Abstract
Purpose
Adrenocortical carcinoma (ACC) is an endocrine malignancy with a poor prognosis. The association of adult-onset ACC with inherited cancer predisposition syndromes is poorly understood. Our study sought to define the prevalence of Lynch syndrome (LS) among patients with ACC.
Patients and Methods
One hundred fourteen patients with ACC were evaluated in a specialized endocrine oncology clinic and were prospectively offered genetic counseling and clinical genetics risk assessment (group 1). In addition, families with known mismatch repair (MMR) gene mutations that were recorded in the University of Michigan Cancer Genetics Registry were retrospectively reviewed for the presence of ACC (group 2). ACC tumors from patients with LS were tested for microsatellite instability and immunohistochemistry (IHC) to evaluate for MMR deficiency.
Results
Ninety-four (82.5%) of 114 patients with ACC underwent genetic counseling (group 1). Three individuals (3.2%) had family histories suggestive of LS. All three families were found to have MMR gene mutations. Retrospective review of an additional 135 MMR gene–positive probands identified two with ACC (group 2). Four ACC tumors were available (group 1, 3; group 2, 1). All four tumors were microsatellite stable; three had IHC staining patterns consistent with germline mutation status.
Conclusion
The prevalence of LS among patients with ACC is 3.2%, which is comparable to the prevalence of LS in colorectal and endometrial cancer. Patients with ACC and a personal or family history of LS tumors should be strongly considered for genetic risk assessment. IHC screening of all ACC tumors may be an effective strategy for identifying patients with LS.
INTRODUCTION
Lynch syndrome (LS) is an inherited cancer predisposition syndrome associated with elevated cancer risks. The lifetime colorectal cancer risk is estimated to be as high as 35% to 80% and the endometrial cancer risk in women is estimated to be elevated to 34% to 71%.1–4 Identifying individuals with LS and subsequently enrolling them in screening and surveillance protocols significantly decreases cancer-related morbidity and mortality.5 Focus has thus shifted to consider other potentially related cancers that have previously gone unrecognized owing to a lower prevalence than the classic LS-associated cancers. Elevated risks of other cancers, including gastric, ovarian, urinary tract, pancreas, brain tumors, and sebaceous tumors have been established in patients with LS.6 However, when considering tumors that are rare in the general population, recognizing an association with an inherited cancer predisposition syndrome can be difficult. The ability to recognize an association of a rare tumor with an inherited cancer predisposition syndrome increases the ability to diagnose the syndrome and to provide appropriate interventions for patients and their families.
Adrenocortical carcinoma (ACC) is a rare cancer with an incidence of 0.72 cases per million individuals per year (300 new diagnoses are made in the United States per year).7 While the association of childhood ACC with hereditary cancer syndromes is well described, the association of ACC in adult patients and inherited predisposition syndromes is far less well understood. ACC is known to be a core tumor in Li Fraumeni syndrome (LFS) because of germline TP53 mutations, and a diagnosis of ACC in childhood is strongly associated with this condition. Fifty percent to 80% of all children diagnosed with ACC have an underlying diagnosis of LFS.8–11 This association is not as strong in adult-onset ACC. LFS has been identified as the underlying cause in 3.9% to 5.8% of patients with adult-onset ACC.12,13 Adult-onset ACC has also been described in multiple endocrine neoplasia type 114,15 and has been reported in patients with familial adenomatous polyposis and LS.16 Our aim was to uncover the prevalence of inherited cancer syndromes in patients with ACC.
PATIENTS AND METHODS
Patients
Prospective analysis (group 1).
Our study population included patients who presented with a diagnosis of ACC to the University of Michigan Multidisciplinary Endocrine Oncology program between December 1, 2009 and October 31, 2011 (probands).13 This clinic is a national and international referral center, with expertise in the diagnosis and interdisciplinary management of patients with ACC. Patients met with a genetic counselor who obtained a four-generation cancer-focused pedigree and performed a clinical genetics risk assessment. Family cancer histories were confirmed where records were available. All patients were offered TP53 germline genetic testing for LFS through a Clinical Laboratory Improvement Amendments–certified clinical lab based on the Chompret criteria as previously described.13,17 Other genetic testing was offered based on the clinical cancer genetics risk assessment.
Retrospective chart review (group 2).
The University of Michigan Cancer Genetics Registry was queried for all probands with mismatch repair (MMR) gene mutations and a personal diagnosis of ACC. In this retrospective group, probands are defined as the first person presenting to the University of Michigan Cancer Genetics program. This registry was initiated in 2002, and participation in the registry is offered to all probands and their family members who are evaluated in the University of Michigan Cancer Genetics program. Probands enrolled in the Cancer Genetics Registry through the Multidisciplinary Endocrine Oncology program were excluded from the retrospective chart review.
Formalin-fixed paraffin-embedded (FFPE) tumor samples of ACC were obtained for those patients identified as having LS.
Permission for human research was approved through the University of Michigan institutional review board.
DNA Extraction
Hematoxylin and eosin–stained slides cut from FFPE tissues were evaluated by a pathologist (L.V.F.), and the areas of the slide representing tumor and normal (no malignant tissue) were identified and manually dissected from 10-μm FFPE tissue sections using Pinpoint Slide DNA Isolation System (Zymo Research Corporation, Irvine, CA). Areas selected for dissection ranged from 2 mm to 1 cm in diameter. The proportion of tumor cells in the material used for the extraction of DNA exceeded 50% in all of the patients. Corresponding normal tissue was available in two of the four selected patients (patients 3 and 4; Appendix Fig A2, online only) Tissue samples were digested with 5 μL of Proteinase K (Zymo Research Corporation) in 50-μL extraction buffer (Zymo Research Corporation) for 4 hours at 55°C. Samples were then heated to 95°C for 10 minutes and vortexed. No additional purification of the DNA was performed.
Genomic DNA from peripheral blood lymphocytes was obtained and used as control for the two patients in whom only tumor tissue was identified by histologic examination (patients 1 and 2; Appendix Fig A1, online only). DNA from peripheral blood lymphocytes was extracted using standard procedures according to the manufacturer's instructions (Gentra Puregene Cell kit, Qiagen, Valencia, CA).
Microsatellite Instability Assay
Microsatellite instability (MSI) testing was performed with the MSI Analysis System, version 1.2, according to the manufacturer's protocol, (Promega Corporation, Madison, WI). In short, five mononucleotide repeat markers are used for MSI determination (BAT-26, NR-21, BAT-25, MONO-27, and NR-24) and two pentanucleotide markers for establishing sample identity (Penta C and Penta D).18 Amplicons were separated by capillary electrophoresis using an automated ABI Prism 3130XL Genetic Analyzer (Applied Biosystems, Carlsbad, CA). A positive amplification control and a no-template control were set up with each run. Data were analyzed using GeneMapper 4.0 software (Applied Biosystems, Carlsbad, CA). For analysis, allelic profiles of normal versus tumor tissues were compared, and MSI was scored as the presence of alterations in the length of short tandem repeats in tumor DNA compared with normal DNA.19–21 Alleles present in the tumor sample that were not present in the corresponding normal tissue indicate MSI. MSI was defined as instability (≥ one of five mononucleotide microsatellite markers) in tumor DNA compared with normal DNA. Tumors with instability in ≥ two of five mononucleotide microsatellite markers was defined as MSI-high. MSI-low was defined as instability in one of five mononucleotide markers in tumor DNA compared with normal DNA. Tumors with no instability (none of five mononucleotide markers altered) were defined as microsatellite stable (MSS).22,23
Immunohistochemical Staining
Immunoperoxidase staining was performed on FFPE tissues as previously described.24 The primary antibodies were MLH1 predilute mouse monoclonal M1 from Ventana Medical Systems, MSH2 predilute mouse monoclonal G219-1129 from Ventana Medical Systems, MSH6 predilute mouse monoclonal 44 from Ventana Medical Systems, and PMS2 predilute rabbit monoclonal ERP3974 from Ventana Medical Systems. Positive and negative controls were stained appropriately, and any convincing nuclear staining was considered positive.
RESULTS
One hundred fourteen patients with ACC were evaluated in the University of Michigan Endocrine Oncology Program during the study period. Ninety-four patients (82.5%) met with a genetic counselor. Thirty-three patients (35.1%) were men and 61 patients (64.9%) were women. The majority of patients were white (87.2%). Average age at ACC diagnosis was 44.7 years, with a range of three to 82 years old at diagnosis (Table 1). Fifty-three patients underwent germline TP53 mutation analysis, and four patients (7.4%) were positive for a germline TP53 mutation as previously described.13
Table 1.
Characteristics of Patients in the Endocrine Oncology Program (n = 94)
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex | ||
| Female | 61 | 64.9 |
| Male | 33 | 35.1 |
| Race | ||
| White | 82 | 87.2 |
| African American | 8 | 8.5 |
| Asian | 2 | 2.1 |
| Other | 2 | 2.1 |
| Age at diagnosis, years | ||
| Mean | 44.7 | |
| Range | 3-82 | |
| Median | 46 | |
In the prospective analysis (group 1), after completion of a four-generation cancer genetics pedigree, three probands were identified as having family histories suggestive of LS (three of 94; 3.2%); two probands had family histories that met clinical diagnostic criteria for LS (Amsterdam I criteria; Table 2), and a third proband had a family history suggestive of LS (Table 3). All three probands underwent germline TP53 analysis and were negative for any germline mutations by sequencing and deletion/duplication analysis. Two of three probands underwent germline MMR genetic testing and were positive for MMR gene mutations (proband 1, MSH2; proband 3, MSH6). LS work-up in the third family (proband 2) was completed after the proband died and the brother tested positive for an MLH1 mutation.
Table 2.
Clinical Diagnostic Criteria for LS
| Amsterdam I Criteria: |
Three or more individuals diagnosed with colorectal cancer plus all of the following
|
| Amsterdam II Criteria: |
Three or more individuals diagnosed with an LS-associated tumor, including colorectal, endometrial, small bowel, ureter, or renal pelvis cancer plus all of the following
|
Abbreviation: LS, Lynch syndrome.
Table 3.
Characteristics of Patients With ACC and LS and Results of MSI and IHC Screenings for LS in ACC Tumors
| Group and Proband | Sex | Age at Diagnosis (years) | Right or Left Side | ACC Stage | MSI | IHC | Germline Mutation | Other LS Cancer History* and Age (years) | Family History of LS Cancer* in First- and Second-Degree Relatives and Their Ages (years)† | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | ||||||||||
| Proband 1 | M | 52 | Right | III | MSS | Absence of MSH2/MSH6; retention of MLH1/PMS2 | MSH2 | c.2131C>T p.R711X | SebCa; 53 | Amsterdam I |
| Proband 2 | M | 47 | Right | III | MSS | Absence of MLH1/PMS2; retention of MSH2/MSH6 | MLH1‡ | c.2246T>C p.L749P | — | Amsterdam I |
| Proband 3§ | M | 39 | Right | II | MSS | Normal | MSH6 | c.2141C>G p.S714C | — | F: CRC; 45 |
| Group 2 | ||||||||||
| Proband 4 | F | 42 | Right | I | MSS | Absence of MSH2/MSH6; retention of MLH1/PMS2 | MSH2 | c.792+1G>A | SebAd; 47 | PGF: CRC; late 40s |
| Proband 5 | F | 23 | Left | III | — | — | MSH2 | c.942+3A>T | SebCa; 49 | M: OvCa, 54; MA: CRC, 83 |
NOTE. Group 1 refers to patients recruited prospectively through the endocrine oncology program. Group 2 refers to patients identified through retrospective review of the Cancer Genetics Registry. ACC tumor was not available for Proband 5. Full clinical description of patients, including details of endocrine evaluation, are available in Appendix Table A1 (online only).
Abbreviations: ACC, adrenocortical carcinoma; CRC, colorectal cancer; F, father; FDG-PET, fluorodeoxyglucose–positron emission tomography; IHC, immunohistochemistry; LS, Lynch syndrome; M, mother; MA, maternal aunt; MSI, microsatellite instability; MSS, microsatellite stable; OvCa, ovarian cancer; PGF, paternal grandfather; SebAd, sebaceous adenoma; SebCa, sebaceous carcinoma.
LS cancers include the following cancers: colorectal, endometrial, ovary, stomach, small intestine, urinary tract, renal pelvis, bile duct, pancreas, glioblastoma multiforme, and sebaceous gland tumors.
First- and second-degree relatives include children, parents, siblings, aunts, uncles, and grandparents.
Mutation identified in proband's brother; germline testing not available for proband.
Pathology review revealed oncocytic adrenocortical tumor of uncertain malignant potential. Tumor grew significantly over a 2-year course and was FDG-PET–positive; thus, patient is receiving follow-up with a diagnosis of ACC.
In the retrospective chart review (group 2), an additional 135 probands from independently ascertained families with MMR gene mutations enrolled onto the University of Michigan Cancer Genetics Registry (MLH1, n = 44; MSH2, n = 66; MSH6, n = 18; PMS2, n = 7) and revealed two probands with personal histories of ACC (MSH2, n = 2; probands 4 and 5; Table 3). At initial consultation, proband 4 tested negative for germlineTP53 mutations by sequencing and deletion/duplication analysis in accordance with Chompret guidelines to exclude the possibility of second inherited predisposition syndrome.
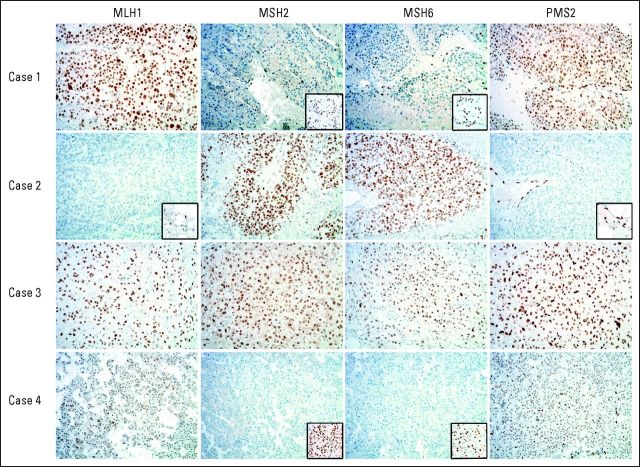
ACC tumor tissue was available for four of five patients (Table 3). All of the tested samples were MSS (Appendix Figs A1 and A2). The ACC tumor from the two probands with MSH2 mutations had immunohistochemical (IHC) staining demonstrating the absence of MSH2 and MSH6 with retention of MLH1 and PMS2. The ACC tumor from the proband whose brother tested positive for an MLH1 mutation had IHC staining demonstrating the absence of MSH2 and MSH6 with retention of MLH1 and PMS2. The ACC tumor from the proband whose brother tested positive for an MLH1 mutation had IHC staining, demonstrating absence of MLH1 and PMS2 with retention of MSH2 and MSH6. The ACC tumor from the proband with an MSH6 mutation had a normal IHC staining pattern with retention of all four proteins (Fig 1; Table 3).
Fig 1.
Immunohistochemistry stains of adrenocortical carcinoma (ACC) tumors. Patient 1 demonstrates absence of nuclear staining for MSH2 and MSH6 in the ACC tumor with positive nuclear staining in the adjacent normal adrenal tissue as shown in the inset photo. Retention of nuclear staining of MLH1 and PMS2 in the ACC tumor is shown. Patient 2 demonstrates absence of nuclear staining in the ACC tumor for MLH1 and PMS2 and positive nuclear staining in the adjacent normal adrenal tissue as shown in the inset photo. Retention of nuclear staining of MSH2 and MSH6 in the ACC tumor is shown. Patient 3 demonstrates retention of the nuclear staining in the ACC tumor tissue for all four proteins (MLH1, MSH2, MSH6, and PMS2). Patient 4 demonstrates absence of nuclear staining for MSH2 and MSH6 in the ACC tumor with positive nuclear staining in the adjacent normal adrenal tissue as shown in the inset photo. Retention of nuclear staining of MLH1 and PMS2 in the ACC tumor is shown.
DISCUSSION
Childhood ACC is strongly associated with LFS, an inherited cancer predisposition syndrome, and ACC is a core malignancy of LFS.9 Though the majority of adult-onset ACC has been thought to be sporadic in nature, there is increasing evidence of an association between adult ACC and inherited conditions, including LFS and multiple endocrine neoplasia type 1.12–14,16 Our current study defines LS as another hereditary cancer syndrome to be considered in the evaluation of patients with ACC.
Identification of individuals with LS is important in routine medical care and oncologic care.1,25 The National Comprehensive Cancer Network26 advocates for tumor screening of colorectal cancers and endometrial cancers to analyze for high levels of MSI and absent IHC staining in an effort to identify those individuals and subsequently their families who are at elevated risk for cancer, which has been shown to decrease morbidity and mortality5 and has been demonstrated as cost-effective.1
In this prospective series of patients with ACC, three (3.2%) of 94 patients were subsequently diagnosed with LS. This prevalence is significantly higher than observed in the general population (one of 440 patients; P = .0185; Fisher's exact test).27 The prevalence of LS among patients with ACC is similar to the prevalence of LS among all patients with colorectal cancer (2% to 4%) and endometrial cancer (1% to 5%).28–32 Furthermore, the identification of two patients with ACC among 135 probands in the Cancer Genetics Registry suggests a significant relative risk increased over the general population (approximately 0.72 diagnoses per million persons per year). Case reports of ACC in patients with LS date back to the initial reports defining the syndrome. One of the first families described by Lynch et al,33 Family N, was ascertained through a proband who died at age 44 years with a history of ACC with a striking family history of young-onset endometrial cancer and young-onset, multiple primary colorectal cancer. In addition, there have been four single case reports of ACC in conjunction with LS and germline MSH2 mutations.34–37
The description of the five patients presented in our study brings the total number of reports of ACC and LS to 10 and expands the spectrum of germline mutations to include MLH1 and MSH6. These findings give weight to the consideration of including ACC as an LS-associated tumor. Including ACC as an LS-associated tumor in the Amsterdam II criteria (Table 2) for a clinical diagnosis of LS would increase the diagnostic yield. It also holds the potential to increase the true-positive rate, while reducing the false-negative rate. In our prospective series, the clinical diagnostic yield would increase from two of five patients to three of five patients (40% v 60%; Table 3) with proband 5 meeting these enhanced Amsterdam II criteria.
In an attempt to understand a relationship with LS, we analyzed MSI and loss of MMR gene protein expression by IHC. MSI analysis revealed all four ACC tumors to be MSS. This is in contrast with findings in the classic LS cancer, colorectal cancer, in which the majority of these cancers in patients with LS demonstrate high levels of MSI.29 Although intriguing, our findings concur with those of previous studies that report a lower incidence of MSI on polymerase chain reaction testing in rare cancers shown to be associated with LS.34–37 These observations suggest that DNA MMR gene deficiency in these tumors may be a secondary event that occurs in a later stage in carcinogenesis, therefore preventing the accumulation of detectable MSI.35 In our series, IHC analysis identified loss of expression for MSH2 and MSH6 in the ACC tumor from both MSH2 mutation carriers and loss of MLH1 and PMS2 expression in the ACC tumor from the MLH1 family, consistent with the molecular phenotype of LS. However, staining for MSH6 was retained in the ACC tumor from the proband with the MSH6 mutation. This normal staining pattern in the MSH6 mutation carrier is not an unexpected finding and is consistent with the IHC findings in colorectal and endometrial cancers, in which not all patients with LS have informative tumor screening.28,29 Furthermore, IHC analysis has been shown to be inconsistent in other LS tumors in MSH6 mutation carriers.38,39 In the combined data set of eight patients with ACC with tumor analysis, four patients presented here and four single case reports,34–37 IHC was informative and indicated a lack of expression corresponding with the gene harboring the mutation in six (75%) of eight. MSI analysis was not informative in any of the eight patients.
ACC is a cancer diagnosis with a very low incidence in the general population and a low incidence within our retrospective chart review. Screening for ACC in MMR mutation carriers may not necessarily be recommended, however, in patients with LS, incidentally found adrenal tumors and symptoms and signs of adrenal hormone excess should be clinically evaluated and treated with an increased suspicion for malignancy.
Our study is a prospective, unselected series of patients with ACC. Patients who underwent a genetic evaluation for LS had personal and/or family histories that were suggestive of LS. The prevalence of 3.2% may indeed be an underestimate. Future work to understand the overall prevalence of LS in ACC is needed. This can be accomplished via routine IHC screening of ACC tumors as has been carried out in colorectal cancer tumors and endometrial cancer tumors.28,29
Genetic evaluation for LS should be considered in all patients with ACC and personal or family histories of LS-associated tumors including, but not limited to, young onset, multiple primary colorectal, endometrial, ovarian, gastric, small intestine, urinary tract, renal pelvis, pancreas, and bile duct cancers; glioblastoma multiforme; and sebaceous gland tumors. On the basis of the tumor IHC results, we suggest IHC for the MMR proteins as the primary molecular screening tool for ACC tumors.
Given the relatively few cases of ACC diagnosed annually, approximately 300 cases in the United States, it would be reasonable to consider IHC screening for all patients with ACC to help identify patients with LS, similar to the recommendations for colorectal cancers, endometrial cancers, and sebaceous tumors.31,40,41 In addition, given the prevalence of ACC in this prospective series, ACC should be considered an LS-associated tumor and should be included in the spectrum of cancers in the Amsterdam II clinical diagnostic criteria for LS. The identification of MMR mutations and LS as the underlying cause of the development of ACC would have significant implications on cancer screening as well as aiding in the identification of other relatives at risk for this autosomal dominant cancer predisposition condition.
Appendix
Table A1.
Full Clinical Data of the Five Probands With Adrenocortical Carcinoma and Lynch Syndrome
| Proband | Size (cm) | Grade | Stage | Clinical Features |
Biochemical Evaluations |
||||
|---|---|---|---|---|---|---|---|---|---|
| Hypercortisolism | Androgen Secretion | Other | Hypercortisolism | Androgen Secretion | Other | ||||
| 1 | 14.5 | High | III | No | No | HTN | NA | NA | Normokalemia |
| 2 | 11 | High | III | Possible | No | HTN | Elevated AM cortisol | No | Hypokalemia; normal aldosterone |
| 3 | 7 | Low | II | No | No | NA | No | NA | No |
| 4 | 5 | Low | I | No | No | NA | No | No | No |
| 5 | 14 | NA | III | Yes | Yes | NA | NA | NA | NA |
Abbreviation: AM, morning; HTN, hypertension; NA, not assessed.
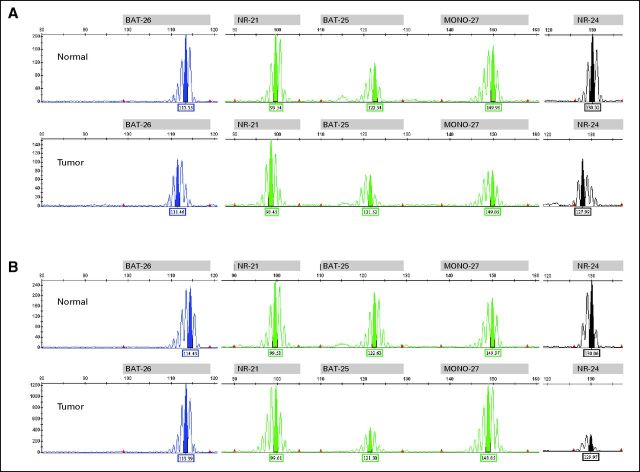
Fig A1.
Microsatellite instability assay profiles of adrenocortical carcinomas of (A) Proband 1 and (B) Proband 2 demonstrating a microsatellite stable pattern in normal versus tumor.
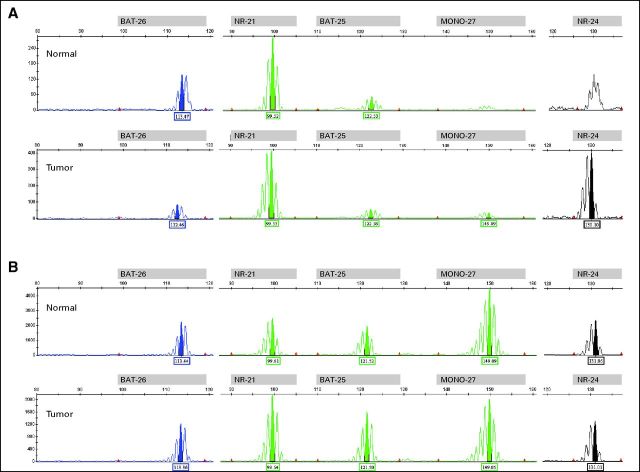
Fig A2.
Microsatellite instability assay profiles of adrenocortical carcinomas of (A) Proband 4 and (B) Proband 3 demonstrating a microsatellite stable pattern in normal and tumor.
Footnotes
Supported by Grants No. P30 CA014089 (S.B.G.), P30 CA046592 (S.B.G.), T32-DK007245 from the National Institutes of Health (T.E.), and K07CA120448-5 from the National Institutes of Health National Cancer Institute (E.M.S.).
Presented at the 15th Annual Meeting of the Collaborative Group of the Americas, on Inherited Colorectal Cancer, Montreal, Canada, October 10-11, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Gary D. Hammer, Atterocor (C), Orphagen (C) Consultant or Advisory Role: Jessica N. Everett, Myriad Genetics (C); Shanna L. Gustafson, Myriad Genetics (C); Stephen B. Gruber, Myriad Genetics (C); Gary D. Hammer, Orphagen (C) Stock Ownership: Gary D. Hammer, Atterocor, Orphagen Honoraria: None Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Victoria M. Raymond, Jessica N. Everett, Tobias Else
Provision of study materials or patients: Victoria M. Raymond, Jessica N. Everett, Shanna L. Gustafson, Chelsy R. Jungbluth, Elena M. Stoffel
Collection and assembly of data: Victoria M. Raymond, Jessica N. Everett, Chelsy R. Jungbluth, Gary D. Hammer, Thomas J. Giordano, Tobias Else
Data analysis and interpretation: Victoria M. Raymond, Jessica N. Everett, Larissa V. Furtado, Shanna L. Gustafson, Stephen B. Gruber, Elena M. Stoffel, Joel K. Greenson, Thomas J. Giordano, Tobias Else
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Dinh TA, Rosner BI, Atwood JC, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarnio M, Mecklin JP, Aaltonen LA, et al. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- 4.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 6.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: A prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: Have we made progress? World J Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro RC, Sandrini F, Figueiredo B, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraumeni JF, Jr, Miller RW. Adrenocortical neoplasms with hemihypertrophy, brain tumors, and other disorders. J Pediatr. 1967;70:129–138. doi: 10.1016/s0022-3476(67)80179-3. [DOI] [PubMed] [Google Scholar]
- 10.Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat. 2003;21:313–320. doi: 10.1002/humu.10185. [DOI] [PubMed] [Google Scholar]
- 11.Varley JM, McGown G, Thorncroft M, et al. Are there low-penetrance TP53 alleles? Evidence from childhood adrenocortical tumors. Am J Hum Genet. 1999;65:995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann LJ, Heinze B, Fassnacht M, et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97:E476–E485. doi: 10.1210/jc.2011-1982. [DOI] [PubMed] [Google Scholar]
- 13.Raymond VM, Else T, Everett JN, et al. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:E119–E125. doi: 10.1210/jc.2012-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldmann J, Fendrich V, Habbe N, et al. Screening of patients with multiple endocrine neoplasia type 1 (MEN-1): A critical analysis of its value. World J Surg. 2009;33:1208–1218. doi: 10.1007/s00268-009-9983-8. [DOI] [PubMed] [Google Scholar]
- 15.Langer P, Cupisti K, Bartsch DK, et al. Adrenal involvement in multiple endocrine neoplasia type 1. World J Surg. 2002;26:891–896. doi: 10.1007/s00268-002-6492-4. [DOI] [PubMed] [Google Scholar]
- 16.Else T. Association of adrenocortical carcinoma with familial cancer susceptibility syndromes. Mol Cell Endocrinol. 2012;351:66–70. doi: 10.1016/j.mce.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chompret A, Abel A, Stoppa-Lyonnet D, et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet. 2001;38:43–47. doi: 10.1136/jmg.38.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacher JW, Flanagan LA, Smalley RL, et al. Development of a fluorescent multiplex assay for detection of MSI-high tumors. Dis Markers. 2004;20:237–250. doi: 10.1155/2004/136734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 20.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 21.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 22.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 23.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa H, Lockman JC, Frankel WL, et al. Mismatch repair gene PMS2: Disease-causing germline mutations are frequent in patients whose tumors stain negative for PMS2 protein, but paralogous genes obscure mutation detection and interpretation. Cancer Res. 2004;64:4721–4727. doi: 10.1158/0008-5472.CAN-03-2879. [DOI] [PubMed] [Google Scholar]
- 25.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. National Comprehensive Cancer Network Web site. http://www.nccn.org.
- 27.Chen S, Wang W, Lee S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296:1479–1487. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 29.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 30.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: Genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: Clinical and pathologic considerations. Cancer Control. 2009;16:14–22. doi: 10.1177/107327480901600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch HT, Shaw MW, Magnuson CW, et al. Hereditary factors in cancer: Study of two large Midwestern kindreds. Arch Intern Med. 1966;117:206–212. [PubMed] [Google Scholar]
- 34.Berends MJ, Cats A, Hollema H, et al. Adrenocortical adenocarcinoma in an MSH2 carrier: Coincidence or causal relation? Hum Pathol. 2000;31:1522–1527. doi: 10.1053/hupa.2000.20409. [DOI] [PubMed] [Google Scholar]
- 35.Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: A report of new cases and review of the literature. Hum Pathol. 2012 doi: 10.1016/j.humpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Medina-Arana V, Delgado L, González L, et al. Adrenocortical carcinoma, an unusual extracolonic tumor associated with Lynch II syndrome. Fam Cancer. 2011;10:265–271. doi: 10.1007/s10689-010-9416-8. [DOI] [PubMed] [Google Scholar]
- 37.Broaddus RR, Lynch PM, Lu KH, et al. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol. 2004;17:981–989. doi: 10.1038/modpathol.3800150. [DOI] [PubMed] [Google Scholar]
- 38.Okkels H, Lindorff-Larsen K, Thorlasius-Ussing O, et al. MSH6 mutations are frequent in hereditary nonpolyposis colorectal cancer families with normal pMSH6 expression as detected by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2012;20:470–477. doi: 10.1097/PAI.0b013e318249739b. [DOI] [PubMed] [Google Scholar]
- 39.Hendriks YM, Wagner A, Morreau H, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: Impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 40.Weissman SM, Burt R, Church J, et al. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J Genet Couns. 2012;21:484–493. doi: 10.1007/s10897-011-9465-7. [DOI] [PubMed] [Google Scholar]
- 41.Plocharczyk EF, Frankel WL, Hampel H, et al. Mismatch repair protein deficiency is common in sebaceous neoplasms and suggests the importance of screening for lynch syndrome. Am J Dermatopathol. 2012 doi: 10.1097/DAD.0b013e31825f7efe. [DOI] [PubMed] [Google Scholar]