Abstract
Muscle metabolic by-products during exercise, such as K+, lactic acid, ATP, H+, and phosphate, are well established to be involved in the reflex cardiovascular response to static muscle contraction. However, the role of muscle reactive oxygen species (ROS), a metabolic by-product during muscle contraction, in the exercise pressor reflex (EPR) has not been investigated in detail. In the present study, we evaluated the role of muscle ROS in the EPR in a decerebrate rat model. We hypothesized that muscle NADPH oxidase-derived ROS contributes to sensitization of the EPR. Thus the rise in blood pressure and heart rate in response to a 30-s static contraction induced by electrical stimulation of L4/L5 ventral roots was compared before and after hindlimb arterial infusion of the redox agents: diethyldithiocarbamate, a superoxide dismutase inhibitor; the superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl (tempol); the free radical scavenger dimethylthiourea; a NADPH oxidase inhibitor, apocynin; and a xanthine oxidase inhibitor, allopurinol. The EPR-induced pressor response was augmented after treatment with diethyldithiocarbamate and was attenuated after treatment with tempol, dimethylthiourea, and apocynin. Treatment with allopurinol did not affect the EPR function. None of the drug's affected the EPR heart rate response. In addition, neither the pressor response to electrical stimulation of the central end of dorsal roots, nor femoral blood flow was affected by any treatment. These data suggest that NADPH oxidase-derived muscle ROS plays an excitatory role in the EPR control of blood pressure.
Keywords: static contraction, sympathetic outflow, blood pressure, decerebration
static exercise evokes sympathetic activation and increases blood pressure and heart rate (HR) (27, 29, 33). The two neural mechanisms that cause the exercise-induced increase in sympathetic discharge are central command (10, 11) and the exercise pressor reflex (EPR); the afferent arm of which consists of group III and IV muscle afferents (8, 20, 21, 29). To a large extent, activation of these afferents occurs in response to local accumulation of metabolic by-products of muscle contraction (1). Group IV afferents, in response to metabolic stimuli, can be activated by the accumulation of metabolic by-products (12, 13, 20, 26, 33). Although group III afferents primarily respond to mechanical stimuli, they can also be sensitized by metabolic by-products of contracting muscle (24, 39).
Skeletal muscle contraction-induced metabolic by-products, such as K+, lactic acid, ATP, H+, and phosphate, have been well documented to be involved in the modulation of the EPR (23, 26). The role of muscle reactive oxygen species (ROS) (5–7, 34) in modulating the EPR had been largely overlooked. In 1996, Bonigut et al. (4) first reported that muscle ROS, mainly via hydroxyl radicals, exerted an inhibitory effect on the EPR in anesthetized cats. However, recent studies have shown that the EPR is compromised by anesthesia, which is especially a concern in rats (42). The latter study showed that, after decerebration, the effects of anesthesia on EPR were largely abolished (42). The role of muscle ROS in the EPR in a decerebrate animal model has not been investigated. In addition, although the study of Bonigut et al. provided important evidence that muscle ROS modulated the EPR during muscle contraction, the source of skeletal muscle ROS remains unclear.
In the present study, using a decerebrate rat model (41), we reevaluated the role of skeletal muscle ROS production in the EPR by modulating the muscle redox state with acute hindlimb intra-arterial infusion of redox agents. Furthermore, we examined the contributions of muscle NADPH oxidase-derived ROS and xanthine oxidase-derived ROS on the EPR.
METHODS
Experiments were performed on male Sprague-Dawley rats weighing 370–420 g. These experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical Preparation
The rats were placed in a plastic box into which isoflurane (Halocarbon Laboratories, River Edge, NY) (5%) and O2 mixture were introduced. After the rats were anesthetized, they were removed from the box, and anesthesia was maintained by placing a nose cone over the face. A jugular vein and the trachea were cannulated. After tracheal cannulation, the lungs were ventilated with an anesthetic mixture of 2–3% isoflurane and oxygen. The right carotid artery was catheterized for measurement of mean arterial pressure (MAP) and HR. Body temperature was maintained between 37 and 38°C by a heating pad. A catheter was placed in the right iliac artery with its tip advanced to the abdominal aortic bifurcation, ensuring that the drugs were delivered to the left hindlimb through the left iliac artery without interrupting flow. The EPR function was compared before and after administration of the drugs. At the end of the experiment, rats were killed by intravenous (IV) administration of pentobarbital sodium (120 mg/kg).
Decerebration
In the present study, rats were decerebrated under isoflurane anesthesia. The decerebration procedure was performed as described by Smith et al. (41). Briefly, Rats were placed in a stereotaxic apparatus (Stoelting, Chicago, IL) and customized spinal frame. The head and pelvis were stabilized. Before decerebration, the lungs were ventilated with the isoflurane-oxygen mixture. Dexamethasone (0.2 mg IV) was given to reduce brain edema and inflammatory responses from the decerebration (46). The remaining intact carotid artery was isolated and ligated to reduce bleeding during decerebration. Subsequently, a portion of bone superior to the central sagittal sinus was removed. The dura mater was breached and reflected. The cerebral cortex was gently aspirated to visualize the superior and inferior colliculi. Using a blunt instrument (5 mm posterior to the bregma), the brain was perpendicularly sectioned precollicularly, and the transected forebrain aspirated. The cranial vault was filled with warm agar (37°C). After the decerebration had been completed, the lungs were ventilated with a mixture of room air and oxygen instead of the anesthesia gas. Arterial blood gas values were measured at regular intervals. Arterial blood gas values were maintained at normal levels for rats (arterial Po2 >85 Torr; arterial Pco2 35–45 Torr; pH 7.3–7.4) by ventilating the lungs mechanically (model 683, Harvard Apparatus) and by supplementing the inspired gas with O2. A minimum recovery period of 1.25 h was employed postdecerebration before data collection began.
Procedures for Static Contraction
In order to activate both mechanically and metabolically sensitive skeletal muscle afferent fibers, static hindlimb contraction was induced using electrical stimulation of ventral roots (41). A laminectomy exposing the lower lumbar portions of the spinal cord (L2–L6) was performed. The dura of the cord was cut and reflected, allowing visual identification of the L4–L6 spinal roots. The dorsal and ventral roots of L4 and L5 were carefully separated. The ventral roots were sectioned, and the cut peripheral ends were positioned on insulated bipolar platinum electrodes. The exposed neural tissue was covered in a pool of warm mineral oil (37°C). The animals were secured within the spinal adaptor (Stoelting, Wood Dale, IL) by clamps placed on rostral lumbar vertebrae. Furthermore, the pelvis was stabilized with steel posts within the frame, and the hindlimb containing the triceps surae muscles under study was fixed in one position with clamps. The angle of the hip and knee was 120 and 80°, respectively. The calcaneal bone was sectioned, and the Achilles' tendon connected to a force transducer (model FT-03, Grass Instruments, West Warwick, RI) for the measurement of muscle tension. Electrical stimulation was performed using a Grass Instruments S88 stimulator. Electrically induced static muscle contraction of the triceps surae was performed by stimulating the L4/L5 ventral roots for 30–35 s. Constant-current stimulation was used at three times motor threshold (defined as the minimum current required to produce a muscle twitch), with a pulse duration of 0.1 ms at 40 Hz (41). In the experiments, all muscles of the hindlimb-undergoing study were denervated, except for the triceps surae muscle. At the end of this experiment, the neuromuscular blocking agent pancuronium bromide (200 μg/kg) was administered IV. Electrical activation of the ventral roots was repeated using the stimulus parameters described previously. This maneuver was instituted to eliminate the possibility that cardiovascular responses were mediated by direct activation of sensory afferent fibers during stimulation protocols.
Electrical Stimulation of the Central End of L4/L5 Dorsal Roots
In the acute animal experiments, hindlimb intra-arterial infusion of drugs may recirculate and influence the EPR by a central or efferent mechanism. Therefore, an independent experiment was designed to test this possibility by stimulating the central end of left L4/L5 dorsal roots for 20 s (frequency: 5–7 Hz; pulse duration, 0.1 ms; voltage: 6 V). These parameters achieved a similar pressor response as static contraction. We compared the pressor response to electrical stimulation of dorsal roots before and after hindlimb arterial infusion of redox agents. If treatment with these drugs had no effect on the pressor response induced by stimulation of the central end of the dorsal root, this would suggest that these drugs only modulate the EPR via affecting muscle afferents.
Measurement of Femoral Blood Flow
In an independent experiment, left femoral blood flow will be measured to determine whether the drugs used in these experiments modulate the EPR via an effect on blood flow. After decerebration, the left femoral artery will be isolated. A perivascular flow probe (0.5 VB587; Transonic Systems), connected to a flow meter (T106, small-animal flow meter; Transonic Systems), was placed around the left femoral artery (free perfusion) to allow continuous recording of femoral blood flow during infusion of drugs.
Drugs and Injected Solutions
Hindlimb infusion of the superoxide dismutase (SOD) inhibitor diethyldithiocarbamate (DETC) was expected to increase superoxide concentration in skeletal muscle, whereas the SOD mimetic 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl (tempol) or the free radical scavenger dimethylthiourea (DMTU) was expected to decrease muscle ROS. The NADPH oxidase inhibitor apocynin was used to decrease NADPH oxidase-derived ROS in skeletal muscle. Similarly, the xanthine oxidase inhibitor allopurinol was used to reduce xanthine oxidase-derived ROS production in skeletal muscle.
All drugs were purchased from Fisher Scientific. DETC, tempol, and DMTU were dissolved in saline before use. Apocynin was dissolved in 95% alcohol and diluted in saline. Allopurinol was dissolved in 1 N NaOH and then diluted in normal saline. The pH of the allopurinol solution was adjusted to 7.4 by using 1 N HCl.
All of the drugs above were administrated via hindlimb intra-arterial infusion using a syringe pump delivery system (model 310; Stoelting, Wood Dale, IL). The infusion volume was 0.15–0.20 ml for 8–10 min. Three doses of DETC or tempol (1, 5, and 10 mg/kg) were used to determine the dose-dependent effects of the drugs on the EPR. The dose of DMTU (10 mg/kg) was determined based on a previous study (4). Two doses of apocynin and allopurinol (1 and 10 mg/kg) were used for determining dose-response effects. Saline was administrated as the control of the drugs.
Experiment Protocols
Protocol 1.
In this protocol, we tested the hypothesis that muscle ROS production modulated the EPR. Three redox agents (DETC, tempol, and DMTU) were used to modulate the hindlimb muscle redox state via arterial infusion. The effect of each drug on the EPR was investigated in an independent group (n = 6). In addition, to determine whether each drug affects the EPR via a central mechanism, we employed two control groups for each drug (n = 4–6), in which 1) we compared the effect of each drug on the pressor response to direct stimulation of the central end of the dorsal root; and 2) we investigated the effect of bolus IV administration of redox drugs on the EPR. Finally, to determine whether the drugs used in this protocol modulate the EPR via an effect on blood flow, we measured femoral blood flow and femoral vascular conductance before and after hindlimb arterial infusion of drugs (n = 4).
Protocol 2.
In this protocol, we determined whether NADPH oxidase-derived ROS or xanthine oxidase-derived ROS was involved in the modulation of the EPR. Two redox agents (apocynin and allopurinol) were used to inhibit the activity of muscle NADPH oxidase or xanthine oxidase. We compared the EPR function before and after administration of these drugs. Control groups, similar to those described in protocol 1 were used to determine whether apocynin or allopurinol affects the EPR via a central mechanism. In order to exclude the possibility that the drugs modulate the EPR by affecting blood flow, femoral blood flow and femoral vascular conductance were measured before and after the administration of these two agents.
Measurement of Superoxide Production and NAD(P)H Oxidase Activity in Skeletal Muscle
In an independent in vitro experiment, using the lucigenin-enhanced chemiluminescence method (17), we investigated the effects of redox agents on basal superoxide production and the activity of NADPH oxidase in triceps surae muscle. The rats were euthanized by an overdose of pentobarbital sodium (120 mg/kg). Muscles were immediately removed and immersed in cold Krebs-HEPES buffer containing the following (in mM): 99 NaCl, 4.7 KCl, 1.9 CaCl2, 1.2 MgSO4, 1 K2HPO4, 25 NaHCO3, 10 glucose, and 10 HEPES (pH 7.4) on ice. The muscle mass was excised into small strips. Each strip was placed in a polypropylene tub containing 5 μmol/l lucigenin in a preheated Krebs-HEPES buffer (37°C) and then read in a Sirius luminometer (FB12, Berthold, Pforzheim, Germany) in a dark room. The chemiluminescence was reported by relative light units at 30-s intervals for 5 min. Data were corrected for background activity and normalized to tissue weight. Superoxide production was measured under the conditions of preincubation of muscle with a SOD inhibitor DETC (1 mM) or the SOD mimetic tempol (1 mM), or the free radical scavenger DMTU (1 mM) for 30 min.
NADPH (10 μmol/l) was used to stimulate NADPH oxidase. To determine the contribution of NADPH oxidase to superoxide production, muscle was preincubated for 30 min with one of the following agents: apocynin (1 mM), allopurinol (300 μM), tempol (1 mM), or DMTU (1 mM). Thus NAD(P)H-dependent superoxide generation represents NAD(P)H oxidase activity (25).
Data Acquisition and Statistical Analysis
MAP, HR, blood flow, and muscle tension were acquired using PowerLab software (AD Instruments). Baseline values were determined by analyzing at least 30 s of the data immediately before the interventions (i.e., arterial injections or ventral root stimulation). The peak response was determined in the period of the greatest change from baseline. MAP is expressed in millimeters of mercury, and HR in beats per minute. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilograms times seconds. Peak developed tension was calculated by subtracting the resting tension from the peak tension and is expressed in grams. All values are expressed as means ± SE. Differences between groups were determined by a two-way ANOVA followed by the Tukey post hoc test. Changes in MAP, HR, blood flow, TTI, and peak developed tension before and after arterial administration of chemicals were determined by paired t-test. P < 0.05 was considered statistically significant.
RESULTS
After decerebration, baseline MAP and HR were maintained at physiological levels in all animals (102.7 ± 3.1 mmHg; 367.7 ± 10.1 beats/min; n = 66). Baseline values for MAP and HR were not significantly affected by hindlimb arterial infusion of any agents. Hindlimb arterial infusion of low and middle doses of DETC and tempol (1 and 5 mg/kg) did not affect baseline MAP and HR, whereas treatment with high doses of tempol and DETC (10 mg/kg) slightly but significantly changed the baseline MAP (DETC: 101.3 ± 3.4 vs. 107.2 ± 3.6 mmHg, n = 6, P < 0.05; tempol: 103.8 ± 2.7 vs. 98.3 ± 2.8 mmHg; n = 6, P < 0.05) without affecting the baseline HR.
Effects of DETC
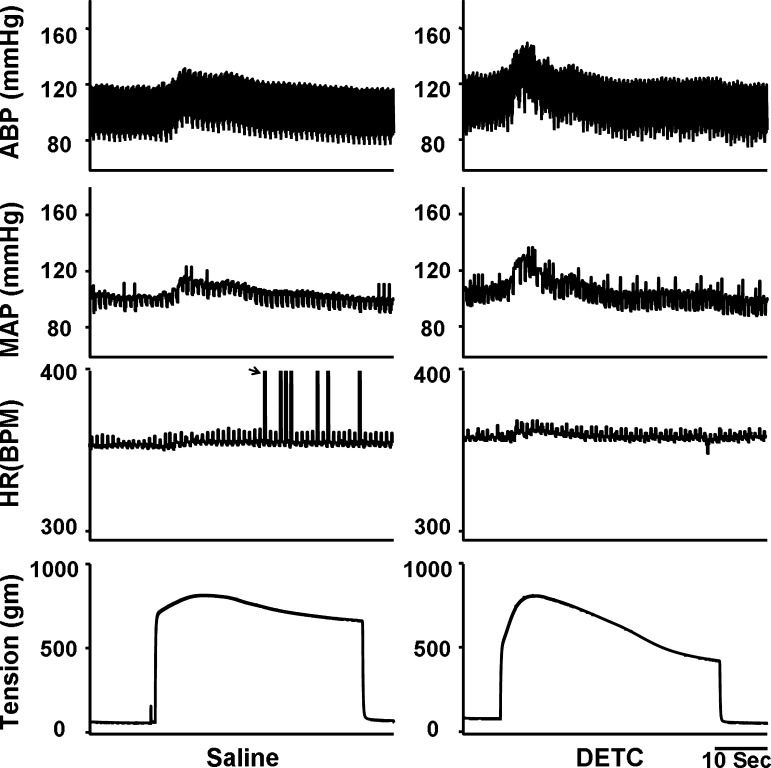
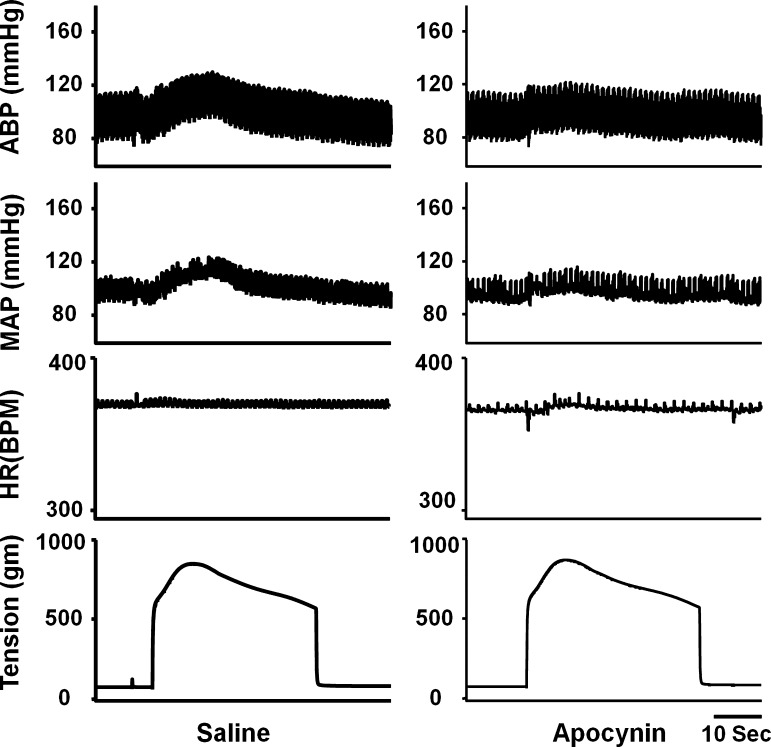
Figure 1 shows a representive recording that hindlimb arterial infusion of a SOD inhibitor DETC [10 mg/kg, 0.15 ml/10 min, intra-arterially (IA)] dramatically enhanced the pressor response to static contraction induced by electrical stimulation of L4/L5 ventral roots in a decerebrate rat. As shown in Fig. 2A, the effect of DETC on the EPR-evoked pressor response was dose dependent. Treatment with DETC did not alter the HR response to static contraction (Fig. 2A). IV administration of DETC (10 mg/kg, 0.15 ml) slightly but significantly enhanced the EPR response to static contraction (18.5 ± 0.9 vs. 23.3 ± 1.5 mmHg, saline vs. DETC, n = 5, P < 0.05). However, compared with IA administration at the same dose, the effect was weaker (+5 vs. +10 mmHg, IV vs. IA), indicating that a local effect of DETC existed during IA administration.
Fig. 1.
Original record of the exercise pressor reflex (EPR) control of blood pressure and heart rate (HR) in response to static contraction induced by electrical stimulation of L4/L5 ventral roots before and after hindlimb arterial infusion of diethyldithiocarbamate (DETC; 10 mg/kg, 0.15 ml, 10 min) in decerebrate rats. In HR panel, black arrow points to the artifacts. ABP, arterial blood pressure; MAP, mean arterial pressure; BPM, beats/min.
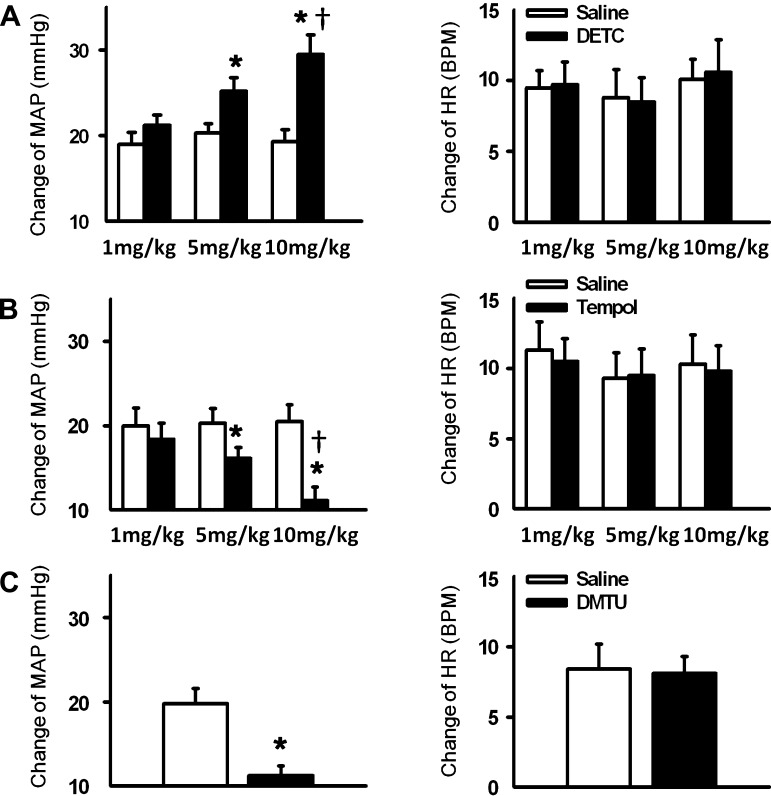
Fig. 2.
Mean changes in MAP and HR in response to 30-s static contraction before and after hindlimb arterial infusion of DETC (A), 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl (tempol; B), and dimethylthiourea (DMTU; C). Saline was infused as the control. Values are means ± SE; n = 6 in each group. *P < 0.05 vs. saline; †P < 0.05 vs. the lowest dose group (1 mg/kg).
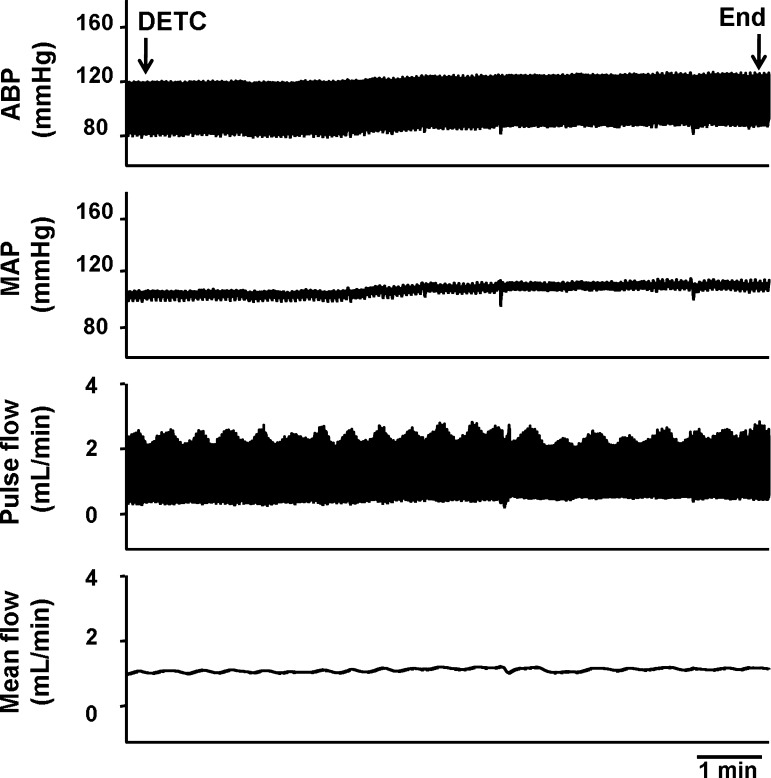
In four rats, left femoral blood flow, as well as blood pressure and HR, were measured before and during muscle contraction in the absence and presence of DETC (10 mg/kg). Although infusion of DETC (10 mg/kg) slightly increased baseline MAP, there was no effect on hindlimb femoral blood flow and femoral vascular conductance at rest or during contraction (Fig. 3 and Table 1), indicating that the effect of DETC on the EPR is not related to changes in hindlimb blood flow.
Fig. 3.
Original record showing that intra-arterial infusion of DETC (10 mg/kg) slightly increased blood pressure without affecting femoral blood flow in a decerebrate rat.
Table 1.
The effect of the redox drugs (10 mg/kg) on the femoral blood flow and femoral vessel conductance at rest and during contraction in decerebrate rats
| FBF, ml/min |
FVC, ml·min−1·mmHg−1 ×10−3 | |||
|---|---|---|---|---|
| At rest | Contraction | At rest | Contraction | |
| DETC | ||||
| Before | 1.8±0.4 | 3.2±0.6* | 17.7±0.4 | 26.9±4.9* |
| After | 1.8±0.4 | 3.2±0.7* | 17.2±4.0 | 24.0±5.3* |
| Tempol | ||||
| Before | 1.5±0.3 | 2.8±0.8* | 16.1±4.0 | 24.5±7.6* |
| After | 1.6±0.4 | 2.9±0.6* | 17.9±5.5 | 28.4±7.8*† |
| DMTU | ||||
| Before | 1.6±0.3 | 3.1±0.4* | 15.7±3.7 | 26.0±4.4* |
| After | 1.6±0.4 | 3.1±0.4* | 15.3±4.2 | 27.0±4.1* |
| Apocynin | ||||
| Before | 1.7±0.4 | 3.0±0.6* | 16.3±3.2 | 24.8±4.8* |
| After | 1.7±0.4 | 3.0±0.7* | 16.2±2.8 | 26.1±5.1* |
Values are means ± SE; n = 4 rats in each group. FBF, femoral blood flow; FVC, femoral vessel conductance; DETC, diethyldithiocarbamate; tempol, 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl; DMTU, dimethylthiourea.
P < 0.05 vs. at rest;
P < 0.05 vs. after.
In four rats, pretreatment with DETC (10 mg/kg) did not increase the pressor response induced by the stimulation of the central end of L4/L5 dorsal roots (20.8 ± 2.6 vs. 21.3 ± 2.8 mmHg, saline vs. DETC, n = 4, P > 0.05), indicating that the effect of DETC on the EPR in decerebrate rats is unlikely to be completely mediated by a central mechanism.
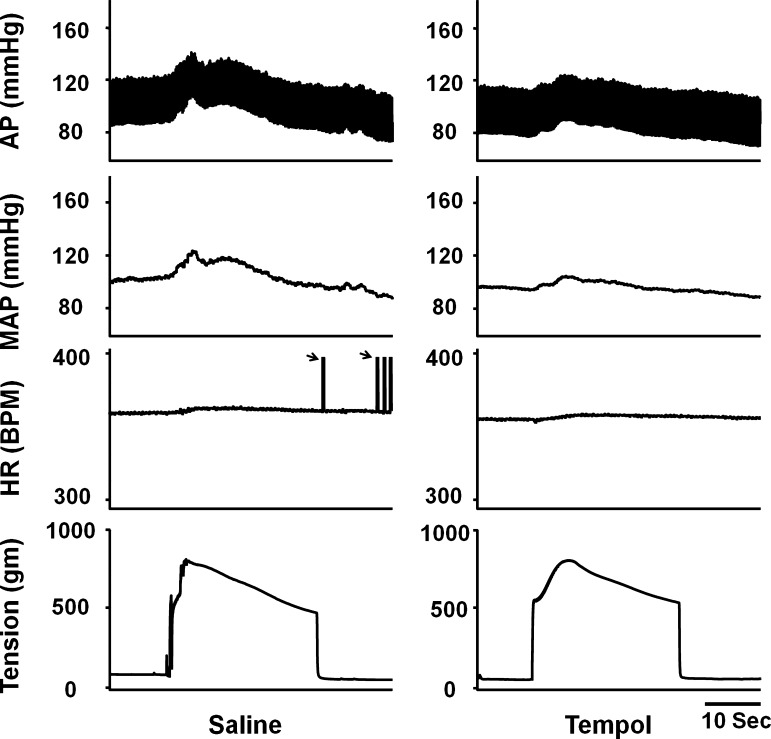
Effects of Tempol and DMTU
A representative tracing (Fig. 4) shows that hindlimb arterial infusion of the SOD mimetic tempol (10 mg/kg) attenuated the EPR-evoked pressor response to static contraction. Figure 2B shows the dose-dependent effect of tempol on the EPR-evoked pressor response. Treatment with each dose of tempol had no effect on the HR response to static contraction. Using another free radical scavenger, DMTU, a similar effect as with tempol on the EPR was observed (Fig. 2C). In addition, IV administration of both tempol and DMTU (10 mg/kg, 0.15 ml) slightly attenuated the EPR-evoked pressor response (21.1 ± 2.0 vs. 16.2 ± 1.0 mmHg, saline vs. tempol, n = 5, P < 0.05; 19.7 ± 1.3 vs. 15.3 ± 0.6 mmHg, saline vs. DMTU, n = 6, P < 0.05).
Fig. 4.
Original record of EPR control of blood pressure and HR in response to static contraction induced by electrical stimulation of L4/L5 ventral roots before and after hindlimb arterial infusion of tempol (10 mg/kg, 0.15 ml, 10 min) in decerebrate rats. In HR panel, black arrow points to the artifacts. AP, arterial pressure.
Both tempol and DMTU did not change the pressor response to stimulation of the central end of L4/L5 dorsal roots (22.3 ± 2.3 vs. 22.8 ± 2.7 mmHg, saline vs. tempol, n = 4; 23.3 ± 3.0 vs. 23.8 ± 3.7 mmHg, saline vs. DMTU, n = 4), indicating that the effect of tempol and DMTU on the EPR is not likely via a central mechanism. Hindlimb arterial infusion of tempol (10 mg/kg) did not change femoral blood flow at rest and during contraction, but slightly and significantly increased femoral vascular conductance during contraction (Table 1). DMTU (10 mg/kg) had no effect on femoral blood flow and femoral vessel conductance at rest or during contraction (Table 1), suggesting that it is unlikely that tempol and DMTU attenuated the EPR via affecting hindlimb blood flow.
Effects of Apocynin and Allopurinol
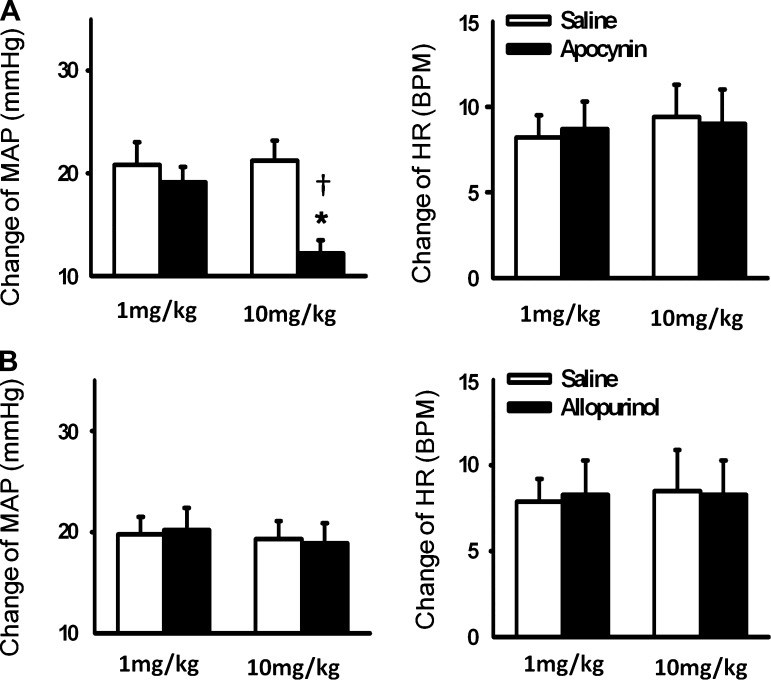
We investigated the effects of NADPH oxidase-derived ROS and xanthine oxidase-derived ROS on EPR function. Hindlimb arterial infusion of the NADPH oxidase inhibitor apocynin (10 mg/kg) significantly reduced the EPR-induced pressor effect compared with saline pretreatment (Figs. 5 and 6A). However, pretreatment with a xanthine oxidase inhibitor allopurinol did not affect EPR function (Fig. 6B). Both apocynin and allopurinol pretreatment did not alter the EPR-induced HR response (Fig. 6). IV administration of apocynin (10 mg/kg, 0.15 ml) tended to attenuate the EPR-evoked pressor response, but did not reach a significant difference (20.1 ± 1.8 vs. 17.1 ± 1.5 mmHg, saline vs. apocynin, n = 5, P > 0.05).
Fig. 5.
Original record of EPR control of blood pressure and HR in response to static contraction induced by electrical stimulation of L4/L5 ventral roots before and after hindlimb arterial infusion of apocynin (10 mg/kg, 0.15 ml, 10 min) in a decerebrate rat.
Fig. 6.
Mean changes in MAP and HR in response to a 30-s static contraction before and after hindlimb arterial infusion of apocynin (A) and allopurinol (B). Saline was infused as the control. Values are means ± SE; n = 6 in each group. *P < 0.05 vs. saline; †P < 0.05 vs. the lowest dose group (1 mg/kg).
Pretreatment with apocynin did not affect femoral blood flow or femoral vascular conductance at rest and during contraction (Table 1) or the central pressor effect induced by electrical stimulation of L4/L5 dorsal roots (20.5 ± 2.8 vs. 21.0 ± 2.9 mmHg, saline vs. apocynin, n = 4), excluding the possibility of a blood flow dependency or central mechanism.
Muscle Tension Developed By Static Contraction
In the present study, muscle peak developed tension induced by static contraction ranged from 700 to 750 g in all groups. There was no significant difference in TTI before and after treatment with redox agents in all groups (Table 2), indicating that the acute administration of the redox drugs does not affect the muscle contractility. In all groups, the IV administration of neuromuscular blocking agent pancuronium bromide abolished muscle contraction induced by electrical activation of ventral root as well as the contraction-induced pressor response, which eliminates the possibility that cardiovascular responses were mediated by direct activation of sensory afferent fibers during stimulation protocols.
Table 2.
Tension-time indexes for static contraction before and after administration of the redox drugs in decerebrate rats
| Group | n | TTI, kg ×s |
|
|---|---|---|---|
| Before | After | ||
| DETC | |||
| 1 mg/kg | 6 | 15.2±2.1 | 15.1±1.9 |
| 5 mg/kg | 6 | 15.6±2.4 | 15.2±2.1 |
| 10 mg/kg | 6 | 15.0±1.5 | 14.6±1.3 |
| Tempol | |||
| 1 mg/kg | 6 | 15.3±2.2 | 15.3±2.3 |
| 5 mg/kg | 6 | 14.8±1.9 | 15.1±2.0 |
| 10 mg/kg | 6 | 15.4±2.1 | 15.6±2.3 |
| DMTU | 6 | 15.2±2.2 | 15.3±2.4 |
| Apocynin | |||
| 1 mg/kg | 6 | 15.8±2.7 | 15.8±2.7 |
| 10 mg/kg | 6 | 15.2±2.6 | 15.3±2.7 |
| Allopurinol | |||
| 1 mg/kg | 6 | 15.8±3.1 | 15.6±2.9 |
| 10 mg/kg | 6 | 15.1±2.3 | 15.2±2.5 |
Values are means ± SE; n, no. of rats. TTI, tension-time index. There were no significant differences (P > 0.05) between means in any one group.
Effect of Drugs on Muscle ROS Metabolism and NADPH Oxidase Activity
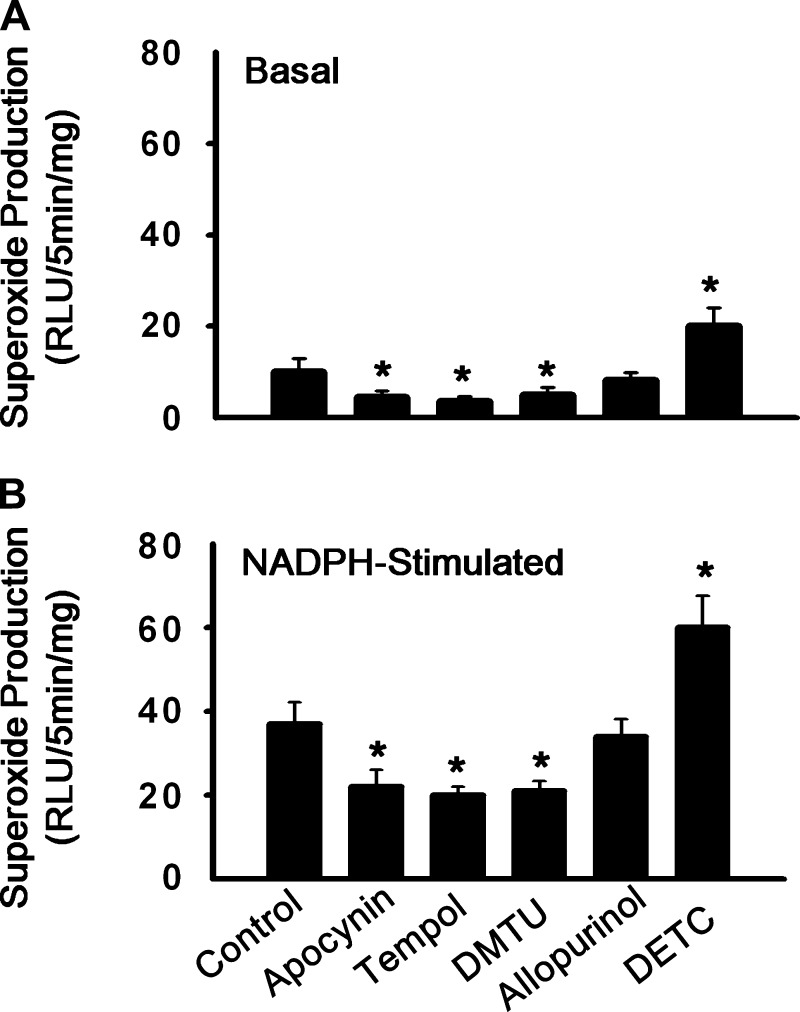
In an independent in vitro experiment, we investigated the effects of the redox drugs on the basal muscle superoxide production and NADPH-stimulated muscle superoxide production. As shown in Fig. 7A, incubation with DETC significantly increased the basal muscle superoxide production, whereas treatment with tempol or DMTU or apocynin significantly decreased the basal muscle superoxide production. Treatment with allopurinol had little effect on the basal muscle superoxide production. Incubation of apocynin but not allopurinol significantly decreased the NADPH-stimulated muscle ROS production (Fig. 7B), indicating that apocynin decreased the muscle NAPDH oxidase activity. Incubation of tempol or DMTU also reduced the NADPH-stimulated muscle superoxide production (Fig. 7B). In contrast, incubation of DETC enhanced the NADPH-stimulated muscle superoxide production (Fig. 7B). The data above confirmed that the redox drugs affected muscle ROS metabolism as expected.
Fig. 7.
Influences of the redox agents (DETC, tempol, DMTU, apocynin, and allopurinol) on superoxide production in the absence (basal) and presence of NADPH (10 μmol/l) in the triceps surae muscle of normal rats. RLU, relative light units. Values are means ± SE; n = 8. *P < 0.05 vs. control.
DISCUSSION
The primary findings of the present study demonstrated 1) that muscle ROS production exerts an excitatory role in the EPR response to a 30-s static contraction induced by electrical stimulation of L4/L5 ventral roots in decerebrate rats; and 2) that the NADPH oxidase-derived ROS production contributes to the modulation of the EPR function.
The Role of Skeletal Muscle ROS Production in the EPR
Strenuous exercise increases free radical content in skeletal muscle. This phenomenon became widely appreciated after Davies et al. (9) and Jackson et al. (16) used electron paramagnetic resonance spectroscopy to demonstrate increased free radicals after exercise in animal and human experiments. Initially, muscle-derived radicals were thought only to be a contraction-induced metabolic by-product without any biological effects. Subsequent studies demonstrated that muscle-derived ROS are involved in the regulation of muscle contraction-evoked force production (34–38). At the present time, the role of muscle ROS in muscle fatigue has been well established. The above studies raise an important question: Is the role of muscle ROS only limited to the mechanism of muscle fatigue? In the present study, we evaluated the potential role of muscle ROS production on the EPR in a decerebrate rat model. We found that 1) hindlimb arterial infusion of the SOD inhibitor DETC, which is believed to increase superoxide anions in skeletal muscle, dose-dependently increased the blood pressure response after evoking the EPR. 2) In contrast, pretreatment with free radical scavengers (tempol and DMTU) reduced EPR function. 3) Compared with IV, IA administration exhibited a stronger effect on the EPR, indicating that the local effects of redox drugs on the EPR existed during IA administration. 4) Neither the pressor response to central dorsal stimulation, nor blood flow was affected by IA administration of these drugs, indicating the unlikely possibility that these drugs affected the EPR function exclusively via a central or blood flow mechanism. Taken together, the present study suggests that muscle ROS production plays an excitatory role in the EPR response.
Surprisingly, our finding is contrary to that of Bonigut et al. (4), who reported an inhibitory effect of muscle ROS production on the EPR response to 5-min intermittent static contraction induced by stimulation of the intact sciatic nerve in anesthetized cats. On closer inspection, one possible explanation for the discrepant results might be different experiment protocols between the two studies. First, there are differences in anesthesia and animal species between the two studies. However, the effect of anesthesia on EPR in cats was weaker compared with that in rats (15, 41). Therefore, anesthesia is most likely not the only explanation for these differences. In our opinion, the discrepancy is most likely due to the difference in experimental protocol, such as the time used to active the EPR, between the two studies. In the present study, the EPR was induced by a 30-s static contraction method, which activates both mechanically and metabolically sensitive skeletal muscle afferent fibers (40, 41, 43). However, in the experiment performed by Bonigut et al. (4), a 5-min intermittent static contraction protocol was used to induce the EPR. The 5-min intermittent static contraction protocol may produce much more metabolic by-products than a 30-s static contraction, which may lead to different results due to 1) a greater amount of muscle ROS; and 2) a predominant activation of the group IV afferents to induce the metaboreflex. Previous studies showed that mild or moderate increase in muscle ROS played an excitatory role in muscle force production, whereas high levels of muscle ROS (oxidative stress) promote contractile dysfunction, resulting in muscle weakness and fatigue (34–36, 38). Therefore, it is reasonable to speculate that mild or moderate increases in muscle ROS might enhance EPR function, whereas high levels of muscle ROS inhibit EPR function. Nonetheless, because we lack reliable data to quantify in vivo muscle ROS level during contraction, the speculation remains to be tested in future work. In addition, during 5-min contraction, excessive accumulation of muscle metabolic by-products might predominantly activate the metaboreflex, whereas a 30-s contraction might activate both mechano- and metaboreflex. Differential activation of EPR during the two protocols might also contribute to the discrepant findings.
The mechanisms by which muscle ROS production modulates the EPR remains to be determined. Contraction-induced metabolites (e.g., ATP, lactic acid) may either directly activate or indirectly sensitize metabo- and mechanoreceptors in muscle (24, 39). Therefore, we speculated that muscle ROS affects the EPR via a similar mechanism. Unlike other metabolites, muscle ROS has no receptors. What is the mechanism by which muscle ROS affects the EPR? Muscle ROS can act on muscle redox-sensitive targets (usually cysteine sites) to modulate the force production (30). Similarly, it is reasonable to speculate that ROS might also act on the mechano- or/and metaboreceptor redox-sensitive target sites to active or sensitize these receptors. The cellular mechanism by which ROS sensitize EPR remains to be determined.
The Sources of Muscle ROS Involved in EPR Activation
Under normal physiological conditions, the generation of skeletal muscle ROS at rest is low. However, during aerobic contractions, skeletal muscle produces significant amounts of superoxide anion due to its increase in oxygen uptake that reaches up to 90 times the values obtained at rest (18, 19, 22, 28). Although increased production of ROS during muscle activity is generally accepted, the source of the ROS remains unclear. Mitochondria, nonphagocytic NAD(P)H oxidase, xanthine oxidase, and lipoxygenases are several potential sources of muscle ROS production at rest and during exercise (2, 3, 14, 17, 44). In the present study, we showed that an NADPH oxidase inhibitor, apocynin, attenuated the EPR, suggesting that the NADPH-derived ROS in skeletal muscle plays an important role in modulating the EPR. We also showed that a xanthine oxidase inhibitor, allopurinol, had no effect on EPR function, indicating that xanthine oxidase is not a main source of the ROS that modulated the ERP in this protocol. However, because the present study did not verify that a large enough volume of allopurinol remained in the hindlimb to have an effect on muscle afferents, we cannot completely rule out the possibility that xanthine oxidase-derived ROS is involved in the EPR modulation. In addition, we did not exclude the possibility that other sources of muscle ROS (i.e., mitochondria) might also be involved in the ROS-mediated EPR sensitization.
The Effect of Redox Agents on Muscle ROS Metabolism
In the present study, we confirmed whether the redox drugs used did indeed affect muscle ROS metabolism as expected. Our data showed that these agents modulated the basal ROS production and NADPH oxidase activity in vitro. However, the inability to measure ROS production in vivo remains a limitation of this study.
The present study shows that muscle ROS exerts a tonic effect on the EPR in normal rats. However, one should also consider the role this mechanism may play in pathological conditions, such as chronic heart failure (CHF) and hypertension. Previous studies have well established that the EPR is exaggerated in the CHF state (31, 32, 40, 42, 43). It has also been reported that there is increased skeletal muscle ROS production in CHF mice (47). Thomas and colleagues (45, 48) further provided evidence that skeletal muscle oxidative stress in the CHF and hypertensive states contribute to impaired sympathetic vasoconstriction. It is still not completely clear from this or previous work if there is a cause-effect relationship between the exaggerated EPR and increased muscle ROS production in CHF. This issue requires further investigation.
Limitations
A major concern in the present study is that IA infusion of redox drugs can potentially enter the systemic circulation and affect the central nervous system or efferent trafficking. Therefore, we compared the effects of IA and IV administration on EPR function. The evidence that the effect of IA administration on EPR function is stronger than following IV administration at the same dose supports the view that IA administration affects the EPR via a peripheral mechanism. Nonetheless, there remains a possibility that central effects of these drugs exist and cannot be absolutely excluded. In another control experiment, the pressor response to stimulation of the central end of dorsal roots was compared before and after drug administration to rule out the possibility that the drugs affect EPR function via central or efferent mechanisms. Although dorsal root stimulation is helpful in the present study, it is possible that the dorsal root stimulation might activate not only muscle afferents (groups III and IV), but also other fiber types arising from joint, bone, and skin. It would be useful to directly record the afferent input before and after drug administration to address this issue definitely.
Summary
In conclusion, we have shown that muscle ROS plays an excitatory role in the EPR response to a 30-s static contraction in decerebrate rats. The NADPH oxidase-derived ROS in skeletal muscle plays an important role in modulating the EPR function.
Acknowledgments
The authors thank Richard Robinson for technical assistance.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem 253: 307–312, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87: 465–470, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bonigut S, Stebbins CL, Longhurst JC. Reactive oxygen species modify reflex cardiovascular responses to static contraction. J Appl Physiol 81: 1207–1212, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappa B activation. J Physiol 586: 3979–3990, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clanton TL Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol 102: 2379–2388, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Close GL, Jackson MJ. The use of in vivo microdialysis techniques to detect extracellular ROS in resting and contracting skeletal muscle. Methods Mol Biol 477: 123–136, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982. [DOI] [PubMed] [Google Scholar]
- 10.Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96: 1166–1169, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidalgo C, Sanchez G, Barrientos G, Racena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem 281: 26473–26482, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto GA, Botterman BR. Peripheral factors influencing expression of pressor reflex evoked by muscular contraction. J Appl Physiol 58: 1676–1682, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta 847: 185–190, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 165: 412–418, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Ji LL Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 18: 1079–1086, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci 854: 102–117, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol 549: 645–652, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem 278: 12094–12100, 2003. [DOI] [PubMed] [Google Scholar]
- 26.MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K(+), lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Mastaloudis A, Yu TW, O'Donnell RP, Frei B, Dashwood RH, Traber MG. Endurance exercise results in DNA damage as detected by the comet assay. Free Radic Biol Med 36: 966–975, 2004. [DOI] [PubMed] [Google Scholar]
- 29.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol 97: 1477–1485, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol 90: 1714–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 34.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid MB Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol 90: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Reid MB Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc 33: 371–376, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Reid MB Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med 44: 169–179, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol 75: 1081–1087, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Stofan DA, Callahan LA, DiMARCOAF, Nethery DE, Supinski GS. Modulation of release of reactive oxygen species by the contracting diaphragm. Am J Respir Crit Care Med 161: 891–898, 2000. [PubMed] [Google Scholar]
- 45.Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111: 178–186, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsui H, Ide T, Hayashidani S, Suematsu N, Shiomi T, Wen J, Nakamura K, Ichikawa K, Utsumi H, Takeshita A. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of heart failure. Circulation 104: 134–136, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W, Thomas GD. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension 48: 637–643, 2006. [DOI] [PubMed] [Google Scholar]