Abstract
Technological limitations have prevented the interrogation and manipulation of cellular activity in response to bioactive molecules within model and living systems that is required for the development of diagnostic and treatment modalities for diseases, such as cancer. In this work, we demonstrate that gold-coated liposomes are capable of encapsulation and on-demand release of signaling molecules with a spatial and temporal resolution leading to activation of individual cells. As a model system, we used cells modified to overexpress a certain G-protein coupled receptor, the CCK2 receptor, and achieved its activation in a single cell via the localized release of its agonist. This content release was triggered by illumination of the liposomes at wavelengths corresponding to the plasmon resonance of the gold coating. The use of plasmon resonant liposomes may enable on-demand release of a broad range of molecules using biologically safe near infrared light and without molecule chemical modification. In combination with the spectral tunability of plasmon resonant coating, this technology may allow for multiplexed interrogation of complex and diverse signaling pathways in model or living tissues with unprecedented spatial and temporal control.
Keywords: Controlled release, nanoparticles, liposomes, plasmon resonance, cell signaling
Precise on-demand delivery of biologically active agents is critical for examining cellular responses, conducting in vitro single cell manipulation, and developing effective diagnostics and therapeutics, particularly in the area of cancer. Experimental advances and clinical observations of the past decade support the view that the tumor microenvironment forms a complex network of signaling pathways between cellular and noncellular components, and actively participates in cancer initiation, propagation and metastasis.1–3 Nontumor cells can contribute both inhibitory and proliferative signals to epithelial cancer cells, and communication between tumor environment and epithelium is bidirectional, involving multiple, often redundant, signaling pathways.4–6 It therefore appears that successful strategies for cancer treatment, producing lasting remission, may depend on the ability to identify and manipulate these communication pathways, and to precisely target cells implicated in the activation of these pathways. A broad platform for selective stimulation of cellular receptors by small molecules, evocative of photochemical “uncaging” of neurotransmitters and similar methods developed in neurobiology,7–12 will allow for activating and monitoring individual cells acting within a complex tumor environment.
Controlled stimulation of cellular activity can be accomplished by light-activated content release from liposomes. We previously introduced plasmon resonant gold-coated liposomes with plasmon resonance peaks tunable in the near infrared (NIR) range and capable of controlled release of fluorescent molecules using a laser light stimulus.13–15 The liposomal structure allows for the encapsulation of a variety of agents and the plasmon resonant gold coating allows for light-mediated release of those contents via a photothermal conversion process. Light-mediated release is achieved by illuminating the gold-coated liposomes with laser light. The spectral tunability of these gold-coated liposomes allows for wavelength-selective light-mediated release from these nanocapsules, where encapsulated contents are only released from gold-coated liposomes having a resonance peak matching the wavelength of the illuminating laser; gold-coated liposomes having a different resonance peak and uncoated liposomes retain their encapsulated content.15 Furthermore, because of the range of spectral tunability, NIR laser light is used for release, increasing the penetration depth of the release stimulus through biological samples and reducing the likelihood of photothermal or photochemical damage.
Here we present the first demonstration of activating cellular responses with single-cell spatial and high temporal resolution through controlled ligand release from plasmon resonant gold-coated liposomes triggered by a beam of NIR laser light. To demonstrate spatially and temporally controlled release resulting in activation of individual cells, we employ agonist-mediated activation of a membrane-bound receptor. Specifically, we use previously characterized HEK293 cells overexpressing the CCK2 G-protein-coupled receptor (HEK293/CCK2R cells),16 and load CCK8, a peptide derivative of the endogenous cholecystokinin ligand for that receptor, within gold-coated liposomes. Upon illumination with laser light directed through an inverted microscope, the hydrophilic ligand is released in proximity to cells, where it can bind extracellular receptor domains. In order to achieve the high spatial resolution of release required for single-cell activation, we focus the laser light to a spot size corresponding to about the footprint of a cell and direct the beam to specifically activate areas of interest. G-protein-coupled receptor (GPCR) activation in single cells is monitored using a calcium sensitive fluorescent dye.
RESULTS AND DISCUSSION
Encapsulation of Ligands
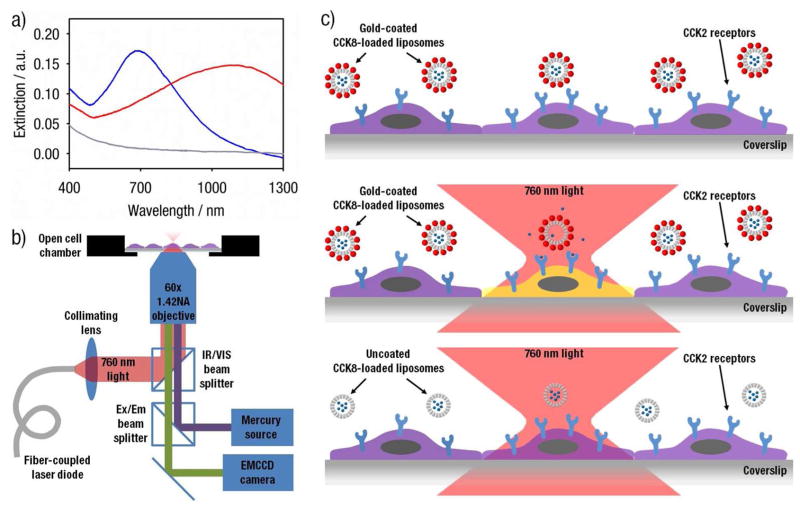
Gold-coated liposomes are prepared by reducing gold onto the surface of 100 nm diameter liposomes (Figure 1) composed of a temperature-sensitive lipid composition.17,18 The gold-coating process does not significantly alter the size or the zeta potential of CCK8-loaded liposomes (Figure 1). As shown in dynamic light scattering and TEM (Figure 1), plasmon resonant liposomes retain an average diameter around 100 nm after the gold-coating process, similar to findings reported in previous work.15 Gold-coated liposomes, encapsulating the ligand CCK8, exhibit their characteristic plasmon resonance peaks and tunability.13–15 Gold-coated liposomes with a marked resonance at 760 nm (Figure 2a) were used with the 760 nm laser diode directed through the microscope objective for light-mediated release. Illumination of such gold-coated liposomes results in localized heating, increased liposome membrane permeability, and the release of encapsulated agents, in a manner described previously.14,15 Conversely, uncoated liposomes demonstrate no extinction at or around 760 nm and are not expected to release content in response to 760 nm laser illumination.
Figure 1.
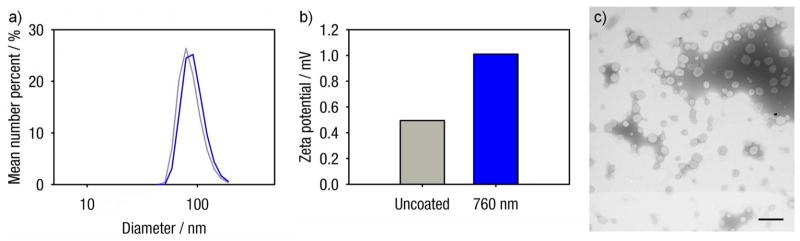
Sizing (a) and zeta potential (b) for uncoated (grey) and 680 nm gold-coated (blue) liposomes encapsulating CCK8. Sizing data is number weighted. Intensity weighted sizing data is available in Figure S1. Both uncoated and gold-coated liposomes have average diameters around 100 nm. The zeta potentials for both uncoated and gold-coated liposomes are minimal, with absolute values around 1 mV or lower. (c) Negative stain transmission electron micrographs of gold-coated liposomes resonant at 680 nm. Scale bar represents 500 nm.
Figure 2.
(a) Extinction spectra of liposome preparations: uncoated liposomes (grey) and gold-coated liposomes with a plasmon resonance peak at 680 nm (blue) and at 1100 nm (red). Experimental samples were prepared and measured with equal quantities of lipids in solution and, therefore, presumably an equal number of liposomes per unit volume. (b) Schematic drawing of the inverted microscope setup for light-induced release and calcium monitoring. The 760 nm beam for light-induced release is produced by a pulsed fiber-coupled laser diode and is directed though a 60x objective to illuminate HEK293/CCK2R cells through an IR/VIS beam splitter. Indo-1 intensity from HEK293/CCK2R cells is monitored through the same 60x objective and imaged using an EMCCD camera. (c) Schematic drawing of light-induced release from gold-coated liposomes. HEK293/CCK2R cells are incubated with gold-coated liposomes, which only release and induce cellular activation when illuminated with 760 nm light. The microscope objective focuses the laser to obtain an activation area comparable to the surface area of the cell. Uncoated liposomes do not respond to the laser stimulus and do not induce cellular activation.
Spatially Controlled Ligand Release
To demonstrate spatially controlled release, we chose to monitor the CCK2 receptor activation in stably transfected HEK293 cells. The CCK2 receptor belongs to the GPCR family, which is a family of transmembrane receptors containing members that are recognized as crucial arbitrators of tumor growth and metastasis, participate in autocrine and paracrine signaling in the tumor microenvironment, and represent the direct or indirect targets of over 50% of current therapeutics.19,20 The CCK2 receptor alone has been implicated in a number of cancers, including pancreatic and small-cell lung cancer.21–23 The interaction of the CCK8 ligand with the HEK293/CCK2R cells is well characterized; CCK8 binds to the CCK2 receptor with reported dissociation constants around 30 nM, and nonspecific binding to cells not expressing the CCK2 receptor is insignificant.16,24 As activation of GPCRs by extracellular ligands results in increases to intracellular calcium concentration, we loaded cells with the ratiometric calcium indicator Indo-1 and monitored changes in the 405/485 nm fluorescence intensity ratio. For the duration of the controlled release experiment, Indo-1-loaded cells were incubated with gold-coated or uncoated liposomes, encapsulating or not encapsulating CCK8 at a 50 μM concentration, in an open cell chamber set on the stage of an inverted microscope. The incubation temperature of the setup and all added sample aliquots were maintained at 10°C to ensure that liposomal contents would not be inadvertently released in response to environmental thermal stresses. Our previous experience with thermosensitive liposomes prepared according to the Needham Dewhirst recipe17 and subsequently coated with gold indicated some content leakage occurring at sub-physiological temperatures.14 The experimental design in this work demonstrates the principle of localized ligand release, without the interference of that leakage. A cell was selected and illuminated over a duration of 2 minutes using a pulsed 760 nm laser diode beam delivering 10 mW average power (measured before the microscope objective) and focused to a 20 μm diameter at the focus plane of a 60x objective (Figure 2b). Laser light was pulsed at a frequency of 200 kHz and a pulse width of 0.5 μs. The illumination time was chosen based on previous release studies indicating that 2 min of illumination with this pulsing regimen ensures at least 75% content release from gold-coated liposomes.15
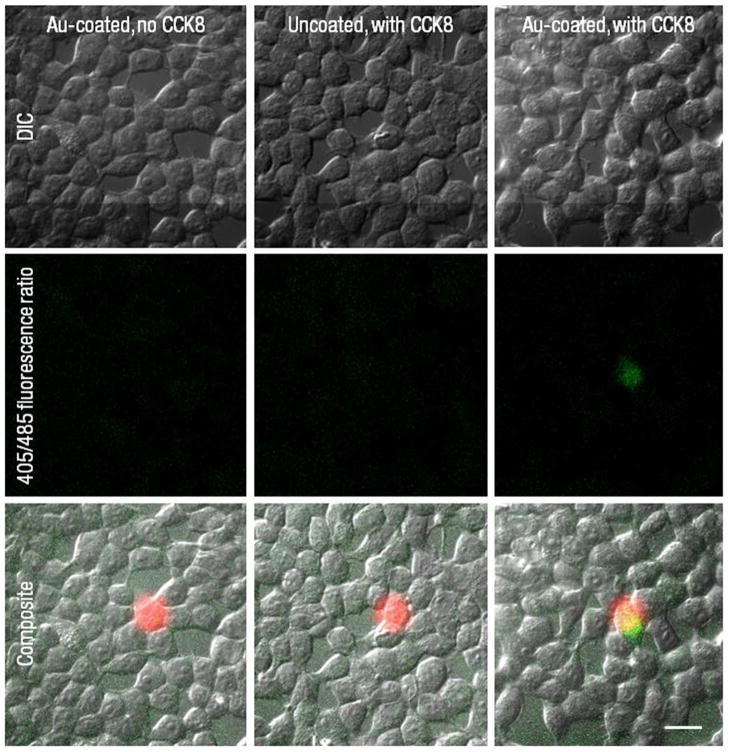
We hypothesized that extracellular release of CCK8 from liposomes results in activation of GPCR signaling pathways in nearby cells (Figure 2c). This activation mechanism does not involve internalization of gold-coated liposomes by cells; rather, it entails release of ligand in proximity to a cell, followed by binding of that ligand to extracellular domains of GPCRs. Indeed, local release of CCK8 resulted in an increase of the 405/485 nm fluorescence ratio of intracellular Indo-1. Figure 3 shows differential interference contrast (DIC) and fluorescence intensity images, which are indicative of activation of HEK293/CCK2R cells incubated with various liposome compositions. Cell activation upon laser illumination occurs only with gold-coated liposomes loaded with the CCK8 ligand. As discussed later, in control experiments where cells were incubated with uncoated liposomes with encapsulated CCK8 or gold-coated liposomes without CCK8, no significant changes in calcium concentration were noted. From these observations, it appears that cell activation occurs following photothermal release of CCK8 from gold-coated liposomes, and that this photothermal activation does not compromise the activity of the released ligand. Furthermore, ligand release and subsequent cellular activation is limited to the single cell selected for illumination, demonstrating unprecedented spatial control of the release process.
Figure 3.
Differential interference contrast images of HEK293/CCK2R cells (top row), 405/485 ratiometric images derived from fluorescence imaging of Indo-1 (middle row), and composite images of DIC, ratiometric, and 760 nm laser spot images (bottom row). Ratiometric images represent intracellular calcium concentration after 1.5 minutes of illumination; green color is indicative of increases in intracellular calcium levels from baseline ratios around 0.43; quantitative representation of intracellular calcium is presented in Figure 4. Cells incubated with gold-coated liposomes (right column) demonstrate an increase in calcium response due to CCK2 receptor activation in the cell co-localized with the laser spot (shown in red), as evident by the overlapping green and red color (bottom row). Cells incubated with gold-coated blank liposomes (left column) and uncoated CCK8-loaded liposomes (middle column) do not demonstrate significant change in calcium response due to laser illumination (middle and bottom rows). Scale bar applies to all panels and corresponds to 20 μm.
GPCR Response to Ligand Release
The duration of the intracellular calcium increase is shorter when eliciting a cellular response with CCK8 released from gold-coated liposomes than when adding free CCK8 to the system. As shown in Figure 4 (left column), the spike in intracellular calcium concentration observed when a cell is stimulated via light-activated ligand release from gold-coated liposomes; this spike lasts for less than two minutes before calcium returns to baseline levels (Figure 5). This localized activation is followed by secondary calcium responses, up to 3–5 minutes after light-mediated activation of gold-coated liposomes (Figure 4, left column). These transient changes in intracellular calcium concentration are evocative of the oscillations in intracellular calcium previously reported in pancreatic acinar cells exposed to low pM concentrations of CCK8, similar to normal endogenous levels.25,26 They are also similar in duration to intracellular calcium increases experienced by the HEK293/CCK2R cells when exposed to 0.5 nM free CCK8 (right column). We attribute the transient calcium concentration spikes following light-activated ligand release to the stochastic character of receptor activation associated with very low concentrations of ligands.27 Following their local release from gold-coated liposomes, the diffusion of CCK8 ligand may result in concentration of this peptide that is close to the receptor activation threshold, and the resulting GPCR activation is driven by chance.26,27 An alternative explanation for the secondary calcium response is the transactivation of another receptor whose downstream effects result in increases in intracellular calcium, such as the epidermal growth factor receptor (EGFR). Downstream signaling of the CCK2 receptor has been shown to stimulate metalloproteinase cleavage of membrane-bound EGFR ligand precursors from the cell surface, which then leads to activation of EGFR.28,29
Figure 4.
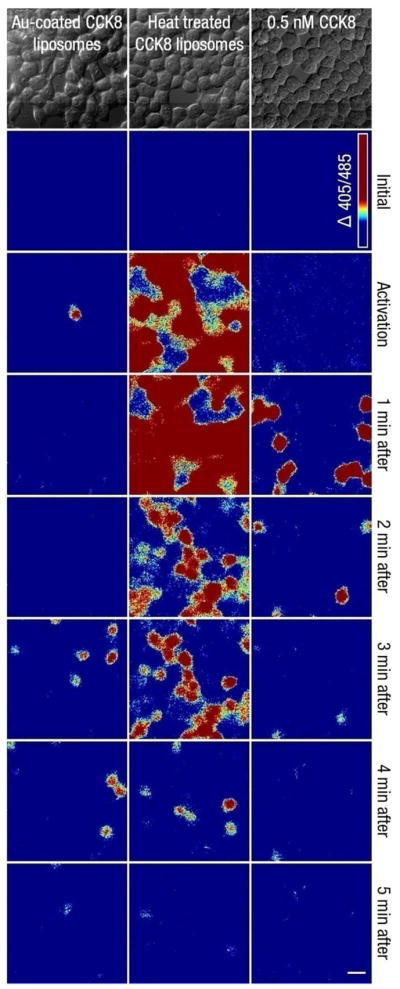
DIC images and time-lapse intracellular calcium concentration changes within HEK293/CCK2R cells following: laser-induced release from gold-coated CCK8-loaded liposomes (left column), exposure to CCK8-loaded gold-coated liposomes preheated at 55 °C for 10 minutes (middle column), and exposure to 0.5 nM free CCK8 (right column). The “activation” time point signifies 1.5 minutes of laser illumination (left column) or addition of free CCK8 from either heat-treated gold-coated liposomes or CCK8 stock solution (middle and right columns). Images are derived from subtracting a baseline 405/485 ratiometric image (taken directly prior to the start of illumination or CCK8 addition) from those of each represented time point. Following the initial single cell response to light-mediated CCK8 release, after 1.5 minutes of illumination (column 1, row 3), described in Figure 3 5, there is a second flux of calcium that occurs approximately 3.5 minutes later (column 1, row 6) and spreads to neighboring cells. In cells exposed to preheated gold-coated liposomes (middle column), calcium levels increase in cells throughout the field of view right after exposure and return to baseline about 5 minutes later. Calcium increases in cells exposed to 0.5 nM free CCK8 (right column) are much shorter in duration and intracellular calcium levels return to baseline about 2 minutes after CCK8 exposure. The false color scale at the top right corner applies to all panels and extends over a range of 0 to 0.24 (representing the increase in the Indo1 405/485 ratio). The scale bar at the lower right corner corresponds to 20 μm.
Figure 5.
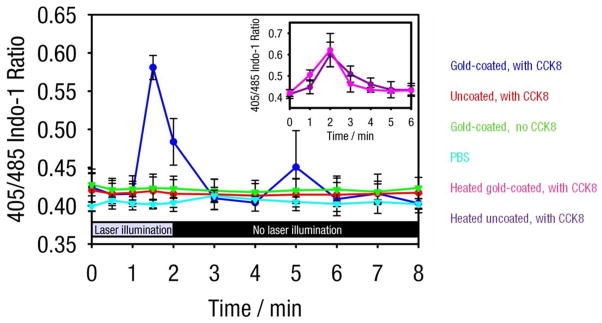
Time dependence of the fluorescence emission intensity ratio (405 nm to 485 nm). Intensity counts obtained with HEK293/CCK2R cells incubated with: gold-coated liposomes containing CCK8 (blue), uncoated liposomes containing CCK8 (red), gold-coated blank liposomes (green), and PBS (cyan). This figure provides a quanititative representation of data provided in Figures 3 and 5. Time 0 indicates the initiation of 760 nm laser illumination and time 2 indicates the end of laser illumination. For gold-coated CCK8-loaded liposomes, fluorescence ratios were collected from the single cell in the laser beam path; averages and standard deviations are derived from two trials. For all other samples, measurements were collected from cells in the beam path and from four other randomly selected cells; averages and standard deviations are collected from two trials with five points from each trial. The fluorescence ratio for gold-coated liposomes loaded with CCK8 and in the path of the 760 beam increased significantly following 1.5 minutes of 760 nm illumination, indicating an increase in calcium concentration in these cells; the calcium concentration was then restored within about 1.5 minutes. The increase in the fluorescence ratio corresponds to the maximum anticipated change represented by gold-coated and uncoated CCK8-loaded liposomes heat treated at 55 °C for 10 minutes (inset, pink and purple, respectively). All other preparations showed no significant changes in intracellular calcium levels during or following illumination.
In comparison, when free CCK8 from gold-coated CCK8-loaded liposomes that have been heat-treated is introduced into solution, to achieve a free CCK8 concentration of about 100 nM (determined using a fluorescamine assay), the increase in intracellular calcium encompasses most of the cells in the field of view and lasts about 2.5 times longer, between 4 and 5 minutes, before returning to baseline (Figure 4, middle column), an effect reminiscent of acinar cells hyperstimulated with CCK8.26 Increases in intracellular calcium concentrations resulting from introduction of free CCK8 are greater than those following release from gold-coated liposomes, as can be seen in the quantitative results provided in Figure 5.
Quantitative changes in the Indo-1 fluorescence ratio (405/485 nm) over several experimental conditions are compared in Figure 5. The calcium response for the single cell located in the area of laser illumination (see Figure 3) is provided for cells incubated with gold-coated CCK8-loaded liposomes (Figure 5, blue); this cell shows an increase in the Indo-1 fluorescence ratio during illumination with laser light, indicating the controlled release of encapsulated CCK8 within the area of laser illumination. Notably, the magnitude of the Indo-1 fluorescence ratio observed in this cell is just below the average peak Indo-1 signal observed in cells exposed to heat-treated (10 min at 55°C) CCK-loaded gold-coated liposomes (Figure 4 inset, pink) and to ionomycin (with a peak value of 0.602).
Controls for Light-Induced Release
In a series of control experiments, HEK293/CCK2R cells incubated with either uncoated liposomes containing CCK8, gold-coated liposomes without CCK8, or PBS, and subsequently exposed to the same laser illumination regimen, did not produce significant changes in fluorescence intensity (Figure 5, red, green, and cyan, respectively); this trend is corroborated when examining only cells in the path of the laser, as well (Supporting Information Figure S2). Uncoated liposomes containing CCK8 do not exhibit any extinction at 760 nm (Figure 2a) and expectedly did not cause significant changes in intracellular calcium concentration. Gold-coated liposomes not containing CCK8 were tested to ensure that, upon laser illumination, gold-coated liposomes do not compromise cell viability, as described below. Incubation in PBS at 10°C was tested to examine the presence of nonspecific intracellular calcium transients and the effects of laser light exposure on intracellular calcium response; neither 10°C incubation nor exposure to 760 nm laser light is accompanied by measurable changes in intracellular calcium (see Figure 5 and Supporting Information Figures S3 and S4).
In examining the effect of reduced temperature on the experimental GPCR response reported, it should be noted that CCK8 can bind to and activate cells expressing the CCK2 receptor at temperatures as low as 4°C.24,30 However, these lower temperatures may affect cellular processes, such as receptor-mediated endocytosis, which has been shown to occur at rates about 10x slower at 4°C than 37°C.24,30 Indeed, we determined that HEK293/CCK2R exposure to 2 nM free CCK8 at 10°C or 37°C generates a similar magnitude of intracellular calcium concentration increase (Supporting Information, Figure S3). However, their temporal responses vary, with cells at 37°C demonstrating a longer response (Supporting Information, Figure S3). The reduced setup temperature allows us to demonstrate the concept of spatially and temporally controlled release of a bioactive molecule and subsequent activation of cellular pathways, while minimizing any background due to non-negligible liposome permeability.14 New compositions of plasmon resonant liposomes currently developed in this laboratory will improve stable encapsulation of molecules at physiological temperatures and extend the range of possible applications of this technology in biological assays.
Cell Viability
Viability of cells used in light-activated release experiments was assessed by monitoring cell membrane integrity and intracellular esterase activity. An early indicator of cell death due to photothermal heating of gold nanoparticles adjacent to cell membranes is compromised membrane integrity, which can be monitored by the resulting influx of extracellular calcium into cells.31 As shown in Figure 5 (green trace), incubation and 760 nm laser illumination with gold-coated liposomes not containing CCK8 did not change the Indo-1 405/485 fluorescence ratio in HEK293/CCK2R cells immersed in HBSS (which includes 1.26 mM Ca2+), confirming the integrity of the cellular membrane. At the completion of the controlled release process, we examined cell viability using calcein AM. Calcein AM was added at a 5 μM concentration to HEK293/CCK2R cells following incubation and 760 nm laser light release with unloaded gold-coated liposomes. Setup temperature and laser light release conditions were as described previously for Figures 2–5. The strong and uniform calcein fluorescence in images taken following the release process indicates normal enzymatic activity of cells and provides further evidence of cell membrane integrity (Figure 6). Together, these tests demonstrate that the conditions of light-activated CCK8 ligand release and GPCR activation do not interfere with normal cellular functions, and that the gold-coated liposomes and their subsequent light-induced heating do not affect cell viability.
Figure 6.
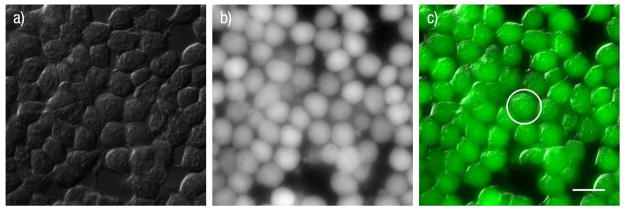
Cell viability assay following the laser illumination procedure with gold-coated blank liposomes (not containing CCK8). Calcein AM was added following the completion of the illumination procedure. Laser illumination consisted of 2 minutes of 0.5 μs pulses delivered at a frequency of 200 kHz. Laser light was supplied by a 760 nm laser diode operating at an average power of 10 mW and focused to a spot size of about 20 μm in diameter; the point of laser illumination within the field of view is marked by a white circle in panel c. The location of cells, as shown by the DIC image taken at the beginning of the release process (a), co-localizes with calcein fluorescence (b), as shown in the composite image (c). Over 2 trials, 100% of cells in the field of view remained viable after the laser release process with gold-coated liposomes. Scale bar applies to all panels and corresponds to 20 μm.
CONCLUSIONS
Using plasmon resonant gold-coated liposomes in combination with focused laser light, we were able to elicit localized ligand release with subsequent GPCR activation with a single-cell spatial and high temporal resolution. This system improves upon the photochemical uncaging technology32–35 and overcomes a number of its limitations. Photochemical uncaging requires chemical modification of compounds, which has restrictions on the size and type of the usable compound, and often diminishes its binding affinity. Also, UV light frequently used in these applications has limited penetration through and may cause damage to biological samples, and two-photon uncaging is limited to certain compounds. Lastly, photochemical uncaging does not allow for the release of large payloads. Polymeric,36–41 liposomal,42–44 and other stimuli-sensitive nanoparticles45–50 are able to encapsulate agents without additional chemical modification, release larger payloads, and can be targeted to specific cell types, but have not yet been shown to be able to induce cellular responses in a single cell or with high temporal resolution.
We envision that this spatially controlled release technology can be employed in connection with a highly collimated beam and a spatial light modulator or spatial scanning to stimulate release of encapsulated signaling molecules in user defined spatial patterns or shapes, expanding on an earlier concept of spatially patterned photolysis of caged neurotransmitters.33,51 The spatial control of this process may also be used to examine individual cells within mixed cultures, an approach that is particularly enticing for interrogating cancer stem cells within their preferred microenvironments.52 As we demonstrated here, GPCR activation by localized extracellular release of GPCR ligands from gold-coated liposomes results in a short calcium response, similar to the response expected to physiologically relevant CCK8 concentrations.26,53 Gold-coated liposomes can potentially encapsulate a number of signaling molecules and peptides, including neurotransmitters such as dopamine and serotonin, with the precise amount of payload delivered controlled by illumination time, as previously described.15 When used in combination with spectrally-selective release allowed by gold-coated liposomes, it may also be possible to encapsulate and release different agents to examine cellular response to multiple ligands released in a spatially and temporally controlled manner and mimic cellular microenvironments. If encapsulating receptor agonists and antagonists, this system can perform similarly to optical switching, expanding the existing array of tools for manipulation of cells with light.7 Lastly, PEGylated lipids already present in these gold-coated liposomes facilitate the addition of ligands targeting molecular receptors known to internalize nanoparticles of this size.54–58 Receptor-mediated endocytosis followed by light-induced release from gold-coated liposomes may enable in situ hybridization, delivery of siRNA, or tracking of intracellular signaling through well conducting monolayers, like endothelium.
In conclusion, we demonstrated spatial and temporal control of ligand release and subsequent cell activation using a combination of gold-coated liposomes and laser-mediated release. Using a model of the general process of agonist-mediated receptor activation, we were able to release CCK8 from gold-coated liposomes to activate a single selected HEK293/CCK2R cell growing in vitro upon application of a light stimulus. The light-activated nanocapsules introduced here can be used for investigating and mapping the time-dependent response of cells to a signaling peptide and perhaps the spread of cell signals via intercellular and intracellular communication. Full development and application of this technology will lead to a better understanding of intercellular signaling in cancer and to new diagnostic and therapeutic approaches not available at present time.
MATERIALS AND METHODS
Liposome Preparation and Encapsulation of CCK8
Liposomes were prepared from synthetic lipids using a lipid composition similar to one previously demonstrated to exhibit temperature-sensitive controlled release;17 the logic supporting this composition is that the instability that occurs during the gel to liquid–crystalline phase transition of lipids sufficiently perturbs the liposome membrane to induce the leakage of contents. The membrane was composed of dipalmitoylphosphatidylcholine (DPPC), monopalmitoylphosphatidylcholine (MPPC), and dipalmitoylphosphatidylethanolamine-[N-methoxy(polyethylene glycol)-2000] (DPPE-PEG2000, all lipids from Avanti Polar Lipids; Alabaster, AL) in a 90:10:4 molar ratio. The proper proportions of dry lipids were dispersed in chloroform and dried by convection with N2; this process was followed by overnight evaporation under vacuum. Dry lipids (60 mM lipid concentration) were then dispersed in phosphate buffered saline (PBS) or PBS containing a 50 μM concentration of a cholecystokinin peptide derivative, Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH2 (CCK8) (Sigma Aldrich, St. Louis, MO), prepared from a 1 mM stock solution of CCK8 in DMSO. Liposomes were prepared by the standard freeze/thaw cycle method and subsequent extrusion through 100 nm polycarbonate membranes, as detailed in previous publications.13–15 Following extrusion, the liposome preparation (2 mL) was subjected to one stage of dialysis against PBS (2 L) at 4°C using cellulose membrane with a 100,000 molecular weight cut-off (Spectrum Laboratories; Rancho Dominguez, CA) to remove excess CCK8. All liposome preparations were stored at 4°C to minimize content leakage.
Reduction of Gold
The process for the reduction of gold onto the surface of liposomes was similar to the technique previously reported.13–15 To summarize, aqueous solutions of gold chloride (100 mM) and of ascorbic acid (500 mM) were prepared. These solutions were added to the previously prepared liposome sample diluted with PBS (1 mL, 10 mM). For resonance wavelengths approximately matched to a 760 nm laser diode, the gold chloride solution (18 μL) was added and gently swirled until uniformly distributed; this was followed by the addition of the ascorbic acid solution (27 μL) and gentle swirling until color, a feature characteristic of the presence of plasmon resonance, developed. Following reduction, the gold-coated liposomes (1 mL) were dialyzed twice against PBS (1.5 L) at 4 °C. Extinction spectra of gold-coated liposomes were taken with a Cary 5 spectrophotometer in double beam mode. Samples were diluted (0.25 mM lipids) in PBS for measurement.
Particle Sizing and Zeta Potential
A Zeta Sizer Nano-ZS particle sizer from Malvern Instruments was used to measure the size and zeta potential of intact (as prepared) uncoated and gold-coated liposomes.
TEM Imaging
A Phillips CM-12 transmission electron microscope (TEM) operating at an accelerating voltage of 100 keV was used to observe the morphology of the liposomes. The sample imaged was unloaded gold-coated liposomes with a plasmon resonance peak at 680 nm. Sample preparation followed the liposome preparation and reduction of gold procedures described earlier. Liposome samples were diluted (100 μM lipid concentration). Samples were prepared for TEM by placing a droplet of the liposome solution (5–6 μL) on a mica-carbon support film; then, the film was floated onto a solution of water and 8% ammonium molybdate, and a stain introduced to visualize the surface of lipid bilayers. A nickel grid was subsequently used as a deposition surface and the excess solution was wicked away using filter paper.
Cell Culture
The cells used in this experiment were from a HEK293 cell line stably transfected with CCKR2 (HEK293/CCK2R), as previously described.16 Cells were maintained in DMEM supplemented with 10% fetal bovine serum at 37 °C and 5% CO2.
Dye Loading
For release studies, HEK293/CCK2R cells were incubated on 25 mm round coverslips and loaded with a 6 μM concentration of cell-permeant Indo-1 (Invitrogen, Carlsbad, CA) for 25 minutes following a 10 minute wash in Hank’s Balanced Salt Solution (HBSS). Following loading, the cells were washed twice in HBSS, for 10 minutes each wash.
In Vitro Release
To monitor changes in calcium levels in response to liposomes and released CCK8, cell cultures were observed under epi-illumination using an inverted Olympus IX71 microscope equipped with a 60x 1.42 NA objective and a 100 W mercury lamp excitation source. Indo-1 fluorescence was excited with at 345 nm and emission intensities were collected at 405 nm and 485 nm wavelengths; the 405 nm peak of Indo-1 increases in intensity and the 485 nm peak decreases in intensity in response to increasing calcium concentrations.
Coverslips with HEK293/CCK2R cells loaded with Indo-1 were placed in a low-profile open bath chamber (Warner Instruments, Hamden, CT) mounted on the microscope stage and immersed in 300 μL of HBSS at 10°C. Following five baseline images taken at 1 minute intervals, 200 μL of HBSS and 100 μL one of the following, gold-coated CCK8-loaded liposomes, uncoated CCK8-loaded liposomes, gold-coated liposomes without encapsulated CCK8 (10 mM lipids), or PBS, were added to the chamber and five more images were taken at 1 minute intervals. The cells were then illuminated through the objective of the inverted microscope with a 760 nm laser diode (RPMC lasers, O’Fallon, MO) delivering an average power of 10 mW (measured before the microscope objective) and focused to a spot size about 20 μm in diameter. The laser diode was pulsed at a frequency of 200 kHz and with a 0.5 μs laser pulse width, an illumination scheme previously shown to elicit content release from gold-coated liposomes.15 The duration of illumination was 2 minutes. Cells were imaged at 30 second intervals during illumination and at 1 minute intervals following the end of laser illumination for 6 minutes. To examine the response to full content release from gold-coated CCK8-loaded liposomes, 100 μL of gold-coated CCK8-loaded liposomes were heat treated at 55°C for 10 minutes. Heat treated uncoated and gold-coated liposomes were then added to cells in the manner described above, resulting in a free CCK8 concentration of about 100 nM, as determined using fluorescamine (see below); cells were then imaged at 1 minute intervals for 6 minutes. Following in vitro release, 6 μL of 1 mM ionomycin was added to cells to achieve a 10 μM concentration and the cells imaged for Indo-1 intensity. An air-cooled 512×512 pixel back-thinned EM-CCD digital camera was used to collect images (Hamamatsu, Bridgewater, NJ).
Fluorescamine Assay of Encapsulation Efficiency
Encapsulation of CCK8 within liposomes was measured by heat treating 500 μL of uncoated and gold-coated liposomes at 55°C for 10 minutes. The heated solutions were then individually dialyzed against 5 mL of PBS overnight and the dialyzate collected. A 500 μL aliquot of each dialyzate was then added to 500 μL of fluorescamine in acetone at a 500 μM concentration. Fluorescence emission from both resulting solutions were then measured at 495 nm using a back-thinned CCD array spectrometer (Ocean Optics, Dunedin, FL) and a 390 nm LED excitation source. CCK8 concentrations were determined by comparison of emission intensity to that of a standard curve developed by diluting free CCK8 in PBS.
Cell Esterase Activity
Cell viability was determined using a calcein AM (Invitrogen, Carlsbad, CA) live cell assay following the in vitro release process with blank gold-coated liposomes not containing CCK8. Calcein AM was added to the HEK293/CCK2R cells at a 5 μM concentration following the release process described above. Following a 2 minute incubation, the cells were washed with HBSS (1 mL) twice. Calcein fluorescence was then monitored by epi-fluorescence with an illumination wavelength of 485 nm and an emission wavelength of 525 nm. Viability was determined by correlating calcein fluorescence with the presence of cells, as delineated by differential interference contrast (DIC) images taken at the initiation of the in vitro release process.
Data Analysis
Fluorescence images were analyzed using ImageJ software. For each in vitro release study, the 405 nm and 485 nm intensities of cells located at the point of 760 nm illumination and four other random cells in the field of view were measured and the 405/485 ratio was calculated for each monitored cell. Ratiometric images were obtained by dividing images of fluorescence emission intensities at 405 nm by those at 485 nm, and corrected by subtracting the ratio images of cells prior to illumination.
Supplementary Material
Acknowledgments
The authors acknowledge R. Lynch and C. Weber for enlightening discussions and assistance in cell work and maintenance. The authors also acknowledge financial support for this research by NIH (CA120350 and HL007955) and NSF (CBET 0853921).
ABBREVIATIONS
- NIR
near-infrared
- HEK293/CCK2R
HEK293 cells overexpressing the CCK2 receptor
- GPCR
G-protein-coupled receptor
- CCK8
cholecystokinin peptide derivative, Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH2
- PBS
phosphate buffered saline
- EGFR
epidermal growth factor receptor
- DPPC
dipalmitoylphosphatidylcholine
- MPPC
monopalmitoylphosphatidylcholine
- DPPE-PEG2000
dipalmitoylphosphatidylethanolamine-[N-methoxy(polyethylene glycol)-2000]
- TEM
transmission electron microscope
- HBSS
Hank’s Balanced Salt Solution
Footnotes
Supporting Information Available: Supplemental Figures S1 – S4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Liotta LA, Kohn EC. The Microenvironment of the Tumour-Host Interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 2.Bierie B, Moses HL. Tumour Microenvironment: TGFβ: The Molecular Jekyll and Hyde of Cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 3.Derynck R, Akhurst RJ, Balmain A. TGF-beta Signaling in Tumor suppression and Cancer Progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 4.Tlsty TD, Coussens LM. Tumor Stroma and Regulation of Cancer Development. Annu Rev Pathol Mech Dis. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 5.Ingber DE. Can Cancer Be Reversed by Engineering the Tumor Microenvironment? Seminars in Cancer Biology. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo F, Sahai E. Cell Communication Networks in Cancer Invasion. Curr Op Cell Biol. 2011;23:621–629. doi: 10.1016/j.ceb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Gorostiza P, Isacoff EY. Optical Switches for Remote and Noninvasive Control of Cell Signaling. Science. 2008;322:395–399. doi: 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis-Davies GCR. Caged Compounds: Photorelease Technology for Control of Cellular Chemistry and Physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miesenbock G. The Optogenetic Catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 10.Scanziani M, Hausser M. Electrophysiology in the Age of Light. Nature. 2009;461:930–939. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- 11.Kramer RH, Chambers JJ, Trauner D. Photochemical Tools for Remote Control of Ion Channels in Excitable Cells. Nat Chem Biol. 2005;1:360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- 12.Haydon PG, Ellis-Davies GCR. Ultrahigh-Speed Photochemical Stimulation of Neurons. Nat Methods. 2005;2:811–812. doi: 10.1038/nmeth1105-811. [DOI] [PubMed] [Google Scholar]
- 13.Troutman TS, Barton JK, Romanowski M. Biodegradable Plasmon Resonant Nanoshells. Adv Mater. 2008;20:2604–2608. doi: 10.1002/adma.200703026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troutman TS, Leung SJ, Romanowski M. Light-Induced Release from Plasmon-Resonant Liposomes. Adv Mater. 2009;21:2334–2338. doi: 10.1002/adma.200900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung SJ, Kachur XM, Bobnick MC, Romanowski M. Wavelength-Selective Light-Induced Release from Plasmon Resonant Liposomes. Adv Funct Mater. 2011;21:1113–1121. doi: 10.1002/adfm.201002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Vagner J, Josan J, Lynch RM, Morse DL, Baggett B, Han H, Mash EA, Hruby VJ, Gillies RJ. Enhanced Targeting with Heterobivalent Ligands. Mol Cancer Ther. 2009;8:2356–2365. doi: 10.1158/1535-7163.MCT-08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A New Temperature-Sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 18.Tai LA, Tsai PJ, Wang YC, Wang YJ, Lo LW, Yang CS. Thermosensitive Liposomes Entrapping Iron Oxide Nanoparticles for Controllable Drug Release. Nanotechnology. 2009;20:135101–135109. doi: 10.1088/0957-4484/20/13/135101. [DOI] [PubMed] [Google Scholar]
- 19.Dorsam RT, Gutkind JS. G-Protein-Coupled Receptors and Cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum DM, Rasmussen SGF, Kobilka BK. The Structure and Function of G-Protein-Coupled Receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aly A, Shulkes A, Baldwin GS. Gastrins, Cholecystokinins and Gastrointestinal Cancer. Biochim Biophys Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Rozengurt E, Walsh JH. Gastrin, CCK, Signaling, and Cancer. Annu Rev Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Matters GL, McGovern C, Harms JF, Markovic K, Anson K, Jayakumar C, Martenis M, Awad C, Smith JP. Role of Endogenous Cholecystokinin on Growth of Human Pancreatic Cancer. Int J Oncology. 2011;38:593. doi: 10.3892/ijo.2010.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Andrea LD, Testa I, Panico MR, Stasi RD, Caraco C, Tarallo L, Arra C, Barbieri A, Romanelli A, Aloj L. In Vivo and In Vitro Characterization of CCK8 Bearing a Histidine-Based Chelator Labeled with (99m)Tc-Tricarbonyl. Pept Sci. 2008;90:707–712. doi: 10.1002/bip.21041. [DOI] [PubMed] [Google Scholar]
- 25.Tsunoda Y, Stuenkel EL, Williams JA. Oscillatory Model of Calcium Signaling in Rat Pancreatic Acinar Cells. Am J Physiol Cell Physiol. 1990;258:C147–C155. doi: 10.1152/ajpcell.1990.258.1.C147. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MGT, Ghaneh P, et al. Direct Activation of Cytosolic Ca2+ Signaling and Enzyme Secretion by Cholecystokinin in Human Pancreatic Acinar Cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Perc M, Rupnik M, Gosak M, Marhl M. Prevalence of Stochasticity in Experimentally Observed Responses of Pancreatic Acinar Cells to Acetylcholine. Chaos. 2009;19:037113. doi: 10.1063/1.3160017. [DOI] [PubMed] [Google Scholar]
- 28.Noble PJM, Wilde G, White MRH, Pennington SR, Dockray GJ, Varro A. Stimulation of Gastrin-CCKB Receptor Promotes Migration of Gastric AGS Cells via Multiple Paracrine Pathways. Am J Physiol Gastrointest Liver Physiol. 2003;284:G75–G84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 29.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF Receptor Transactivation by G-Protein-Coupled Receptors Requires Metalloproteinase Cleavage of ProHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 30.Aloj L, Aurilio M, Rinaldi V, D’ambrosio L, Tesauro D, Peitl PK, Maina T, Mansi R, von Guggenberg E, Joosten L, et al. Comparison of the Binding and Internalization Properties of 12 DOTA-Coupled and 111In-Labelled CCK2/Gastrin Receptor Binding Peptides: A Collaborative Project under COST Action BM0607. Eur J Nucl Med Mol Imaging. 2011;38:1417–1425. doi: 10.1007/s00259-011-1816-y. [DOI] [PubMed] [Google Scholar]
- 31.Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng JX. Gold Nanorods Mediate Tumor Cell Death by Compromising Membrane Integrity. Adv Mater. 2007;19:3136–3141. doi: 10.1002/adma.200701974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H, Ellis-Davies GCR. Two-Color Two-Photon Uncaging of Glutamate GABA. Nat Methods. 2009;7:123–125. doi: 10.1038/nmeth.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolenko V, Poskanzer KE, Yuste R. Two-Photon Photostimulation and Imaging of Neural Circuits. Nat Methods. 2007;4:943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- 34.Shoham S, O’Connor DH, Sarkisov DV, Wang SSH. Rapid Neurotransmitter Uncaging in Spatially Defined Patterns. Nat Methods. 2005;2:837–843. doi: 10.1038/nmeth793. [DOI] [PubMed] [Google Scholar]
- 35.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal Control of Cell Signaling Using a Light-Switchable Protein Interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedard MF, De Geest BG, Skirtach AG, Mohwald H, Sukhorukov GB. Polymeric Microcapsules with Light Responsive Properties for Encapsulation and Release. Adv Colloid Interface Sci. 2010;158:2–14. doi: 10.1016/j.cis.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Palankar R, Skirtach AG, Kreft O, Bedard M, Garstka M, Gould K, Mohwald H, Sukhorukov GB, Winterhalter M, Springer S. Controlled Intracellular Release of Peptides from Microcapsules Enhances Antigen Presentation on MHC Class 1 Molecules. Small. 2009;5:2168–2176. doi: 10.1002/smll.200900809. [DOI] [PubMed] [Google Scholar]
- 38.Abidian MR, Kim DH, Martin DC. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv Mat. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, et al. Gold Nanocages Covered by Smart Polymers for Controlled Release with Near-Infrared Light. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrera AP, Rodriguez M, Torres-Lugo M, Rinaldi C. Multifunctional Magnetite Nanoparticles Coated with Fluorescent Thermo-Responsive Polymeric Shells. J Mater Chem. 2008;18:855–858. [Google Scholar]
- 41.Skirtach AG, Javier AM, Kreft O, Kohler K, Alberola AP, Mohwald H, Parak WJ, Sukhorukov GB. Laser-Induced Release of Encapsulated Materials inside Living Cells. Angew Chem Int Ed. 2006;118:4728–4733. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]
- 42.Volodkin DV, Skirtach AG, Mohwald H. Near-IR Laser Remote Release from Liposome Complexes. Angew Chem Int Ed. 2009;48:1807–1809. doi: 10.1002/anie.200805572. [DOI] [PubMed] [Google Scholar]
- 43.Yashchenok AM, Delcea M, Videnova K, Jares-Erijman EA, Jovin TM, Konrad M, Mohwald H, Skirtach AG. Enzyme Reaction in the Pores of CaCO3 Particles upon Ultrasound Disruption of Attached Substrate-Filled Liposomes. Angew Chem Int Ed. 2010;49:8116–8120. doi: 10.1002/anie.201003244. [DOI] [PubMed] [Google Scholar]
- 44.Anderson LJE, Hansen E, Lukianova-Hleb EY, Hafner JH, Lapotko DO. Optically Guided Controlled Release from Liposomes with Tunable Plasmonic Nanobubbles. J Controlled Release. 2010:151–158. doi: 10.1016/j.jconrel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delcea M, Mohwald H, Skirtach AG. Stimuli-Responsive Lbl Capsules and Nanoshells for. Drug Delivery. Adv Drug Delivery Rev. 2011;63:730–747. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Lee SE, Liu GL, Kim F, Lee LP. Remote Optical Switch for Localized and Selective Control of Gene Interference. Nano Lett. 2009;9:562–570. doi: 10.1021/nl802689k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally Precise In Vivo Control of Intracellular Signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 48.Hong H, Yang Y, Zhang Y, Engle JW, Nickels RJ, Wang X, Cai W. Cancer-Targeted Optical Imaging with Fluorescent Zinc Oxide Nanowires. Nano Lett. 2011;11:3744–3750. doi: 10.1021/nl201782m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Choi E, Tamanoi F, Zink JI. Light-Activated Nanoimpeller-Controlled Drug Release in Cancer Cells. Small. 2008;4:421–426. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF, Zink JI, Nel AE. Autonomous In Vitro Anticancer Drug Release from Mesoporous Silica Nanoparticles by pH-Sensitive Nanovalves. J Am Chem Soc. 2010;132:12690–12697. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutz C, Otis TS, DeSars V, Charpak S, DiGregorio DA, Emiliani V. Holographic Photolysis of Caged Neurotransmitters. Nat Methods. 2008;5:821–827. doi: 10.1038/nmeth.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hope K, Bhatia M. Clonal Interrogation of Stem Cells. Nat Methods Supplement. 2011;8:S36–S40. doi: 10.1038/nmeth.1590. [DOI] [PubMed] [Google Scholar]
- 53.Spiller DG, Wood CD, Rand DA, White MRH. Measurement of Single-Cell Dynamics. Nature. 2010;465:736–745. doi: 10.1038/nature09232. [DOI] [PubMed] [Google Scholar]
- 54.Lian T, Ho RJY. Trends and Developments in Liposome Drug Delivery Systems. J Pharm Sci. 2001;90:667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 55.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-Targeted Liposomes: Doxorubicin-Loaded Long-Circulating Liposomes Modified with Anti-Cancer Antibody. J Controlled Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Kumar S, Harrison N, Richards-Kortum R, Sokolov K. Plasmonic Nanosensors for Imaging Intracellular Biomarkers in Live Cells. Nano Lett. 2007;7:1338–1343. doi: 10.1021/nl070365i. [DOI] [PubMed] [Google Scholar]
- 57.Cressman S, Dobson I, Lee JB, Tam YYC, Cullis PR. Synthesis of a Labeled RGD-Lipid, Its Incorporation into Liposomal Nanoparticles, and Their Trafficking into Cultured Endothelial Cells. Bioconjugate Chem. 2009;20:1404–1411. doi: 10.1021/bc900041f. [DOI] [PubMed] [Google Scholar]
- 58.Turk MJ, Waters DJ, Low PS. Folate-Conjugated Liposomes Preferentially Target Macrophages Associated with Ovarian Carcinoma. Cancer Lett. 2004;213:165–172. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.