Abstract
Objective
The transcription factor 7-like 2 (TCF7L2) gene has been suggested to play an important role in the pathogenesis of cancer. However, the results have been inconsistent. In this study, we performed a meta-analysis to clarify the associations between TCF7L2 polymorphism and cancer risk.
Methods
Published literature from PubMed and EMBASE were retrieved. Pooled odds ratios (ORs) with 95% confidence interval (CIs) were calculated using fixed- or random-effects model.
Results
A total of 19 studies (14,814 cases and 33,856 controls) were identified for the analysis of the association between TCF7L2 polymorphism and cancer risk. The results showed that TCF7L2 polymorphism was associated with breast cancer (Homogeneous model: OR = 1.17, 95%CI = 1.02–1.35, I 2 = 21.8%, p for heterogeneity = 0.276; Heterogeneous model: OR = 1.11, 95%CI = 1.03–1.20, I 2 = 0.0%, p for heterogeneity = 0.543), prostate cancer (Homogeneous model: OR = 0.89, 95%CI = 0.84–0.96, I 2 = 0.0%, p for heterogeneity = 0.640; Heterogeneous model: OR = 0.89, 95%CI = 0.84–0.95, I 2 = 0.0%, p for heterogeneity = 0.871), and colon cancer (Heterogeneous model: OR = 1.15, 95%CI = 1.01–1.31, I 2 = 0.0%, p for heterogeneity = 0.658), but not with colorectal cancer, lung cancer, and ovarian cancer.
Conclusions
The present meta-analysis indicated that there were significantly associations between the TCF7L2 rs7903146 polymorphism and risk of breast, prostate and colon cancers, rather than colorectal cancer, lung cancer, and ovarian cancer.
Introduction
The transcription factor 7-like 2 (TCF7L2) gene, previously reported as TCF-4, has been found to be associated with type 2 diabetes. Rs7903146 variant of TCF7L2 gene was firstly identified as one susceptibility marker of type 2 diabetes by genome-wide association study [1]. The following studies further confirmed the association between TCF7L2 rs7903146 variant and type 2 diabetes [2]. In addition, individuals carrying T alleles of TCF7L2 rs7903146 variant demonstrated high risk of insulin resistance [3]. Alternatively, TCF7L2 may affect cancer independently of diabetes, as the TCF7L2 gene product is involved the Wnt/β-catenin signaling pathway. TCF7L2 forms an active nuclear complex with β-catenin that binds and induces the expression of target genes involved in cellular proliferation, evasion of apoptosis, and tissue invasion and metastasis.
To date, many studies have been published investigating the association between TCF7L2 rs7903146 or rs12255372 (it is in high linkage disequilibrium with rs7903146) and several types of cancer, including breast cancer [4]–[8], prostate cancer [5], [9]–[12], colorectal cancer [5], [13]–[15], colon cancer [5], [16], lung cancer [5] and ovarian cancer [6]. However, the conclusions have been conflicting. Therefore, we performed a meta-analysis to clarify the association between TCF7L2 rs7903146 variant and cancer risk.
Materials and Methods
Literature and Search Strategy
We searched the PubMed and EMBASE literature databases. The search strategy to identify all possible studies involved the use of the following key words: (TCF7L2 or transcription factor 7-like 2 or TCF-4) and (variant or variation or polymorphism or genotype) and (cancer or carcinoma or tumor). All related studies published in English language were included. The reference lists of retrieved articles were hand-searched. If more than one article were published using the same case series, only the study with the latest data was included. The literature search was updated on February 18, 2013.
Inclusion Criteria and Data Extraction
The studies included in the meta-analysis must meet all the following inclusion criteria: (1) evaluates the associations of TCF7L2 polymorphism with cancer risk; (2) uses case–control or cohort design; and (3) provides sufficient data for calculation of odds ratio (OR) with 95% confidence interval (CI). The following information was extracted from each study: (1) name of the first author; (2) year of publication; (3) country of origin; (4) ethnicity; (5) cancer type; (6) sample size of cases and controls; (7) covariates’ adjusted OR with 95%CI under co-dominant model; (8) minor allele frequency in cases and controls; (9) p for Hardy Weinberg Equilibrium test in controls; and (10) studied polymorphism. The two authors independently assessed the articles for compliance with the inclusion/exclusion criteria, resolved disagreements and reached a consistent decision.
Statistical Analysis
The association of TCF7L2 polymorphism with cancer risk was estimated by calculating pooled ORs and 95%CIs under a co-dominant model. The significance of pooled OR was determined by Z test (P<0.05 was considered statistically significant). Q test was performed to evaluate the between-study heterogeneity. A random- (DerSimonian-Laird method [17] or fixed- (Mantel–Haenszel method) [18] effects model was used to calculate pooled OR in the presence (P≤0.10) or absence (P>0.10) of heterogeneity, respectively. Subgroup analysis by cancer type was performed to address the between-study heterogeneity. Publication bias was assessed by Begg’s test [19] and Egger’s test [20] (P<0.05 was considered statistically significant). Data analysis was performed using STATA version 11 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the Studies
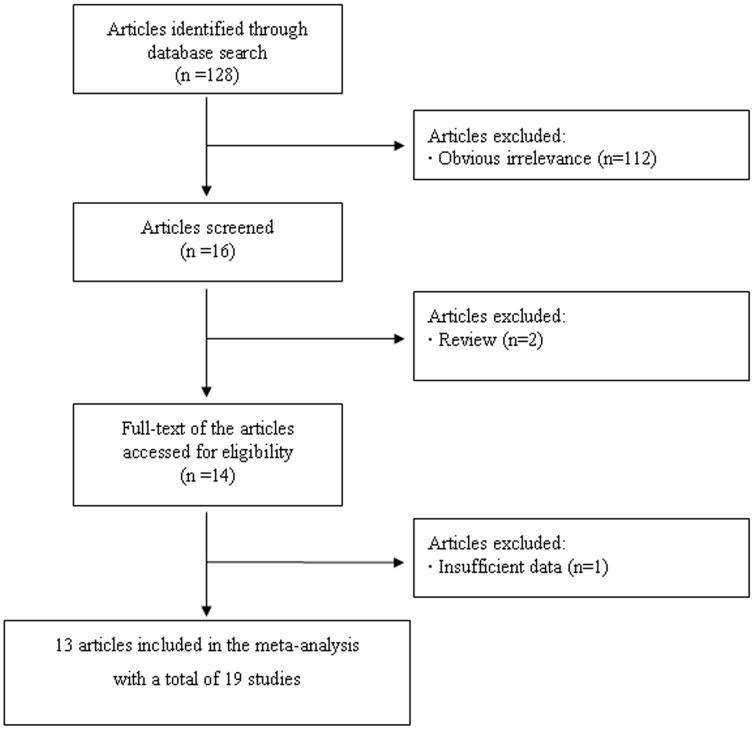
In this study, we followed the PRISMA Statement (Checklist S1). A flow chart describing the process of inclusion/exclusion of study is presented in Fig. 1. The literature search identified a total of 128 potentially relevant papers. Of them, 112 papers were excluded because of obvious irrelevance by reading the titles and abstracts. In addition, two papers were excluded because they were reviews. Then, 14 papers met the primary inclusion criteria. However, one paper was excluded because it did not provide sufficient data to calculate OR with 95%CI [21]. Since more than one study was included in two articles by Folsom et al [5] and Goode et al [6], they were considered as separate studies in the meta-analysis. In addition, since rs7903146 was in high LD with rs12255372, we combined all the included studies together. At last, 19 studies for the association between TCF7L2 polymorphism and cancer risk were included in the final meta-analysis. Of them, five were on breast cancer, five were on prostate cancer, four were on colorectal cancer, two were on colon cancer, two were on lung cancer, and one was on ovarian cancer. The characteristics of the included studies are listed in Table 1. It should be noted that because most studies provided covariates’ adjusted OR with 95%CI under a co-dominant model, we calculated the pooled estimate under this model only.
Figure 1. Flow chart of meta-analysis for exclusion/inclusion of individual articles (or studies).
Table 1. Characteristics of included studied of association between TCF7L2 rs7903146 (or its proxy rs12255372) polymorphism and cancer risk.
| Study | Country | Ethnicity | Cancer type | Sample size | Homogeneous co-dominant model | Heterogeneous co-dominant model | MAF | P for HWE | Studied SNP | ||||||
| Cases | Controls | ORa | 95% CI | ORa | 95% CI | Cases | Controls | ||||||||
| Burwinkel,2006 | Germany | European | Breast cancer | 592 | 735 | 1.37 | 0.91 | 2.08 | 1.21 | 0.97 | 1.53 | 0.29 | 0.26 | 0.643 | rs12255372 |
| Agalliu,2008 | USA | European | Prostate cancer | 582 | 540 | 0.73 | 0.44 | 1.20 | 0.94 | 0.72 | 1.23 | 0.27 | 0.29 | 0.777 | rs12255372 |
| Folsom,2008a | USA | Mixed | Colorectal cancer | 180 | 11410 | 1.56 | 0.97 | 2.53 | 1.17 | 0.85 | 1.61 | 0.34 | 0.29 | 0.806 | rs7903146 |
| Folsom,2008b | USA | Mixed | Colon cancer | 128 | 2.15 | 1.27 | 3.64 | 1.25 | 0.85 | 1.83 | 0.36 | 0.29 | 0.806 | rs7903146 | |
| Folsom,2008c | USA | European | Lung cancer | 177 | 1.59 | 0.96 | 2.63 | 1.63 | 1.17 | 2.25 | 0.37 | 0.29 | 0.806 | rs7903146 | |
| Folsom,2008d | USA | Black | Lung cancer | 62 | 0.62 | 0.22 | 1.76 | 0.75 | 0.44 | 1.3 | 0.26 | 0.29 | 0.806 | rs7903146 | |
| Folsom,2008e | USA | Mixed | Breast cancer | 342 | 0.87 | 0.57 | 1.32 | 0.98 | 0.78 | 1.23 | 0.28 | 0.29 | 0.806 | rs7903146 | |
| Folsom,2008f | USA | Mixed | Prostate cancer | 366 | 1.04 | 0.72 | 1.50 | 0.80 | 0.64 | 0.99 | 0.27 | 0.29 | 0.806 | rs7903146 | |
| Hazra,2008 | USA | European | Colorectal cancer | 357 | 814 | 0.63 | 0.37 | 1.08 | 0.90 | 0.68 | 1.18 | 0.25 | 0.29 | 0.497 | rs12255372 |
| Slattery,2008 | USA | Mixed | Colon cancer | 1573 | 1962 | 1.03 | 0.80 | 1.33 | 1.14 | 0.99 | 1.31 | 0.29 | 0.28 | 0.878 | rs7903146 |
| Goode,2009a | USA | European | Breast cancer | 779 | 830 | 0.92 | 0.62 | 1.36 | 1.16 | 0.93 | 1.43 | NA | 0.28 | 0.23 | rs12255372 |
| Goode,2009b | USA | European | Ovarian cancer | 391 | 458 | 0.97 | 0.56 | 1.68 | 0.95 | 0.71 | 1.26 | NA | 0.26 | 0.96 | rs12255372 |
| Tsilidis,2009 | USA | Mixed | Colorectal cancer | 202 | 354 | 1.44 | 0.81 | 2.57 | 1.13 | 0.78 | 1.66 | 0.32 | 0.28 | 0.196 | rs7903146 |
| Wang,2009 | USA | Mixed | Prostate cancer | 249 | 249 | 0.62 | 0.32 | 1.20 | 0.94 | 0.62 | 1.42 | 0.27 | 0.30 | 0.151 | rs7903146 |
| Meyer,2010 | USA | Mixed | Prostate cancer | 365 | 5757 | 0.88 b | 0.75 | 1.03 | 0.88 b | 0.75 | 1.03 | NA | 0.30 | >0.05 | rs7903146 |
| Machiela,2012 | USA | European | Prostate cancer | 2782 | 4458 | 0.90 b | 0.83 | 0.97 | 0.90 b | 0.83 | 0.97 | NA | 0.28 | >0.05 | rs7903146 |
| Naidu,2012 | Malaysia | Southeast Asian | Breast cancer | 387 | 252 | 1.574 | 0.829 | 2.987 | 1.329 | 0.948 | 1.862 | 0.31 | 0.26 | 0.518 | rs12255372 |
| Sainz,2012 | Germany | European | Colorectal cancer | 1764 | 1749 | 1.28 | 1.01 | 1.63 | 1.13 | 0.98 | 1.30 | 0.31 | 0.28 | 0.435 | rs7903146 |
| Connor,2012 | USA | Mixed | Breast cancer | 3523 | 4209 | 1.24 | 1.03 | 1.49 | 1.09 | 0.99 | 1.20 | 0.26 | 0.23 | 0.121 | rs7903146 |
Covariates’ adjusted estimate b Estimate under an additive model.
MAF, minor allele frequency; NA, not available; HWE, Hardy Weinberg Equilibrium.
Meta-analysis Results
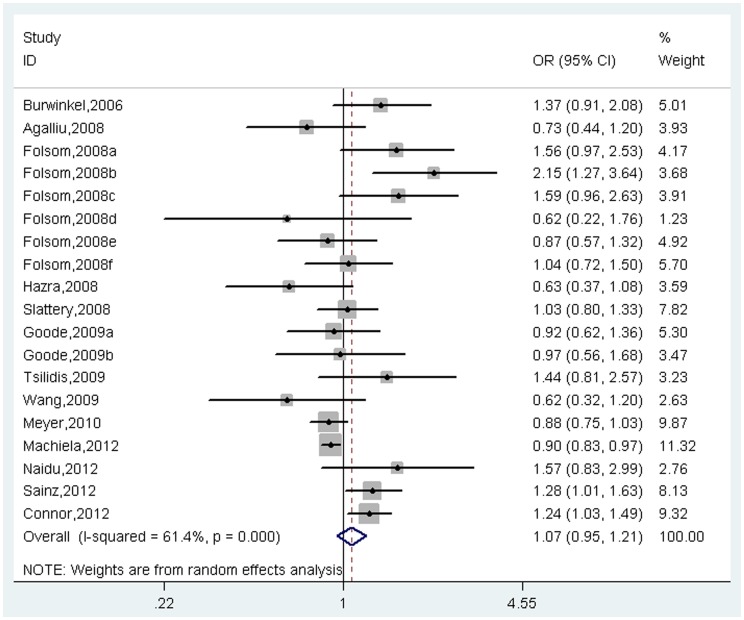
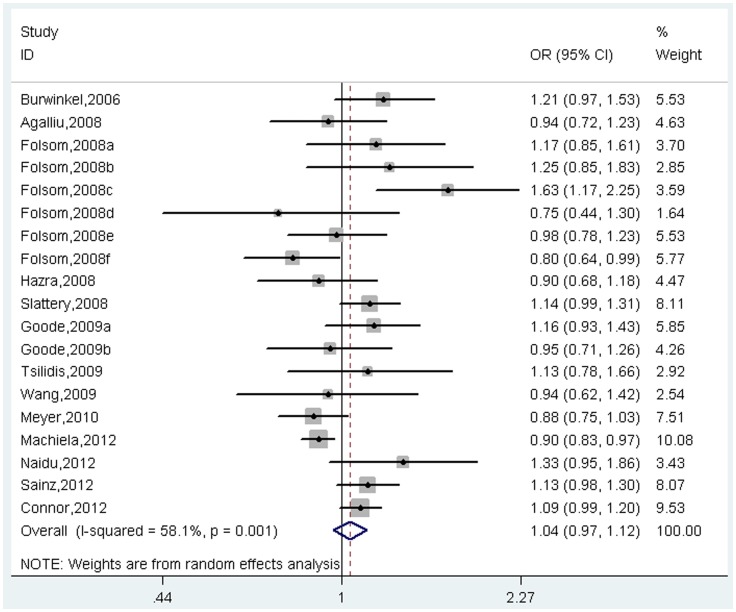
A total of 14814 cases and 33856 controls were identified for the analysis of the association between TCF7L2 polymorphism with cancer risk. The results indicated that TCF7L2 polymorphism might not be associated with cancer risk under co-dominant model (Homogeneous model: OR = 1.07, 95%CI = 0.95–1.21, I 2 = 61.4%, p for heterogeneity<0.001, Figure 2; Heterogeneous model: OR = 1.04, 95%CI = 0.97–1.12, I 2 = 58.1%, p for heterogeneity = 0.001, Figure 3). However, further subgroup analysis by cancer type suggested that the effect size was significant for breast cancer (Homogeneous model: OR = 1.17, 95%CI = 1.02–1.35, I 2 = 21.8%, p for heterogeneity = 0.276; Heterogeneous model: OR = 1.11, 95%CI = 1.03–1.20, I 2 = 0.0%, p for heterogeneity = 0.543), prostate cancer (Homogeneous model: OR = 0.89, 95%CI = 0.84–0.96, I 2 = 0.0%, p for heterogeneity = 0.640; Heterogeneous model: OR = 0.89, 95%CI = 0.84–0.95, I 2 = 0.0%, p for heterogeneity = 0.871), and colon cancer (Heterogeneous model: OR = 1.15, 95%CI = 1.01–1.31, I 2 = 0.0%, p for heterogeneity = 0.658), but not for colorectal cancer, lung cancer, and ovarian cancer (all p>0.05, Table 2).
Figure 2. Meta-analysis of association between TCF7L2 rs7903146 polymorphism and cancer risk under homogeneous co-dominant model.
Figure 3. Meta-analysis of association between TCF7L2 rs7903146 polymorphism and cancer risk under heterogeneous co-dominant model.
Table 2. Pooled ORs and 95%CIs of the association between TCF7L2 rs7903146 (or its proxy rs12255372) polymorphism and cancer risk.
| Contrasts | No. of studies (cases/controls) | Homogeneous co-dominant model | Heterogeneous co-dominant model | ||||||
| OR | 95%CI | I 2 (%) | P H | OR | 95%CI | I 2 (%) | P H | ||
| All | 19 (14814/33856) | 1.07 | 0.95–1.21 | 61.4 | <0.001 | 1.04 | 0.97–1.12 | 58.1 | 0.001 |
| Cancer type | |||||||||
| Breast cancer | 5 (5623/17436)a | 1.17 | 1.02–1.35 | 21.8 | 0.276 | 1.11 | 1.03–1.20 | 0.0 | 0.543 |
| Prostate cancer | 5 (4358/22493)a | 0.89 | 0.84–0.96 | 0.0 | 0.640 | 0.89 | 0.84–0.95 | 0.0 | 0.871 |
| Colorectal cancer | 4 (2502/14327)a | 1.18 | 0.84–1.66 | 59.1 | 0.062 | 1.09 | 0.98–1.22 | 0.0 | 0.507 |
| Colon cancer | 2 (1710/13372)a | 1.43 | 0.70–2.94 | 83.6 | 0.014 | 1.15 | 1.01–1.31 | 0.0 | 0.658 |
| Lung cancer | 2 (239/11410)a | 1.11 | 0.45–2.73 | 60.8 | 0.110 | 1.14 | 0.53–2.44 | 82.7 | 0.016 |
| Ovarian cancer | 1 (391/458) | 0.97 | 0.56–1.68 | – | – | 0.95 | 0.71–1.27 | – | – |
Shared the same number of controls (n = 11410).
Potential Publication Bias
No publication bias could be detected under homogeneous co-dominant model (p = 0.780 for Begg’s test and p = 0.123 for Egger’s test) and heterogeneous co-dominant model (p = 0.889 for Begg’s test and p = 0.274 for Egger’s test).
Discussion
To the best of our knowledge, our meta-analysis represents the first one investigating the association between TCF7L2 rs7903146 polymorphism and cancer risk. The results suggested that TCF7L2 rs7903146 polymorphism might not be associated with cancer risk. However, further stratified analysis demonstrated the significant association with breast cancer, prostate cancer and colon cancer, rather than colorectal cancer, lung cancer, and ovarian cancer.
Interestingly, the T allele of rs7903146 polymorphism, which has been associated with increased risk of type 2 diabetes, showed the inverse association with prostate cancer based on our meta-analysis. The finding was consistent with those from the individual studies by Folsom et al. [5] and Machiela et al [12]. However, the other three included studies did not suggest any association [9]–[11]. In addition, we found positive association between rs7903146 polymorphism and breast cancer risk. Recently, Michailidou K et al. [22] have found rs7904519 in intron 4 of TCF7L2 (r2 = 0.37 with rs7904519/rs12255372), to be associated with breast cancer. Further studies are necessary to examine the potential different mechanisms of TCF7L2 polymorphism in various cancers.
Heterogeneity between studies is common in meta-analysis of genetic association studies. Although heterogeneity can be taken into account by performing a random-effects model, it would increase the odds of type I error [23]. We found significant between-study heterogeneity in the association of TCF7L2 rs7903146 polymorphism with cancer risk. Therefore, subgroup analysis by cancer types was performed to explore the source of heterogeneity. The results showed that the between-study heterogeneity disappeared in several subgroups, but remained in other subgroups suggesting other covariates might confound the association.
The current meta-analysis has several strengths. First, OR (95% CI) after covariates adjustment from individual study was used to calculate the pooled the estimate, which increased the accuracy of effect estimate. Second, statistical power was greatly improved for the association study of TCF7L2 rs7903146 polymorphism in the pooled analysis. However, several limitations should also be noted. First, the case – control study design does not allow for the inference of causality between the gene polymorphism and the outcome. Second, the effect of gene – gene/gene – environment interactions was not addressed in this meta-analysis. Third, although ethnicity plays an important role in the association of TCF7L2 rs7903146 polymorphism with cancer risk, we did not perform the further subgroup analysis by ethnicity because of limited studies for each cancer type.
In conclusion, the results indicated that there was a significantly association between TCF7L2 rs7903146 polymorphism and the risk of breast cancer, prostate cancer and colon cancer, rather than colorectal cancer, lung cancer, and ovarian cancer. Further well-designed large-scale studies with the consideration of gene–gene and gene–environment interactions should be conducted to investigate the association in future.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Salonen JT, Uimari P, Aalto JM, Pirskanen M, Kaikkonen J, et al. (2007) Type 2 diabetes wholegenome association study in four populations: the DiaGen consortium. Am J Hum Genet 81: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peng S, Zhu Y, Lü B, Xu F, Li X, et al. (2013) TCF7L2 gene polymorphisms and type 2 diabetes risk: a comprehensive and updated meta-analysis involving 121,174 subjects. Mutagenesis 28: 25–37. [DOI] [PubMed] [Google Scholar]
- 3. Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, et al. (2007) Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burwinkel B, Shanmugam KS, Hemminki K, Meindl A, Schmutzler RK, et al. (2006) Transcription factor 7-like 2 (TCF7L2) variant is associated with familial breast cancer risk: a case-control study. BMC Cancer 6: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folsom AR, Pankow JS, Peacock JM, Bielinski SJ, Heiss G, et al. (2008) Variation in TCF7L2 and increased risk of colon cancer: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 31: 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goode EL, Szabo C, Prokunina-Olsson L, Vierkant RA, Fredericksen ZS, et al. (2009) No association between a candidate TCF7L2 variant and risk of breast or ovarian cancer. BMC Cancer 9: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naidu R, Yip CH, Taib NA (2012) Genetic variations in transcription factor 7-like 2 (TCF7L2) gene: association of TCF7L2 rs12255372(G/T) or rs7903146(C/T) with breast cancer risk and clinico-pathological parameters. Med Oncol 29(2): 411–417. [DOI] [PubMed] [Google Scholar]
- 8. Connor AE, Baumgartner RN, Baumgartner KB, Kerber RA, Pinkston C, et al. (2012) Associations between TCF7L2 polymorphisms and risk of breast cancer among Hispanic and non-Hispanic white women: the Breast Cancer Health Disparities Study. Breast Cancer Res Treat 136: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agalliu I, Suuriniemi M, Prokunina-Olsson L, Johanneson B, Collins FS, et al. (2008) Evaluation of a variant in the transcription factor 7-like 2 (TCF7L2) gene and prostate cancer risk in a population-based study. Prostate 68: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang MH, Helzlsouer KJ, Smith MW, Hoffman-Bolton JA, Clipp SL, et al. (2009) Association of IL10 and other immune response- and obesity-related genes with prostate cancer in CLUE II. Prostate 69: 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyer TE, Boerwinkle E, Morrison AC, Volcik KA, Sanderson M, et al. (2010) Diabetes genes and prostate cancer in the Atherosclerosis Risk in Communities study. Cancer Epidemiol Biomarkers Prev 19: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Machiela MJ, Lindström S, Allen NE, Haiman CA, Albanes D, et al. (2012) Association of type 2 diabetes susceptibility variants with advanced prostate cancer risk in the Breast and Prostate Cancer Cohort Consortium. Am J Epidemiol 176: 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hazra A, Fuchs CS, Chan AT, Giovannucci EL, Hunter DJ (2008) Association of the TCF7L2 polymorphism with colorectal cancer and adenoma risk. Cancer Causes Control 19: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsilidis KK, Helzlsouer KJ, Smith MW, Grinberg V, Hoffman-Bolton J, et al. (2009) Association of common polymorphisms in IL10, and in other genes related to inflammatory response and obesity with colorectal cancer. Cancer Causes Control 20: 1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sainz J, Rudolph A, Hoffmeister M, Frank B, Brenner H, et al. (2012) Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. J Clin Endocrinol Metab 97: E845–8451. [DOI] [PubMed] [Google Scholar]
- 16. Slattery ML, Folsom AR, Wolff R, Herrick J, Caan BJ, et al. (2008) Transcription factor 7-like 2 polymorphism and colon cancer. Cancer Epidemiol Biomarkers Prev 17: 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 18. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 19. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ. 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Domenyuk VP, Litovkin KV, Verbitskaya TG, Dubinina VG, Bubnov VV (2007) Identification of new DNA markers of endometrial cancer in patients from the Ukrainian population. Exp Oncol 29: 152–155. [PubMed] [Google Scholar]
- 22.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J,et al. (2013) Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet 45: 353–61, 361e1–2. [DOI] [PMC free article] [PubMed]
- 23. Munafò MR, Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20: 439–444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)