Abstract
A compact genome and a tiny brain make Drosophila the prime model to understand the neural substrate of behavior. The neurogenetic efforts to reveal neural circuits underlying Drosophila vision started about half a century ago, and now the field is booming with sophisticated genetic tools, rich behavioral assays, and importantly, a greater number of scientists joining from different backgrounds. This review will briefly cover the structural anatomy of the Drosophila visual system, the animal’s visual behaviors, the genes involved in assembling these circuits, the new and powerful techniques, and the challenges ahead for ultimately identifying the general principles of biological computation in the brain.
A typical brain utilizes a great many compact neural circuits to collect and process information from the internal biological and external environmental worlds and generates motor commands for observable behaviors. The fruit fly Drosophila melanogaster, despite of its miniature body and tiny brain, can survive in almost any corner of the world.1 It can find food, court mate, fight rival conspecific, avoid predators, and amazingly fly without crashing into trees. Drosophila vision and its underlying neuronal machinery has been a key research model for at least half century for neurogeneticists.2 Given the efforts invested on the visual system, this animal model is likely to offer the first full understanding of how visual information is computed by a multi-cellular organism. Furthermore, research in Drosophila has revealed many genes that play crucial roles in the formation of functional brains across species. The architectural similarities between the visual systems of Drosophila and vertebrate at the molecular, cellular, and network levels suggest new principles discovered at the circuit level on the relationship between neurons and behavior in Drosophila shall also contribute greatly to our understanding of the general principles for how bigger brains work.3 I start with the anatomy of Drosophila visual system, which surprisingly still contains many uncharted areas.
Keywords: Drosophila, vision, neural circuits, behavior, neurogenetics
Overall View of Drosophila Visual System
As most insects, the adult fly visual system is composed of several ganglionic relays, the retina, medulla, lobula, and lobula plate. The retina, an optically compound eye, is composed of regularly arranged ommatidia, each of which contains eight photoreceptors (R1-R8) in addition to supporting cells, to detect light ranging from UV to green.4 Although the red eyes are the two largest structures on the head, mutant flies without the eyes can live and propagate inside small vials with food. The obvious visibility of eyes and their dispensability for survival made the Drosophila eye a perfect target for genetic research. In fact, the first mutant in Drosophila, identified by T. Morgan over a hundred years ago, was white, an ABC transporter, which when missing results in white-eyed flies instead of the wild type red eye flies.5 Now we know White is responsible for carrying precursors of the eye color pigments into the developing eyes.
Each ommatidium observes a certain solid angle of the visual field and conveys information of that particular region. Equal number parallel units downstream of photoreceptor cells, called visual columns or cartridges, process visual information from corresponding regions of the visual field. As visual information is propagated in a topographic manner, the spatial location of objects in the visual field is preserved until leaving the lobula complex, beyond which massive integration occurs. Before that point, information is not passively relayed inside each column either. In addition to various columnar neurons to process information, there are many types of wide-field tangential/horizontal cells connecting columns at various levels to presumably integrate or compare information across a sizable region of the visual field.6 Communications between visual columns are the basis of many types of visual information processing, such as motion detection, object-background discrimination, sensitivity enhancement, and color perception.
Vision begins when photons hit the light capture structure, the rhabdomere, and initiate visual transduction cascades of rhodopsins in the photoreceptor cells of the eye.7,8 There are five types of rhodopsins expressed in the eye, and their peaks of absorption range from 345–508 nm.8 Each photoreceptor cell expresses only one type of rhodopsin; all R1-R6 cells express Rh1, and R7 express either Rh3 or Rh4, and R8 express either Rh5 or Rh6.9,10 These rhodopsins render a range of preferred light sensitivity from UV to green, which is different from the spectrum of visible light in human (400–700 nm).
The mosaic arrangement of photoreceptors containing different rhodopsins, similar to that in human retina, suggests Drosophila has color vision.9,10 It has been shown that the fruit flies are capable of spectral discrimination, although Drosophila has not been rigorously tested for true color vision.11 On the other hand, structural and behavioral evidences suggest that Drosophila can sense polarized light.10,12 A group of specialized ommatidia located near the dorsal rim area can detect polarized light. It was shown recently that Drosophila can utilize the sky's natural polarization pattern for active orientation during flight.13 Furthermore, a walking fly also exhibits polarotactic behavior, aligning itself with the e-vector of linearly polarized light shining from below.14 Besides the dorsal rim, the photoreceptors from different regions of retina are responsible for detecting distinct wavelengths and directions of polarized light.14,15
Structures downstream of the eye, including the lamina, medulla, and lobula complex, are more complicated (Fig. 1). It was estimated that, in the medulla, each column has contributions from more than 60 types of neurons. Besides going centripetally into the brain or spreading horizontally across neighboring visual columns, signals also move centrifugally from the central brain to the peripheral, presumably for feedback controls.
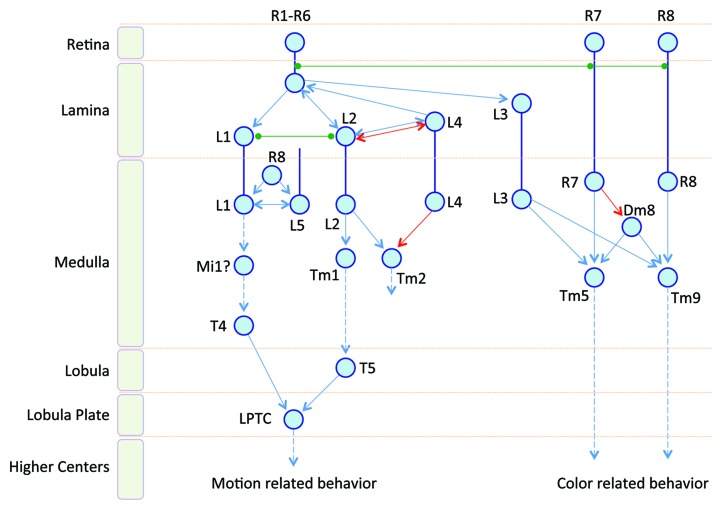
Figure 1. A diagram of connectivity in the Drosophila visual system. The visual system relies on hundreds of repeated units, the visual columns, to process information across the visual field in parallel. Shown here are some known components of a single visual column and their inter-connectivity. Motion-related behaviors depend on the R1-R6 pathway to compute motion signals, while the R7/R8 pathway is responsible for color perception and color-related behaviors. In the diagram, each neuron is simplified to one or more blue circles, which also depict the sites of connections. The direct synaptic connections are shown as arrows. When direct connection is unknown, an arrow with a dotted line is used to indicate the flow direction of information. Reciprocal connections are shown as a line with two arrow heads. Electrically coupled connections via gap junctions are depicted as green lines with green circles. The red color arrows indicate synaptic inputs coming from other visual columns. Most connections in the diagram were only revealed by reconstruction of serial EM sections, and their functions have yet been studied or confirmed by electrophysiology or behavioral assays.
In addition to R1–R6, the lamina column contains neuronal processes from five types of monopolar cells (L1-L5), C2, C3, and T1 neurons.16 The lamina also contains wide-field amacrine cells. L1 and L2 are the main postsynaptic targets of R1-6 and were demonstrated, together with other L neurons, playing a critical role in motion detection.17,18 L neurons form connections in the medulla. Axons of R7 and R8 pass through the lamina, without forming synapses there, and form connections in the medulla as well. The medulla has 10 layers; the L neurons and R7/R8 ramify in one or several distinct layers of the outer six layers. Each visual column in the medulla consists of processes from much more neurons (about 60).19 Those neurons were classified according to their morphologies and the connections they made: intrinsic medulla neurons (Mi), transmedulla neurons (Tm, connecting the medulla and lobula), and Y shaped transmedulla neurons (TmY, connecting the medulla, lobula, and lobula plate).20 There are also bushy T neurons connecting different layers of the medulla and the lobula. Each of T4 and T5 cells has four different subtypes in every column. In both large insects and Drosophila, T4 and T5 were suggested to feed motion inputs to the lobula plate.21 While the functions of most of the above cells are still unknown, it’s reasonable to speculate that many of the local computations occur there. Additionally, they might also serve to split the signals from photoreceptors into several parallel pathways that specialize in different processing properties, such as motion, color, and figure.
The knowledge on the last two visual neuropils, the lobula, and the lobula plate, offers an interesting contrast. We know much more about the organization and function of the lobula plate due to its simpler structure and extensive studies on motion processing, while the exact composition and functions of the lobula are still scarce. The lobula plate contains some large field tangential cells, lobula plate tangential cells (LPTC), integrating signals from hundreds of R1–6 pathways with their tremendous dendrites, and is responsible for computing the direction of optic flow.22 The LPTC neurons can be grouped into horizontal (HS) and vertical (VS) systems based on their overall preferred directions.
On the contrary, the lobula was predicted mainly sensitive to object features, such as orientation, texture, and color. The lobula is a cortex-like neuropil with lobula columnar neurons (LCNs) forming many “palisades”; the LCNs are comparable to the pyramidal cells in a mammalian cortex.23 The cell types and functions of the lobula neurons are just begun to be revealed. Among several classes of lobula-specific visual projection neurons bringing information to ventrolateral protocerebrum,24 LT10 and LT11 were required for proper response to certain second-order motion, suggesting the shared functions in motion detection or interactions between the lobula and the lobula plate.25
Motion circuits
The prominent model of motion detection is EMD (elementary motion detector), first proposed by Hassenstein and Reichardt half a century ago, explaining the neural mechanism of biological motion computation in animals ranging from insects to mammals.22 According to the model, the basic unit of motion detection in the visual system is a Reichardt detector, each of which utilizes two channels to sample changes in luminance at two distinct locations in the visual field. The output of one channel is delayed and then multiplied with that of another channel; a subsequent subtraction of the two channels yields the direction of motion. EMD is probably the single most successful model in biological computation; however, mapping such a simple algorithm onto the neural hardware turned out to be a rather difficult task.22
Linking Drosophila's visual motion input to its behavioral output, the optomotor response, has provided most of our knowledge on neural implementation of motion perception. The primary input of visual motion is the photoreceptors R1–6. Downstream of R cells, the two most prominent pathways, L1 and L2, are involved in motion detection.17 Interestingly, although there is no evidence to suggest that R1–6 function differently, the L1 and L2 neurons, while receiving seemingly similar inputs from R1–6, were shown recently to play different roles in motion processing.19 Neurogenetic experiments suggested that L1 and L2 mediate motion responses of opposite polarity at intermediate contrast: L1 for back-to-front motion and L2 for front-to-back motion, respectively. However, at low contrast, L1 and L2 rely on each other for motion detection.17 L2, but not L1, can also differentially modulate translational and rotational walking behaviors.26 Electrophysiological studies further indicated that L1 and L2 were selective for dark-bright transitions (L1-ON-pathway) and for bright-dark transitions (L2-OFF-pathway), respectively.27 Furthermore, these two pathways form two types of Reichardt detectors operating in parallel.28
Starting from the L cells, the motion circuit quickly becomes complicated (Fig. 1). In the lamina, L2 and L4 of the same column and the neighboring columns are reciprocally connected, and L4 is implicated in motion detection as well.16,18 Single cell transcript profiling identified L1 is glutamatergic, while L2 and L4 are cholinergic, providing important clues about the receptor types of their post-synaptic targets.29 High-resolution studies by serial-sectioning transmission electron microscopy (ssTEM) of the retina, lamina, and portion of medulla depicted a very intriguing interwoven connection diagram, while far from complete, that rivals a general purpose electronic circuits.16,19,30 First, high interconnectivity occurs among R, L, and C cells besides R1–6 connect to L1-3 (Fig. 1). R8 form connections on R7, both of their targets receive inputs from L3. In the lamina, C2 and C3 provide inputs to L1. However, in the medulla, L1 connects to C2 and C3, while both of which connect to L2. L1 and L5 in the medulla, as well as L2 and L4 in the lamina, are reciprocally connected. L1 and L2 receive matched inputs from R1–6, and there is a strong electrical coupling between L1 and L2. The L2 and the L4 of adjacent visual columns form reciprocally synaptic connections directly in the lamina and indirectly in the medulla via an unknown cell type.27 Furthermore, EM study also showed that L2 connects to two medulla neurons, Tm1 and Tm2, while Tm2 also receives input from L4 of the neighboring visual columns responsible for anterior location in visual space. Last but not the least, direct electrical coupling through gap junctions occurs in multiple places, and likely plays crucial roles in visual processing, such as those linking R7/R8 to R6 in a specific zone of the lamina22,31(see below).
Combining data from Drosophila and other insects, it was speculated that the L1 signal goes through Mi1 to T4, while the L2 signal goes through Tm1 to T5, and both pathways finally reach the LPTC in the lobula plate, which is well characterized and known to integrate local motion signals into global optic flow and play a key role in visual course control.21,32
Color circuits
It is generally believed that R7 and R8, with their distinct rhodopsins, are the inputs for color-sensitive circuits. The information about the neurons beyond R7 and R8 is rather limited except the immediate downstream targets (Fig. 1). Serial EM indicated that Tm5 and Tm9 receive direct synaptic input from R7 and R8, respectively.11 They also receive input from L3, therefore, indirectly from R1–R6. In parallel, the wide-field Dm8 amacrine neurons pool inputs from 13–16 R7 neurons and then feed into Tm5 and Tm9 as well.11 Such intricate connections make these neurons the prefect candidates as color-opponent neurons. Behavioral analysis demonstrated that Dm8 neurons are both necessary and sufficient for preferential response toward UV light.11 With systematic clonal analysis to reconstruct the neural network underlying color vision in the medulla, more candidate neurons were revealed as having immediate contacts with R7 and/or R8 within the same column.33 Importantly, the analysis also identified other neurons that would be important for processing color information further, including neurons connecting R7 and R8 from other columns, third order neurons, and local neurons.33
The motion and color pathways in Drosophila were commonly considered to be separated. The R1–6-based motion pathway is regarded as achromatic because motion perception is independent of R7/8 and color-information.26,34,35 Given the inter-connectivity between the R1–6 pathway and R7/R8 pathway in the medulla, it will be interesting to see whether R1–6 contribute to color perception in Drosophila. On the other hand, a recent study strongly suggested that R7/R8 supply information into motion pathways to improve the size, speed, and spectral range of optomotor response.36 This is likely through gap junction-mediated electrical interactions between R7/R8 and R6.31,36
Beyond the optic lobes
In addition to the local neurons making connections within and between various optical lobes, there are also visual projection neurons (VPNs) connecting the medulla, lobula, and lobula plate to the central brain.24 Together, they both convey visual information to the central brain and send the command signals back to the optic lobes. For example, each palisade of the lobula columnar neurons gives rise to a unique axon bundle to target a specific region in the lateral protocerebrum.24 Interestingly, this deepest part of the visual system has similar neural organization as the glomerular antennal lobes; therefore, the assembly of the local interneurons and projection neurons was called optic glomerulus.37 Recently, electrophysiological analysis demonstrated that optic glomeruli enabled reliable responses by converging sensory inputs, thus to further reconstruct the fly’s visual world.23 A morphology-based screen of all VPNs identified 44 pathways, and a detailed analysis of 14 lobula-specific pathways suggested that at least the ventrolateral protocerebrum could be divided into several functional subdomains.24 A behavioral study demonstrated that two VPNs, LT10, and LT11 are required for processing certain types of second order motion.25
Deeper brain centers also participate in visual information processing, directly or indirectly. The central brain structures such as the mushroom body, ellipsoid body, and fan-shaped body have been suggested to be involved in vision related behaviors.38-41 Not only they are structurally complicated, but also, since the signals feeding into these centers come from multi-senses and are presumably highly “processed,” their roles are inherently difficult to point out. In many instances, it is the visual system and the higher brain centers together determine a suitable reaction.
Visual Behaviors in Drosophila
A single ray of light has properties of intensity, propagation direction, wavelength spectrum, and polarization. The rays of light coming from the world surrounding a Drosophila in the nature convey much more information. Lights of different properties distribute spatially along the visual field to form patterns, and both the organization and the properties of these rays could undergo dynamic changes in a short period; yet flies are equipped with massive, parallel neural circuits handling such complex visual information, to react with instantaneous responses.42
Throughout the long history of Drosophila vision research, two innate behaviors have been broadly and extensively studied for both the neural basis of visual processing and the molecular basis of neural circuit formation: the phototactic response, turning toward a light source, and the optomotor response, moving in react to visual motion cues.
Phototactic behaviors
Over half a century ago, T-mazes and countercurrent devices were developed to fractionate populations of Drosophila according to their phototactic abilities. The behavioral mutants, blind flies or flies with reduced vision, exhibited abnormal response and were identified and/or isolated from the normal behaving animals.43,44 From then on, utilizing genetic tricks to generate Drosophila eyes composed exclusively of a single mutation while keeping the rest of the animal wild-type, powerful genetic screens with light-seeking behavior as the phenotypic readout were conducted in multiple laboratories. These studies provided much information of photoreceptors and the general cell biology of neurons.45-47
The variant, “two-color choice” assay, where the flies were evaluated based on their selection of either green light or UV light, were so highly optimized that one person could screen through hundreds of lines in a morning.48 From these screens, multiple genes responsible for R7 and R8 development were identified.45,49-51 Combined with the neurogenetic approach, the downstream targets of R7 were also revealed recently.11
It’s intriguing that, for such a simple, quick, and robust response, there is much variability that can’t be accounted for. For one, there were evidences suggesting a long-term plasticity of phototaxis in Drosophila,52 highlighting that this innate response is not a simple reflex. By training, Drosophila can learn to suppress their innate preference toward light.53 Furthermore, when repeatedly assessing phototactic responses in single animals with a high-throughput automatic device, “FlyVac,” surprising variability was found even within isogenic strains that were identically reared.54 The finding of such “phototactic personality” indicates that rich new information could still be extracted from a detailed observation of behaviors as simple as phototaxis.
In a phototaxis assay, despite the physical nature of the device, flies response to the relative static, structurally simple light source ranging from a white fluorescent light tube to a single color LED. As noted previously, Drosophila is also capable of polarotaxis when linearly polarized-light, from either the sky or a mercury lamp with a filter, is provided from above or below of the body plane.15
Motion-related behaviors
The stimulus to induce a visual motion response, unlike that for a phototactic response, requires more sophisticated setups, which come in various forms: moving papers painted with alternate black and white strips, programmable LED arrays, and high refresh-rate computer displays as well as projectors. Most of the time flies were tested individually for its response to visual motion, by measuring continuously the locomotion of the whole body or the movement of body parts (head, wings, legs, and antennae). The Drosophila under test can be either tethered or freely moving, in the form of flight or walk.
In the 1970s, Gotz and Heisenberg developed a very complicated optomotor maze composed of a series of interconnected units. Each unit has one entrance and two exits. In a unit, flies sorted themselves out, based on their reactions to the surrounding motion stimuli, to different exits and subsequently entered the next units to repeat the sorting process again.55 Using this maze, the early efforts of genetic screens resulted in identification of the first gene related to motion perception, the optomotor-blind (omb).55 A series of genetic screens combining mosaic techniques with behavioral analysis in the “Benzer’s machine,” which generated moving light bars on a dark surface as the visual motion cue, uncovered N-Cadherin, a cell surface adhesion molecule, and LAR, a receptor tyrosine phosphatase.49,51
The recent “circuit breaking” strategy, aiming at zooming in the neurons or circuits responsible for different behaviors, evaluates a fly’s motion response after modulating directly the target neurons’ activity by genetic methods. With a “virtual” flight simulator, the flight arena, where steering responses of a tethered fly in react to motion stimuli can be recorded and analyzed, Rister et al. showed that L1 and L2, the major synaptic targets of R1–6, were necessary and mostly sufficient for motion-induced behaviors.17,51 When free moving flies were given panorama visual motion in a U-shaped hallway, they rapidly walked against the direction of motion, and accumulated at the origin of the motion, so the setup was named “flystampede.”18 Blocking lamina neurons, including L4, rendered the flies non-responsive to such motion stimuli, while they still exhibited robust phototactic response, suggesting a functional segregation of motion and phototactic pathways right after photoreceptors.18 Katsov et al. examined the trajectories of flies reacting to motion stimuli presented by a computer screen, which allowed systematically varying the velocity, contrast, luminance, spatial density, and coherence of the visual stimulus.26 Interestingly, two distinct instantaneous responses to motion were identified. While the flies immediately moved opposite to the direction of visual motion containing sparse stimuli, dense stimuli evoked locomotion in the same direction of the motion.26 Genetic dissection of these responses suggested two parallel pathways, a L2-dependent pathway and a Foma1-dependent pathway, are sensitive to different visual features and coupled to distinct behavioral outputs.26
In the past few years, new hardware, such as patch-clamp recording devices and two-photon imaging modules, was integrated to behavior setups in order to measure the activities of neurons of a behaving fly. These devices provided additional dimensions to correlate neural circuits with visual motion behaviors. Detailed discussion of these will be in the section of new techniques.
Second-order motion and visual illusion
Playing with visual motion stimuli has lead to astonishing discoveries to help gaining insight of the mechanism of motion detection in Drosophila.56 One example is the works on second order motion. The common motion perception is through spatio-temporal correlation of luminance, this is called first order motion or Fourier motion, such as a black bar moving on a random-dot background. In second-order motion, the moving contour is defined by contrast, texture, flicker, or some other quality, rather than luminance. It was believed previously only animals with advanced cortex can detect high-order motions, until it was demonstrated that Zebrafish and Drosophila could see second-order motion.57 Further study revealed that fruit flies could track a moving object or figure containing both elementary and higher-order signals, such as theta motion.58,59 Through a novel white-noise analysis method, the response toward such object was efficiently decoupled into an elementary motion component and a higher-order figure motion component.59 The elementary motion component was velocity-dependent, whereas the higher-order figure motion component was driven by retinal position.59 Both components could be linear superposed, and their combination was both necessary and sufficient to predict the full range of figure tracking behaviors, which is beyond the successful range of a simple EMD model.59 These findings inspire interesting questions of the neural substrate of the second order motion and the differences, at the circuit level, between the first and second order motion.
Genetic dissections revealed LT10 and LT11, two lobula projection neurons connecting the lobula to the central brain, were involved in perception of theta motion,25 one type of second-order motion, while being unlikely required for first-order or flicker-defined second-order stimuli.58 This entailed an interesting possibility that the lobula, commonly associated with processing object features, might participate in motion detection, although the lobula plate with its LPTC neurons was widely considered the downstream center of EMDs for spatial integration of motion in the visual field. Given the numerous interconnections between the lobula and the lobula plate by various types of neurons, it is also very likely that certain motion processing in the lobula plate requires modulation from the lobula.58
Besides second-order motion, it seems that human and flies share more common features in visual processing, such as visual illusion.56 When presented by a classic illusion, the reverse-phi motion, a flying Drosophila exhibited a response inverse to the direction of motion.56 The reverse-phi stimulus can be deconstructed into individual components: motion and flicker. By varying the relative strength of these components dynamically, an interesting interaction between the motion and flicker components was revealed. A stimulus with equal strength of reverse-phi and standard motion made flies turn against the direction of stimulus motion, suggesting a nonlinear combination of the response toward the two stimuli.56 A classic EMD would not explain this response, although it could predict the reverse-phi response mostly.56 Whether second-order motion or visual motion illusion is mediated by EMD or not still remains an open question. At least, correlation-type EMD models can explain some non-Fourier motion responses through amendment.
Other visual behaviors
Besides phototaxis and motion detection, a fly needs more visual strategies to survive the rather complex and dynamic environment in the wild. Some of the essential survival skills can now be studied in laboratory settings, for example, to walk and fly forward and straight, to avoid an incoming branch or pray, to take off promptly, and land safely. Flight simulators played a prominent role in vision research in Drosophila, besides contributing to the motion studies discussed previously. Recent years see booming behavioral studies with various innovative paradigms based on the versatile flight simulators. Limited by space, I will only summarize a small numbers of works here, although much more deserved to be mentioned.
Drosophila takes a looming stimulus seriously, which indicates oncoming danger, and jumps into air to escape promptly.60 This visually evoked jump escape is rapid: within less than 300 ms after the onset of looming stimulation, the fly is already airborne.61 However, close examination with high-speed videography suggested that there was a “planning” stage. Two hundred milliseconds prior to takeoff, the fly performed a series of postural adjustments to guide its escape directly away from the looming threat.61 The loom-sensitive neurons were identified, and silencing these neurons by genetic manipulation reduced the frequency of the loom escape response, while activating these neurons in blind flies with optogenetic stimulation sufficiently elicited the escape response.62
The readily available computer-controllable displays, combined with increasingly sophisticated video-tracking software, also promoted novel behavioral paradigms for walking flies. While determining the position and orientation of a single fly within a field is mostly straightforward, interactions, especially body contacts between flies, make tracking a group of flies automatically a much more demanding task. Dankert et al. developed a software system for monitoring and analyzing a pair of flies, which focused on detecting behavioral features exhibited during aggression and courtship.63 Ctrax was developed as a general purpose offline tracking tool with two goals.64,65 It can accurately track many individuals without swapping identities, but also detect behavioral patterns with classification algorithms.64 In the study of Drosophila spatial memory, the ability to remember a location based on surrounding visual cues, the controllable display and multi-fly tracking software were two essential prerequisites.66
With a mult-camera system, Grover et al. not only tracked robustly the movement of Drosophila in real-time, but also constructed the three-dimensional visual hull of each fly, making more detailed analysis of fly behaviors possible.67 Similar automated hull reconstruction approaches have been applied to track the maneuvers of freely flying insects, leaving computer-aided manual tracing of body features from thousands of frames a distant past.68,69 Tethering a fly in a flight arena might have unknown effects due to the highly constrained experimental conditions. A new tool was developed to enable tracking the freely flying flies in a wind tunnel outfitted with virtual reality display technology, thus finally allowing researchers to investigate the “natural” form of flight in detail.70
Multisensory integration
A fly utilizes multiple sensory modalities to gather information from the feature-rich environment to guide its behavior. Visual responses were known in the past to be modulated by mechanical and olfactory senses.71
The importance of halteres to insect flight is well known, but it is not the only mechanosensory input used in flight.72,73 Recently it was reported that Drosophila actively moved its antennae during visual-guided steering maneuver.74 Normally, the forward flying Drosophila would experience expending visual stimulus, a strong aversive visual cue in tethered flight, and a wind. Antennae might help the fly to detect and orient toward the wind to counteract the inhibitory effect of visual expansion, therefore to maintain forward flight.75
Multi-model sensory integration, particular between vision and olfaction, was typically investigated in free flight setups76,77 and flight simulators.78 There was a new “loose” tether setup that allows a fly to freely rotate in a horizontal plane.79-81 A fly in free flight responds to odors by turning upwind and increasing its flight speed. Interestingly, it needed appropriate visual feedback to locate a hidden odor source.76 While a fly tracked the plume of an attractive odor, the olfactory cue modulated the gains of the optomotor response to yaw rotation and sideslip optic flow, resulting in better tracking of the odor plume.82
The advantage of using a “loose” tether setup is to manipulate the visual and olfactory stimuli precisely. When a fly freely rotates horizontally to choose different odor sources in a panorama arena, its heading can be readily calculated from the video images recorded by a camera.79 Genetic manipulation revealed that minimal activity of a single type of odorant receptor neurons is sufficient to trigger rapid odor evoked flight modulation.81 A study on “anti-tracking” noxious odors in aversive flight revealed shared features of olfactory modulation by both attractive and aversive odors in a loose tether assay.83
High-center modulation
Not much is known about how cross sensory modulation is implemented in Drosophila. In a hungry fly, olfaction sensitivity was increased directly through action of sNPF and insulin on specific odorant receptor neurons.84 In the visual system, a group of interneurons of the lobula vertical system showed boosted activity toward visual motion during flight. Ectopic application of biogenic amine octopamine evoked similar responses in quiescent flies.41 Furthermore, the octopamine neurons projecting to the optic lobes showed elevated activities during flight, while inactivation of octopamine neurons abolished the flight-induced effect.41 This suggestes that the state-dependent modulation of visual interneurons is through endogenous release of octopamine.41 How flight induces the activity of the octopamine neurons is still unclear. Additionally, octopamine might play multiple roles in regulating flight, as silencing octopamine neurons inverted the response to CO2 from attractive to aversive in flight.40
In contrast to the luxuriation of highly quantitative behavioral assays, the investigation on the neural basis of those seemingly “simple” responses has been far more challenging. Identifying neurons beyond the primary sensory inputs and their synaptic targets is still relatively slow despite the multiple genetic tools available. However, this did not deter the curious minds from peeking into the deeper brain to study complicated behaviors related to vision, such as salience,85 novelty and attention,86-88 choice,89 memory,39 and courtship, while some of these seem to exceed the abilities of typical Drosophila by the traditional viewpoint. Detailed accounts of these findings, although fascinating, are beyond this review.
A walking fly relies on visual cues to maintain its course in order to walk straight, as shown in the classic Buridan's Paradigm, which requires higher brain centers.90 When the guidance cue temporally disappears and re-appears, the fly acts on it accordingly. Further genetic manipulation suggested that protein kinase S6KII in the ring neurons of the ellipsoid body was necessary for such spatial orientation memory.91,92 Moving animals experience self-generated reafferent optic flow, which is useful to provide information about the stationary environment and ego motion. However, since the reafferent optic flow could also confound image motion, a fly would have difficulties detecting moving objects from the optic flow field. Studies revealed that a walking fly used a mechanism called “regressive motion salience” to handle a mismatch between predicted reafferent retinal motion and externally caused motion by selectively responding to the back-to-front moving object.93
The action of a fly walking across a gap of widths exceeding its body length provided a unique opportunity to probe the neural circuits of decision and motor planning in Drosophila.94,95 By visual width estimation through parallax motion, a fly decided to cross surmountable gaps while avoiding attempts at insurmountable gaps. The complex maneuver of gap-crossing displays modularity of motor controls.94 Two genetic mutations causing defects in the protocerebral bridge (PB) of the central complex rendered flies with errant gap-crossing behavior. The mutant flies were able to initiate gap-crossing attempts, but could not aim their maneuvers to the correct directions, suggesting PB transmits directional clues to the motor output and is an essential part of the visual targeting network.95
From Molecules to Circuits and Behavior
Thanks to the efforts of generations of Drosophila geneticists, numerous genetic tools are established to manipulate the Drosophila genome and screen for corresponding phenotypes. Using the Drosophila eye as a model organ, almost the full spectrum of cell biology of neurons has been studied. Genetic screens have identified important genes with conserved functions in the processes of phototransduction, cell fate determination, cell adhesion and sorting, proliferation and programmed cell death, axon path-finding, target selection, and synapse formation. Besides structural abnormalities, the pioneers also investigated how genetic mutations in the eye would elicit visual deficits by electro-retinogram (ERG) and behavioral tests. As more investigators shifted research focus to the inner neurons, which are similar to the neurons in our brain, essential clues about neural circuits and biological computation were gradually obtained.
The study of optical lobe development in Drosophila has a long history.96 Because of the relative ease in observing certain behaviors, the fruit fly has been a favored model for investigating molecular basis of behaviors since the early days of behavioral genetics. Pioneer works by Benzer led to famous discoveries of period, a circadian rhythm gene, and dunce, a gene essential for learning and memory.97 A motion-based screen identified optomotor-blind, which mutation caused insensitive to motion stimuli. Behavioral screens also revealed that N-Cadherin and LAR were necessary for motion detection ability.49,51
Combining powerful genetic approaches, such as mosaic analysis, with the modern imaging techniques, such as confocal microscopy, allows us to characterize the effects of genetic mutations on the morphology of specific neurons and to deduce the connectivity of a local circuit.98,99 Important molecules were identified in different parts of the visual system. For example, in the retina, 2-color choice screens identified N-Cadherin50 and NF-YC;100 in the lamina and medulla, Dscam1 and Dscam2 were shown to regulate synaptic specificity and mediate tiling;101,102 and in the lobula plate, Mosaic analysis with a repressible cell marker (MARCM) revealed Cdc42, a GTPase, played important roles in the development of the VS neurons.103,104 With a great number of genes identified and mutations available, one step further would be to go from molecules to behaviors, correlating the affected neurons by genetic mutations with abnormal behaviors. Analyzing the functional role of specific components of synaptic connections, for example, subtypes of neurotransmitter receptors as well as gap junction proteins, in vision seems the low-hanging fruit for linking molecules to behaviors.30
Cell surface molecules play pivotal roles throughout the life of a neuron, from fate determination to synapse formation and plasticity.105,106 Therefore, it is probably not surprising that most genes identified so far, from genetic screens on the developmental events leading to visual circuit assembly, were cell surface molecules. Several recent reviews provided a step-by-step guide to those rather complicated events and summarized a broad range of cell surface molecules.107,108 Additionally, readers interested in comparing the fruit fly with other species for similar developmental events and molecules are encouraged to read Sanes and Zipursky (2010)3 and Huberman et al. (2010).109 In terms of visual circuit development, especially in the R cells and L neurons, the functions of cell surface molecules were well established. However, it is not clear whether any of those molecules function in maintaining synaptic connections. Additionally, are there molecules involved directly in a specific neural computation process instead of acting as a generic structural component?
It is often desirable to identify genes or neurons with specific behavioral functions without being complicated by the secondary effects of abnormal development. However, as cells frequently utilize the same set of molecular events again and again from developmental stages to the adulthood, we might not have adequate tools to bypass developmental defects to only focus on their roles in circuits and behavior in adults. Moreover, how a functional circuit is assembled and which molecules are involved in the process are too important to overlook.
Advance of Neurogenetic Tools
Three categories of tools have accelerated our understanding of circuits and behavior in Drosophila in the past decade. One is the behavioral paradigms to reveal what a fly can do or think; this topic was covered in the previous section. The second is the genetic tools to manipulate individual neurons, preferably while a fly is performing a task, with precise spatial and temporal resolutions to establish the casual basis of behaviors. The third is the tools allowing us to peek into the fly brains, observing directly the activities of neurons or a network during an action.
We are fortunate to possess diverse sophisticated genetic tools to control the expression level of specific genes and genetic effectors. For a thorough review of the current genetic tools to manipulate genes and neurons for neurogenetic study of behavior, readers are recommended to read Venken et al. (2011);110 I only provide a brief description here. Built on top of the popular GAL4-UAS binary system, the use of Gal80 and split-Gal4 further narrows down the population of affected neurons through combination. New activator-element pairs, such as the lexA-lexAop system and the Q-system, permit orthogonally control of two sets of expressions within neurons. However, placing many genetic components into the genome would potentially increase the chance of breaking or interfering with “important” genes, thus lead to undesirable behavioral consequences. Methods for site-directed integration of multiple genes into a well-characterized chromosomal location would offer great advantages. Additionally, the Integrase Swappable In Vivo Targeting Element (InSITE) system helped to replace existing GAL4 insertions with newer genetic effectors, making reuse of the old genetic “handles” a less painful process.111 Furthermore, a new large scale promoter fusion approach promised to provide better genetic “handles” for various neurons throughout the nervous system.112
There are numerous genetic-encoded reagents for manipulating neural circuits and observing behavioral consequences. The commonly used belong to three categories: (1) eliminating target neurons by killing them (Diphtheria and Ricin), (2) lowering neurons excitability or silence their synaptic transmission (Kir, TNT, Shibiriets, and Halorhodopsin) or (3) increasing activities of the target neurons (TrpA1, NaChBac, and Channelrhodopsin). Channelrhodopsin-2, an optogenetic reagent, has been shown to sufficiently induce looming response in blind flies by controlling light.113-115
The highly effective RNAi libraries, which empower systematically knocking-down the expression of targeted genes, open the door for genome-wide RNAi screens to identify molecular players underlying behaviors.116,117 However, large scale RNAi screens for visual phenotypes have not been reported yet.
Besides genetic tools for precise manipulation of target neurons, biophysical approaches, including neural activity imaging and electrophysiological recording, are being adapted to monitor the activities of neurons of interest in behaving flies responding to visual stimuli to provide the unparalleled details of signal processing. Recording field potentials with multielectrodes in the brain of tethered flies revealed a brain activity of 20–30 Hz, which amplitude increased when the fly was presented with salience cues.85 Patch recording from R cells and L neurons in head-fixed flies demonstrated that the chromatic inputs, R7 and R8, contributed to motion perception through improving motion discrimination.36 Additionally, whole cell patch recording revealed that the activity of VS cells, the known motion-processing neurons in the lobula plate, increased in the flying flies, suggesting the gain of the VS neurons change with locomotor state.118,119 Recording from those LPTC neurons as measurement of motion detection, while genetically blocking L1 and/or L2 neurons, revealed L1 and L2 channels in lamina were ON and OFF sensitive, respectively.27 Further recording with different combinations of ON-OFF signals suggested there are two, instead of four, kinds of independent motion detectors in the fly motion detection circuit.28 Likewise visually guided whole cell recording from three types of horizontal neurons (HSN, HSE, and HSS) in the lobula plate were conducted to study the optomotor response elicited by large-field horizontal motion.120 Moreover, recording the activity of visual interneurons also helped to elucidate the inner processing mechanisms of visual illusion.56,121
Using multiphoton imaging to detect sensitive fluorescent indicators also proved to be informative as it directly visualized the activities of the neurons deep in the brain. Imaging calcium signals in L1 and L2 neurons, while presenting visual stimuli to the fly, revealed that L1 and L2 preferentially responded to light and dark moving edges, respectively.122 When a tethered fly walking on a floating ball was presented with visual motion, calcium transients in the horizontal LPTCs corresponded closely to robust optomotor behavior.123 The amplification of calcium signals was correlated with walking speed in response to visual motion, suggesting these cells facilitate motion processing in behavioral contexts.124 Among various genetic encoded indicators, a popular reagent in recent years is the GCaMP series. GCaMP is a calcium indicator that emits fluorescence in response to increased calcium levels resulting from neuronal activities. The development and molecular optimization of GCAMP to achieve brighter, faster, and higher signal-to-noise ratio detections led to high-performance GCaMPs, including GCaMP3, GCaMP5, and GCaMPJ.125,126 Alternatively, there were new developments on visualization of neural circuits based on activities, such as the CaLexA (calcium-dependent nuclear import of LexA) system.127
In addition to traditional genetic screens, “omic” approaches have also been incorporated into behavioral research. Whole genome comparison with microarray analysis of gene expression levels in the “neutral” flies and the “aggressive” flies, derived from multi-generations of artificial selection, identified genes independently contributing to aggresion,128 however, similar approaches have not been utilized in vision study. The nascent connectomic approach to reconstruct the wiring diagram of the fly brain at the synaptic level also attracted much attention.30,129,130 Electron microscopy has been a valuable tool for studying the eyes of Drosophila in the past century. However, the increased complexity of the deeper visual system hindered the progress there, which traditionally depended on “brute force” reconstruction of circuits from hundreds of serial ultrathin sections. Aided by new developments such as serial block face scanning electron microscopy (SBFSEM),131 focused ion beam milling and scanning electron microscopy (FIB-SEM),132 and semi-automated reconstruction serial-section transmission electron microscopy (ssTEM),133 a high-throughput connectomic reconstruction of a large volume of the fly brain is now possible. I expect that, after the adult retina,134 lamina,16 and part of medulla,19,29 the synaptic connectivity of the entire medulla will soon be revealed.133
Challenges Ahead
This is certainly a great time to work on neural circuits and behavior in Drosophila. In addition to the growing numbers of genetic methods and reagents available, new advances and technologies from other disciplines such as computer science, engineering, and microscopy are quickly becoming accessible for neurogenetics and behavior research. However, understanding how a tiny brain actually works faces at least another decade of challenge. Nevertheless, the question at hand is fundamental and holds the promise of providing generalized insight to understand bigger brains like ours. We will need to expend the genetic toolbox, develop new behavioral paradigms, and refine the concepts of biological circuitry and behavior.
Because of the inherent intricacy of even the tiny fly brain, we still lack a consistently robust and effective interrogating tool for simple behaviors. One particular issue is the shortage of clean genetic “handles,” which would allow us to exclusively manipulate specific neurons, but not others, with sufficient strength and at the right time in the life history of the animal related to their behavioral function. Systematic approaches ranging from enhancer trapping to promoter bashing have not yet delivered sufficiently. A game-changing tool would enable researchers to generate custom designed genetic elements to accomplish controlled expression at desired times with predetermined strengths in predetermined subsets of neurons.
The second challenge is regarding behavioral paradigms and analysis. Drosophila exhibits a large repertoire of behaviors, and about 50 of them have been characterized by different studies in laboratory settings. The “Fly Olympiad Project” at the Janelia Farm Research Campus of HHMI planned to develop high-throughput assays for a wide range of known behaviors, and using these assays to generate a database of behavioral phenotypes after manipulating activities of populations of neurons with the new generation of promoter-fusion lines as handles.135 As the interest in Drosophila behavior grows, the number of new behaviors that have not been previously reported soars. In most of the assays discussed previously, in order to have the precision for body position and signal location, the flies to be tested need to be fixed, thus presenting an abnormal situation for the fly. On the other hand, a Drosophila in free flight or a group of flies exchanging social information is obviously not compatible with current implementation of brain activity imaging or electrophysiological recordings.
Furthermore, there is a time-frame for any behavioral assays. While constantly exposed to the environment throughout its life, a fly constantly reacts to it, with multi-model sensory cues, each of which might have unique temporal effects. As most vision research focused on the instantaneous response of a fly, for convenience and efficiency, behaviors that manifest over a large time scale, although probably equally import, were largely ignored. Automated systems to periodically document specific behavior throughout the life of Drosophila have emerged, although are somehow limited to locomotion activities with an interval from 20 min to 2 h.136,137 However, suppose with cameras of high resolution and high speed, we can record every move to the finest detail and accumulate terabytes or petabytes of video data, how, then, can the data be analyzed? What is a meaningful move and what is not?
When comparing the diagram of a biological circuit to that of an electric circuit, the difference is tremendous at this moment, mainly due to the fact that we have far from enough information to fill the gaps in the biological circuit. On the other hand, should they be comparable any way? At the basic level, the building blocks of biological computation are different from that of silicon-based processors. The high-level principles of information processing by the “carbon-based” lifeform like us remain to be discovered. Furthermore, bridging the wide gap between neural circuits and behavior would require knowing the intermediate level elements—the neural computations at the level of populations of neurons.138 The static circuit diagrams building from the best approach available, serial EM reconstruction, will provide clues, but much of the dynamic processes would be far beyond what this tool was designed for.
The role of a neuron might be multiple. Neurons far away from sensory inputs, such as those in the mushroom body, might participate in vision, olfactory, and learning/memory. Giving it any single label, drawing from a corresponding behavioral paradigm, would not sufficiently reflect its roles. Another issue is the functional redundancy of circuits. Certain behavioral responses, which are too critical for survival to fail, might be implemented with multiple pathways to a “fail-proof” degree. Simply blocking one pathway might exhibit only little or no sign of behavioral deficit. As we navigate deeper into the brain to probe complex circuits and behavior, the golden standard of “necessary and sufficient” might not be expected to be the case from time to time.
Once we can freely modulate a neuron or different classes of neurons in Drosophila and observe any circuit activity or behavioral consequence at will, can it be declared that we understand a tiny brain? How about the mind?139
Acknowledgments
Research in the author’s laboratory is supported by National Basic Research Program of China (2012CB825504), NSFC grants (31070925 and 91232720), and 100-Talents Program of the Chinese Academy of Sciences.
Glossary
Abbreviations:
- EMD
elementary motion detector
- LCN
lobula columnar neurons
- LPTC
lobula plate tangential cells
- ssTEM
serial-sectioning transmission electron microscopy
- VPN
visual projection neurons
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/25521
References
- 1.Keller A. Drosophila melanogaster’s history as a human commensal. Curr Biol. 2007;17:R77–81. doi: 10.1016/j.cub.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 2.Heisenberg M, Wolf R. Vision in Drosophila: genetics of microbehavior. Berlin; New York: Springer-Verlag, 1984. [Google Scholar]
- 3.Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heisenberg M, Buchner E. Role of Retinula Cell-Types in Visual Behavior of Drosophila-Melanogaster. J Comp Physiol. 1977;117:127–62. doi: 10.1007/BF00612784. [DOI] [Google Scholar]
- 5.Morgan TH. Sex Limited Inheritance in Drosophila. Science. 1910;32:120–2. doi: 10.1126/science.32.812.120. [DOI] [PubMed] [Google Scholar]
- 6.Bausenwein B, Dittrich AP, Fischbach KF. The optic lobe of Drosophila melanogaster. II. Sorting of retinotopic pathways in the medulla. Cell Tissue Res. 1992;267:17–28. doi: 10.1007/BF00318687. [DOI] [PubMed] [Google Scholar]
- 7.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–93. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 8.Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35:356–63. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wernet MF, Desplan C. Building a retinal mosaic: cell-fate decision in the fly eye. Trends Cell Biol. 2004;14:576–84. doi: 10.1016/j.tcb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Morante J, Desplan C. Building a projection map for photoreceptor neurons in the Drosophila optic lobes. Semin Cell Dev Biol. 2004;15:137–43. doi: 10.1016/j.semcdb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Gao S, Takemura SY, Ting CY, Huang S, Lu Z, Luan H, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–42. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf R, Gebhardt B, Gademann R, Heisenberg M. Polarization Sensitivity Of Course Control In Drosophila-Melanogaster. J Comp Physiol. 1980;139:177–91. doi: 10.1007/BF00657080. [DOI] [Google Scholar]
- 13.Weir PT, Dickinson MH. Flying Drosophila orient to sky polarization. Curr Biol. 2012;22:21–7. doi: 10.1016/j.cub.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wernet MF, Velez MM, Clark DA, Baumann-Klausener F, Brown JR, Klovstad M, et al. Genetic dissection reveals two separate retinal substrates for polarization vision in Drosophila. Curr Biol. 2012;22:12–20. doi: 10.1016/j.cub.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie RC. Polarization vision: Drosophila enters the arena. Curr Biol. 2012;22:R12–4. doi: 10.1016/j.cub.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–63. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 17.Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–70. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Nern A, Zipursky SL, Frye MA. Peripheral visual circuits functionally segregate motion and phototaxis behaviors in the fly. Curr Biol. 2009;19:613–9. doi: 10.1016/j.cub.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takemura SY, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischbach KF, Dittrich APM. The Optic Lobe of Drosophila-Melanogaster. 1. A Golgi Analysis of Wild-Type Structure. Cell Tissue Res. 1989;258:441–75. doi: 10.1007/BF00218858. [DOI] [Google Scholar]
- 21.Bahl A, Ammer G, Schilling T, Borst A. Object tracking in motion-blind flies. Nat Neurosci. 2013;16:730–8. doi: 10.1038/nn.3386. [DOI] [PubMed] [Google Scholar]
- 22.Borst A, Haag J, Reiff DF. Fly motion vision. Annu Rev Neurosci. 2010;33:49–70. doi: 10.1146/annurev-neuro-060909-153155. [DOI] [PubMed] [Google Scholar]
- 23.Mu L, Ito K, Bacon JP, Strausfeld NJ. Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. J Neurosci. 2012;32:6061–71. doi: 10.1523/JNEUROSCI.0221-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol. 2006;497:928–58. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Liu H, Lei Z, Wu Z, Guo A. Lobula-specific visual projection neurons are involved in perception of motion-defined second-order motion in Drosophila. J Exp Biol. 2013;216:524–34. doi: 10.1242/jeb.079095. [DOI] [PubMed] [Google Scholar]
- 26.Katsov AY, Clandinin TR. Motion processing streams in Drosophila are behaviorally specialized. Neuron. 2008;59:322–35. doi: 10.1016/j.neuron.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A. ON and OFF pathways in Drosophila motion vision. Nature. 2010;468:300–4. doi: 10.1038/nature09545. [DOI] [PubMed] [Google Scholar]
- 28.Eichner H, Joesch M, Schnell B, Reiff DF, Borst A. Internal structure of the fly elementary motion detector. Neuron. 2011;70:1155–64. doi: 10.1016/j.neuron.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Takemura SY, Karuppudurai T, Ting CY, Lu Z, Lee CH, Meinertzhagen IA. Cholinergic circuits integrate neighboring visual signals in a Drosophila motion detection pathway. Curr Biol. 2011;21:2077–84. doi: 10.1016/j.cub.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinertzhagen IA, Lee CH. The genetic analysis of functional connectomics in Drosophila. Adv Genet. 2012;80:99–151. doi: 10.1016/B978-0-12-404742-6.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw SR, Fröhlich A, Meinertzhagen IA. Direct connections between the R7/8 and R1-6 photoreceptor subsystems in the dipteran visual system. Cell Tissue Res. 1989;257:295–302. doi: 10.1007/BF00261833. [DOI] [PubMed] [Google Scholar]
- 32.Gabbiani F, Jones PW. A genetic push to understand motion detection. Neuron. 2011;70:1023–5. doi: 10.1016/j.neuron.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–65. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci USA. 2008;105:4910–5. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi S, Desplan C, Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc Natl Acad Sci USA. 2010;107:5634–9. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardill TJ, List O, Li X, Dongre S, McCulloch M, Ting CY, et al. Multiple spectral inputs improve motion discrimination in the Drosophila visual system. Science. 2012;336:925–31. doi: 10.1126/science.1215317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strausfeld NJ, Sinakevitch I, Okamura JY. Organization of local interneurons in optic glomeruli of the dipterous visual system and comparisons with the antennal lobes. Dev Neurobiol. 2007;67:1267–88. doi: 10.1002/dneu.20396. [DOI] [PubMed] [Google Scholar]
- 38.Tang S, Guo A. Choice behavior of Drosophila facing contradictory visual cues. Science. 2001;294:1543–7. doi: 10.1126/science.1058237. [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–6. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 40.Wasserman S, Salomon A, Frye MA. Drosophila tracks carbon dioxide in flight. Curr Biol. 2013;23:301–6. doi: 10.1016/j.cub.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suver MP, Mamiya A, Dickinson MH. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr Biol. 2012;22:2294–302. doi: 10.1016/j.cub.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 42.Borst A. Drosophila’s view on insect vision. Curr Biol. 2009;19:R36–47. doi: 10.1016/j.cub.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci USA. 1967;58:1112–9. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Götz KG. Fractionation of Drosophila populations according to optomotor traits. J Exp Biol. 1970;52:419–36. doi: 10.1242/jeb.52.2.419. [DOI] [PubMed] [Google Scholar]
- 45.Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–9. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–33. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neuroscience. 2008;28 doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zipursky SL. [Google Scholar]
- 49.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–50. doi: 10.1016/S0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 50.Nern A, Nguyen LV, Herman T, Prakash S, Clandinin TR, Zipursky SL. An isoform-specific allele of Drosophila N-cadherin disrupts a late step of R7 targeting. Proc Natl Acad Sci USA. 2005;102:12944–9. doi: 10.1073/pnas.0502888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, et al. Drosophila LAR regulates R1-R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–48. doi: 10.1016/S0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 52.Willmund R, Emanns A, Eusemann B, Roos W. Mutants Affecting Plasticity of Phototactic Behavior in Drosophila-Melanogaster. J Insect Physiol. 1984;30:431–6. doi: 10.1016/0022-1910(84)90021-0. [DOI] [Google Scholar]
- 53.Le Bourg E, Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav. 2002;30:330–41. doi: 10.3758/BF03195958. [DOI] [PubMed] [Google Scholar]
- 54.Kain JS, Stokes C, de Bivort BL. Phototactic personality in fruit flies and its suppression by serotonin and white. Proc Natl Acad Sci USA. 2012;109:19834–9. doi: 10.1073/pnas.1211988109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchner E, Wu CF. Drosophila neurogenetics--the Heisenberg impact. J Neurogenet. 2009;23:1–2. doi: 10.1080/01677060802687701. [DOI] [PubMed] [Google Scholar]
- 56.Tuthill JC, Chiappe ME, Reiser MB. Neural correlates of illusory motion perception in Drosophila. Proc Natl Acad Sci USA. 2011;108:9685–90. doi: 10.1073/pnas.1100062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theobald JC, Duistermars BJ, Ringach DL, Frye MA. Flies see second-order motion. Curr Biol. 2008;18:R464–5. doi: 10.1016/j.cub.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 58.Theobald JC, Shoemaker PA, Ringach DL, Frye MA. Theta motion processing in fruit flies. Front Behavl Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aptekar JW, Shoemaker PA, Frye MA. Figure tracking by flies is supported by parallel visual streams. Curr Biol. 2012;22:482–7. doi: 10.1016/j.cub.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 60.Dewell RB, Gabbiani F. Escape behavior: linking neural computation to action. Curr Biol. 2012;22:R152–3. doi: 10.1016/j.cub.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 61.Card G, Dickinson MH. Visually mediated motor planning in the escape response of Drosophila. Curr Biol. 2008;18:1300–7. doi: 10.1016/j.cub.2008.07.094. [DOI] [PubMed] [Google Scholar]
- 62.de Vries SE, Clandinin TR. Loom-sensitive neurons link computation to action in the Drosophila visual system. Curr Biol. 2012;22:353–62. doi: 10.1016/j.cub.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–7. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon JC, Dickinson MH. A new chamber for studying the behavior of Drosophila. PLoS One. 2010;5:e8793. doi: 10.1371/journal.pone.0008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474:204–7. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grover D, Tower J, Tavaré S. O fly, where art thou? J R Soc Interface. 2008;5:1181–91. doi: 10.1098/rsif.2007.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ristroph L, Berman GJ, Bergou AJ, Wang ZJ, Cohen I. Automated hull reconstruction motion tracking (HRMT) applied to sideways maneuvers of free-flying insects. J Exp Biol. 2009;212:1324–35. doi: 10.1242/jeb.025502. [DOI] [PubMed] [Google Scholar]
- 69.Straw AD, Branson K, Neumann TR, Dickinson MH. Multi-camera real-time three-dimensional tracking of multiple flying animals. J R Soc Interface. 2011;8:395–409. doi: 10.1098/rsif.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fry SN, Rohrseitz N, Straw AD, Dickinson MH. Visual control of flight speed in Drosophila melanogaster. J Exp Biol. 2009;212:1120–30. doi: 10.1242/jeb.020768. [DOI] [PubMed] [Google Scholar]
- 71.Frye MA, Dickinson MH. Closing the loop between neurobiology and flight behavior in Drosophila. Curr Opin Neurobiol. 2004;14:729–36. doi: 10.1016/j.conb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Bender JA, Dickinson MH. A comparison of visual and haltere-mediated feedback in the control of body saccades in Drosophila melanogaster. J Exp Biol. 2006;209:4597–606. doi: 10.1242/jeb.02583. [DOI] [PubMed] [Google Scholar]
- 73.Sherman A, Dickinson MH. Summation of visual and mechanosensory feedback in Drosophila flight control. J Exp Biol. 2004;207:133–42. doi: 10.1242/jeb.00731. [DOI] [PubMed] [Google Scholar]
- 74.Mamiya A, Straw AD, Tómasson E, Dickinson MH. Active and passive antennal movements during visually guided steering in flying Drosophila. J Neurosci. 2011;31:6900–14. doi: 10.1523/JNEUROSCI.0498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Budick SA, Reiser MB, Dickinson MH. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J Exp Biol. 2007;210:4092–103. doi: 10.1242/jeb.006502. [DOI] [PubMed] [Google Scholar]
- 76.Frye MA, Tarsitano M, Dickinson MH. Odor localization requires visual feedback during free flight in Drosophila melanogaster. J Exp Biol. 2003;206:843–55. doi: 10.1242/jeb.00175. [DOI] [PubMed] [Google Scholar]
- 77.Fry SN, Rohrseitz N, Straw AD, Dickinson MH. TrackFly: virtual reality for a behavioral system analysis in free-flying fruit flies. J Neurosci Methods. 2008;171:110–7. doi: 10.1016/j.jneumeth.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Frye MA, Dickinson MH. Motor output reflects the linear superposition of visual and olfactory inputs in Drosophila. J Exp Biol. 2004;207:123–31. doi: 10.1242/jeb.00725. [DOI] [PubMed] [Google Scholar]
- 79.Duistermars BJ, Frye MA. Crossmodal visual input for odor tracking during fly flight. Curr Biol. 2008;18:270–5. doi: 10.1016/j.cub.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 80.Bender JA, Dickinson MH. Visual stimulation of saccades in magnetically tethered Drosophila. J Exp Biol. 2006;209:3170–82. doi: 10.1242/jeb.02369. [DOI] [PubMed] [Google Scholar]
- 81.Bhandawat V, Maimon G, Dickinson MH, Wilson RI. Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J Exp Biol. 2010;213:3625–35. doi: 10.1242/jeb.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chow DM, Theobald JC, Frye MA. An olfactory circuit increases the fidelity of visual behavior. J Neurosci. 2011;31:15035–47. doi: 10.1523/JNEUROSCI.1736-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wasserman S, Lu P, Aptekar JW, Frye MA. Flies dynamically anti-track, rather than ballistically escape, aversive odor during flight. J Exp Biol. 2012;215:2833–40. doi: 10.1242/jeb.072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–44. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Swinderen B, Greenspan RJ. Salience modulates 20-30 Hz brain activity in Drosophila. Nat Neurosci. 2003;6:579–86. doi: 10.1038/nn1054. [DOI] [PubMed] [Google Scholar]
- 86.van Swinderen B. Attention-like processes in Drosophila require short-term memory genes. Science. 2007;315:1590–3. doi: 10.1126/science.1137931. [DOI] [PubMed] [Google Scholar]
- 87.van Swinderen B. Attention in Drosophila. Int Rev Neurobiol. 2011;99:51–85. doi: 10.1016/B978-0-12-387003-2.00003-3. [DOI] [PubMed] [Google Scholar]
- 88.Sareen P, Wolf R, Heisenberg M. Attracting the attention of a fly. Proc Natl Acad Sci USA. 2011;108:7230–5. doi: 10.1073/pnas.1102522108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang K, Guo JZ, Peng Y, Xi W, Guo A. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science. 2007;316:1901–4. doi: 10.1126/science.1137357. [DOI] [PubMed] [Google Scholar]
- 90.Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–61. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453:1244–7. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- 92.Kahsai L, Zars T. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol. 2011;99:139–67. doi: 10.1016/B978-0-12-387003-2.00006-9. [DOI] [PubMed] [Google Scholar]
- 93.Zabala F, Polidoro P, Robie A, Branson K, Perona P, Dickinson MH. A simple strategy for detecting moving objects during locomotion revealed by animal-robot interactions. Curr Biol. 2012;22:1344–50. doi: 10.1016/j.cub.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pick S, Strauss R. Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr Biol. 2005;15:1473–8. doi: 10.1016/j.cub.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 95.Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila. Curr Biol. 2010;20:663–8. doi: 10.1016/j.cub.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 96.Fischbach KF, Hiesinger PR. Optic lobe development. Adv Exp Med Biol. 2008;628:115–36. doi: 10.1007/978-0-387-78261-4_8. [DOI] [PubMed] [Google Scholar]
- 97.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–8. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–60. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–17. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–9. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Millard SS, Lu Z, Zipursky SL, Meinertzhagen IA. Drosophila dscam proteins regulate postsynaptic specificity at multiple-contact synapses. Neuron. 2010;67:761–8. doi: 10.1016/j.neuron.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Millard SS, Zipursky SL. Dscam-mediated repulsion controls tiling and self-avoidance. Curr Opin Neurobiol. 2008;18:84–9. doi: 10.1016/j.conb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scott EK, Reuter JE, Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J Neurosci. 2003;23:3118–23. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scott EK, Raabe T, Luo L. Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J Comp Neurol. 2002;454:470–81. doi: 10.1002/cne.10467. [DOI] [PubMed] [Google Scholar]
- 105.Ting CY, Lee CH. Visual circuit development in Drosophila. Curr Opin Neurobiol. 2007;17:65–72. doi: 10.1016/j.conb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 106.Schwabe T, Gontang AC, Clandinin TR. More than just glue: the diverse roles of cell adhesion molecules in the Drosophila nervous system. Cell Adh Migr. 2009;3:36–42. doi: 10.4161/cam.3.1.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol. 2011;21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 108.Melnattur KV, Lee CH. Visual circuit assembly in Drosophila. Dev Neurobiol. 2011;71:1286–96. doi: 10.1002/dneu.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huberman AD, Clandinin TR, Baier H. Molecular and cellular mechanisms of lamina-specific axon targeting. Cold Spring Harb Perspect Biol. 2010;2:a001743. doi: 10.1101/cshperspect.a001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–30. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gohl DM, Silies MA, Gao XJ, Bhalerao S, Luongo FJ, Lin CC, et al. A versatile in vivo system for directed dissection of gene expression patterns. Nat Methods. 2011;8:231–7. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–63. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 114.de Vries SE, Clandinin T. Optogenetic stimulation of escape behavior in Drosophila melanogaster. J Vis Exp. 2013;71:e50192. doi: 10.3791/50192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–84. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mathey-Prevot B, Perrimon N. Drosophila genome-wide RNAi screens: are they delivering the promise? Cold Spring Harb Symp Quant Biol. 2006;71:141–8. doi: 10.1101/sqb.2006.71.027. [DOI] [PubMed] [Google Scholar]
- 117.Neumüller RA, Perrimon N. Where gene discovery turns into systems biology: genome-scale RNAi screens in Drosophila. Wiley Interdiscip Rev Syst Biol Med. 2011;3:471–8. doi: 10.1002/wsbm.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13:393–9. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 119.Clark DA, de Vries SE, Clandinin TR. Watching the fly brain in action. Nat Methods. 2010;7:505–6. doi: 10.1038/nmeth0710-505. [DOI] [PubMed] [Google Scholar]
- 120.Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, Ito K, et al. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol. 2010;103:1646–57. doi: 10.1152/jn.00950.2009. [DOI] [PubMed] [Google Scholar]
- 121.Srinivasan MV. Even Insects Experience Visual Illusions. Curr Sci India. 1993;64:649–54. [Google Scholar]
- 122.Clark DA, Bursztyn L, Horowitz MA, Schnitzer MJ, Clandinin TR. Defining the computational structure of the motion detector in Drosophila. Neuron. 2011;70:1165–77. doi: 10.1016/j.neuron.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seelig JD, Chiappe ME, Lott GK, Dutta A, Osborne JE, Reiser MB, et al. Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nat Methods. 2010;7:535–40. doi: 10.1038/nmeth.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. Walking modulates speed sensitivity in Drosophila motion vision. Curr Biol. 2010;20:1470–5. doi: 10.1016/j.cub.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–40. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Y, Song X, Ye S, Miao L, Zhu Y, Zhang RG, et al. Structural insight into enhanced calcium indicator GCaMP3 and GCaMPJ to promote further improvement. Protein Cell. 2013;4:299–309. doi: 10.1007/s13238-013-2103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Masuyama K, Zhang Y, Rao Y, Wang JW. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J Neurogenet. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–31. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 129.DeFelipe J. From the connectome to the synaptome: an epic love story. Science. 2010;330:1198–201. doi: 10.1126/science.1193378. [DOI] [PubMed] [Google Scholar]
- 130.Lichtman JW, Sanes JR. Ome sweet ome: what can the genome tell us about the connectome? Curr Opin Neurobiol. 2008;18:346–53. doi: 10.1016/j.conb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knott G, Marchman H, Wall D, Lich B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci. 2008;28:2959–64. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chklovskii DB, Vitaladevuni S, Scheffer LK. Semi-automated reconstruction of neural circuits using electron microscopy. Curr Opin Neurobiol. 2010;20:667–75. doi: 10.1016/j.conb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 134.Bate M, Martinez Arias A. The Development of Drosophila melanogaster. Plainview, N.Y.: Cold Spring Harbor Laboratory Press, 1993. [Google Scholar]
- 135.Korff W, Anderson D, Branson K, Card G, Chen N, Hayes S, et al. The Fly Olympiad: A series of high-throughput, quantitative behavioral experiments in Drosophila neurobiology. Front Behav Neurosci Conference Abstract: Tenth International Congress of Neuroethology, 2012. [Google Scholar]
- 136.Zou S, Liedo P, Altamirano-Robles L, Cruz-Enriquez J, Morice A, Ingram DK, et al. Recording lifetime behavior and movement in an invertebrate model. PLoS One. 2011;6:e18151. doi: 10.1371/journal.pone.0018151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carey JR, Papadopoulos N, Kouloussis N, Katsoyannos B, Müller HG, Wang JL, et al. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp Gerontol. 2006;41:93–7. doi: 10.1016/j.exger.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carandini M. From circuits to behavior: a bridge too far? Nat Neurosci. 2012;15:507–9. doi: 10.1038/nn.3043. [DOI] [PubMed] [Google Scholar]
- 139.Vosshall LB. Into the mind of a fly. Nature. 2007;450:193–7. doi: 10.1038/nature06335. [DOI] [PubMed] [Google Scholar]