vivo in the absence of host cell factors and in diverse species including chicken (Sherman et al. 1998) and mouse embryonic cells (Luo et al. 1998) and indeed Mos-1 mariner appears to be transpositionally active in S. mansoni (YN Alrefaei et al., unpublished observations).
Retroviral based transgenesis
Retroviral transgenesis offers a tractable method to introduce foreign genes into schistosomes. In general, viral vectors are capable of transducing ≥90% of the cells to which they are exposed while other non-viral transfection methods as those described above are often much less proficient (Burns et al. 1993). Critically retroviruses such as gammaretroviruses [e.g. murine leukemia virus (MLV)] and lentiviruses [e.g. human immunodeficiency virus (HIV-1)] integrate into the host cell chromosomes (Coffin et al. 1997). Retroviruses have been used to transfect, integrate and express reporter genes in various complex organisms including zebrafish, rhesus macaque, Xenopus (oocytes), newts, mollusks, parasitic amoebae, mosquitoes and fruit flies (Burns et al. 1994, 1996, Lin et al. 1994, Lu et al. 1996, Matsubara et al. 1996, Franco et al. 1998, Jordan et al. 1998, Que et al. 1999, Boulo et al. 2000, Calmels et al. 2005). Moreover, transgenic cell lines from zebrafish, dwarf surfclams and other complex species have been established through this method (Lin et al. 1994, Lu et al. 1996). These successes indicated that retroviruses would be good candidates as vectors to insert transgenes into schistosome chromosomes.
Kines et al. (2006) demonstrated that the infectious replication incompetent MLV retrovirus pseudotyped with vesicular stomatitis virus glycoprotein (VSVG) was capable of transfecting S. mansoni (Fig. 3). An MLV derived vector, pLNHX, was modified to include a reporter gene under the control of an endogenous schistosome gene promoter. The constructs along with a plasmid encoding the VSVG gene were used to transfect GP2-293 packaging cells modified to express the MLV Gag and Pol gene (Kines et al. 2006). Sporocysts, schistosomules and adults of S. mansoni were exposed to the resulting virions. Analysis by two-colour immunofluorescence, southern hybridization and reverse transcription-polymerase chain reaction (RT-PCR) indicated successful transduction of these schistosome developmental stages by this retrovirus. A follow-up study confirmed that VSVG-MLV could transduce blood-stage forms of S. mansoni and demonstrated that the provirus integrates into the schistosome genome with possible target site preferences (Kines et al. 2008). This study also showed that the S. mansoni actin promoter (Correnti et al. 2007) can drive the reporter luciferase transgene.
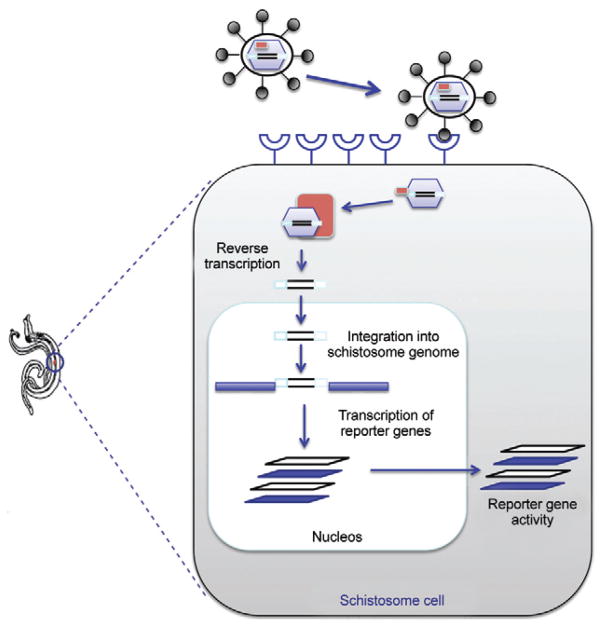
Fig. 3.
schematic representation of retroviral replication and integration into a schistosome cell. After entering the cell, the retrovirus RNA genome is reverse transcribed into double-stranded DNA by reverse transcriptase (RT) present in the virion. The DNA copy migrates to the cell nucleus and integrates into the host genome as the provirus. Viral mRNAs are transcribed from proviral DNA by host cell enzymes in the nucleus. Both spliced and unspliced mRNAs are translated into viral proteins in the cytoplasm. The capsid precursor protein, Gag, and RT are translated from full-length RNA. The glycoproteins are translated from spliced mRNA and transported to the cell plasma membrane. Immature virions containing Gag, RT and the genome RNA assemble near the modified cell membrane. [Adapted from Strauss and Strauss (2002)].
In the studies of Kines et al. (2006) and Kines et al. (2008), it is likely that the cells transduced by the virions were tegumental and/or intestinal cells (Mann et al. 2008). In order for heritable transmission to occur, germline transduction would have to have taken place. The schistosome egg represents a potentially advantageous developmental stage at which to direct transgenes (Mann et al. 2011). Accordingly Kines et al. (2010) proceeded to transduce schistosome eggs with VSVG-pseudotyped MLV facilitated by electroporation. These workers showed that square wave electroporation was more effective in delivering VSVG-pseudotyped Moloney MLV (MMLV) into schistosome eggs then passive soaking. Quantitative PCR analysis revealed that schistosome eggs electroporated with virions had several fold more copies of provirus than eggs simply soaked in virions (Kines et al. 2010). This analysis confirmed the potential of the schistosome egg as a target for germ line transgenesis.
Although the studies by Kines et al. (2006, 2008, 2010) reported successful transduction and integration of transgenes in several S. mansoni developmental stages, the reporter genes were not especially large (< 2 kb). To investigate the cargo capacity of MLV in schistosomes, Yang et al. (2010) transduced S. japonicum schistosomules with a longer transgene, human telomerase reverse transcriptase (hTERT) gene that is 3.5 kb in length. RT-PCR, in situ hybridization immunohistochemistry and immunoblot analysis determined that S. japonicum can be successfully transduced with VSVG-pseudotyped MMLV and that the MLV vector can transport sizeable genes as cargo (Yang et al. 2010). These findings also suggested a possible route to establishing immortalized cell lines from schistosome tissues utilizing the oncogenic potential of hTERT. Retroviral transgenesis for schistosomes is reviewed in greater detail in Mann et al. (2008, 2010, 2011).
In summary, these findings confirmed that VSVG-pseudotyped MMLV is capable of integrating transgenes into schistosomes. Although the studies only demonstrated somatic transgenesis, these findings markedly enhance the likelihood that transgenic lines of schistosomes will be a reality. In addition to MMLV, other retroviral vectors can be investigated for transducing schistosomes, including lentiviruses, which have the ability to transduce non-dividing cells making them attractive for transgenesis approaches for schistosomes (Mann et al. 2008). Lentiviral vectors derived from HIV-1 and feline immunodeficiency viruses have been developed (Dull et al. 1998, Poeschla 2008) and are commercially available.
RNAi and vector based RNAi
Along with the now available draft genome sequences of two of the three medically important schistosome species, S. japonicum and S. mansoni (Berriman et al. 2009, Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium 2009), RNAi (Fire et al. 1998) has revolutionized the world of functional genomics for schistosomes. This allows for among other things the ability to identify potentially novel drug targets in a variety of medically important organisms. It has been determined that schistosomes possess the various components of the RNAi machinery (Krautz-Peterson & Skelly 2008a), (Verjovski-Almeida et al. 2003) and that RNAi is active in schistosomes. Previous studies in RNAi in schistosomes targeted various enzymes and biochemical pathways in an effort to elucidate their function and garner an overall better understanding of these organisms. For instance, RNAi has been used to disrupt the haemoglobin degradation pathway by specifically targeting enzymes involved in this process, cathepsin B (Correnti et al. 2005) and cathepsin D (Morales et al. 2008), both of which resulted in the stunting of the growth of the parasites, underscoring the importance of the pathway to the survival of the organism and utility as a potential drug target (Correnti et al. 2005, Delcroix et al. 2006, Krautz-Peterson & Skelly 2008b). Other areas of schistosome biology investigated using RNAi include water movement and drug uptake across the parasite tegument, as well as various aspects of parasite physiology and development (Faghiri & Skelly 2009). As versatile a tool as RNAi is, its utility in functional genomics is hampered by the fact that it is transitory in nature (resulting in a temporary downregulation of gene expression). In addition, it may be inaccessible to some developmental stages and/or tissues of schistosomes.
Conventional RNAi using dsRNA frequently leads to transient gene silencing and, in addition, may be inaccessible to some developmental stages and/or tissues of schistosomes. Vector based RNAi approaches that lead to integration of transgene encoding cassettes that express short interfering RNAs can circumvent deficiencies with exogenous RNAi approaches by providing continuous and/or conditional gene silencing (Brummelkamp et al. 2002, ter Brake et al. 2006). It was recently demonstrated that MLV encoding long hairpin RNAs, ~120 bp long, driven by a RNA polymerase II promoter (S. mansoni actin) targeting S. mansoni cathepsin B in adult worms delivers strong silencing (Tchoubrieva et al. 2010). On the other hand, in many species, including insects, mammals, birds and pathogenic protozoa, Pol III promoter-based DNA vectors have been employed to express small interfering RNA or short hairpin RNA (shRNA) (~21 bp long) (Brummelkamp et al. 2002, Wakiyama et al. 2005). Aiming to establish vector-based RNAi driven by a Pol III promoter, Ayuk et al. (2011) cloned S. mansoni and human U6 gene promoters (~270 bp) into pLNHX driving shR-NA targeting firefly luciferase. These workers targeted luciferase because the effect of RNAi against luciferase can be readily discerned (in contrast to many endogenous genes) (Rinaldi et al. 2008, Ayuk et al. 2011). The findings reveal that the putative S. mansoni U6 gene promoter of 270 bp in length is active in schistosomes. Given that the U6 gene promoter drove expression of shRNA from an episome, the findings also indicate the potential of this putative RNA polymerase III dependent promoter as a component regulatory element in vector-based RNAi for functional genomics of schistosomes. Together the recent reports from Tchoubrieva et al. (2010) and Ayuk et al. (2011) demonstrate that vector based RNAi utilizing integration-competent and/or episomal vectors that carry transgene cassettes that include Pol II or Pol III promoters can provide sustained endogenous gene knockdown in schistosomes. This technology can be expected to find wide utility in functional genomics for schistosomes.
Outlook
Significant progress has been made in schistosome transgenesis (Grevelding 2006, Beckmann et al. 2007, Morales et al. 2007, Kines et al. 2008, 2010, Yang et al. 2010, Tchoubrieva et al. 2010, Ayuk et al. 2011, Rinaldi et al. 2011). Although the establishment of transgenic schistosome lines remains to be reported, transgene integration in the germ cells holds the promise for development of lines of transgenic schistosomes. Strategies for integrating constructs into the germ cells still need further development. Two methods now proven to insert transgenes into schistosome chromosomes, MLV retroviral mediated transgenesis and piggyBac transposon mediated transgenesis, have been successfully utilized for stable germ line transgenesis in vertebrate and invertebrate organisms (Burns et al. 1994, Franco et al. 1998, Jasinskiene et al. 1998, Jordan et al. 1998, Boulo et al. 2000, González-Estévez et al. 2003, Balu et al. 2005). These findings suggest that establishment of stable heritable lines of transgenic schistosomes could be achieved in the not too distant future. If so, schistosome transgenesis can be expected contribute substantially to functional genomics analysis of schistosomes.
Acknowledgments
Financial support: NIAID (RO1 AI072773, RO1 AI072773/S1), PAAET (Kuwait)
To Gabriel Rinaldi, Victoria Mann, Sutas Suttiprapa and Mary Ayuk, for informative discussions.
References
- Ayuk MA, Suttiprapa S, Rinaldi G, Mann VH, Lee CM, Brindley PJ. Schistosoma mansoni U6 gene promoter-driven short hairpin RNA induces RNA interference in human fibrosarcoma cells and schistosomules. Int J Parasitol. 2011;41:783–789. doi: 10.1016/j.ijpara.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu B, Shoue DA, Fraser MJ, Jr, Adams JH. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proc Natl Aca Sci USA. 2005;102:16391–16396. doi: 10.1073/pnas.0504679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann S, Wippersteg V, El-Bahay A, Hirzmann J, Oliveira G, Grevelding CG. Schistosoma mansoni: germ-line transformation approaches and actin-promoter analysis. Exp Parasitol. 2007;117:292–303. doi: 10.1016/j.exppara.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulo V, Cadoret JP, Shike H, Shimizu C, Miyanohara A, Burns JC. Infection of cultured embryo cells of the pacific oyster, Crassostrea gigas, by pantropic retroviral vectors. In Vitro Cell Dev Biol Anim. 2000;36:395–399. doi: 10.1290/1071-2690(2000)036<0395:IOCECO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, Pearce EJ. Genetic manipulation of schistosomes. Int J Parasitol. 2007;37:465–473. doi: 10.1016/j.ijpara.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Matsubara T, Lozinski G, Yee JK, Friedmann T, Washabaugh CH, Tsonis PA. Pantropic retroviral vector-mediated gene transfer, integration and expression in cultured newt limb cells. Dev Biol. 1994;165:285–289. doi: 10.1006/dbio.1994.1253. [DOI] [PubMed] [Google Scholar]
- Burns JC, McNeill L, Shimizu C, Matsubara T, Yee JK, Friedmann T, Kurdi-Haidar B, Maliwat E, Holt CE. Retrovirol gene transfer in Xenopus cell lines and embryos. In Vitro Cell Dev Biol Anim. 1996;32:78–84. doi: 10.1007/BF02723038. [DOI] [PubMed] [Google Scholar]
- Calmels B, Ferguson C, Laukkanen MO, Adler R, Faulhaber M, Kim HJ, Sellers S, Hematti P, Schmidt M, von Kalle C, Akagi K, Donahue RE, Dunbar CE. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood. 2005;106:2530–2533. doi: 10.1182/blood-2005-03-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capy P, Maruyama K, David JR, Hartl DL. Insertion sites of the transposable element mariner are fixed in the genome of Drosophila sechellia. J Mol Evol. 1991;33:450–456. doi: 10.1007/BF02103137. [DOI] [PubMed] [Google Scholar]
- Cary LC, Goebel M, Corsaro BG, Wang HG, Rosen E, Fraser MJ. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; New York: 1997. p. 843. [PubMed] [Google Scholar]
- Correnti JM, Brindley PJ, Pearce EJ. Long-term suppression of cathepsin B levels by RNA interference retards schistosome growth. Mol Biochem Parasitol. 2005;143:209–215. doi: 10.1016/j.molbiopara.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Correnti JM, Jung E, Freitas TC, Pearce EJ. Transfection of Schistosoma mansoni by electroporation and the description of a new promoter sequence for transgene expression. Int J Parasitol. 2007;37:1107–1115. doi: 10.1016/j.ijpara.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Correnti JM, Pearce EJ. Transgene expression in Schistosoma mansoni: introduction of RNA into schistosomula by electroporation. Mol Biochem Parasitol. 2004;137:75–79. doi: 10.1016/j.molbiopara.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Cort WW, Olivier L. The development of the sporocysts of a schistosome, Cercaria stagnicolae Talbot, 1936. J Parasitol. 1943;29:164–176. [Google Scholar]
- Davis RE, Parra A, LoVerde PT, Ribeiro E, Glorioso G, Hodgson S. Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proc Natl Acad Sci USA. 1999;96:8687–8692. doi: 10.1073/pnas.96.15.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcroix M, Sajid M, Caffrey CR, Lim KC, Dvorák J, Hsieh I, Bahgat M, Dissous C, McKerrow JH. A multienzyme network functions in intestinal protein digestion by a platyhelminth parasite. J Biol Chem. 2006;281:39316–39329. doi: 10.1074/jbc.M607128200. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Dinguirard N, Yoshino TP. Potential role of a CD36-like class B scavenger receptor in the binding of modified low-density lipoprotein (acLDL) to the tegumental surface of Schistosoma mansoni sporocysts. Mol Biochem Parasitol. 2006;146:219–230. doi: 10.1016/j.molbiopara.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elick TA, Bauser CA, Fraser MJ. Excision of the piggyBac transposable element in vitro is a precise event that is enhanced by the expression of its encoded transposase. Genetica. 1996;98:33–41. doi: 10.1007/BF00120216. [DOI] [PubMed] [Google Scholar]
- Faghiri Z, Skelly PJ. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake. FASEB J. 2009;23:2780–2789. doi: 10.1096/fj.09-130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschotte C, Pritham EJ. DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet. 2007;41:331–368. doi: 10.1146/annurev.genet.40.110405.090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Franco M, Rogers ME, Shimizu C, Shike H, Vogt RG, Burns JC. Infection of lepidoptera with a pseudotyped retroviral vector. Insect Biochem Mol Biol. 1998;28:819–825. doi: 10.1016/s0965-1748(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Fraser MJ, Ciszczon T, Elick T, Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol. 1996;5:141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Fraser MJ, Smith GE, Summers MD. Acquisition of host cell DNA sequences by Baculoviruses: relationship between host DNA insertions and FP mutants of Autographa californica and Galleria mellonella nuclear polyhedrosis viruses. J Virol. 1983;47:287–300. doi: 10.1128/jvi.47.2.287-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas TC, Jung E, Pearce EJ. TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni. PLoS Pathog. 2007;3:e52. doi: 10.1371/journal.ppat.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldhof P, Visser A, Clark D, Saunders G, Britton C, Gilleard J, Berriman M, Knox D. RNA interference in parasitic helminths: current situation, potential pitfalls and future prospects. Parasitology. 2007;134:609–619. doi: 10.1017/S0031182006002071. [DOI] [PubMed] [Google Scholar]
- Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ, Bell JB, Largaespada DA, Hackett PB. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003;8:108–117. doi: 10.1016/s1525-0016(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Schaack S, Pace JK, 2nd, Brindley PJ, Feschotte C. A role for host-parasite interactions in the horizontal transfer of transposons across phyla. Nature. 2010;464:1347–1350. doi: 10.1038/nature08939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Estévez C, Momose T, Gehring WJ, Saló E. Transgenic planarian lines obtained by electroporation using transposon-derived vectors and an eye-specific GFP marker. Proc Natl Acad Sci USA. 2003;100:14046–14051. doi: 10.1073/pnas.2335980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevelding CG. Transgenic flatworms in parasitic flatworms. In: Maule AG, Marks NJ, editors. Molecular biology, biochemistry, immunology and physiology. CABI; Wallingford: 2006. pp. 149–179. [Google Scholar]
- Handler AM. Use of the piggyBac transposon for germ-line transformation of insects. Insect Biochem Mol Biol. 2002;32:1211–1220. doi: 10.1016/s0965-1748(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Handler AM, McCombs SD, Fraser MJ, Saul SH. The lepidopteran transposon vector, piggyBac, mediates germ-line transformation in the Mediterranean fruit fly. Proc Natl Acad Sci USA. 1998;95:7520–7525. doi: 10.1073/pnas.95.13.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyers O, Walduck AK, Brindley PJ, Bleiss W, Lucius R, Dorbic T, Wittig B, Kalinna BH. Schistosoma mansoni miracidia transformed by particle bombardment infect Biomphalaria glabrata snails and develop into transgenic sporocysts. Exp Parasitol. 2003;105:174–178. doi: 10.1016/j.exppara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Jacobson JW, Medhora MM, Hartl DL. Molecular structure of a somatically unstable transposable element in Drosophila. Proc Natl Acad Sci USA. 1986;83:8684–8688. doi: 10.1073/pnas.83.22.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan TV, Shike H, Boulo V, Cedeno V, Fang Q, Davis BS, Jacobs-Lorena M, Higgs S, Fryxell KJ, Burns JC. Pantropic retroviral vectors mediate somatic cell transformation and expression of foreign genes in dipteran insects. Insect Mol Biol. 1998;7:215–222. doi: 10.1111/j.1365-2583.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- Jurberg AD, Gonçalves T, Costa TA, de Mattos AC, Pascarelli BM, de Manso PP, Ribeiro-Alves M, Pelajo-Machado M, Peralta JM, Coelho PM, Lenzi HL. The embryonic development of Schistosoma mansoni eggs: proposal for a new staging system. Dev Genes Evol. 2009;219:219–234. doi: 10.1007/s00427-009-0285-9. [DOI] [PubMed] [Google Scholar]
- Kalinna BH, Brindley PJ. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends Parasitol. 2007;23:197–204. doi: 10.1016/j.pt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kapitonov VV, Jurka J. Rolling-circle transposons in eukaryotes. Proc Natl Acad Sci USA. 2001;98:8714–8719. doi: 10.1073/pnas.151269298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitonov VV, Jurka J. Self-synthesizing DNA transposons in eukaryotes. Proc Natl Acad Sci USA. 2006;103:4540–4545. doi: 10.1073/pnas.0600833103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsi A, Moav B, Hackett P, Liu Z. Effects of insert size on transposition efficiency of the sleeping beauty transposon in mouse cells. Mar Biotechnol (NY) 2001;3:241–245. doi: 10.1007/s101260000072. [DOI] [PubMed] [Google Scholar]
- Kines KJ, Mann VH, Morales ME, Shelby BD, Kalinna BH, Gobert GN, Chirgwin SR, Brindley PJ. Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus. Exp Parasitol. 2006;112:209–220. doi: 10.1016/j.exppara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kines KJ, Morales ME, Mann VH, Gobert GN, Brindley PJ. Integration of reporter transgenes into Schistosoma mansoni chromosomes mediated by pseudotyped murine leukemia virus. FASEB J. 2008;22:2936–2948. doi: 10.1096/fj.08-108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines KJ, Rinaldi G, Okatcha TI, Morales ME, Mann VH, Tort JF, Brindley PJ. Electroporation facilitates introduction of reporter transgenes and virions into schistosome eggs. PLoS Negl Trop Dis. 2010;4:e593. doi: 10.1371/journal.pntd.0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautz-Peterson G, Skelly PJ. Schistosoma mansoni: the dicer gene and its expression. Exp Parasitol. 2008a;118:122–128. doi: 10.1016/j.exppara.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautz-Peterson G, Skelly PJ. Schistosome asparaginyl endo-peptidase (legumain) is not essential for cathepsin B1 activation in vivo. Mol Biochem Parasitol. 2008b;159:54–58. doi: 10.1016/j.molbiopara.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe DJ, Churchill ME, Robertson HM. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO J. 1996;15:5470–5479. [PMC free article] [PubMed] [Google Scholar]
- Lampe DJ, Grant TE, Robertson HM. Factors affecting transposition of the Himar1 mariner transposon in vitro. Genetics. 1998;149:179–187. doi: 10.1093/genetics/149.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, Haase J, Charles I, Maskell DJ, Peters SE, Dougan G, Wain J, Parkhill J, Turner AK. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009;19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Gaiano N, Culp P, Burns JC, Friedmann T, Yee JK, Hopkins N. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science. 1994;265:666–669. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- Lu JK, Chen TT, Allen SK, Matsubara T, Burns JC. Production of transgenic dwarf surfclams, Mulinia lateralis, with pantropic retroviral vectors. Proc Natl Acad Sci USA. 1996;93:3482–3486. doi: 10.1073/pnas.93.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ivics Z, Izsvák Z, Bradley A. Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc Natl Acad Sci USA. 1998;95:10769–10773. doi: 10.1073/pnas.95.18.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VH, Morales ME, Kines KJ, Brindley PJ. Transgenesis of schistosomes: approaches employing mobile genetic elements. Parasitology. 2008;135:141–153. doi: 10.1017/S0031182007003824. [DOI] [PubMed] [Google Scholar]
- Mann VH, Morales ME, Rinaldi G, Brindley PJ. Culture for genetic manipulation of developmental stages of Schistosoma mansoni. Parasitology. 2010;137:451–462. doi: 10.1017/S0031182009991211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VH, Suttiprapa S, Rinaldi G, Brindley PJ. Establishing transgenic schistosomes. PLoS Negl Trop Dis. 2011;5:e-1230. doi: 10.1371/journal.pntd.0001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Beeman RW, Shike H, Besansky NJ, Mukabayire O, Higgs S, James AA, Burns JC. Pantropic retroviral vectors integrate and express in cells of the malaria mosquito, Anopheles gambiae. Proc Natl Acad Sci USA. 1996;93:6181–6185. doi: 10.1073/pnas.93.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Maruyama K, Hartl DL. Molecular and functional analysis of the mariner mutator element Mos-1 in Drosophila. Genetics. 1991;128:311–318. doi: 10.1093/genetics/128.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskey C, Izsvák Z, Kawakami K, Ivics Z. DNA transposons in vertebrate functional genomics. Cell Mol Life Sci. 2005;62:629–641. doi: 10.1007/s00018-004-4232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales ME, Mann VH, Kines KJ, Gobert GN, Fraser MJ, Jr, Kalinna BH, Correnti JM, Pearce EJ, Brindley PJ. piggyBac transposon mediated transgenesis of the human blood fluke, Schistosoma mansoni. FASEB J. 2007;21:3479–3489. doi: 10.1096/fj.07-8726com. [DOI] [PubMed] [Google Scholar]
- Morales ME, Rinaldi G, Gobert GN, Kines KJ, Tort JF, Brindley PJ. RNA interference of Schistosoma mansoni cathepsin D, the apical enzyme of the hemoglobin proteolysis cascade. Mol Biochem Parasitol. 2008;157:160–168. doi: 10.1016/j.molbiopara.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T, Bower TM, Brown AE, Crisanti A, Catteruccia F. piggyBac-mediated germline transformation of the malaria mosquito Anopheles stephensi using the red fluorescent protein dsRED as a selectable marker. J Biol Chem. 2002;277:8759–8762. doi: 10.1074/jbc.C100766200. [DOI] [PubMed] [Google Scholar]
- Olivier L, Mao CP. The early larval stages of Schistosoma mansoni Sambon, 1907 in the snail host, Australorbis glabratus (Say, 1818) J Parasitol. 1949;35:267–275. [PubMed] [Google Scholar]
- Osman A, Niles EG, Verjovski-Almeida S, LoVerde PT. Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathog. 2006;2:e54. doi: 10.1371/journal.ppat.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan SC. The fine structure of the miracidium of Schistosoma mansoni. J Invertebr Pathol. 1980;36:307–372. doi: 10.1016/0022-2011(80)90040-3. [DOI] [PubMed] [Google Scholar]
- Pan SC. Schistosoma mansoni: the ultrastructure of larval morphogenesis in Biomphalaria glabrata and of associated host-parasite interactions. Jpn J Med Sci Biol. 1996;49:129–149. doi: 10.7883/yoken1952.49.129. [DOI] [PubMed] [Google Scholar]
- Peloquin JJ, Thibault ST, Staten R, Miller TA. Germline transformation of pink bollworm (Lepidoptera: gelechiidae) mediated by the piggyBac transposable element. Insect Mol Biol. 2000;9:323–333. doi: 10.1046/j.1365-2583.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- Plasterk RH, Izsvák Z, Ivics Z. Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends Genet. 1999;15:326–332. doi: 10.1016/s0168-9525(99)01777-1. [DOI] [PubMed] [Google Scholar]
- Poeschla EM. Integrase, LEDGF/p75 and HIV replication. Cell Mol Life Sci. 2008;65:1403–1424. doi: 10.1007/s00018-008-7540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritham EJ, Putliwala T, Feschotte C. Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene. 2007;390:3–17. doi: 10.1016/j.gene.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Que X, Kim D, Alagon A, Hirata K, Shike H, Shimizu C, Gonzalez A, Burns JC, Reed SL. Pantropic retroviral vectors mediate gene transfer and expression in Entamoeba histolytica. Mol Biochem Parasitol. 1999;99:237–245. doi: 10.1016/s0166-6851(99)00021-3. [DOI] [PubMed] [Google Scholar]
- Rinaldi G, Morales ME, Cancela M, Castillo E, Brindley PJ, Tort JF. Development of functional genomic tools in trematodes: RNA interference and luciferase reporter gene activity in Fasciola hepatica. PLoS Negl Trop Dis. 2008;2:e260. doi: 10.1371/journal.pntd.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi G, Suttiprapa S, Brindley PJ. Quantitative retrotransposon anchored PCR confirms transduction efficiency of transgenes in adult Schistosoma mansoni. Mol Biochem Parasitol. 2011;177:70–76. doi: 10.1016/j.molbiopara.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium . The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hayflick, his limit and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Sherman A, Dawson A, Mather C, Gilhooley H, Li Y, Mitchell R, Finnegan D, Sang H. Transposition of the Drosophila element mariner into the chicken germ line. Nat Biotechnol. 1998;16:1050–1053. doi: 10.1038/3497. [DOI] [PubMed] [Google Scholar]
- Skelly PJ, Da’dara A, Harn DA. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int J Parasitol. 2003;33:363–369. doi: 10.1016/s0020-7519(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. Viruses and human disease. Academic Press; San Diego: 2002. p. 383. [Google Scholar]
- Sumitani M, Yamamoto DS, Oishi K, Lee JM, Hatakeyama M. Germline transformation of the sawfly, Athalia rosae (Hymenoptera: Symphyta), mediated by a piggyBac-derived vector. Insect Biochem Mol Biol. 2003;33:449–458. doi: 10.1016/s0965-1748(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Tamura T, Thibert C, Royer C, Kanda T, Abraham E, Kamba M, Komoto N, Thomas JL, Mauchamp B, Chavancy G, Shirk P, Fraser M, Prudhomme JC, Couble P. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Tchoubrieva EB, Ong PC, Pike RN, Brindley PJ, Kalinna BH. Vector-based RNA interference of cathepsin B1 in Schistosoma mansoni. Cell Mol Life Sci. 2010;67:3739–3748. doi: 10.1007/s00018-010-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Brake O, Konstantinova P, Ceylan M, Berkhout B. Silencing of HIV-1 with RNA interference: a multiple shRNA approach. Mol Ther. 2006;14:883–892. doi: 10.1016/j.ymthe.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Verjovski-Almeida S, DeMarco R, Martins EA, Guimarães PE, Ojopi EP, Paquola AC, Piazza JP, Nishiyama MY, Jr, Kitajima JP, Adamson RE, Ashton PD, Bonaldo MF, Coulson PS, Dillon GP, Farias LP, Gregorio SP, Ho PL, Leite RA, Malaquias LC, Marques RC, Miyasato PA, Nascimento AL, Ohlweiler FP, Reis EM, Ribeiro MA, Sá RG, Stukart GC, Soares MB, Gargioni C, Kawano T, Rodrigues V, Madeira AM, Wilson RA, Menck CF, Setubal JC, Leite LC, Dias-Neto E. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–157. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- Wakiyama M, Matsumoto T, Yokoyama S. Drosophila U6 promoter-driven short hairpin RNAs effectively induce RNA interference in Schneider 2 cells. Biochem Biophys Res Commun. 2005;331:1163–1170. doi: 10.1016/j.bbrc.2005.03.240. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Coates CJ, George AL., Jr piggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Kaminski JM, George AL., Jr Functional zinc finger/sleeping beauty transposase chimeras exhibit attenuated overproduction inhibition. FEBS Lett. 2005;579:6205–6209. doi: 10.1016/j.febslet.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wippersteg V, Kapp K, Kunz W, Grevelding CG. Characterisation of the cysteine protease ER60 in transgenic Schistosoma mansoni larvae. Int J Parasitol. 2002;32:1219–1224. doi: 10.1016/s0020-7519(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Yang S, Brindley PJ, Zeng Q, Li Y, Zhou J, Liu Y, Liu B, Cai L, Zeng T, Wei Q, Lan L, McManus DP. Transduction of Schistosoma japonicum schistosomules with vesicular stomatitis virus glycoprotein pseudotyped murine leukemia retrovirus and expression of reporter human telomerase reverse transcriptase in the transgenic schistosomes. Mol Biochem Parasitol. 2010;174:109–116. doi: 10.1016/j.molbiopara.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino TP, Laursen JR. Production of Schistosoma mansoni daughter sporocysts from mother sporocysts maintained in synxenic culture with Biomphalaria glabrata embryonic (Bge) cells. J Parasitol. 1995;81:714–722. [PubMed] [Google Scholar]
- Zayed H, Izsvák Z, Walisko O, Ivics Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther. 2004;9:292–304. doi: 10.1016/j.ymthe.2003.11.024. [DOI] [PubMed] [Google Scholar]