Abstract
The host genotype has been proposed to contribute to individually composed bacterial communities in the gut. To provide deeper insight into interactions between gut bacteria and host, we associated germ-free C3H and C57BL/10 mice with intestinal bacteria from a C57BL/10 donor mouse. Analysis of microbiota similarity between the animals with denaturing gradient gel electrophoresis revealed the development of a mouse strain-specific microbiota. Microarray-based gene expression analysis in the colonic mucosa identified 202 genes whose expression differed significantly by a factor of more than 2. Application of bioinformatics tools demonstrated that functional terms including signaling/secretion, lipid degradation/catabolism, guanine nucleotide/guanylate binding and immune response were significantly enriched in differentially expressed genes. We had a closer look at the 56 genes with expression differences of more than 4 and observed a higher expression in C57BL/10 mice of the genes coding for Tlr1 and Ang4 which are involved in the recognition and response to gut bacteria. A higher expression of Pla2g2a was detected in C3H mice. In addition, a number of interferon-inducible genes were higher expressed in C3H than in C57BL/10 mice including Gbp1, Mal, Oasl2, Ifi202b, Rtp4, Ly6g6c, Ifi27l2a, Usp18, Ifit1, Ifi44, and Ly6g indicating that interferons may play an essential role in microbiota regulation. However, genes coding for interferons, their receptors, factors involved in interferon expression regulation or signaling pathways were not differentially expressed between the two mouse strains. Taken together, our study confirms that the host genotype is involved in the establishment of host-specific bacterial communities in the gut. Based on expression differences after colonization with the same bacterial inoculum, we propose that Pla2g2a and interferon-dependent genes may contribute to this phenomenon.
Introduction
The intestine is colonized by a complex community of bacteria. These bacteria convert indigestible food components into absorbable fermentation products and modify non-nutritive plant metabolites and drugs [1]. Deep-sequencing analyses of the gut microbiome demonstrated that a well-defined set of bacterial genes and functions is widely shared between human individuals and that a common core of bacterial species may exist [2–5]. Changes in microbiota composition or function are often observed in patients suffering from chronic disorders including inflammatory bowel diseases [6] and obesity and obesity-associated metabolic disorders [7]. It is therefore important to better understand how microbiota is shaped under physiological and pathological conditions. Diet has been considered one of the most important environmental regulators of microbiota [8]. However it can be deduced from studies in twins and less related human study subjects that the host genotype may contribute to the development of individual bacterial populations in the intestine [9,10]. The notion that the host genotype is at least in part responsible for the selection of a host-specific microbiota is supported by a study in six different genetically distinct inbred mouse strains [11].
Many genes that have been proposed to influence microbiota composition are involved in immune functions such as the pattern recognition receptors (PRRs) including Toll-like receptors (TLRs) and the nucleotide-binding oligomerization domain-containing protein 2 (NOD2). For instance, NOD2-deficient and wild-type control mice differ in microbiota composition [12,13]. Effects of TLR2-dependent mechanisms on bacterial gut colonization have been concluded from studies with a Bacteroides fragilis mutant strain that does not produce the capsular polysaccharide A. This strain failed to establish in the gut of germ-free mice. In contrast, the wild-type strain induced tolerance via TLR2 and successfully colonized the murine gut [14]. However, no clear effect of TLR-dependent mechanisms on gut colonization was observed when germ-free TLR2/TLR4-deficient and wild-type mice were associated with a complex bacterial inoculum from a single donor mouse [15]. Recognition of bacterial antigens by TLRs induces the expression of antimicrobial substances by Paneth cells in the small intestine [16] and Paneth cell products, namely defensins, have been shown to modulate microbiota composition [17]. Interestingly, differences in the numbers of small intestinal Paneth cells and the profiles of antimicrobial peptides produced were observed in mice which differed in their genetic background. These differences were associated with a mouse strain-specific microbiota composition [18].
Taken together, there is good indication that host-specific sensing of bacterial antigens by PRRs and the subsequent production of antimicrobial compounds is involved in the establishment of an individual microbiota. However, a large intercross study in mice suggested that complex interactions between polygenic host traits and environmental factors are responsible for microbiota individuality [19]. In order to identify candidate genes the relevance of which can later be tested in hypothesis-driven approaches, we associated germ-free inbred C57BL/10 and C3H mice with the fecal microbiota from one single conventional donor mouse. The microbiota that developed in the experimental animals was analyzed with denaturing gradient gel electrophoresis. Differences between the two mouse strains in mucosal responses towards the bacterial colonization were addressed at the gene expression level with a microarray approach and the expression of selected genes was measured with quantitative PCR.
Materials and Methods
Ethic statement
The protocol for the animal experiment was approved by the Animal Welfare Committee of the Ministry of Environment, Health and Consumer Protection of the Federal State of Brandenburg (Germany), State Office of Environment, Health and Consumer Protection (approval number: AZ 32-44456+1). The regulations of the German Animal Welfare Act (TierSchG, §8, Abs.1) were strictly followed.
Animal experiment
The study was conducted in 12 week-old male C3HHeOuJ (C3H) and C57BL/10ScSn (C57BL/10) mice (12 mice per group). Animals were bred and maintained germ-free in Trexler-type isolators under highly controlled conditions (22.2 °C, 55.5% relative air humidity, 12 h light-dark cycle). Animals had free access to irradiated (25 kGy) standard chow (Altromin 1314, Altromin Spezialfutter GmbH, Lage, Germany) and autoclaved distilled drinking water. Conventional C3H and C57BL/10 (C57BL/10-MRL) mice from our breeding facilities and C57BL/10 (C57BL/10-Harlan) and C57BL/6 (C57BL/6-Harlan) mice purchased from the Harlan Laboratories (Rossdorf, Germany) were tested for mutations in the Pla2g2a-encoding gene (see below). Conventional animals were housed in individually ventilated cages under standard housing conditions.
Germ-free C3H and C57BL/10 mice were associated with the unspecified microbiota of a single C57BL/10 donor mouse. A fresh fecal sample was diluted 1:50 with sterile phosphate buffered saline (8.00 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4) and 100 µl of this suspension containing ~107 bacterial cells was intragastrically applied to the experimental animals. During the experimental period, the associated mice were housed in one single isolator in polycarbonate cages on irradiated wood chips (one mouse per cage). After 13 weeks, animals were killed by cervical dislocation and colonic contents were collected for microbiota analysis. Colonic mucosa was carefully scratched, homogenized and immediately frozen in liquid nitrogen and stored at -80 °C until further processing.
Microbiota analysis
Bacterial DNA extraction and DGGE were performed as described earlier [15]. Briefly, colonic contents and mucosa were freeze-dried and 15 mg, each, was subjected to DNA extraction with the Fast DNA SpinKit (Qbiogene, Morgan Irvine, USA). PCR was performed with the universal 16S rRNA gene-targeting primers U968-GC-f and L1401-r. DGGE was carried out with a denaturing-gradient gel electrophoresis system (C.B.S. Scientific, Del Mar, USA) using gels with a 40% to 60% denaturing gradient. The gels were subsequently silver stained [20].
Mucosal RNA extraction and gene expression analysis
RNA was extracted from the colonic mucosa with the NucleoSpin RNA II Kit (Machery-Nagel, Duren, Germany) and shipped on dry ice to ServiceXS (Leiden, the Netherlands). At ServiceXS, the quality of the RNA was checked with the Agilent Bioanalyzer (Agilent, Santa Clara, USA). Biotin-labelled cDNA was synthesized using the Affymetrix One-Cycle Target Labeling. After testing the cDNA quality (Agilent Bioanalyzer), hybridization was performed using 12.5–20 µg of cDNA on a customized Affymetrix nugomm 1a520177 chip [21]. For unknown reasons, the RNA from one C57BL/10 mouse failed the quality tests and the mucosa obtained from this animal was excluded from all further analysis. Affymetrix protocols were strictly followed for all procedures including hybridization, washing, staining and scanning of the chips. One chip was used per experimental animal.
The relative expression of the following genes was determined by quantitative real-time PCR: interferon-induced guanylate-binding protein 1 (Gbp1), Cluster of differentiation 14 (Cd14), angiopoietin-4 (Ang4), and phospholipase A2, group IIA (Pla2g2a). Interferon-induced guanylate-binding protein 1, cluster of differentiation 14, and phospholipase A2, group IIA were selected because differences between C3H and mice with a C57 genetic background in the expression of these genes have already been published [22]. We therefore considered these genes appropriate controls. Angiopoietin-4 was selected because it is a known Paneth cell marker [23] and we aimed at verifying its expression in the colonic mucosa of our experimental animals. Hypoxanthine-guanine phosphoribosyltransferase (Hprt1) and ribosomal protein L13a (Rpl13a) were selected as the reference genes. For quantitative real-time PCR analysis, RNA from three single mice per group and one pooled sample of the remaining mice per group were used. The RNA (0.7 µg) was transcribed with the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) and quantitative real-time PCR was performed with the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, USA) using the SYBR Green PCR kit (Qiagen). All reactions were carried out in triplicates. The primer sequences for Gbp1 were taken from the literature [22]. The primer sequences for Cd14 (5'- CGA ACA AGC CCG TGG AAC CT-3' and 5'-CAA GCA CAC GCT CCA TGG TC-3'), for Ang4 (5'-TGG CCA GCT TTG GAA TCA CTG-3' and 5'-GCT TGG CAT CAT AGT GCT GAC G-3'), and for Pla2g2a (5'-GGC CTT TGG CTC AAT ACA GGT C-3' and 5'-ACA GTG GCA TCC ATA GAA GGC A-3') were designed with the PerlPrimer software [24]. Primer sequences for Hprt1 and Rpl13a were 5'-CGT TGG GCT TAC CTC ACT GCT-3', 5'-CAT CAT CGC TAA TCA CGA CGC T-3' and 5'-GTT CGG CTG AAG CCT ACC AG-3', 5'-TTC CGT AAC CTC AAG ATC TGC T-3', respectively.
Pla2g2a genotyping
Genomic DNA was extracted from the tail tip of three mice per group (C3H, C57BL/6, C57BL/10-MRL, C57BL/10-Harlan). The exon 3 of the Pla2g2a gene was amplified with the primers 5’-CTG GCT TTC CTT CCT GTC AGC CTG GCC-3’ and 5’-GGA AAC CAC TGG GAC ACT GAG GTA GTG-3’ [25]. The amplicons were subsequently sequenced (Eurofins MWG Operon, Ebersberg, Germany). In addition, Southern blotting using the FastDigest BamHI restriction enzyme (Fermentas, St. Leon-Rot, Germany) was applied.
Data analysis and statistics
DGGE gels were analyzed with the software GelCompar II (Applied Maths, Sint-Martens-Latem, Belgium). Differences in bacterial community structure were evaluated using the Dice similarity coefficient and bottom-up cluster analysis using the Unweighted Pair Group Method with Arithmetic mean (UPGMA). ANOVA followed by the Scheffé test was performed using SPPS 16.0 (IBM, New York, USA) in order to identify significant differences (p ≤ 0.05) between mice with the same genetic background (intra-strain differences in microbiota composition) and between the C3H and C57BL/10 mice (inter-strain differences in microbiota composition).
Mucosal affymetrix microarray gene expression data (nugomm1a520177 affymetrix arrays analyzed with nugomm1a520177mmentrezg custom cdf) was quality checked and evaluated in R (version 2.15.2) with Bioconductor (version 2.11) tools [26]. The microarray data was quality checked with affy/affyplm and normalised with Robust Multichip Average (RMA) [27]. Differential gene expression was evaluated with Linear Models For Microarray Data (limma) [28] using the false discovery rate (FDR) of 5% as the threshold for statistical significance. Gene enrichment analysis was performed with DAVID [29]. Microarray data was submitted to Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; GEO accession number: GSE45876).
The qRT-PCR data was analyzed with the 7500 software version 2.0.5 (Applied Biosystems). The relative target gene expression levels were determined with the relative standard curve method after normalization to the reference gene expression. Differences in gene expression were tested for statistical significance (p < 0.05) with the Mann-Whitney test. Prism 5.0 (Graph Pad Software, La Jolla, USA) was used for graphical data presentation and re-testing of statistical significances.
Results
Intestinal microbiota composition
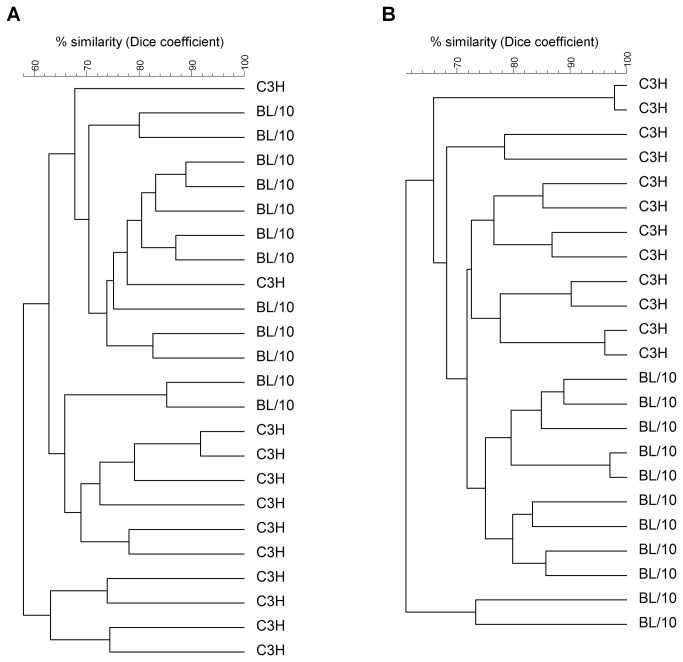
Thirteen weeks after association of germ-free C3H and C57BL/10 mice with the unspecified intestinal microbiota from a single conventional C57BL/10 mouse, we analyzed the luminal and mucosa-associated microbiota composition in the colon of the recipients using a DGGE approach. The observed band patterns were used as an indicator for differences between C3H and C57BL/10 mice in microbiota composition. C3H and C57BL/10 mice grouped according to their luminal (Figure 1A) and mucosa-associated (Figure 1B) microbiota composition when cluster analysis was performed. Luminal and mucosal microbiota similarity between the C3H mice was 65.29 ± 8.93% and 72.64 ± 7.95%, respectively. The similarity between the C57BL/10 mice was 72.37 ± 8.21 for the luminal and 73.26 ± 9.78% for the mucosa-associated microbiota. The higher similarity of the luminal microbiota composition in the C57BL/10 mice was statistically significant (p < 0.001). When the band patterns of the C57BL/10 was compared with the patterns of the C3H mice, the similarity in microbiota composition was 61.40 ± 8.86% in the lumen and 67.70 ± 7.55% at the colonic wall. The statistical analysis revealed that the intra-strain similarity in the lumen (p = 0.011) and at the mucosal wall (p < 0.001) was significantly higher than the inter-strain similarity. A similarity matrix showing the microbiota similarity in per-cent between the experimental animals is presented in Table S1.
Figure 1. Cluster formation of C3H and C57BL/10 mice according to their luminal (A) and mucosa-associated (B) microbiota.
Microbiota similarity in previously germ-free mice was calculated 13 weeks after association with the same bacterial inoculum using the Dice similarity coefficient and bottom-up cluster analysis (UPGMA) based on band patterns in DGGE gels.
Differences in mucosal gene expression
We compared the expression of ~16.000 genes in the colonic mucosa of previously germ-free C3H and C57BL/10 mice 13 weeks after association with intestinal bacteria. The expression of 210 genes significantly (FDR ≤ 0.05) differed by a factor of ≥ 2 (Table S2). Twenty-six of these genes were > 4-fold higher expressed in C57BL/10 (Table 1) and 30 genes were > 4-fold higher expressed in C3H mice (Table 2). DAVID functional gene enrichment analysis was performed on all 210 differentially expressed genes. Significantly enriched functional terms identified were signaling/secretion, lipid degradation/catabolism, guanine nucleotide/guanylate binding and immune response. These terms included 66 genes associated with signaling/secretion (Table S3), 7 with peptidase inhibition (Table S4), 6 with guanine binding (Table S5), 18 with response to bacteria/defense (Table S6), 7 with hormone activity (Table S7) and 14 lipoprotein-associated genes (Table S8).
Table 1. Genes > 4-fold higher expressed in C57BL/10 than in C3H mice (FDR ≤ 0.05).
| Gene symbol | Gene name | Fold change |
|---|---|---|
| Ang4 | angiogenin, ribonuclease A family, member 4 | 33.06 |
| Lin7c | lin-7 homolog C (C. elegans) | 32.64 |
| Nxpe4 | neurexophilin and PC-esterase domain family, member 4 | 30.51 |
| Pla2g4c | phospholipase A2, group IVC (cytosolic, calcium-independent) | 23.41 |
| Pnliprp2 | pancreatic lipase-related protein 2 | 15.17 |
| Spna1 | spectrin alpha 1 | 13.64 |
| Rpgrip1 | retinitis pigmentosa GTPase regulator interacting protein 1 | 13.09 |
| Pdxdc1 | pyridoxal-dependent decarboxylase domain containing 1 | 12.12 |
| Fam199x | family with sequence similarity 199, X-linked | 11.43 |
| Pcdh17 | protocadherin 17 | 11.37 |
| B4galnt2 | beta-1,4-N-acetyl-galactosaminyl transferase 2 | 9.31 |
| Cbr3 | carbonyl reductase 3 | 8.45 |
| Trim30d | tripartite motif-containing 30D | 8.14 |
| Mep1a | meprin 1 alpha | 7.77 |
| Trim34a | tripartite motif-containing 34A | 7.19 |
| Ceacam12 | carcinoembryonic antigen-related cell adhesion molecule 12 | 6.10 |
| Gsdmc4 | gasdermin C4 | 5.76 |
| P2ry6 | pyrimidinergic receptor P2Y, G-protein coupled, 6 | 5.27 |
| Msi2 | Musashi homolog 2 (Drosophila) | 5.02 |
| 1810030J14Rik | RIKEN cDNA 1810030J14 gene | 4.66 |
| Myl7 | myosin, light polypeptide 7, regulatory | 4.40 |
| Tlr1 | toll-like receptor 1 | 4.34 |
| Itln1 | intelectin 1 (galactofuranose binding) | 4.27 |
| Gsdmc2 | gasdermin C2 | 4.21 |
| 2210407C18Rik | RIKEN cDNA 2210407C18 gene | 4.21 |
| Fam73a | family with sequence similarity 73, member A | 4.10 |
Table 2. Genes > 4-fold higher expressed in C3H than in C57BL/10 mice (FDR ≤ 0.05).
| Gene symbol | Gene name | Fold change |
|---|---|---|
| Pla2g2a | phospholipase A2, group IIA (platelets, synovial fluid) | 70.80 |
| Nxpe5 | neurexophilin and PC-esterase domain family, member 5 | 33.03 |
| Slpi | secretory leukocyte peptidase inhibitor | 28.44 |
| Qpct | glutaminyl-peptide cyclotransferase (glutaminyl cyclase) | 18.57 |
| Gbp1 | guanylate binding protein 1 | 18.38 |
| Plscr2 | phospholipid scramblase 2 | 16.62 |
| Lpo | lactoperoxidase | 11.25 |
| Ppy | pancreatic polypeptide | 10.39 |
| Apoc2 | apolipoprotein C-II | 8.96 |
| Abhd1 | abhydrolase domain containing 1 | 8.87 |
| Mal | myelin and lymphocyte protein, T cell differentiation protein | 7.13 |
| Oasl2 | 2'-5' oligoadenylate synthetase-like 2 | 6.67 |
| Ifi202b | interferon activated gene 202B | 6.51 |
| Afm | afamin | 6.47 |
| Hddc3 | HD domain containing 3 | 6.46 |
| Rtp4 | receptor transporter protein 4 | 5.64 |
| BC064078 | cDNA sequence BC064078 | 5.64 |
| Ly6g6c | lymphocyte antigen 6 complex, locus G6C | 5.46 |
| Tff2 | trefoil factor 2 (spasmolytic protein 1) | 5.45 |
| Amn | amnionless | 5.44 |
| Ifi27l2a | interferon, alpha-inducible protein 27 like 2A | 5.23 |
| Usp18 | ubiquitin specific peptidase 18 | 5.00 |
| Tmem87a | transmembrane protein 87A | 4.69 |
| Ifit1 | interferon-induced protein with tetratricopeptide repeats 1 | 4.56 |
| 2610305D13Rik | RIKEN cDNA 2610305D13 gene | 4.55 |
| Ifi44 | interferon-induced protein 44 | 4.34 |
| Cd14 | CD14 antigen | 4.17 |
| 2210010C17Rik | RIKEN cDNA 2210010C17 gene | 4.13 |
| Ly6g | lymphocyte antigen 6 complex, locus G | 4.11 |
| Eno3 | enolase 3, beta muscle | 4.05 |
We had a closer look at the functions of genes with fold changes of more than 4. Many of these genes are involved in the synthesis of antibacterial factors and in immune functions Genes with (possible) antibacterial functions include the phospholipase A2, group IIA, secretory leukocyte peptidase inhibitor, and lactoperoxidase all of which were higher expressed in C3H mice. In contrast, the angiogenin, ribonuclease A family , member 4 was higher expressed in the BL/10 mice. The LPS receptor cluster of differentiation 14 was higher expressed in C3H mice and the toll-like receptor 1 was higher expressed in the BL/10 animals. Interferon-inducible genes were higher expressed in C3H than in C57BL/10 mice. These genes included guanylate nucleotide binding protein 1, myelin and lymphocyte protein, 2'-5' oligoadenylate synthetase-like 2, interferon activated gene 202B, receptor transporter protein 4, lymphocyte antigen 6 complex, locus G6C, interferon alpha-inducible protein 27 like 2A, ubiquitin-specific peptidase 18, interferon-induced protein with tetratricopeptide repeats 1, interferon-induced protein 44, and lymphocyte antigen 6 complex, locus G.
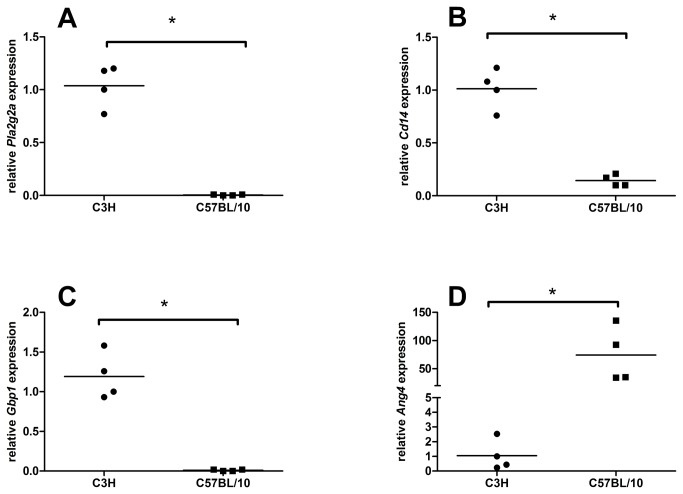
The expression at the mRNA level of four selected genes was measured with qRT-PCR to confirm the microarray gene data. The relative expression of phospholipase A2, group IIA (Pla2g2a), cluster of differentiation 14 (CD14) and guanylate nucleotide binding protein 1 (Gbp1) was higher in the colonic mucosa of the C3H than in C57BL/10 mice. In contrast, the expression of Ang4 was significantly higher in C57BL/10 than in C3H mice (Figure 2). These findings are in line with the results from the microarray experiment.
Figure 2. Quantitative real-time results for the expression of Pla2g2a (A), CD14 (B) Gbp1 (C) and Ang4 (2D) in the colonic mucosa of previously germ-free C3H and C57BL/10 mice 13 weeks after association with the same bacterial inoculum.
RNA from three single mice per group and pooled RNA from the remaining C3H and C57BL/10 mice, respectively were used. Hypoxanthine-guanine phosphoribosyltransferase (Hprt1) and ribosomal protein L13a (Rpl13a) expression were selected for data normalization and tested for tested for statistical significance (* < 0.05) with the Mann-Whitney test.
Testing for frameshift mutations in the Pla2g2a-encoding gene
C57BL/10 and C57BL/6 mice share a considerable proportion of their genetic background. Since the Pla2g2a gene is naturally disrupted in C57BL/6 mice by a frameshift mutation in exon 3, we tested whether this was also the case in our experimental C57BL/10. In addition, we sequenced the gene in the experimental C3H mice and in conventional C57BL/10 mice purchased from the Harlan laboratories. C57BL/6 mice of the same origin were used as a reference. We did not detect the frameshift mutation in our experimental C57BL/10 mice or in any C3H mouse when the exon 3 was sequenced. This result was confirmed by Southern blotting which clearly demonstrated that both (sub-) strains used are homozygous for the wild-type Pla2g2a genotype. In contrast, C57BL/6 mice and C57BL/10 purchased from Harlan laboratories were homozygous for the defective Pla2g2a gene.
Discussion
Interactions between intestinal bacteria and the host at the mucosal interface influence host health and disease. For instance, excessive host responses towards commensal gut bacteria are associated with the onset and perpetuation of chronic gut inflammation [6]. On the other hand, host factors may influence gut bacteria since differences in the recognition of and in responses towards intestinal bacteria are implicated in the selection of individual intestinal microbiota [30]. However, the exact nature and the mechanisms of such interactions are poorly understood. To provide deeper insight into host-specific responses to commensal gut bacteria, we associated germ-free C3H and C57BL/10 mice with the very same bacterial inoculum. Upon association, the mice developed a strain-specific intestinal microbiota composition which is in line with our previous observations in these mouse strains and with findings by others [15,31–33].
To identify genotype-specific responses towards bacterial colonization of the intestine, we analyzed with a microarray approach the mucosal gene expression in our experimental animals. This technique has been previously applied by others to describe differences in intestinal gene expression between germ-free and conventional C3H mice revealing that more than 50% of the differentially regulated genes were higher expressed in the conventional mice. The majority of these genes grouped in Gene Ontology biological processes involved in water transport across the gut wall and immune responses. Since mainly immunoglobulin-associated genes were identified in the “defense/immunity protein activity molecular function cluster”, it can be assumed that presence of microbiota influenced adaptive immune responses in this model [34]. The category “immune responses” was also enriched in differentially expressed genes when mucosal gene expression in our experimental animals was evaluated with bioinformatics approaches. We had a closer look at single genes whose expression differed by a factor of greater than 4. Amongst these genes, we found higher expression in C57BL/10 than in C3H mice of toll-like receptor 1 (TLR1, 4-fold) and angiogenin 4 (Ang4, 33-fold). Direct effects of these two genes on colonic microbiota composition have so far not been demonstrated. However, differences in Ang4 expression have been reported between germ-free mice and mice monoassociated with Bacteroides thetaiotaomicron [35]. The antibacterial peptide Ang4 is very effective against Gram positive bacteria including Enterococcus faecalis and Listeria monocytogenes. Gram negative bacteria such as Bacteroides thetaiotaomicron and Escherichia coli are less sensitive. Interestingly, intestinal Ang4 mRNA expression is induced by complex microbiota and by B . thetaiotaomicron alone in previously germ-free mice [23].
In contrast to Ang4, phospholipase A2, group IIA (Pla2g2a), guanylate nucleotide binding protein 1 (Gbp1), and cluster of differentiation 14 (Cd14) were higher expressed in C3H than in C57BL/10 mice. The same observation has been reported in C3H and C57BL/6 mice and was associated with a strain-specific susceptibility to microbiota-driven colitis: interleukin 10-deficient mice with a C3H background are susceptible whereas the C57BL/6 background renders the mice colitis resistant [22]. The latter strain shares a considerable proportion of its genetic background with the C57BL/10 mice that were used in our experiment because C57BL/6 and C57BL/10 are sub-strains of C57BL origin. For Cd14, which is involved in the detection of lipopolysaccharides by TLR4, it was later demonstrated that a higher expression by gut epithelial cells is responsible for a lower colitis susceptibility in IL-10-/- mice with a C3H than with a C57BL/6 background [36]. Whether or not differences in intestinal Cd14 expression are also involved in the selection of resident gut bacteria remains to be clarified.
The high genetic homogeneity that can be assumed for C57BL/6 and C57BL/10 mice suggests that our experimental mice were affected by a known frameshift mutation in exon 3 of the Pla2g2a gene in C57BL/6 mice which results in a defective gene product [25]. This would have been the most plausible explanation for a more than 70-fold higher expression of this gene in C3H mice. However, gene sequence and Southern blot analyses revealed that this mutation was absent in our experimental animals. Differences in intestinal Pla2g2a activities have been shown for different mouse strains with the wild-type alleles. It has been proposed in one study that nucleotide substitutions and resulting amino acid changes may influence the substrate binding properties of the enzyme [37]. In conjunction with the fact that Pla2g2a gene expression is equal in germ-free and conventional mice [23], these findings suggest that this gene is a good candidate for genetically fixed host factors that may influence microbiota composition. In fact, Pla2g2a exerts strong antibacterial effects against Gram positive and to a lesser extend against Gram negative bacteria and there is evidence that it plays an important role in the intestinal defense against pathogenic bacteria [38].
The expression in human bronchioepithelial and nasal epithelial cells of type IIA phospholipase A2 is up-regulated by IFN-gamma [39] indicating that interferons may regulate the expression of genes with possible functions in host-microbe interactions. This notion is supported by the fact that many interferon (IFN)-inducible genes were > 4-fold higher expressed in C3H than in C57BL/10 mice. In addition to the aforementioned Gbp1, these genes included Mal, Oasl2, Ifi202b, Rtp4, Ly6g6c, Ifi27l2a, Usp18, Ifit1, Ifi44, and Ly6g. Interestingly, the interferon regulatory factor 9-encoding gene (IRF9) was 2-fold higher expressed in C3H mice. It may therefore well be that IFN-dependent epithelial responses towards gut bacteria differ in a host-specific manner. However, whether individual IFN-dependent immune responses are the cause or the consequence of differences in intestinal microbiota composition remains elusive. On the one hand mouse strain specific Gbp1 expression has been demonstrated. Among the 46 inbred strains tested, mice with a C3H but not with a C57BL background displayed IFN-inducible GBP-1 synthesis [40]. The importance of genes that are regulated by interferon-alpha or interferon-beta has been concluded from experiments with IRF9-deficient mice. These mice with an impaired IFN-alpha and IFN-beta signaling show higher temporal variations in and lower individuality of intestinal microbiota composition than wild-type control mice and STAT1-deficient mice with defective IFN-gamma signaling [41]. On the other hand intestinal bacteria influence the expression of IFN-inducible genes. For instance, differential expression profiles of IFN-responsive genes in response to monoassociation with Bacteroides thetaiotaomicron and Bifidobacterium longum have been observed in mice. Based on gene interaction network analysis it has been concluded that host responses to B . thetaiotaomicron are centered on tumor necrosis factor alpha triggered gene expression but that the gene expression network associated with Bifidobacterium longum is centered on INF-gamma responsive genes [42]. Taken together, there is good indication that interferons are important players in host-bacteria interactions. However, we did not observe differences between C3H and C57BL/10 mice in the expression of genes coding for interferons, their receptors or for molecules involved in interferon signaling.
Differentially expressed genes with functions in the immune system included SLPI encoding the secretory leucocyte protease inhibitor (28-fold higher expressed in C3H) and Mep1a coding for meprin 1 α (8-fold higher expressed in C57BL/10). SLPI expression is up-regulated by bacterial antigens and under inflammatory conditions. The protein protects the tissue from immune cell-derived proteases and is implicated in the priming of innate immunity and tissue repair. It also acts as an antibacterial protein and is active against both Gram positive and Gram negative bacteria [43]. Meprins have been implicated in dysregulated immune responses towards gut bacteria in chronic gut inflammation because the expression of meprins is down-regulated in patients with ileal Crohn’s disease. Pre-treatment with meprin α and meprin β decreased the ability of E. coli to bind to host receptors and to induce the expression of pro-inflammatory IL-8 [44]. These findings suggest that SLPI and meprin 1 α may act as microbiota regulators.
The intestinal mucus layer in the colon consists of a dense inner bacteria-free and a loose outer layer that is colonized by commensal bacteria. The mucins that form this layer are mainly composed of the densely O-glycosylated mucus protein MUC2. The glycans may serve as substrates and, in addition as adhesion sites for gut bacteria. Therefore, different mucin glycosylation pattern may be involved in the selection of a host specific microbiota [45]. Interestingly, a recent study clearly demonstrated that intestinal expression of β-1,4-N-acetylgalactosaminyltransferase 2 (B4galnt2) which may contribute to mucin decoration with N‑acetylgalactosamine influences gut microbiota in mice [46]. Using a 16S rRNA gene sequencing approach, the authors identified numerous bacterial taxa or operational taxonomic units that were influenced by B4galnt2 expression. Since closely related bacterial species appeared to replace each other in B4galnt2 -/- and B4galnt2 +/+ mice, respectively, it is difficult to interpret whether B4galnt2-induced changes influence microbiota function. Interestingly, Helicobacter spp. were rarely detected in the intestine of B4galnt2 -/- mice indicating that adhesion of the pathogen and possibly by other adherent bacteria to N-acetogalactosamine residues in the intestine is impaired in these animals.
Genes with functions in plasma membrane structure or functions (Lin7c, Plscr2, AMN) and cell adhesion and cell signaling (Pcdh17, P2ry6, Ceacam12) were also differentially regulated in the experimental mice but their role in intestinal host-microbe interactions was not obvious from literature research.
In summary, differences between inbred mouse strains in microbiota composition suggest that genetic host factors are involved in the selection of host-specific bacteria. From our findings we conclude that Pla2ga2 and interferon-responsive genes are good candidates for the identification of host factors that play a role in host-microbe interactions. The specific role of these candidate genes may be addressed in hypothesis-driven experiments taking advantage of gnotobiotic knockout mouse models. In addition, it may be possible in such studies to clarify whether differential expression of selected genes is cause or consequence of differences in intestinal microbiota composition.
Supporting Information
Similarity of the intestinal microbiota in the colonic lumen (A) and at the clonic mucosa (B).
(PDF)
Genes differentially expressed (fold change >2) between C57BL/10 and C3H mice.
(PDF)
DAVID functional gene list: signaling/secretion.
(PDF)
DAVID functional gene list: peptidase inhibition.
(PDF)
DAVID functional gene list: guanine binding.
(PDF)
DAVID functional gene list: response to bacteria/defense.
(PDF)
DAVID functional gene list: hormone activity.
(PDF)
DAVID functional gene list: lipoprotein-associated.
(PDF)
Acknowledgments
We thank Ines Grüner and Ute Lehman for animal care and Marion Urbich and Sarah Schaan for excellent technical assistance.
Funding Statement
This work was supported by a grant from the German Research Foundation (LO1184/1-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blaut M (2011) Ecology and Physiology of the Intestinal Tract. Curr Top Microbiol Immunol: Epub ahead of print. PubMed: 22120885. [DOI] [PubMed] [Google Scholar]
- 2. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T et al. (2011) Enterotypes of the human gut microbiome. Nature 473: 174-180. doi:10.1038/nature09944. PubMed: 21508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59-65. doi:10.1038/nature08821. PubMed: 20203603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sekelja M, Berget I, Naes T, Rudi K (2011) Unveiling an abundant core microbiota in the human adult colon by a phylogroup-independent searching approach. ISME J 5: 519-531. doi:10.1038/ismej.2010.129. PubMed: 20740026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY et al. (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105-108. doi:10.1126/science.1208344. PubMed: 21885731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loh G, Blaut M (2012) Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes 3: 544-555. doi:10.4161/gmic.22156. PubMed: 23060017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tremaroli V, Bäckhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489: 242-249. doi:10.1038/nature11552. PubMed: 22972297. [DOI] [PubMed] [Google Scholar]
- 8. Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH (2012) The influence of diet on the gut microbiota. Pharmacol Res, 69: 52–60. PubMed: 23147033. [DOI] [PubMed] [Google Scholar]
- 9. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A et al. (2009) A core gut microbiome in obese and lean twins. Nature 457: 480-484. doi:10.1038/nature07540. PubMed: 19043404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zoetendal EG, Akkermans AD, Akkermans-van Vliet WM, de Visser AGM, de Vos WM (2001) The Host Genotype Affects the Bacterial Community in the Human Gastrointestinal Tract. Microb Ecol Health Dis 13: 129-134. doi:10.1080/089106001750462669. [Google Scholar]
- 11. Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B et al. (2010) Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLOS ONE 5: e8584. doi:10.1371/journal.pone.0008584. PubMed: 20052418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T et al. (2009) Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A 106: 15813-15818. doi:10.1073/pnas.0907722106. PubMed: 19805227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rehman A, Sina C, Gavrilova O, Häsler R, Ott S et al. (2011) Nod2 is essential for temporal development of intestinal microbial communities. Gut 60: 1354-1362. doi:10.1136/gut.2010.216259. PubMed: 21421666. [DOI] [PubMed] [Google Scholar]
- 14. Round JL, Lee SM, Li J, Tran G, Jabri B et al. (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332: 974-977. doi:10.1126/science.1206095. PubMed: 21512004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loh G, Brodziak F, Blaut M (2008) The Toll-like receptors TLR2 and TLR4 do not affect the intestinal microbiota composition in mice. Environ Microbiol 10: 709-715. doi:10.1111/j.1462-2920.2007.01493.x. PubMed: 18036181. [DOI] [PubMed] [Google Scholar]
- 16. Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A 105: 20858-20863. doi:10.1073/pnas.0808723105. PubMed: 19075245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J et al. (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11: 76-83. doi:10.1038/ni.1825. PubMed: 19855381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gulati AS, Shanahan MT, Arthur JC, Grossniklaus E, von Furstenberg RJ et al. (2012) Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLOS ONE 7: e32403. doi:10.1371/journal.pone.0032403. PubMed: 22384242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benson AK, Kelly SA, Legge R, Ma F, Low SJ et al. (2010) Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A 107: 18933-18938. doi:10.1073/pnas.1007028107. PubMed: 20937875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanguinetti CJ, Dias Neto E, Simpson AJ (1994) Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. BioTechniques 17: 914-921. PubMed: 7840973. [PubMed] [Google Scholar]
- 21. De Groot PJ, Reiff C, Mayer C, Müller M (2008) NuGO contributions to GenePattern. Genes Nutr 3: 143-146. doi:10.1007/s12263-008-0093-2. PubMed: 19034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Buhr MF, Mähler M, Geffers R, Hansen W, Westendorf AM et al. (2006) Cd14, Gbp1, and Pla2g2a: three major candidate genes for experimental IBD identified by combining QTL and microarray analyses. Physiol Genomics 25: 426-434. doi:10.1152/physiolgenomics.00022.2005. PubMed: 16705022. [DOI] [PubMed] [Google Scholar]
- 23. Hooper LV, Stappenbeck TS, Hong CV, Gordon JI (2003) Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4: 269-273. doi:10.1038/ni888. PubMed: 12548285. [DOI] [PubMed] [Google Scholar]
- 24. Marshall OJ (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20: 2471-2472. doi:10.1093/bioinformatics/bth254. PubMed: 15073005. [DOI] [PubMed] [Google Scholar]
- 25. Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W et al. (1995) A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem 270: 22378-22385. doi:10.1074/jbc.270.38.22378. PubMed: 7673223. [DOI] [PubMed] [Google Scholar]
- 26. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. doi:10.1186/gb-2004-5-10-r80. PubMed: 15461798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu ZJ, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F (2004) A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc 99: 909-917. doi:10.1198/016214504000000683. [Google Scholar]
- 28. Diboun I, Wernisch L, Orengo CA, Koltzenburg M (2006) Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics 7: 252. doi:10.1186/1471-2164-7-252. PubMed: 17029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44-57. PubMed: 19131956. [DOI] [PubMed] [Google Scholar]
- 30. Spor A, Koren O, Ley R (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9: 279-290. doi:10.1038/nrmicro2540. PubMed: 21407244. [DOI] [PubMed] [Google Scholar]
- 31. Hufeldt MR, Nielsen DS, Vogensen FK, Midtvedt T, Hansen AK (2010) Variation in the gut microbiota of laboratory mice is related to both genetic and environmental factors. Comp Med 60: 336-347. PubMed: 21262117. [PMC free article] [PubMed] [Google Scholar]
- 32. Jussi V, Erkki E, Paavo T (2005) Comparison of cellular fatty acid profiles of the microbiota in different gut regions of BALB/c and C57BL/6J mice. Antonie Van Leeuwenhoek 88: 67-74. doi:10.1007/s10482-004-7837-9. PubMed: 15928978. [DOI] [PubMed] [Google Scholar]
- 33. Kovacs A, Ben-Jacob N, Tayem H, Halperin E, Fa Iraqi et al. (2011) Genotype is a stronger determinant than sex of the mouse gut microbiota. Microb Ecol 61: 423-428. doi:10.1007/s00248-010-9787-2. PubMed: 21181142. [DOI] [PubMed] [Google Scholar]
- 34. Mutch DM, Simmering R, Donnicola D, Fotopoulos G, Holzwarth JA et al. (2004) Impact of commensal microbiota on murine gastrointestinal tract gene ontologies. Physiol Genomics 19: 22-31. doi:10.1152/physiolgenomics.00105.2004. PubMed: 15226484. [DOI] [PubMed] [Google Scholar]
- 35. Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG et al. (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291: 881-884. doi:10.1126/science.291.5505.881. PubMed: 11157169. [DOI] [PubMed] [Google Scholar]
- 36. de Buhr MF, Hedrich HJ, Westendorf AM, Obermeier F, Hofmann C et al. (2009) Analysis of Cd14 as a genetic modifier of experimental inflammatory bowel disease (IBD) in mice. Inflamm Bowel Dis 15: 1824-1836. doi:10.1002/ibd.21030. PubMed: 19637338. [DOI] [PubMed] [Google Scholar]
- 37. Markova M, Koratkar RA, Silverman KA, Sollars VE, MacPhee-Pellini M et al. (2005) Diversity in secreted PLA2-IIA activity among inbred mouse strains that are resistant or susceptible to Apc Min/+ tumorigenesis. Oncogene 24: 6450-6458. PubMed: 16007193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nevalainen TJ, Graham GG, Scott KF (2008) Antibacterial actions of secreted phospholipases A2. Review. Biochim Biophys Acta 1781: 1-9. doi:10.1016/j.bbalip.2007.12.001. PubMed: 18177747. [DOI] [PubMed] [Google Scholar]
- 39. Lindbom J, Ljungman AG, Lindahl M, Tagesson C (2002) Increased gene expression of novel cytosolic and secretory phospholipase A(2) types in human airway epithelial cells induced by tumor necrosis factor-alpha and IFN-gamma. J Interferon Cytokine Res 22: 947-955. doi:10.1089/10799900260286650. PubMed: 12396716. [DOI] [PubMed] [Google Scholar]
- 40. Staeheli P, Prochazka M, Steigmeier PA, Haller O (1984) Genetic control of interferon action: mouse strain distribution and inheritance of an induced protein with guanylate-binding property. Virology 137: 135-142. doi:10.1016/0042-6822(84)90016-3. PubMed: 6089411. [DOI] [PubMed] [Google Scholar]
- 41. Thompson CL, Hofer MJ, Campbell IL, Holmes AJ (2010) Community dynamics in the mouse gut microbiota: a possible role for IRF9-regulated genes in community homeostasis. PLOS ONE 5: e10335. doi:10.1371/journal.pone.0010335. PubMed: 20428250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sonnenburg JL, Chen CT, Gordon JI (2006) Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLOS Biol 4: e413. doi:10.1371/journal.pbio.0040413. PubMed: 17132046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams SE, Brown TI, Roghanian A, Sallenave JM (2006) SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 110: 21-35. doi:10.1042/CS20050115. PubMed: 16336202. [DOI] [PubMed] [Google Scholar]
- 44. Vazeille E, Bringer MA, Gardarin A, Chambon C, Becker-Pauly C et al. (2011) Role of meprins to protect ileal mucosa of Crohn’s disease patients from colonization by adherent-invasive E. coli. PLOS ONE 6: e21199. doi:10.1371/journal.pone.0021199. PubMed: 21698174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johansson ME, Larsson JM, Hansson GC (2011) The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A 108 Suppl 1: 4659-4665. doi:10.1073/pnas.1006451107. PubMed: 20615996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Staubach F, Künzel S, Baines AC, Yee A, McGee BM et al. (2012) Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J 6: 1345-1355. doi:10.1038/ismej.2011.204. PubMed: 22278669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Similarity of the intestinal microbiota in the colonic lumen (A) and at the clonic mucosa (B).
(PDF)
Genes differentially expressed (fold change >2) between C57BL/10 and C3H mice.
(PDF)
DAVID functional gene list: signaling/secretion.
(PDF)
DAVID functional gene list: peptidase inhibition.
(PDF)
DAVID functional gene list: guanine binding.
(PDF)
DAVID functional gene list: response to bacteria/defense.
(PDF)
DAVID functional gene list: hormone activity.
(PDF)
DAVID functional gene list: lipoprotein-associated.
(PDF)