INTRODUCTION
Foamy viruses (FVs), or spumaviruses, are nonpathogenic retroviruses that have been developed as integrating viral vectors. This protocol presents methods for producing high-titer FV vector stocks, free of contaminating replication-competent retrovirus, to be used for transducing hematopoietic stem cells. FV vector stocks are produced by transfecting 293 cells, harvesting and filtering the culture medium, and concentrating vector virions by ultracentrifugation. The resulting stocks are free of replication-competent helper virus, as indicated by a sensitive marker rescue assay. A typical stock made from 23 10-cm dishes has a final volume of 2 mL with a liter of 107 to 108 transducing units/mL. Potential advantages of FV vectors include a lack of pathogenicity of the wild-type virus, a wide host range, stable virions that can be concentrated by centrifugation, a double-stranded DNA genome that is reverse-transcribed in the vector-producing cells, and the largest packaging capacity of any retrovirus. FV vectors are especially useful for transducing hematopoietic cells. Because hematopoietic stem cells have the ability to self-renew, proliferate, and repopulate the bone marrow after transplantation, efficient transduction of these cells offers the promise to cure many inherited and acquired diseases.
RELATED INFORMATION
The FV vector production protocol presented here is a four-plasmid transient transfection system (Fig. 1; Trobridge et al. 2002a). For additional details on the procedure, see Trobridge and Russell (1998) and Trobridge et al. (2002b).
FIGURE 1.
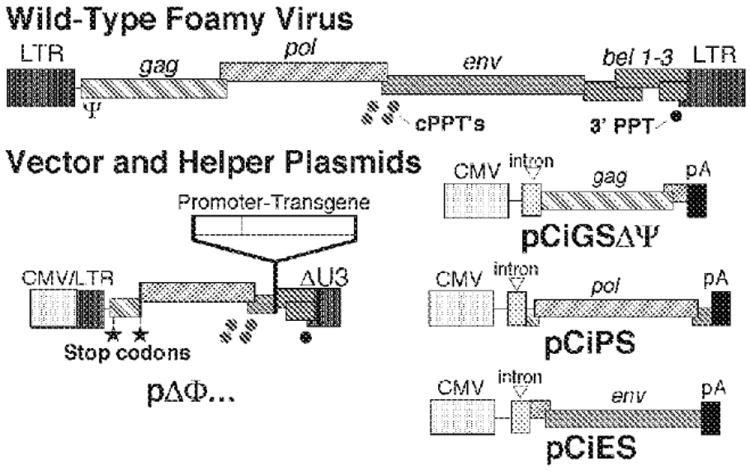
Four-plasmid FV vector production system. Wild-type FV genomes contain gag, pol, and env genes, as do other retroviral genomes, and additional bel genes between env and the viral long terminal repeats (LTRs). The bel1 or tas gene, transcribed from an internal promoter in env (Lochelt et al. 1993), encodes a transcriptional activator required for expression from the LTR promoter (Keller et al. 1991; Rethwilm et al. 1991; Venkatesh et al. 1991). The pΔΦ (deleted foamy) vector plasmid retains only essential cis-acting sequences, including the packaging signal Ψ, a cis-acting region at the 3′ end of pol that includes central polypurine tract (cPPT) sequences, and the 3 PPT sequence. A hybrid cytomegalovirus (CMV)-LTR fusion promoter drives transcription of the vector genome. Stop codons are introduced into the remaining 5 portion of gag so that the vector does not encode any viral gene products. There are separate helper plasmids for gag, pol, and env. The vector transgene is driven by an internal promoter.
MATERIALS
CAUTIONS AND RECIPES: Please see Appendices for appropriate handling of materials marked with <!>, and recipes for reagents marked with <R>.
Reagents
Use sterile technique.
Bovine serum albumin (BSA), Fraction V
<!>CaCl2 (2.0 M)
Filter-sterilize. Store in aliquots at 4 °C.
Cells, 293
Many different subclones of 293 cells are available (Graham et al. 1977); use a subclone known to have high transfection efficiencies. Freeze multiple vials to ensure reproducibility. A single frozen vial can be thawed and kept in growing culture for 4-6 wk for use in transfection. (After this time, thaw a new vial.) Do not allow cells to overgrow when expanding before transfection.
<!>Chloroquine (100 mM)
Filter-sterilize. Store in aliquots at 4 °C.
<R>Cytokine medium
<R>D10
<!>DMSO (dimethyl sulfoxide; 5%) (optional; see Step 11)
Dulbecco’s modified Eagle’s medium (DMEM)
Fetal bovine serum (FBS), heat-inactivated (e.g., 30 min at 56°C)
Hematopoietic cells
Human CD34+ cells from marrow, peripheral blood, or umbilical cord blood can be isolated with magnetic beads using commercially available reagents and protocols (e.g., Miltenyi Biotec). Murine hematopoietic cells can be obtained from bone marrow and lineage-depleted with the Miltenyi system. Stem cells can be enriched for by pretreatment of donor mice with 5-fluorouracil (Vassilopoulos et al. 2001).
<R>HEPES saline (2X)
Human fibronectin fragment CH-296 (1 mg/mL; RetroNectin; Takara Bio)
Sterilize by passing through a 0.22-μm filter. Store at -20°C.
<R>Phosphate mix
<R>Phosphate-buffered saline (PBS), calcium- and magnesium-free
Plasmid DNA
The system (Fig. 1) includes ΔΦ vector plasmid (containing the desired transgene) and the helpher plasmids pCiGSΔΨ, and pCiES. Purify plasmid DNAs with QIAGEN kits or on CsCl gradients, extract with phenol and chloroform, and precipitate with ethanol.
<R>Resuspend in TE buffer (pH 8.0). Plasmids can be heat-treated for 30 min at 68°C to destroy most bacteria that might contaminate the preparation.
Sodium butyrate (500 mM in DMEM)
Filter-sterilize. Store in aliquots at -20°C.
Equipment
Centrifuge, benchtop
Dishes, tissue culture, 10-cm
Filter unit, polyvinylidene fluoride (PVDF), radio-sterilized, 0.45-μm, 250-mL (SCHVU02RE; Millipore)
Incubator, tissue culture
Micropipettor and tips
Microscope, inverted
Plates, nontissue culture-treated, six-well
Slide-A-Lyzer cassette, 10K MW cutoff (Pierce/Thermo Scientific 66450) (optional; see Step 13)
Tubes, centrifuge, conical, several 50-mL or two 250-mL
Ultracentrifuge, equipped with a Beckman SW 28 rotor and tubes
Vortex mixer
Water bath preset to 37°C (optional; see Step 13.i and 15)
METHOD
FV Vector Stock Production
Twenty-four hours before transfection, seed each of 23 tissue culture dishes (10 cm) with 3.25 × 106 293 cells in 10 mL of D10. Grow the cells overnight.
-
Transfect the cells using a calcium phosphate-DNA precipitate:
- Prepare 9.2 mL of DNA solution:
CaCl2 (2.0 M) 1.15 mL ΔΦ vector plasmid (containing the desired transgene) 272 μg pCiGSΔΨ 272 μg pCiPS 34.5 μg pCiES 16.9 μg Sterile H2O 8.05 mL Add 92 μL of phosphate mix to 9.1 mL of 2X HEPES saline.
Add the DNA solution dropwise to the HEPES solution while vortexing gently.
Once mixed, immediately add 64 μL of 100 mM chloroquine (to a final concentration of 25 μM).
Incubate the precipitate for 10 min.
-
Add 800 μL of the precipitate directly to each 10-cm dish. Gently rock the plate to spread the precipitate.
Avoid dislodging the cells.
Incubate the cells for 4-6 h to allow them to take up the DNA.
Add 200 μL of 500 mM sodium butyrate to each dish. Continue incubating overnight.
The following morning, replace the medium with fresh, prewarmed D10 (avoid dislodging the cells). Continue incubating for 48 h.
Collect the culture medium into two 250-mL conical centrifuge tubes (or several 50-mL tubes).
Centrifuge at ~300g for 5 min. Remove the supernatant.
Filter the supernatant through the 250-mL, 0.45-μm filter unit.
Transfer the supernatant to Beckman SW 28 rotor tubes (36 mL/tube). Centrifuge at ~50,000g for 2 h at 20°C.
Carefully aspirate the supernatant, avoiding the bottom of the tube.
Before the pellets dry, add 250 μL of the tissue culture medium (serum-containing medium is acceptable) to be used in subsequent transductions to each centrifuge tube.
Solubilize the vector pellet by pipetting repeatedly while minimizing the generation of foam.
-
Combine the resolubilized pellets by transferring from one tube to the next. Wash the tubes with 500 μL of tissue culture medium; combine this wash with the resuspensions.
The pooled pellets should have a final volume of 2.0 mL. Use the stock fresh (see Step 14) or store in aliquots at -80°C in the presence of 5% DMSO. Minimize direct contact of pure DMSO with stocks by resuspending pellets in medium containing 5% DMSO or by dripping in pure DMSO while agitating.
See Troubleshooting.
Transduction of Hematopoietic Cells
This protocol is optimized for transduction of hematopoietic repopulating cells in nontissue culture-treated six-well plates. If dishes or wells with a different surface area are used, scale the cell density and reagent volumes accordingly.
-
12
Coat nontissue culture-treated six-well plates with RetroNectin:
Dilute the RetroNectin to a final concentration of 50 μg/mL in PBS.
Add 1 mL of the diluted solution to each well.
Incubate the plates for 2 h at room temperature.
Remove the RetroNectin solution. Add 2 mL of PBS containing 2% BSA.
Incubate the plates for 30 min.
-
Wash the wells with 2 mL of PBS.
The coated dishes are now ready to use.
-
13
(Optional) Prepare frozen vector stocks (from Step 11):
Thaw frozen stock quickly in a 37°C water bath.
Reduce the final DMSO concentration to ≤1%, either by dilution or by sterile dialysis in a Slide-A-Lyzer cassette in 500 mL of DMEM for 2 h at room temperature.
-
14
Prepare transduction medium by adjusting the volume of freshly concentrated (from Step 11) or dialyzed/diluted (from Step 13.ii) vector stock with cytokine medium such that the multiplicity of infection in the final transduction mix (see Step 15) is 1-20 transducing particles/cell.
-
15
Resuspend hematopoietic cells in transduction medium prepared in Step 14 at a concentration of 0.5-1.0 × 106 cells/mL. If using cryopreserved cells, prepare them as follows:
Thaw the cryopreserved cells in a 37°C water bath just before transduction.
Wash the cells in 10 mL of DMEM supplemented with 20% heat-inactivated FBS.
Centrifuge at 220g for 5 min.
Resuspend the cells in vector-containing transduction medium.
-
16
Add 1.5 mL of transduction medium with cells (i.e., 0.75-1.5 × 106 cells/well) to the RetroNectin-coated wells (from Step 12.vi). Place the plates in a tissue culture incubator for 10-24 h.
-
17
Following transduction, detach the cells from the plate surface by pipetting the medium to remove weakly attached cells. Harvest the loosened cells.
-
18
Wash each well with 2.0 mL of PBS. Combine the wash with the initial collection from Step 17. Examine the wells under a microscope to confirm that all the cells are collected.
Transduced cells can he grown in liquid culture, plated in progenitor colony assays, or used in animal transplantation experiments.
TROUBLESHOOTING
Problem: Transfection efficiency is low.
[Step 11]
Solution: The pH of the HEPES saline solution can be critical in determining transfection efficiency. If titers are low, prepare a series of solutions with pH values ranging from 7.0 to 7.2. To identify the best pH, test the solutions by transfecting a simple reporter gene plasmid.
References
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Keller A, Partin KM, Lochelt M, Bannert H, Flugel RM, Cullen BR. Characterization of the transcriptional trans-activator of human foamy retrovirus. J Virol. 1991;65:2589–2594. doi: 10.1128/jvi.65.5.2589-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochelt M, Muranyi W, Flugel RM. Human foamy virus genome possesses an internal, Bel-1-dependent and functional promoter. Proc Natl Acad Sci. 1993;90:7317–7321. doi: 10.1073/pnas.90.15.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethwilm A, Erlwein O, Baunach G, Maurer B, ter Meulen V. The transcriptional transactivator of human foamy virus maps to the bel 1 genomic region. Proc Natl Acad Sci. 1991;88:941–945. doi: 10.1073/pnas.88.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Russell DW. Helper-free foamy virus vectors. Hum Gene Ther. 1998;9:2517–2525. doi: 10.1089/hum.1998.9.17-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge G, Josephson N, Vassilopoulos G, Mac J, Russell DW. Improved foamy virus vectors with minimal viral sequences. Mol Ther. 2002a;6:321–328. doi: 10.1006/mthe.2002.0672. [DOI] [PubMed] [Google Scholar]
- Trobridge G, Vassilopoulos G, Josephson N, Russell DW. Gene transfer with foamy virus vectors. Methods Enzymol. 2002b;346:628–648. doi: 10.1016/s0076-6879(02)46082-x. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Trobridge G, Josephson NC, Russell DW. Gene transfer into murine hematopoietic stem cells with helper-free foamy virus vectors. Blood. 2001;98:604–609. doi: 10.1182/blood.v98.3.604. [DOI] [PubMed] [Google Scholar]
- Venkatesh LK, Theodorakis PA, Chinnadurai G. Distinct cis-acting regions in U3 regulate trans-activation of the human spumaretrovirus long terminal repeat by the viral bel1 gene product. Nucleic Acids Res. 1991;19:3661–3666. doi: 10.1093/nar/19.13.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]


