Abstract
Cell adhesion to the extracellular matrix elicits a temporal reorganization of the actin cytoskeleton that is regulated first by Rac1 and later by RhoA. The signaling mechanisms controlling late stage RhoA activation are incompletely understood. Net1A is a RhoA/RhoB-specific guanine nucleotide exchange factor that is required for cancer cell motility. The ability of Net1A to stimulate RhoA activation is negatively regulated by nuclear sequestration. However, mechanisms controlling the plasma membrane localization of Net1A had not previously been reported. Recently we have shown that Rac1 activation stimulates plasma membrane relocalization and activation of Net1A. Net1A relocalization is independent of its catalytic activity and does not require its C-terminal pleckstrin homology or PDZ interacting domains. Rac1 activation during cell adhesion stimulates a transient relocalization of Net1A that is terminated by proteasomal degradation of Net1A. Importantly, plasma membrane localization of Net1A is required for efficient myosin light chain phosphorylation, focal adhesion maturation, and cell spreading. These data show for the first time a physiological mechanism controlling Net1A relocalization from the nucleus. They also demonstrate a previously unrecognized role for Net1A in controlling actomyosin contractility and focal adhesion dynamics during cell adhesion.
Keywords: Rac1, RhoA, Net1A, cell adhesion, focal adhesion, breast cancer
Introduction
Cell adhesion to the extracellular matrix (ECM) is a complex process that is initiated by the binding of membrane spanning ECM receptors such as integrins and syndecans to matrix proteins.1,2 This initiates temporally and spatially coordinated signaling cascades that promote rearrangement of the actin cytoskeleton and ultimately control cell adhesion. Rho family small G proteins are canonical regulators of actin cytoskeletal organization, and their activation is crucially important to this process.3 This is true in all mammalian cell types tested, yet it is clear that the specific signaling events leading to their activation vary between cell types and disease states. Thus, a current challenge is to understand how Rho GTPase signaling is coordinated to control cell adhesion in these cell and disease settings.
The activation state of Rho GTPases is mainly controlled by two families of proteins known as Rho guanine nucleotide exchange factors (RhoGEFs) and Rho GTPase activating proteins (RhoGAPs). RhoGEFs stimulate Rho GTPase activation by decreasing the affinity of Rho proteins for GDP, thereby allowing GTP loading. RhoGAPs accelerate the intrinsic GTPase activity of Rho proteins, thereby stimulating GTP hydrolysis and Rho protein inactivation. There are over 150 RhoGAPs and RhoGEFs in the human genome, while there are only 23 Rho proteins.4-7 This diversity of regulatory proteins is thought be one mechanism by which signaling specificity is generated.
Integrin binding to the ECM stimulates a complex signaling cascade that regulates Rho GTPase activity. These signaling events have been characterized mainly in fibroblasts, and it is unclear whether they are entirely conserved in other cell types, such as cancer cells. Generally, ECM ligation stimulates integrin receptor clustering that promotes recruitment and transphosphorylation of focal adhesion kinase (FAK) on its activating site Y397. Phosphorylation on this site allows for recruitment and activation of the tyrosine kinase Src, which then phosphorylates FAK and other substrates to initiate intracellular signaling.8,9 Activation of the small G proteins Cdc42 and Rac1 are early events in this process, occurring within minutes of integrin binding to the ECM. Their activation is controlled by recruitment and activation of the Rho guanine nucleotide exchange factors (RhoGEF) βPIX and DOCK180, respectively.9,10 Cdc42 and Rac1 activation during spreading are necessary for extension of filopodia and lamellipodia, which allow the cell to establish residency within a specific area of adherence. Activation of these small G proteins stimulates the formation of focal contacts, which are nascent sites of attachment between clustered integrins and the actin cytoskeleton. During this time RhoA activation is suppressed by p190RhoGAP, which is activated by Src phosphorylation.11
Within 30 min of ECM contact this signaling paradigm becomes reversed, with a reduction in Cdc42 and Rac1 activation and a stimulation of RhoA activation. How this is achieved seems to vary between cell types. For example, binding of NIH3T3 mouse fibroblasts to the ECM protein fibronectin stimulates the activity of the RhoA specific GEFs LARG and p115-RhoGEF.12 Alternatively, plating mouse embryo fibroblasts and mouse neuroblastoma cells on fibronectin stimulates the activation of p190RhoGEF.13,14 In fibroblasts the downregulation of p190RhoGAP activity occurs concurrently with RhoGEF activation. This is achieved through inactivation of Src and dephosphorylation of p190RhoGAP by the tyrosine phosphatase PTP-PEST.15,16 Similarly, Rac1 activation may be suppressed by PTP-PEST-dependent dephosphorylation of FAK and p130Cas.15,17,18 Once activated, RhoA promotes phosphorylation of the regulatory myosin light chain (MLC) subunit by direct, ROCK-dependent phosphorylation of MLC and through the phosphorylation-dependent inhibition of myosin light chain phosphatase.19,20 This generates actomyosin contraction necessary for focal adhesion maturation.21 RhoA activation also stimulates cortical actin polymerization and stabilization necessary for solidifying cell adhesion.22
Control of Net1 Isoform Subcellular Localization
The neuroepithelial transforming gene 1 (Net1) is a RhoA/RhoB-specific RhoGEF that is expressed in many tissues and cell types and is overexpressed in human cancers.23-28 Two isoforms exist in most cells, known as Net1 and Net1A, which are identical except for divergent N-terminal regulatory domains.29 Net1 is unusual in that it is one of only two RhoGEFs that localize to the nucleus in resting cells, the other being Ect2. Nuclear localization of Net1 isoforms occurs via multiple nuclear localization signal sequences in their N-terminal regulatory domains.29,30 This is thought to be a negative regulatory mechanism, as RhoA is largely absent from the nucleus and must be activated at the plasma membrane to elicit effects on the actin cytoskeleton. Moreover, truncation of its N-terminus relocalizes Net1 outside the nucleus and stimulates constitutive RhoA activation.24,30 Thus, deciphering mechanisms controlling the subcellular localization of Net1 isoforms is fundamental to understanding how they control RhoA activation and cellular outcomes such as adhesion and motility.
In the recent paper by Carr et al., we examined mechanisms controlling the extranuclear localization of Net1 isoforms.31 To identify proteins that regulated Net1 localization we opted for a candidate approach, reasoning that other Rho family GTPases or their downstream effectors might control Net1 isoform localization. This supposition was based on the temporal nature of Rho GTPase regulation during processes such as cell adhesion. We chose to study this regulation in breast cancer cells because of the potential role for Net1 in promoting metastatic progression in human breast cancer.32,33 Using this approach we found that co-expression of constitutively active Rac1 (V12Rac1) caused a robust relocalization of Net1 isoforms outside the nucleus. This effect was much stronger for Net1A than Net1, perhaps because Net1A has only two of the four NLS sequences present in Net1.29 By subcellular fractionation and confocal microscopy we found that Net1A was relocalized to the plasma membrane, and using a GST-A17RhoA pulldown affinity assay we observed that Net1A was strongly activated by co-expression of V12Rac1. These results indicated that Rac1 was a potent regulator of extranuclear localization of Net1 isoforms, especially Net1A.
Because the small GTPases Rac1 and Cdc42 share many of the same downstream effector proteins we examined whether their activities were interchangeable for regulating Net1A localization. We observed that co-expression of constitutively active Cdc42 was nearly as efficient as active Rac1 at causing Net1A relocalization. However, using an siRNA approach we found that Rac1 knockdown was sufficient to prevent relocalization of transfected Net1A outside the nucleus, both in resting cells and in cells replated on a collagen matrix. We also observed that knockdown of Rac1 expression in the metastatic breast cancer cell line MDA-MB-231 strongly downregulated Net1 isoform localization to the plasma membrane. Thus, the extranuclear localization of Net1A in MCF7 and MDA-MB-231 cells was largely controlled by Rac1 and not by other Rho family small GTPases.
The mechanism by which Rac1 controls Net1A localization was a significant question. We found that Net1A relocalization was independent of its ability to stimulate RhoA activation, and did not require the presence of the pleckstrin homology domain or C-terminal PDZ binding site within Net1A. We next examined whether active Rac1 directly interacted with Net1A to stimulate its relocalization to the plasma membrane, as Rac1 has been shown to localize to the nucleus.34,35 However, we were unable to show that Net1A and Rac1 co-immunoprecipitate. We then tested the requirement for common effector proteins regulated by Rac1. In particular, we assessed whether Pak1 mediated Rac1 effects, as we had previously shown that Pak1 phosphorylated Net1 on two sites in its N-terminus to downregulate its RhoGEF activity.36 However, neither co-expression of constitutively active Pak1 nor alanine or glutamate substitutions of the Pak1 phosphorylation sites in Net1A stimulated Net1A localization. Thus, Pak1 or the related kinases Pak2 or Pak3 were unlikely to mediate Rac1 effects on Net1A localization. We also tested the requirement for PI3K, PI4,5K, and PLD1 as Rac1 effectors and found that they also were not required for Net1A relocalization. Hence, many common Rac1 effectors did not mediate the effects of Rac1 on Net1A localization.
An important aspect of Rac1 stimulated relocalization of Net1A was that it also protected Net1A from proteasome-mediated degradation. We had previously shown that Net1A has a very short half-life in MCF7 cells of 30–40 min, and that interaction with the PDZ domain containing protein Dlg1 protected Net1A from proteasome-mediated degradation.37 Regulation of Net1A stability by the proteasome was later also shown to occur in human keratinocytes, indicating that this might be a common mechanism for regulation of Net1A signaling.38 In accordance with these results, we found that co-expression of constitutively active Rac1 extended Net1A half-life from 40 min to nearly 6 h. However, this effect alone was not sufficient for relocalization, as treatment of cells with the proteasome inhibitor MG132 did not cause Net1A relocalization. Interestingly, we were unable to show that Dlg1 was required for Rac1-mediated stabilization of Net1A, indicating that a distinct mechanism was operative.
Relocalization of Net1A is Necessary for Efficient Cell Spreading and Focal Adhesion Maturation
An important finding of our work was that cell adhesion stimulated Net1A relocalization, and that this was required for proper cell spreading and focal adhesion maturation. Specifically, we showed that plating MCF7 cells on collagen stimulated a transient activation of Rac1 lasting nearly 60 min, and that this was accompanied by a transient relocalization of Net1A outside the nucleus. Moreover, using an siRNA approach we were able to show that Net1A relocalization required Rac1 expression. We also found that Net1A localization outside the nucleus was terminated by the proteasome, since treatment of replated cells with MG132 dramatically extended the duration of Net1A extranuclear localization.
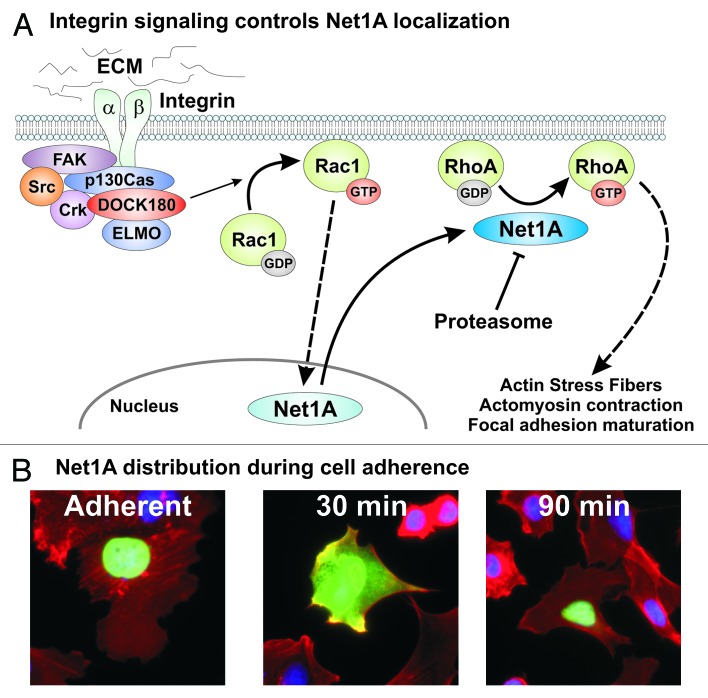
Importantly, we demonstrated that expression of Net1A, but not Net1, was necessary for efficient cell spreading on collagen. Net1A knockdown reduced the number and size of active FAK-containing focal adhesions, indicating that Net1A was necessary for focal adhesion maturation. This was most likely due to effects of Net1A on actomyosin contraction, as Net1A knockdown severely compromised phosphorylation of the regulatory myosin light chain subunit. Actomyosin contraction stimulates focal adhesion maturation by generating tensional forces to aggregate integrins.21 These data support a model in which Rac1 activation stimulates Net1A relocalization to promote RhoA activation and consolidate cell spreading (Fig. 1). Moreover, as cell spreading is completed and Rac1 activation ceases, extranuclear pools of Net1A are degraded by the proteasome to terminate Net1A-mediated RhoA activation. This in turn limits F-actin polymerization and bundling that would ultimately be detrimental to cell adhesion.
Figure 1. (A) Regulation of Net1A localization by integrin ligation. Integrin binding to the ECM promotes recruitment of the FAK/DOCK180 signaling complex, which stimulates Rac1 activation. Rac1 then signals to Net1A in the nucleus, causing Net1A export to the plasma membrane where it activates RhoA. Active RhoA promotes bundling of actin stress fibers, actomyosin contraction, and focal adhesion maturation. After Rac1 activation ceases Net1A is removed from the extranuclear space by proteasome-mediated degradation. (B) Subcellular distribution of Net1A during cell spreading. Shown are MCF7 cells that have been transfected with HA-Net1A, serum starved overnight, and then left adherent or replated on collagen IV for 30 or 90 min. After fixation the cells were stained for HA-Net1A (green), F-actin (red), and DNA (blue).
Conclusions
It is clear that the mechanisms controlling RhoA activation during cell adhesion vary between cell types and physiological settings. By showing that Net1A localization and activation is coupled to Rac1 activation, our studies provide a molecular mechanism for how this may occur in cell types that express significant levels of Net1A, such as MCF7 breast cancer cells. In addition, because the mechanics of cell adhesion and cell motility are innately similar, our studies may partly explain the requirement for Net1 expression in gastric and breast cancer cell motility.26,39,40 Specifically, motile cells require actomyosin contraction to promote focal adhesion maturation in the leading edge and to stimulate focal adhesion dissolution and edge retraction in the rear. The reduced myosin light chain phosphorylation and focal adhesion maturation that we observed in Net1A knockdown cells during adherence indicates that actomyosin contraction was compromised and suggests that this would also be the case in motile cells. In fact, we have observed in wound healing assays that MDA-MB-231 cells lacking Net1A have reduced MLC phosphorylation and become elongated, consistent with defective trailing edge retraction (HSC and JAF, unpublished observations). Although prior studies did not distinguish between Net1 isoforms, our current findings suggest that the Net1A isoform is the primary mediator of cell motility and invasion.
Prior studies identified the RhoA GEFs p115-RhoGEF, LARG, and p190RhoGEF as required for cell adhesion in mouse fibroblasts and neuroblastoma cells.12-14 Thus, an important issue is how these divergent results can be reconciled. A simple explanation may be that different cell types are genetically wired to rely on particular RhoGEFs to control RhoA activation during adhesion, or that particular RhoGEFs respond to activation of specific integrins. However, both of these explanations may be too simplistic. Cells invariably express multiple RhoGEFs, often with similar Rho GTPase specificities. For example, by microarray analysis we observed that MCF7 cells express high levels of the RhoA GEFs ARHGEF10L, PDZ-RhoGEF, LARG, ARHGEF15, and p164-RhoGEF, in addition to Net1 (HSC and JAF, unpublished observations). Thus, our finding that MCF7 cells exhibit a specific requirement for Net1A during adhesion to collagen suggests that these RhoGEFs are not interchangeable. Similarly, the integrin receptors that bind to collagen and fibronectin appear to initiate intracellular signaling by the same FAK-dependent mechanisms, making it unlikely that binding to different ECM proteins would elicit distinct RhoGEF responses.1,8 A more likely explanation is that RhoA signaling is localized within the cell, and that particular RhoGEFs fulfill dedicated functions within specific macromolecular complexes. For example, it has recently been shown that MDA-MB-231 cells require the RhoA GEFs Trio, Net1, and p63RhoGEF for efficient migration, but that only p63RhoGEF contributes to lamellipodia formation.40 Moreover, we have observed that knockdown of both Net1 isoforms in MDA-MB-231 cells only moderately reduces RhoA activation, but almost completely blocks MLC phosphorylation (HSC and JAF, unpublished observations). Since Net1 only functions as a GEF for RhoA and RhoB, but not RhoC, this suggests that Net1 is specifically tasked to control MLC phosphorylation and actomyosin contractility in these cells. Future work will be required to identify the mechanism by which Net1 is predominant in controlling MLC phosphorylation.
Our findings have wider implications for adherence and motility mechanisms in normal and disease cells. For example, leukocytes use integrin-dependent movement to traverse endothelial cells, to arrest at a site of action, and to penetrate a basement membrane.41 All of these actions require Rho GTPase activity and it would be interesting to assess the requirement for Net1A in immune cell migration. Alternatively, metastatic cancer cells invade the ECM through mechanisms that are comparable to leukocyte motility, and plasticity between Rac1-driven mesenchymal and RhoA-driven amoeboid movement is a key feature of invasive cancer cell movement.42-44 Moreover, many cancers are genetically programmed to exhibit elevated Rac1 signaling. Pertinent examples are breast cancers overexpressing the EGF family receptor HER2, the Rac1 GEFs Vav3, Trio, and P-Rex1, or the constitutively active Rac1 splice variant Rac1b.45-49 We predict that these cancers would display elevated Net1A targeting to the plasma membrane. Additionally, measuring Net1A localization in tumor sections may have prognostic value to identify those tumors that are more likely to exhibit metastatic spread. In this regard it will be important to examine how Rac1 coordinates Net1A function during breast cancer cell invasion of the ECM, and to correlate this with expression of known drivers of metastatic progression.
Acknowledgments
This work was supported by grants from the NIH (CA116356) and CPRIT (RP100502) to JAF. Special thanks to members of the Frost, Denicourt, and Dessauer labs for lively and insightful discussions.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/25276
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 4.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 5.Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci. 2005;118:4937–46. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 6.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–72. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–43. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 9.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009;21:676–83. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 11.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–22. doi: 10.1016/S0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 12.Dubash AD, Wennerberg K, García-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–98. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 13.Zhai J, Lin H, Nie Z, Wu J, Cañete-Soler R, Schlaepfer WW, et al. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278:24865–73. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 14.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angers-Loustau A, Côté JF, Charest A, Dowbenko D, Spencer S, Lasky LA, et al. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–31. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sastry SK, Rajfur Z, Liu BP, Cote JF, Tremblay ML, Burridge K. PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J Biol Chem. 2006;281:11627–36. doi: 10.1074/jbc.M600897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garton AJ, Flint AJ, Tonks NK. Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol Cell Biol. 1996;16:6408–18. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sastry SK, Lyons PD, Schaller MD, Burridge K. PTP-PEST controls motility through regulation of Rac1. J Cell Sci. 2002;115:4305–16. doi: 10.1242/jcs.00105. [DOI] [PubMed] [Google Scholar]
- 19.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 20.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 21.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–8. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 23.Chan AM, Takai S, Yamada K, Miki T. Isolation of a novel oncogene, NET1, from neuroepithelioma cells by expression cDNA cloning. Oncogene. 1996;12:1259–66. [PubMed] [Google Scholar]
- 24.Alberts AS, Treisman R. Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J. 1998;17:4075–85. doi: 10.1093/emboj/17.14.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srougi MC, Burridge K. The nuclear guanine nucleotide exchange factors Ect2 and Net1 regulate RhoB-mediated cell death after DNA damage. PLoS One. 2011;6:e17108. doi: 10.1371/journal.pone.0017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leyden J, Murray D, Moss A, Arumuguma M, Doyle E, McEntee G, et al. Net1 and Myeov: computationally identified mediators of gastric cancer. Br J Cancer. 2006;94:1204–12. doi: 10.1038/sj.bjc.6603054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu Y, Lu J, Fu J, Cao Y, Fu G, Kang R, et al. Over-expression of neuroepithelial-transforming protein 1 confers poor prognosis of patients with gliomas. Jpn J Clin Oncol. 2010;40:388–94. doi: 10.1093/jjco/hyp186. [DOI] [PubMed] [Google Scholar]
- 28.Shen SQ, Li K, Zhu N, Nakao A. Expression and clinical significance of NET-1 and PCNA in hepatocellular carcinoma. Med Oncol. 2008;25:341–5. doi: 10.1007/s12032-008-9042-6. [DOI] [PubMed] [Google Scholar]
- 29.Qin H, Carr HS, Wu X, Muallem D, Tran NH, Frost JA. Characterization of the biochemical and transforming properties of the neuroepithelial transforming protein 1. J Biol Chem. 2005;280:7603–13. doi: 10.1074/jbc.M412141200. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J Biol Chem. 2002;277:14581–8. doi: 10.1074/jbc.M111108200. [DOI] [PubMed] [Google Scholar]
- 31.Carr HS, Morris CM, Menon S, Song EH, Frost JA. Rac1 controls the subcellular localization of the Rho guanine nucleotide exchange factor Net1A to regulate focal adhesion formation and cell spreading. Mol Cell Biol. 2012;33:622–34. doi: 10.1128/MCB.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilcrease MZ, Kilpatrick SK, Woodward WA, Zhou X, Nicolas MM, Corley LJ, et al. Coexpression of alpha6beta4 integrin and guanine nucleotide exchange factor Net1 identifies node-positive breast cancer patients at high risk for distant metastasis. Cancer Epidemiol Biomarkers Prev. 2009;18:80–6. doi: 10.1158/1055-9965.EPI-08-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutertre M, Gratadou L, Dardenne E, Germann S, Samaan S, Lidereau R, et al. Estrogen regulation and physiopathologic significance of alternative promoters in breast cancer. Cancer Res. 2010;70:3760–70. doi: 10.1158/0008-5472.CAN-09-3988. [DOI] [PubMed] [Google Scholar]
- 34.Lanning CC, Daddona JL, Ruiz-Velasco R, Shafer SH, Williams CL. The Rac1 C-terminal polybasic region regulates the nuclear localization and protein degradation of Rac1. J Biol Chem. 2004;279:44197–210. doi: 10.1074/jbc.M404977200. [DOI] [PubMed] [Google Scholar]
- 35.Michaelson D, Abidi W, Guardavaccaro D, Zhou M, Ahearn I, Pagano M, et al. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J Cell Biol. 2008;181:485–96. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–61. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- 37.Carr HS, Cai C, Keinänen K, Frost JA. Interaction of the RhoA exchange factor Net1 with discs large homolog 1 protects it from proteasome-mediated degradation and potentiates Net1 activity. J Biol Chem. 2009;284:24269–80. doi: 10.1074/jbc.M109.029439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadimitriou E, Vasilaki E, Vorvis C, Iliopoulos D, Moustakas A, Kardassis D, et al. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-β and miR-24: role in epithelial-to-mesenchymal transition. Oncogene. 2012;31:2862–75. doi: 10.1038/onc.2011.457. [DOI] [PubMed] [Google Scholar]
- 39.Murray D, Horgan G, Macmathuna P, Doran P. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br J Cancer. 2008;99:1322–9. doi: 10.1038/sj.bjc.6604688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi A, Hiatari R, Tsuji T, Ohashi K, Mizuno K. p63RhoGEF-mediated formation of a single polarized lamellipodium is required for chemotactic migration in breast carcinoma cells. FEBS Lett. 2013;587:698–705. doi: 10.1016/j.febslet.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 41.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–9. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 42.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 44.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–9. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Citterio C, Menacho-Márquez M, García-Escudero R, Larive RM, Barreiro O, Sánchez-Madrid F, et al. The rho exchange factors vav2 and vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Signal. 2012;5:ra71. doi: 10.1126/scisignal.2002962. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Guo Z, Chen H, Dong Z, Pan ZK, Ding H, et al. HOXC8-Dependent Cadherin 11 Expression Facilitates Breast Cancer Cell Migration through Trio and Rac. Genes Cancer. 2011;2:880–8. doi: 10.1177/1947601911433129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40:877–92. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, et al. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–20. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 49.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]