Abstract
Background
Increased risk of pancreatic cancer (PC) has been reported in breast cancer (BC) families carrying BRCA1and BRCA2 mutations; however, PC risk in mutation-negative (BRCAX) families has not been explored to date. The aim of this study was to estimate PC risk in high-risk BC families according to the BRCA mutation status.
Methods
A retrospective cohort analysis was applied to estimate standardized incidence ratios (SIR) for PC. A total of 5,799 families with ≥1 BC case tested for mutations in BRCA1 and/or BRCA2 were eligible. Families were divided into four classes: BRCA1, BRCA2, BRCAX with ≥2 BC diagnosed before age 50 (class 3), and the remaining BRCAX families (class 4).
Results
BRCA1 mutation carriers were at increased risk of PC (SIR= 4.11; 95% confidence interval [CI], 2.94-5.76) as were BRCA2 mutation carriers (SIR=5.79; 95% CI, 4.28-7.84). BRCAX family members were also at increased PC risk, which did not appear to vary by number of members with early-onset breast cancer (SIR=1.31; 95%CI, 1.06-1.63 for Class 3 and SIR=1.30; 95%CI, 1.13-1.49 for class 4).
Conclusions
Germline mutations in BRCA1 and BRCA2 are associated with an increased risk of PC. Members of BRCAX families are also at increased risk of PC, pointing to the existence of other genetic factors that increase the risk of both PC and BC.
Impact
This study clarifies the relationship between familial breast cancer and pancreatic cancer. Given its high mortality, PC should be included in risk assessment in familial breast cancer counseling.
Keywords: pancreatic cancer, breast cancer, BRCA1, BRCA2, BRCAX
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer death in the USA and leads to an estimated 227,000 deaths per year worldwide (1). The majority of PCs are sporadic with a median age at diagnosis of 72 years; the male: female ratio is 1.3:1, and cigarette smoking accounts for approximately 20% of tumors (2). It has been estimated that approximately 7-10% of PC patients have one or more close relatives with PC (3) and are therefore classified as having familial pancreatic cancer (FPC). Relatives of FPC cases are at increased risk of developing PC compared to the general population, and the risk rises with an increasing number of affected relatives and younger age at diagnosis (4,5).
It is still unknown what genetic factors cause most familial clustering of PC. Six to 17% of FPC cases are found to carry germline mutations in BRCA2 (6-8), approximately 3% have PALB2 mutations (9-11), and 2% carry deleterious ATM mutations (12). Almost all FPC cases with germline PALB2 mutations have at least one close relative with breast cancer (BC).
BRCA1, BRCA2 and PALB2 form a trimeric complex, which is critical for the maintenance of genomic stability by repairing DNA damage (13). The same genes, in particular BRCA1 and BRCA2, are also found to be mutated in a high percentage of families with hereditary breast and ovarian cancer. Members of these families are at an increased risk of developing PC compared to the general population, that has been estimated to be 2- to 7-fold (14-17) for BRCA2 mutation carriers and 2-fold for BRCA1 mutation carriers (17,18).
Based on these previous reports, the goal of the present study was to estimate the relative risk of PC in a large series of families recruited by the Breast Cancer Family Registry (BCFR) according to their BRCA1 and BRCA2 mutation status. It also aimed to assess, for the first time, the risk of PC among relatives of individuals who test negative for mutations in these genes.
Methods
Selection and Description of Families
The Breast Cancer Family Registry (BCFR) is an international consortium enrolling and studying high-risk breast cancer families from six centers in the USA (Northern California Breast Cancer Family Registry, New York site of the BCFR, Utah site of BCFR, Philadelphia site of the BCFR), Canada (Ontario Familial Breast Cancer Family Registry) and Australia (Australian Breast Cancer Family Registry). The BCFR collects cancer family history, epidemiological data and histopathological data on individuals affected with BC, ascertained through population-based cancer registries (population-based BC families) or family cancer clinics and community outreach (clinic-based BC families) (19). For the present study, families were eligible if the proband (defined in this context as the first family member enrolled in the BCFR and affected with BC) had been screened for pathogenic mutations in BRCA1 and/or BRCA2. According to the mutational status of the proband, families were classified as BRCA1, BRCA2 and BRCAX (proband tested negative for mutations in both genes). BRCAX families were subdivided in two classes: those with at least two early onset (≤50 years) BC cases (Class 3) and those remaining (Class 4). Seven families segregating both BRCA1 and BRCA2 mutations were excluded.
Mutation Analysis Testing
Details on the definition of deleterious BRCA1 and BRCA2 mutations and the techniques used to detect them are provided in the Supplementary Methods.
Data collection
For all included individuals, information on diagnoses of breast, ovarian and pancreatic cancers, ages at diagnosis of these cancers, date of birth and last contact or death, was collected by personal or telephone interview, or by mailed questionnaire. The proband in each family provided information on cancer family history through a family history questionnaire. Overall, documented verification through pathology reports (cancer registries and medical records) was available for 63% of breast cancers (20).
Age at diagnosis of PC was imputed as age at death for 34 cases and as the difference between date of birth and date of last contact for 5 cases. Subjects for which no age was recorded at any event (interview, cancer diagnosis, death or last follow-up) were excluded.
Statistical Analysis
To test if there was any difference in age at diagnosis of PC among the four classes of families we applied one-way ANOVA. To estimate the relative risk of PC for individuals from the four groups of families, we applied a survival analysis considering the time in years from birth to diagnosis of PC, death or last contact.
In total there were 78,820 individuals included from 5,799 eligible families: 12,180 women and 157 men with BC, 1,257 women with ovarian cancer and 417 individuals with PC (219 men and 198 women). Out of the 11,946 individuals in 538 BRCA1 families, 1,094 were genotyped mutation carriers (500 unaffected and 593 affected with breast or ovarian cancer, one affected with PC) and 717 we genotyped non-carriers (655 unaffected, 62 affected). There were 7,773 individuals in 383 BRCA2 families, of which 781 determined to be mutation carriers (360 unaffected and 420 affected with breast or ovarian cancer, one affected with PC) and 523 genotyped non-carriers (479 unaffected, 44 affected).
We estimated the relative risk of PC as a Standardized Incidence Ratio (SIR), defined as the number of PC cases observed divided by the number expected based on incidence rates for the general population. The expected number of cases was calculated by multiplying person-years at risk with population incidence rates of PC. Person-time, SIRs and their 95% confidence intervals (CI) were calculated using the stptime command in STATA version 10 (Stata Corporation, College Station, TX, USA). Population incidence rates specific to country, sex, and 5-year age group for specific 10-year calendar periods were taken from Cancer Incidence in Five Continents Reports (IARC-WHO; update November 2010).
To assess the possible influence of cohort effects, we first based the analysis on decade-specific incidence rates. For this analysis, follow-up began in 1950 because no reliable estimates of pancreas rates are available prior to then. Once we verified that the SIR estimates were not influenced by such cohort-effects, our final analyses were based on population rates specific for each country, sex and 5-year age group averaged from 1950 to 2009 which were applied to all follow-up, regardless of calendar year. Members of each class of family were first analyzed for overall PC risk. We then conducted separate analyses stratified by gender, age (≤50 years vs. > 50 years), degree of relationship to the proband (first-degree relative [FDR]), method of family recruitment (clinic-based vs. population-based) and, for BRCA1 and BRCA2 families, the number of BC cases in the family (≤2BC cases vs. ≥3 BC cases).
Because relatively few family members were tested for mutations in BRCA1 or BRCA2, we performed the analyses using two different approaches. First, we categorized all family members according to the mutational status of the proband. Under the second approach we weighted individuals from BRCA1 and BRCA2 families according to their estimated probability of being a mutation carrier. These probabilities were estimated using the SLINK software (http://linkage.rockefeller.edu/soft/slink.html). SLINK’s algorithm (21) simulates genotypes conditional on any combination of phenotypes and genetic marker data, even if only partially available. If N is the total number of members of a family, x (x1, x2,…,xN) is the vector of their phenotypes, and g= (g1, g2,…,gN) the vector of genotypes to be imputed, then the conditional probability distribution of the genotypes given the phenotypes can be calculated by a series of successive calculations:
BRCA1 and BRCA2 mutation status was imputed for all family members using personal history of breast, ovarian and pancreas cancer as phenotypic data and mutation status, if tested, as genetic data. Age-specific breast, ovarian and pancreas cancer penetrance and incidence estimates for BRCA1 and BRCA2 mutation carriers were also included as liability classes. The liability classes were constructed with seven age brackets for each of the following groups of individuals: affected with BC, affected with ovarian cancer, and unaffected with BC or ovarian cancer. These classes reflected the penetrance and incidence estimates derived from a combined analysis of 22 data sets unselected for family history by Antoniou et al. (22) (Supplementary Table 1). For pancreatic cancer we included an additional liability class with an assumed incidence in non-carriers of 0.005 and a risk in carriers that was initially set to that for non-carriers and then iterated as 0.005 multiplied by the current estimate of the SIR. Genotype simulation conditional on the pedigree phenotypes, relationships, and observed genotypes was conducted 2000 times for the entire BRCA1 and BRCA2 set of families.
For each individual included in this analysis the probability of being a mutation carrier was estimated as the proportion of simulations in which they were imputed to be mutation carriers. This probability was then used as a weight in the estimation of the SIR. This process was repeated until the SIR for pancreatic cancer for BRCA1 and BRCA2 mutation carriers converged, defined in this case to be a change from the previous iteration in the SIR of <0.5%
To compare SIRs obtained in stratified analyses we estimated p-values by applying a rate parameter test (23).
Results
Description of Families
Table 1 summarizes the characteristics of the families included in the study. There were 538 families for which the proband tested positive for a pathogenic mutation in BRCA1. Of these, 61 (11.3%) had at least one relative affected with PC and 4 (0.7%) had two or more affected relatives. There were 383 families found to carry BRCA2 mutations; 49 (12.8%) had at least one relative diagnosed with PC and 7 (1.8%) two or more. Of the BRCAX families, 1,219 had at least two relatives with early onset (≤ 50 years) BC (Class 3); 71 (5.8%) reported at least one relative affected with PC and 10 (0.8%) two or more. Among the 3,659 other BRCAX (Class 4) families, 186 (5.1%) included at least one individual diagnosed with PC and 16 (0.4%) included two or more.
Table 1.
Classes of families and characteristic of pancreatic cancer affected members of BCFR
| Class | BRCA1 | BRCA2 | 3 (BRCAX) | 4 (BRCAX) |
|---|---|---|---|---|
| Selection Criteria | Proband carrier of a BRCA1 mutation |
Proband carrier of a BRCA2 mutation |
Proband tested negative for BRCA1 and BRCA2 mutations; 2 or more BC >=50 years |
Proband tested negative for BRCA1 and BRCA2 mutations; at least 1 BC |
| No. of families | 538 | 383 | 1,219 | 3,659 |
|
No of individuals
(men/women) |
11,946 (5,457/6,490) |
7,773 (3,502/4,271) |
17,037 (7,158/9,879) |
42,064 (18,461/23,603) |
| No. of PC cases | 67 57 families: 1 PC 4 families: ≥2PC |
62 42 families: 1 PC 7 families: ≥2PC |
82 61 families: 1 PC 10 families: ≥2PC |
206 170 families: 1 PC 16 families: ≥2PC |
|
Gender (PC cases
only) |
32 males 35 females |
35 males 27 females |
40 males 42 females |
112 males 94 females |
|
Mean age at
diagnosis of PC |
65.9 years SD (14.9) |
63.1 years SD (11.0) |
66.9 years SD (12.7) |
66.9 years SD (12.5 ) |
Abbreviations; BC: breast cancer, PC pancreatic cancer, SD: standard deviation
The percentage of men and women among PC cases was similar in all classes of families, with a slightly higher number of affected men, except in Class 3 families which showed a higher number of women with PC. The observed mean age at diagnosis of PC was lower for BRCA2 families (63.1 years) compared to the other three classes of families (>65.9), but this difference was not statistically significant (P=0.22). (Table 1)
Pancreatic cancer risk estimates by family class regardless of mutation carrier status
The estimated SIRs for PC by familial class are summarized in Table 2. Overall, the SIR for members of BRCA1 mutation carrier families, was 1.60 (95%CI, 1.26-2.04) and was similar for men (SIR=1.43, 95%CI, 1.01-2.03) and women (SIR=1.80, 95%CI, 1.29-2.51). The SIR was higher for family members aged <50 years than for older ones (4.68, 95%CI, 2.66-8.25 vs. 1.40, 95%CI, 1.08-1.83, P=0.00092). The SIR did not appear to differ by the number of relatives with BC (<2 BCs vs. ≥3BCs, P=0.16) nor by the family recruitment method (clinic-based vs. population-based P=0.38). Overall, PC risk among members of BRCA2 families was 2.20-fold higher (95%CI, 1.71 −2.82) than in the general population with no difference between men and women. The SIR was higher for younger compared to older individuals (SIR=4.77, 95%CI, 2.39-9.55 vs. SIR=2.03, 95%CI, 1.56-2.66, P=0.043). The family recruitment method did not appear to influence the relative risk of PC for members of BRCA2 families (P=0.67). Having fewer relatives with BC appeared to be associated with a greater risk (p=0.0013).
Table 2.
Estimated SIRs and CI for PC by class of family from the BCFR
| Class | BRCA1 | BRCA2 | 3 (BRCAX) | 4 (BRCAX) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Exp | SIR | 95%CI | Obs | Exp | SIR | 95%CI | Obs | Exp | SIR | 95%CI | Obs | Exp | SIR | 95%CI | |
| Overall | 67 | 41.8 | 1.60 | 1.26-2.04 | 62 | 28.2 | 2.20 | 1.71-2.82 | 82 | 62.6 | 1.31 | 1.06-1.63 | 206 | 158 | 1.30 | 1.13-1.49 |
| Men | 32 | 22.3 | 1.43 | 1.01-2.03 | 35 | 14.7 | 2.38 | 1.71-3.31 | 40 | 31.2 | 1.28 | 0.94-1.75 | 112 | 77.6 | 1.44 | 1.20-1.74 |
| Women | 35 | 19.4 | 1.80 | 1.29-2.51 | 27 | 13.5 | 2.00 | 1.37-2.91 | 42 | 31.4 | 1.34 | 0.99-1.81 | 94 | 80.8 | 1.16 | 0.95-1.42 |
| ≤ 50years | 12 | 2.56 | 4.68 | 2.66-8.24 | 8 | 1.67 | 4.77 | 2.39-9.54 | 9 | 3.99 | 2.26 | 1.17-4.34 | 23 | 9.92 | 2.32 | 1.54-3.49 |
| >50 years | 55 | 39.2 | 1.40 | 1.08-1.83 | 54 | 26.6 | 2.03 | 1.56-2.66 | 73 | 58.6 | 1.25 | 0.99-1.57 | 183 | 148 | 1.23 | 1.07-1.42 |
| FDRs | 13 | 5.91 | 2.20 | 1.28-3.79 | 16 | 5.42 | 2.95 | 1.81-4.81 | 22 | 19.0 | 1.16 | 0.76-1.76 | 83 | 67.0 | 1.24 | 1.00-1.54 |
| Population-based | 16 | 8.15 | 1.96 | 1.20-3.20 | 16 | 6.71 | 2.38 | 1.46-3.89 | 30 | 29.4 | 1.02 | 0.71-1.46 | 112 | 95.1 | 1.18 | 0.98-1.42 |
| Clinic-based | 51 | 33.6 | 1.52 | 1.15-2.00 | 46 | 21.5 | 2.14 | 1.60-2.85 | 52 | 33.1 | 1.57 | 1.20-2.06 | 94 | 63.2 | 1.49 | 1.21-1.82 |
| ≤2 BC | 28 | 14.0 | 2.00 | 1.38-2.89 | 34 | 9.84 | 3.45 | 2.47-4.83 | ||||||||
| ≥3 BC | 39 | 27.7 | 1.41 | 1.03-1.92 | 28 | 18.4 | 1.52 | 1.05-2.21 | ||||||||
Abbreviations: Obs, observed number of cases; Exp, expected number of cases; SIR, standardized incidence rate; CI, confidence interval; FDR, first-degree relative; BC, breast cancer
Individuals from BRCAX Class 3 families had an increased risk of developing PC compared to the general population (SIR=1.31, 95%CI, 1.06-1.63) and this relative increase was similar for men and women. The estimated SIR was higher for younger relatives (SIR=2.26, 95%CI 1.17-4.34 vs. SIR=1.24, 95%CI, 0.99-1.57) and for families recruited by clinics (SIR=1.57, 95%CI, 1.20-2.06 vs. SIR=1.02, 95%CI, 0.71-1.46 for population-based families), but these differences were not statistically significant (P=0.12 and 0.056, respectively).
Overall, the SIR for members of Class 4 families was 1.30 (95%CI, 1.13-1.49) and this did not differ by gender. The SIR was higher for younger family members compared to older ones (SIR=2.32, 95%CI, 1.54-3.49 vs. SIR=1.23, 95% CI, 1.07-1.42, respectively, P=0.0085). The estimated SIR was also higher for members of clinic-based families compared to population-based families, but the difference was not statistically significant (SIR: 1.49, 95% CI 1.21-1.82 vs. SIR: 1.18, 95%CI 0.98-1.42, P=0.098).
Pancreatic cancer risk for BRCA1 and BRCA2 mutation carriers: Results using imputed genotypes
Estimated SIRs for BRCA1 and BRCA2 mutation carriers are summarized in Table 3. Based on a weighted analysis as a function of the imputed probability of being a mutation carrier, BRCA1 mutation carriers were estimated to be at increased risk of PC (SIR=4.11, 95%CI, 2.94-5.76), with little evidence of a difference by gender (P=0.090). In the analysis stratified by age group the SIR estimate was greater for individuals aged <50 years (7.75, 95%CI, 3.38-17.7) than for those who were older (SIR=3.77, 95%CI, 2.61-5.44); however, the difference between the two groups was not statistically significant (P=0.15).
Table 3.
Estimated pancreatic cancer SIRs for BRCA1 and BRCA2 mutation carriers from the BCFR
| BRCA1 mutation carriers | BRCA2 mutation carriers | |||||||
|---|---|---|---|---|---|---|---|---|
| Obs | Exp | SIR | 95%CI | Obs | Exp | SIR | 95%CI | |
| Overall | 34.0 | 8.26 | 4.11 | 2.94-5.76 | 41.8 | 7.23 | 5.79 | 4.28-7.84 |
| Men | 14.8 | 4.79 | 3.09 | 1.86-5.15 | 22.5 | 3.88 | 5.81 | 3.84-8.78 |
| Women | 19.2 | 3.47 | 5.52 | 3.53-8.64 | 19.3 | 3.35 | 5.77 | 3.69-9.01 |
| ≤ 50 years | 5.59 | 0.72 | 7.75 | 3.38-17.7 | 5.47 | 0.55 | 9.90 | 4.28-22.9 |
| >50 years | 28.4 | 7.54 | 3.77 | 2.61-5.44 | 36.4 | 6.68 | 5.45 | 3.94-7.54 |
| FDRs | 11.2 | 2.51 | 4.47 | 2.49-8.03 | 13.6 | 2.45 | 5.55 | 3.27-9.45 |
| Population-based | 5.88 | 2.19 | 2.68 | 1.20-6.02 | 11.5 | 2.25 | 5.12 | 2.88-9.12 |
| Clinic-based | 28.1 | 6.07 | 4.63 | 3.20-6.70 | 30.3 | 4.98 | 6.09 | 4.27-8.70 |
| ≤ 2 BCs | 13.8 | 3.31 | 4.18 | 2.47-7.08 | 24.2 | 2.70 | 8.98 | 6.03-13.4 |
| ≥ 3 BCs | 20.1 | 4.95 | 4.07 | 2.63-6.30 | 17.6 | 4.52 | 3.90 | 2.44-6.21 |
Abbreviations: Obs, observed number of cases; Exp, expected number of cases; SIR, standardized incidence rate; CI, confidence interval; FDR, first-degree relative; BC, breast cancer.
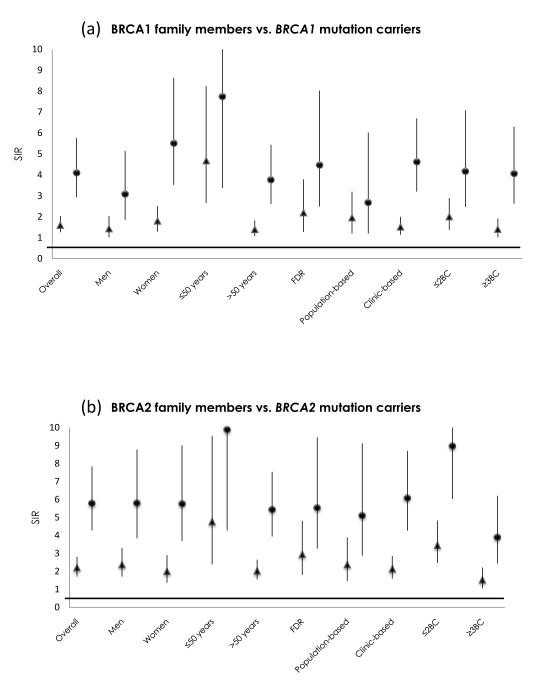
BRCA2 mutation carriers were also found to be at increased risk of PC compared to the general population (SIR=5.79, 95%CI, 4.28-7.84). Results were similar for men and women. BRCA2 mutation carriers <50 years had a higher SIR (9.90, 95%CI, 4.28-22.9) for PC than older ones (SIR=5.45, 95%CI, 3.94-7.54), but the difference was not statistically significant (P=0.22). Having fewer relatives with BC appeared to be associated with a greater relative risk of pancreas cancer (p=0.0073). Figure 1 compares the SIR estimates and 95%CI for PC between BRCA1 and BRCA2 mutation carriers and BRCA1 and BRCA2 families.
Figure 1.
Comparison of SIRs and respective 95% Confidence Intervals (vertical axis) for all family members (triangles) and weighted by imputed carrier probability (circles) in BRCA1 (panel a) and BRCA2 (panel b) families, by class of family (horizontal axis.
Discussion
This study confirms previous reports that BRCA2 mutation carriers have an increased risk of developing PC compared to the general population. It also supports previous evidence that BRCA1 mutation carriers have increased PC risk, but with a higher relative risk than that estimated by other studies. Furthermore, the availability of a large number of high-risk BC families negative for BRCA1 and BRCA2 mutations (approximately 5,200) has provided a unique opportunity to estimate PC risk in non-mutation carriers from families with breast and ovarian cancer.
The association between BRCA2 mutations and PC risk has been previously investigated; individuals from BRCA2 mutation carrier families have been reported to have a PC risk ranging from 2- to 7-fold higher compared to general population (14,16,17,24). In our study, we observed a relative risk of 5.79 (95%CI, 4.28-7.84), slightly higher than most previous estimates. Unlike previous studies, which were mostly limited to first-degree relatives of the index case, we also considered more distant relatives (up to third-degree); however our result was consistent for first-degree relatives (SIR=5.55, 95%CI, 3.27-9.45). Previous reports on BRCA2 mutation carriers aged < 65 years showed from 5- to 37-fold increased risk of PC (14,24). We applied a more extreme phenotype as early onset PC (≤50 years), and estimated a 9.90-fold higher risk compared to the general population (95%CI, 4.28-22.9). We also observed in BRCA2 mutation carrier families a mean age at PC diagnosis of 63.1 years, approximately 9 years earlier than the mean age of PC in the general population (1).
Data from previous studies on the role of germline BRCA1 mutations in PC carcinogenesis are more controversial than those for BRCA2 mutations; some studies reported 2-to 3-fold higher risk of PC in BRCA1 mutation carriers compared with the general population (18, 25). A recently published study reported that Ashkenazi Jewish families with aggregation of BC and PC had a similar percentage of BRCA1 and BRCA2 mutations (26). We also observed a similar percentage of families with BRCA1 and BRCA2 mutations and aggregation of BC and PC (Table 1). Our study, the largest to date assessing PC risk in BRCA1 carriers, estimated a 4.11-fold higher risk of PC, which is higher than estimates from previous reports. The SIR was 4.47 for first-degree relatives of the proband. A previous study estimated that BRCA1 mutation carriers have > 3-fold risk of PC at ages less than 65 years (18); we estimated a SIR of 7.75 (95%CI, 3.38-17.7) for individuals aged less than 50 years. In addition the mean age at diagnosis of PC in members of BRCA1 mutation-carrier families was 65.9 years, which is 6 years earlier than the mean age at PC diagnosis in the general population1. The above-reported relative risks of PC for BRCA1 and BRCA2 mutation carriers were estimated using imputed carrier probabilities for untested family members (Table 3). The same individuals were analyzed for PC risk, regardless of their mutation status (Table 2), with lower SIR estimated both for the overall and all stratified analyses (Figure 1).
A recent study prospectively followed more than 5,000 female carriers of mutations in BRCA1 and BRCA2 for a mean time of 1.95 years. Based on 6 incident PCs in BRCA1 mutation carriers and 2 BRCA2 mutation carriers, they estimated SIRs of SIR=2.55 (95%CI, 1.03-5.31) and 2.13 (95%CI, 0.36-7.03), respectively); furthermore, they estimated an increase risk of PC (OR= 46.5; 95% CI=9.4–230) for female BRCA1 or BRCA2 mutation carriers with a first-degree relative affected with PC compared to those with no first-degree relatives with PC. However this study was limited by the small number of incidence cases and consequent low precision of the relative risk estimates, especially for the subgroup with a first-degree family history of PC. The authors concluded that the presence of a BRCA1 or BRCA2 mutation alone does not justify the adoption of screening for PC, but that this needs to be better investigated in mutation carriers with a first-degree relative affected with PC17.
To assess whether other factors besides BRCA1 and BRCA2 mutations increase the risk of PC in BC families we analyzed a large number of mutation-negative (BRCAX) families after dividing them into two groups, with the first group (Class 3) selected for having a larger predicted genetic component (at least two early onset BC cases). Our study estimated a 30% increased overall PC risk and a more than 2-fold increased risk of early onset PC for BRCAX family members. This latter result is lower, but consistent with that of an earlier study which estimated a 5.5-fold higher risk of PC diagnosed at age ≤50 years in BC families, not due to BRCA1 or BRCA2 mutations (27). Class 4 included the largest number of families and was most diverse in terms of number of BC cases per family and age at diagnosis. Compared to the general population, members of these families were at increased risk both of PC overall and of early onset PC. Results were similar to those for Class 3 families, suggesting that the number of relatives with BC did not affect PC risk.
PALB2, a new BC suppressor gene (11,28,29), has recently been reported as a PC susceptibility gene (9); germline mutations have been found in approximately 3% of families with ≥ 1PC (10). Of note, 4 of the 5 PALB2-related PC families identified to date had >1 relative with BC and in two of those families, mutations were seen in individuals with both BC and PC (30). Recent studies reported a prevalence of about 2% of PALB2 mutations in BRCAX families selected for family history of PC (11,31), suggesting that other, still unknown genetic factors, likely play a role. Thus the increased risk of PC among BRCAX families is unlikey to be due to PALB2 mutations segregating in these families.
To assess whether our estimates of PC risk could be affected by the standardized rates used, we carried out an analysis of the risk of another cancer as a control. After a thorough review of the literature we selected esophageal cancer (EC) as suitable for our purpose for two main reasons: 1) there is little evidence in the literature supporting an association between this cancer and BRCA1 and BRCA2 mutations (32,33) with only a single recent study reporting an increased risk (32) and 2) EC is a cancer with a strong environmental component, being frequently induced by chronic exposure to environmental risk factors like alcohol and smoking (34). Therefore we would not expect an increased incidence in our study sample, compared to the general population. Applying an identical analysis to that for PC, no systematic bias away from 1.0 was observed in the SIR for BRCA1, BRCA2 and BRCAX families in the global nor in the stratified analyses (see Supplementary Table 2).
Our study has some inherent limitations that need to be considered to appropriately interpret our findings. Although the only inclusion criterion applied by sites in the BCFR with clinic-based recruitment was the presence of BC and/or ovarian cancer, there could have been some bias in recruitment, towards families with other cancers (such as PC) previously found associated with genetic predisposition. This could potentially result overestimates of the SIR. However, this is not a major problem since in most of the clinic-based sites, all families that met the study criteria in terms of breast or ovarian cancer, were enrolled in the BCFR. Therefore we believe it is unlikely that enrollment in the BCFR would be dependent on the presence of cancers other than breast and ovarian.
Another important issue concerns the accuracy of reported PC diagnosis in relatives. A family history of cancer was reported by the proband or her first-degree relatives and, in most cases, it was not verified by clinical reports19. Validation studies on cancer family history show a low rate of false-positive and false-negative reports of different cancers by first-degree relatives (35-37). Ziogas et al. showed that the accuracy of reported cancer family history varies by cancer site; for PC the positive predictive value (PPV) was 77.4% (95%CI, 58.9–90.4) for first-degree relatives compared to 53.3& (95%CI, 26.6–78.7) for second-degree relatives (37). Our analysis was robust to the stratification for relationship to the proband.
It has been proposed that the method of ascertainment of the proband is a good predictor of accuracy of reported familial cancer data; probands from clinic-based ascertainment sources have been found to be more accurate in their reporting compared with population-based sources, possibly because they are more informed and more motivated about their risk (37). Our results were consistent when the analyses were limited to families recruited through clinic-based settings, suggesting that misdiagnosis of PC did not give rise to spurious results.
The results of the study have potential implications for the screening of PC in high-risk BC families. Currently, screening programs are directed at BRCA2 mutation carriers and members of BC families with at least one relative affected with PC. Our findings indicate that BRCA1 mutation carriers also have an increased risk and should therefore also be followed up for PC.
Screening provides the best opportunity to reduce mortality from PC by detecting early stage cancers, or high-grade pre-neoplastic lesions, such as intraductal papillary mucinous neoplasm (IPMN) and pancreatic intraepithelial neoplasia (PanIN).
Currently, several centres all over the world are conducting screening programs in high-risk individuals; so far, among 988 patients followed-up for PC, 18 PCs (1.8%) and 23 PanIN3 or high-grade IPMNs (2.32%) have been diagnosed. However, 22 high-risk individuals (2.22%) were over treated for low-grade dysplasia or benign tumors [refs]. These findings demonstrate that screening in high-risk individuals can detect pre-cancerous changes in the pancreas. However, many questions remain, including the identification of the most appropriate screening populations and, most importantly, which criteria for the selection of patients for surgery maximizes benefit and minimizes risk. It is important clarify that early detection screening for pancreas has not a clinical value, but it is limited to well-developed research protocols and/or clinical trials.
We also found that members of BRCAX families with at least one relative affected with BC are at increased of PC, albeit to a lesser degree than BRCA1 and BRCA2 mutation carriers.
In conclusion, carriers of mutations in BRCA1 or BRCA2 have an increased risk of developing PC compared with the general population. Members of BC families that test negative for BRCA1 and BRCA2 mutations are also at increased risk of PC, although more moderate compared with members of mutation-carrying families. Our study suggests that the increased risk of PC in relatives of breast cancer cases is not fully explained by mutations in BRCA1 and BRCA2.
Supplementary Material
Acknowledgements
This work was partially supported by the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, MINECO, Spain (#PI09-02102). The Breast Cancer Family Registry (BCFR) was supported by the National Cancer Institute, National Institutes of Health under RFA # CA-06-503, and through cooperative agreements with members of the BCFR and Principal Investigators, including Cancer Care Ontario (U01 CA69467), Columbia University (U01 CA69398), Fox Chase Cancer Center (U01 CA69631), Huntsman Cancer Institute (U01 CA69446), Cancer Prevention Institute of California (U01 CA69417), University of Melbourne (U01 CA69638) and Research Triangle Institute Informatics Support Center (RFP No. N02PC45022-46). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating BCFR centres, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the BCFR.
Financial support: This work was partially supported by the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Ministry of Science and Innovation, Spain (PI09-02102); Red Temática de Investigación Cooperativa en Cáncer (RTICC).
Footnotes
Conflict of Interest Disclosures: The authors declare they have no conflicts of interest related to this Manuscript.
References
- 1.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. at Rev Gastroenterol Hepatol. 2009 Dec;6(12):699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 2.Blackford A, Parmigiani G, Kensler TW, Wolfgang C, Jones S, Zhang X, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009 Apr 15;69(8):3681–3688. doi: 10.1158/0008-5472.CAN-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korkzac JF, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):704–710. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 4.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004 Apr 1;64(7):2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 5.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. Jan 20;102(2):119–126. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy KM, Brune KA, Griffin C, Sollenberg JE, Petersen GM, Bansal R, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002 Jul 1;62(13):3789–793. [PubMed] [Google Scholar]
- 7.Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003 Feb 5;95(3):214–21. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 8.Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007 Feb;16(2):342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 9.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009 Apr 10;324(5924):217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, et al. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. Nov;78(5):490–494. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 11.Tischkowitz M, Xia B, Sabbaghian N, Reis-Filho JS, Hamel N, Li G, et al. Analysis of PALB2/FANCN-associated breast cancer families. Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6788–793. doi: 10.1073/pnas.0701724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discovery. 2012;2(1):41–6. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):7155–160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005 Sep;42(9):711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer risks in BRCA2 mutation carriers The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999 Aug 4;91(15):1310–316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 16.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006 Dec 6;98(23):1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal J, Ragone A, Lubinski J, Lynch HT, Moller P, Ghadirian P, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012 Dec 4;107(12):2005–9. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002 Sep 18;94(18):1358–365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 19.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–389. doi: 10.1186/bcr801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dite GS, Whittemore AS, Knight JA, John EM, Milne RL, Andrulius IL, et al. Increased cancer risks for relatives of very early-onset breast cancer cases with and without BRCA1 and BRCA2 mutations. Br J Cancer. 2010 Sep 28;103(7):1103–108. doi: 10.1038/sj.bjc.6605876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ott J. Computer-simulation methods in human linkage analysis. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4175–4178. doi: 10.1073/pnas.86.11.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoniou AC, Easton DF. Polygenic inheritance of breast cancer: Implications for design of association studies. Genet Epidemiol. 2003 Nov;25(3):190–202. doi: 10.1002/gepi.10261. [DOI] [PubMed] [Google Scholar]
- 23.Hills M, Clayton D. Statistical Models in Epidemiology. 1993.
- 24.Consortium BCL. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999 Aug 4;91(15):1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 25.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002 Sep 18;94(18):1365–372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 26.Stadler ZK, Salo-Mullen E, Patil SM, Pietanza MC, Vijai J, Saloustros E, et al. Prevalence of BRCA1 and BRCA2 mutations in Ashkenazi Jewish families with breast and pancreatic cancer. Cancer. 2012 May; doi: 10.1002/cncr.26191. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzo Bermejo J, Hemminki K. Risk of cancer at sites other than the breast in Swedish families eligible for BRCA1 or BRCA2 mutation testing. Ann Oncol. 2004 Dec;15(12):1834–1841. doi: 10.1093/annonc/mdh474. [DOI] [PubMed] [Google Scholar]
- 28.Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007 Feb;39(2):165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southey MC, Teo ZL, Dowty JG, Odefrey FA, Park DJ, Tischkowitz M, et al. A PALB2 mutation associated with high risk of breast cancer. Breast Cancer Res. 12(6):R109. doi: 10.1186/bcr2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tischkowitz MD, Sabbaghian N, Hamel N, Borgida A, Rosner C, Taherian N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009 Sep;137(3):1183–1186. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofstatter EW, Domchek SM, Miron A, Garber J, Wang M, Camponeschi K, et al. PALB2 mutations in familial breast and pancreatic cancer. Fam Cancer. Jun;10(2):225–231. doi: 10.1007/s10689-011-9426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005 Jun 29;7(2):60. [PMC free article] [PubMed] [Google Scholar]
- 33.Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2011 doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 34.Taziki MH BN, Tadrisee M, Mansourian AR. Esophageal cancer: 5-year survival rate at south-east of Caspian sea of northern Iran. J Cancer Res Ther. 2011 Apr-Jun;(7):135–137. doi: 10.4103/0973-1482.82923. [DOI] [PubMed] [Google Scholar]
- 35.Novakovic B, Goldstein AM, Tucker MA. Validation of family history of cancer in deceased family members. J Natl Cancer Inst. 1996 Oct 16;88(20):1492–493. doi: 10.1093/jnci/88.20.1492. [DOI] [PubMed] [Google Scholar]
- 36.Airewele G, Adatto P, Cunningham J, Mastromarino C, Spencer C, Sharp M, et al. Family history of cancer in patients with glioma: a validation study of accuracy. J Natl Cancer Inst. 1998 Apr 1;90(7):543–54. doi: 10.1093/jnci/90.7.543. [DOI] [PubMed] [Google Scholar]
- 37.Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med. 2003 Feb;24(2):190–198. doi: 10.1016/s0749-3797(02)00593-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.