Abstract
Background
Botulinum neurotoxin (BoNT) injection into the bladder wall has been shown to be an effective alternative to anticholinergic (antimuscarinic) medications and more invasive surgery in those with multiple sclerosis and spinal cord injury with neurogenic detrusor overactivity (NDO) and urinary incontinence who are not tolerating anticholinergic medications. In August 2011, Botox® (onabotulinumtoxinA) received Food and Drug Administration (FDA) approval for this use. Clinically, intradetrusor injection of BoNT has been found to decrease urinary incontinence and improve quality of life. Its impact on urodynamic parameters is an increase in the maximum cystometric (bladder) capacity and decrease in the maximum detrusor pressures. The most common side effects are urinary tract infections and urinary retention. There have been rare reports and a black box warning of distant spread of BoNT. BoNT has gained popularity because of its effectiveness and long duration of action, relative ease of administration, easy learning curve, reproducibility of results on repeated administration, and low incidence of complications.
Objective
To discuss the structure and function, mechanisms of action, clinical and urodynamic studies, injection technique, potential beneficial and adverse effects, and potential areas of research of BoNT.
Methods
Literature search focused on botulinum toxin in MEDLINE/PubMed. Search terms included botulinum toxin, neurogenic bladder, NDO, botox bladder, botox spinal cord injury, botox, FDA, botox side effects. All papers identified were English language, full-text papers. In addition, English abstracts of non-English papers were noted. The reference list of identified articles was also searched for further papers.
Conclusion
Botulinum toxin is an alternative treatment for individuals with NDO who fail to tolerate anticholinergic medications. Its popularity has increased because of the literature, which has supported its effectiveness, safety, easy use and learning curve, reproducibility of results on repeated use, and recent FDA approval of Botox® (onabotulinumtoxinA).
Keywords: Neurogenic bladder, Spinal cord injuries, Multiple sclerosis, Botulinum neurotoxin, Detrusor overactivity, Anticholinergic drugs, Detrusor sphincter dyssynergia
Introduction
Individuals with overactive bladder (OAB) often complain of one or more of the following problems: urgency (with or without urgency incontinence), urinary frequency, and nocturia. This may be from a neurogenic or idiopathic cause. Urodynamic studies confirm that a person's symptoms are due to detrusor overactivity (DO). The term “neurogenic detrusor overactivity (NDO)” is used to describe a urodynamic finding which is characterized by involuntary detrusor (bladder) contractions during the filling phase, which may be spontaneous or provoked due to a relevant neurological condition.1,2
It is important that individuals with NDO maintain low bladder pressure during bladder storage and voiding with little to no involuntary bladder contractions. Failure to maintain low bladder pressures may lead to upper tract complications due to stasis and poor drainage of the upper tracts. High detrusor pressures may also exacerbate vesico-ureteral reflux. NDO may also cause lower urinary tract complications such as recurrent urinary tract infections (UTIs), bladder stones, fibrosis, trabeculation and a loss of bladder wall compliance, and autonomic dysreflexia.3 In addition, urinary incontinence resulting from NDO has been found to have a significant negative impact on sexuality, cause embarrassment, and decrease a person's quality of life (QoL). In one study, 35.3% of individuals with SCI reported that bladder/bowel issues sometimes or did prevent them from sexual activity. Of this group, 100% of the women and 7.4% of the men were worried about urinary incontinence during sexual activity.4,5
A number of strategies have been developed to treat NDO.6 The mainstay of treatment for NDO when pharmacological therapy is indicated are anticholinergic (antimuscarinic) medications (except for men who use reflex voiding as their method for bladder management).6 Anticholinergic medications have a number of important roles in those with NDO.
An important function of anticholinergic medications is to suppress involuntary bladder contractions. Clinically, this helps to facilitate drainage from the upper tracts by lowering the pressure within the bladder wall. This allows drainage from the ureters through the ureteral vesical junction and into the bladder. While most anticholinergic medication studies focus on the lower urinary tract, Tempkin et al.7 evaluated 12 patients with delayed excretion noted on renal scan before and after administration of oxybutynin. Post-administration of oxybutynin, the mean peak times and 25/5-minute count ratios were significantly decreased, indicating improvement in excretion of isotope from the renal cortex and renal pelvis. While not statistically significant, the excretory index was also increased. From a patient perspective, the ability of anticholinergic medications to inhibit involuntary contractions results in an improved bladder capacity, and helps to prevent urinary frequency, urgency, and urinary incontinence. Meta-analysis in able-bodied individuals has shown a statistically significant improvement in symptoms and moderate improvement in QoL compared to placebo.8,9 In persons with spinal cord injuries (SCI) above T6, anticholinergic medications are important to help control autonomic dysreflexia. Involuntary bladder contractions cause reflex contractions of the urinary sphincter (detrusor sphincter dyssynergia), which in turn may trigger autonomic dysreflexia.10
Despite their effectiveness, a major drawback of anticholinergic medications is their side effects. In those taking oxybutynin, dry mouth develops in at least 50%, constipation in about 15%, drowsiness in about 12%, and blurred vision in approximately 5%. Dry mouth and constipation are particular problems in those with NDO who are trying to limit fluid intake because of their intermittent catheterization program or are already having issues with constipation due to their neurogenic bowel dysfunction. Moreover, those with NDO often need larger doses of anticholinergic medications than able-bodied individuals with idiopathic detrusor overactivity because the goal is not only to minimize frequency and urgency, but to cause urinary retention in order to prevent incontinence between catheterizations. Large doses or combinations of several types of anticholinergic medications are frequently needed.11 Another problem with anticholinergic medications is that they need to be taken on a long term, consistent basis. In an observational study from 2002 to 2007 evaluating the medical and pharmacy bills of individuals with a neurological disease and with ≥2 neurogenic bladder diagnoses, it was noted that persistence with medication remained low, with 81% of neurogenic bladder patients having experienced interrupted OAB treatment as indicated by the discontinuation rates. The reason for the interruption in anticholinergic medications is not known, but may likely be due to side effects, cost, ineffectiveness, or forgetting to take the medications.12
Other traditional bladder management options for those on intermittent catheterization and having problems with NDO include indwelling catheters, which also usually require anticholinergic medication, a switch to reflex voiding with or without a sphincterotomy, bladder augmentation, urinary diversion, or neurostimulation devices such as a sacral cord stimulator.6 These options are frequently not acceptable to many individuals with NDO because they involve surgery or wearing a urinary device. For these reasons, botulinum toxin has emerged as an effective alternative for persons with NDO who are not tolerating or have had poor results with anticholinergic medication.
Botulinum neurotoxin (BoNT) is a potent neurotoxin produced from a gram-positive anaerobic bacterium.13 It was first approved by the Food and Drug Administration (FDA) in 1989 for the treatment of strabismus. Since then, there have been a number of non-urological labeled medical indications including muscular dystonias, focal hyperhidrosis, upper limb spasticity, cosmetic surgery, and most recently, migraine headaches.14 In the past 20 years, BoNT has also been used for a wide variety of off-label uses.15
The first urological application (off-label) was for the treatment of detrusor sphincter dyssynergia in 1988.16 In 2000, BoNT was described as being an effective viable option for treating patients with SCI with urinary incontinence who performed intermittent catheterization.17 Since then, BoNT's popularity has increased as an excellent alternative for those with NDO who are not tolerating anticholinergic medications, because of its effectiveness, safety, easy use and learning curve, and reproducibility of results on repeated use. The two most commonly used preparations of BoNT/A for NDO are Botox® (onabotulinumtoxinA) and Dysport® (abobotulinumtoxinA). Phase III studies have recently been published on Botox®.18 In late August 2011, Botox® received US FDA approval for injection for the treatment of urinary incontinence due to detrusor overactivity (DO) associated with a neurological condition (e.g. SCI, multiple sclerosis (MS)) in adults who have an inadequate response to or are intolerant of an anticholinergic medication.19 The purpose of this article is to give a state-of-the-art review of the use of botulinum in adults with NDO.
Types
BoNT, a potent neurotoxin produced from a gram-positive anaerobic bacterium, Clostridium botulinum, was first isolated by van Ermengem in 1897.13 C. botulinum strains produce seven immunologically different neurotoxins (serotypes A to G). Types A and B have been used to treat medical problems. The most frequently used is serotype A. Only serotype A toxins, specifically Botox® (Allergan, Inc., Irvine, CA, USA) and Dysport® (Ipsen, Inc., Slough, Berkshire, UK) have had a number of studies treating NDO. The only FDA approved BoNT/A serotype for NDO is Botox®.
Other commercially available type A serotypes but not FDA approved for NDO treatment include Xeomin® (Merz Pharmaceuticals, UK Ltd, Herts, UK), Prosigne® (Lanzhou Biological Products, Lanzhou, China), and PurTox® (Mentor Corporation, Madison WI, USA). Myobloc/NeuroBloc (rimabotulinumtoxinB) is the only serotype B neurotoxin commercially available. It is approved for the treatment of cervical dystonias. As will be further discussed in the next section, it is important not to assume that the various serotypes are similar or can be used interchangeably.
Name change
In 2009, to help reduce the potential for dosing errors, the FDA required that botulinum toxin products change their established drug names (‘generic’ names).20 Neither the brand names or the formulations of the products have changed. The revised labels were also implemented to emphasize that the different BoNT products are not interchangeable. The new naming further emphasizes that BoNTs are produced by different biological manufacturing processes, are obtained by different isolation and purification techniques, and are derived from different Clostridium batches.
Differences in the products’ molecular structures and formulations may affect their local migration from the injection site and potency characteristics, which may in turn influence their efficacy, safety profile and antigenic potential. No studies directly compare the different agents for dose, efficacy, and safety for the different brands and formulations.21
The new names were made keeping in mind the recommendation from the World Health Organization that new names meet the criteria for non-proprietary names in that they are “distinctive in sound and spelling and should not be liable to confusion with other names in common use”.22 The non-proprietary names that were assigned to the commonly used BoNT products are listed in Table 1. The assigned non-proprietary names for the two most commonly used BoNTs, Botox® and Dysport®, are onabotulinumtoxinA and abobotulinumtoxinA, respectively.20
Table 1.
FDA-assigned names for botulinum toxin
| Proprietary name | Non proprietary name |
|---|---|
| Botox® | onabotulinumtoxinA |
| Dysport® | abobotulinumtoxinA |
| Myobloc/NeuroBloc® | rimabotulinumtoxinB |
| Xeomin® | incobotulinumtoxinA |
Structure
The BoNT is produced by C. botulinum. The neurotoxin molecule is composed of two chains that are termed the heavy chain (protein molecular weight 100 kDa) and the light chain (protein molecular weight 50 kDa) that are covalently connected by a disulfide bond. This molecule becomes part of a large molecular toxin complex (L toxin complex), which are designated as a progenitor toxin.23 This progenitor toxin is made up of the single neurotoxin molecule (BoNT), a single non-toxic non-hemagglutinin molecule, and a hemagglutinin complex (HA). On food-borne botulism, the non-toxic components have the roles of protecting the toxin protein from degeneration and the degradation action of acids and proteases existing in the gastrointestinal tract. The HA facilitates transport when progenitor toxins cross the epithelial barrier to enter the gastrointestinal tract to enter the systemic circulation. The BoNT molecules then dissociate from the progenitor toxin complexes once they are in the systemic circulation. BoNT is immunologically classified into seven immunologically different serotypes (A to G) which inhibit the release of acetylcholine (ACh) at the neuromuscular junctions and synapses (see Mechanisms of action). Serotypes A, B, E, and F are the causative agents of human botulism.24
Mechanisms of action
Efferent system
Normal neuromuscular function
In vertebrate striated muscle, each muscle fiber is typically innervated by a single motor neuron at a site near the middle of the muscle fiber, often referred to as the motor end plate. Within the neuron terminal are the neuromuscular junctions containing ACh transmitter molecules. The ACh collects within clusters of synaptic vesicles. Each vesicle aligns with a patch of dense material forming an active zone. At this active zone, the vesicles fuse with the plasma membrane causing calcium influx and membrane depolarization, resulting in the release (exocytosis) of the (ACh) transmitter molecules into the synaptic cleft (Fig. 1). The ACh then diffuses across the synaptic cleft, which separates the pre- and post-synaptic cells, and binds to and stimulates the post-synaptic ACh receptors.25 Reuptake of ACh does not occur. However, choline produced by ACh hydrolysis is actively taken up and is important in maintaining continued transmitter synthesis.26
Figure 1.
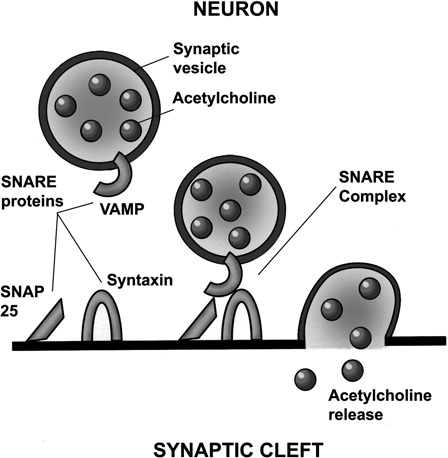
ACh-filled synaptic vesicles with an attached synaptobrevin protein. VAMP combine with SNAP 25 and syntaxin combine to form the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. This complex causes calcium influx, membrane depolarization, and ACh exocytosis from the presynaptic nerve terminal into the synaptic cleft.
The process of the binding of the ACh-filled vesicles to the presynaptic active zone and exocytosis of ACh into the synaptic cleft requires three proteins. The first is synaptobrevin (vesicle-associated membrane protein (VAMP)). This is located directly on the surface of the ACh vesicle membrane. The second protein is synaptosomal-associated protein–molecular weight 25 kilodalton (kDA) (SNAP-25) and the third protein is syntaxin. Both synaptosome and syntaxin are located in the active zone on the internal surface of the plasma membrane of the presynaptic nerve terminal. These three proteins (with the synaptic vesicle) form the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. This complex is essential to allow presynaptic exocytosis of the ACh molecules to occur (Fig. 1).
Action of BoNT at the neuromuscular junction
After dissociation of the progenitor toxin complex in the systemic circulation, the BoNT molecules reach the neuromuscular junction (the peripheral nerve ending). At this point, each BoNT molecule is composed of a light chain and a heavy chain, which are attached by a disulfide bond. The heavy chain is the part of the molecule that recognizes and attaches to the presynaptic nerve terminal and allows the BoNT molecule to enter the nerve cell by endocytosis. Once inside, the disulfide bond is broken and the light chain becomes the active moiety. The light chains of BoNTs (zinc endopeptidases) disrupt the SNARE complex by cleaving the core proteins involved in release of ACh, specifically synaptobrevin (also called the VAMP), SNAP-25, and syntaxin, making attachment of the ACh vesicles to the SNARE complex and exocytosis of the ACh into the synaptic cleft impossible24,27 (see Fig. 2).
Figure 2.
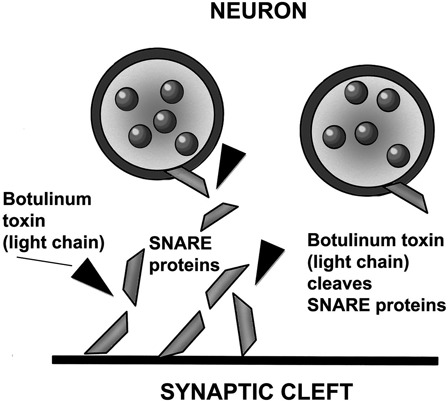
Once the BoNT molecule complex (heavy chain and light chain attached by a disulfide bond) has entered into the neuron, the BoNT light chain disrupts the proteins that form the SNARE complex located at the presynaptic nerve terminal. This prevents the ACh-filled synaptic vesicles from attaching to the SNARE complex so that there is no membrane depolarization or exocytosis of the ACh from the presynaptic nerve terminal. Different serotypes of BoNT cleave different proteins of the SNARE complex.
Different botulinum toxins disrupt different parts of the SNARE complex. BoNT serotypes types A, C, and E cleave the SNAP-25 protein located on the plasma membrane of the presynaptic nerve terminals. Because SNAP-25 is necessary for the fusion of neurotransmitter-filled transmitter vesicles with the plasma membrane and their release during exocytosis, its cleavage causes a highly specific neuromuscular blockade of vesicular ACh release at somatic and autonomic presynaptic nerve terminals. BoNT serotypes B, D, F, and G cleave the synaptobrevin (VAMP) protein, so that the vesicles cannot attach to the SNARE complex.28,29
Botulinum toxin effect – detrusor smooth muscle versus striated skeletal muscle
Traditionally, the effectiveness of botulinum toxin at treating NDO was attributed to its ability to block the presynaptic release of ACh from the parasympathetic efferent nerve, similar to the way it acts on striated muscle. However, there is increasing evidence that BoNT functions differently in detrusor smooth muscle.
An area of particular interest relates to differences in presynaptic nerve recovery. It is well documented that BoNT/A application to striated skeletal muscle is followed by terminal axonal degeneration of cholinergic motor fibers. Post-BoNT/A administration, the axons develop lateral sprouts and this leads to eventual regenerative recovery.30 This process occurs over a 3–4-month period. Haferkamp et al. evaluated detrusor biopsies in 24 SCI patients before and after BoNT/A injection in detrusor muscle. They found that in contrast to striated muscle, there was very little axonal sprouting. There was no correlation of axonal sprouting to clinical recovery.31 Another difference with striated muscle is that there may be a longer direct effect of BoNT/A in the detrusor muscle. Schulte-Baukloh et al. noted that fragments of cleaved SNAP-25 protein remained detectable in the bladder for longer periods post-intradetrusor BoNT/A injections in patients with myelomeningocele than would be expected in striated muscle.32 These differences help explain why clinical effects of BoNT/A are often present for 6 or more months when injected into the detrusor smooth muscle compared to 3 months when injected into skeletal striated muscle.
Afferent system
There is increasing evidence in both animal and human studies that BoNT not only has its effect on the efferent system of the detrusor muscle, but may also alter the afferent nerve input.
Kehera et al. evaluated the in vivo effects of botulinum toxin on visceral sensory function in chronic SCI using T8-9 SCI rats. They found that intravesical BoNT/A inhibited the bladder sensory mechanisms by reducing the frequency of bladder contractions. Another finding with clinical implications on the best method of administration of BoNT/A was their finding that topical intravesical application of BoNT/A did not penetrate the smooth muscle layer even after 1% protamine sulfate disruption of the bladder urothelium.33 Therefore, intravesical injections are needed to for effective delivery of BoNT.
Yokoyama et al. noted that studies on nerve growth factor (NGF) have also supported the concept that BoNT has an impact on the sensory nervous system. NGF is essential both for sensory nerve growth and maintenance. It has been documented that there is a large amount of NGF in those with DO compared to those without DO. However, following the administration of BoNT/A, NGF was found to significantly decrease.34
Conte et al. also investigated whether BoNT/A improved DO by modulating bladder afferent activity. To do so, during urodynamic assessment, they tested the soleus muscle Hoffmann (H) reflex during bladder filling before and after intradetrusor BoNT/A injections in patients with Parkinson's disease (PD) and in patients with complete chronic spinal cord lesion (SCI) and DO refractory to conventional therapy. In healthy subjects and in patients with PD prior to BoNT/A, bladder filling at maximum cystometric capacity (MCC) significantly decreased the H reflex size, whereas in patients with SCI, it slightly facilitated the H reflex size. After BoNT/A injections, at MCC, in patients with PD, BoNT/A significantly reduced the expected H reflex inhibition, whereas in those with SCI, BoNT/A turned the H reflex facilitation at maximum bladder filling into a slight inhibition. The authors concluded that these findings show that BoNT/A injected into the detrusor muscle in patients with PD and SCI modulates bladder afferent activity.35
Apostolidis et al. also provided evidence of the influence of BoNT on the sensory system. They evaluated bladder biopsies in 38 patients (22 with NDO and 16 with idiopathic DO who had intractable DO and were treated with BoNT/A). Biopsies were taken at 4 weeks and 16 weeks post-injection. Urodynamic studies and voiding diaries were also performed. They found that sensory fibers were decreased at 4 weeks and even more significantly decreased at 16 weeks (paired t-test P = 0.0004 and P = 0.0008, respectively), which correlated with significant improvements in clinical and urodynamic parameters. Further evidence of sensory involvement was the decrease in sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human DO.36 In summary, while the exact mechanisms whereby BoNT/A affects the afferent system are not known, there is strong evidence both in animal and human studies that this occurs.
NDO clinical and urodynamic studies of initial treatment
All published series and reviews have supported the use of BoNT/A for the treatment of NDO. This is with respect to clinical findings and urodynamic parameters. There have been no negative open labeled or randomized-controlled studies on the effectiveness of BONT/A in those with NDO. There are also studies evaluating idiopathic detrusor overactivity (IDO) both in adults and children. Some studies have a combination of patients with IDO and NDO. Since this review focuses on the use of BONT in those with NDO, it will just discuss use of BoNT involving adults with NDO. The changes in incontinence and urodynamic findings in these studies are listed in Table 2. The following discussion will highlight some of the unique aspects of these studies.
Table 2.
Clinical and urodynamic findings – neurogenic detrusor overactivity receiving BoNT/A
| First author | Study design | Patients (N) neurogenic disorder | BoNT/A brand dose | Incontinence reduction (%) | MCC (ml) (before–after) | MDP (cm/H2O) (before–after) | RV (ml) |
|---|---|---|---|---|---|---|---|
| Schurch (2000)17 | Prospective Open labeled |
SCI (19) | Botox® 200 U 300 U |
89% overall |
215 → 416 | 66 → 35 | 262 → 490 |
| Reitz (2004)37 | Retrospective | 231 total SCI (67) Spina bifida 22), MS (11) |
Botox® 300 U |
73% overall |
272 → 352 | 61 → 44 At 4 months |
– |
| Schurch (2005)38 | Prospective Randomized placebo controlled Double-blind multi-center |
59 total SCI (53) MS (6) |
Botox® 200 U 300 U |
50% 300 U 50% 6 weeks |
200 U 260 → 448 300 U 293 → 462 6 weeks |
200 U 77 → 40 300 U 92 → 45 6 weeks |
200 U 169 → 234 300 U 254 → 268 |
| Grosse (2005)39 | Prospective Open labeled |
66 total SCI (54) MS (4) Other (3) |
Botox® 300 U Dysport® 750 U |
73% overall | Overall 300 → 400 |
– | – |
| Ehren (2007)41 | Prospective Randomized placebo controlled Double blind |
31 total SCI (20) MS (6) Other (4) |
Dysport® 500 U |
– | 500 U 260 → 460 at 6 weeks |
500 U 68 → 18 at 6 weeks |
– |
| Kalsi (2007)42 | Prospective Open labeled |
MS (45) | Botox® 300 U |
– | 236 → 603 At 4 weeks |
51 → 27 | >100 (98%-IC) |
| Del Popolo (2008)43 | Retrospective | SCI (199) | Dysport® 500 U 750 U 1000 U |
– | Dysport® – all doses 226 → 407 |
– | Dysport® All doses 201 → 300 |
| Giannantoni (2009)44 | Prospective Open labeled |
SCI (17) | Botox® 300 U |
88% – full | 243 → 390 At 4 months |
97.6 → 32.4 At 4 months |
– |
| Deffontaines-Rufin (2011)45 | Prospective Open labeled |
MS (71) | Botox® 300 U | 46% – full 31% – partial 23% – none |
300 U 240 → 328 at 3 months |
300 U 61 → 36 at 3 months |
NA |
| Cruz (2011)18 | Prospective Randomized Placebo-controlled Double-blind multi-center |
275 total SCI (121) MS (154) |
Placebo Botox® 200 U 300 U |
Placebo 13% 200 U – 22% 300 U – 19% |
Placebo-249 → 256 200 U 247 → 404 300 U 247 → 404 |
Placebo- 41 → 48 200 U 52 → 23 300 U 42 → 15 |
Placebo- 57 → 60 200 U 79 → 167 300 U 64 → 248 |
| Kuo (2011)40 | Prospective Open labeled |
24 total SCI (12) CVA (12) |
Botox® 200 U |
SCI 33% – full 58% – partial CVA 83% – full 42% – partial |
SCI 176–374 At 4 weeks CVA 198 → 343 At 4 weeks |
SCI 39 → 21 At 4 weeks CVA 27 → 21 At 4 weeks |
SCI 55 → 242 At 4 weeks CVA 42 → 165 At 4 weeks |
Abbreviations: MCC, mean cystometric capacity; MDP, mean detrusor pressure; RV, residual volume.
In 2000, Schurch et al. published the first paper which evaluated and showed the success of intradetrusor injections of BoNT/A, specifically Botox® (onabotulinumtoxinA) in individuals with NDO. They reported on 21 SCI patients who had NDO and urinary incontinence despite anticholinergics medications. Post-injection, there was a significant increase in the mean maximum bladder (cystometric) capacity (MCC) and a mean decrease of maximum detrusor pressure (MDP). Seventeen of 19 patients were no longer incontinent. Moreover, the investigators noted that the clinical effects and urodynamic improvements lasted much longer than the expected 3-month time course for BoNT/A. These effects were still present at 9 months in the 11 patients who were still being followed.17
Reitz et al. published the largest retrospective multicenter European study consisting of 200 individuals with severe NDO who were performing intermittent catheterization and treated with Botox® (onabotulinumtoxinA).37 Three months after intradetrusor injections of 300 U Botox®, 73% of the patients reported full continence between catheterizations and the others reported some improvement. The MCC and mean volume to the first detrusor contraction increased more than 50%. At the same time the MDP decreased by more than 50%. This study documented the decreased need for anticholinergic medications post-injection. The investigators not only showed the effectiveness of intradetrusor Botox® injections but also showed that they could be used as an alternative to anticholinergic medications. It was also noted that 27% of the patients discontinued their anticholinergic medications completely and the remainder significantly decreased their dose. The beneficial clinical effects persisted for more than 6 months.
Most peer-reviewed articles discuss the impact of Botox® (onabotulinumtoxinA) on NDO. Del Popolo et al. performed a retrospective review of their 8-year experience using Dysport® (abobotulinumtoxinA) in 199 patients with refractory NDO. Patients received a total dose of 500, 750, or 1000 U of Dysport®. In addition to providing information on a different BoNT/A, specifically Dysport®, the results of their study helped to define the doses to use in those with NDO. They found no differences in effectiveness between the higher and lower doses of Dysport®. The authors noted that 20 of the 199 patients showed a poor clinical response to injections. These patients, despite a post-injection increase in cystometric capacity, continued to have reduced compliance without overactive detrusor. The continence rates were not discussed, but the MCC increased by approximately 200 ml and the residual volume increased by approximately 100 ml.43
In addition to performing the first prospective open labeled study of BoNT/A, Schurch et al. also performed the first randomized placebo-controlled study of BoNT/A in individuals with NDO and urinary incontinence. A total of 59 patients were evaluated, 53 with SCI and 6 with MS.38 Individuals receiving Botox® were divided into two groups, one group with 200 U and the other with 300 U injected into the detrusor. The placebo group had normal saline injected into the detrusor. There were significant post-treatment decreases in incontinence episodes (approximately 50%) from the baseline in the two BoNT/A groups but not in the placebo group (P ≤ 0.05). These improvements were seen at the first evaluation at 2 weeks and remained throughout the 6-month period. Unfortunately, there were too few subjects to determine whether there was a statistically significant difference between the 200 and 300 U doses. It was also noted that at 6 months there was a significant improvement in QoL in those receiving Botox® compared to placebo.
Ehren et al. conducted a randomized, placebo-controlled study which compared the effects of Dysport® (500 U) to placebo in 31 patients with NDO. The study ran over a 26-week period. There was a significant decrease in urinary incontinence (P < 0.01), and significant decrease in MDP compared to placebo (P < 0.001). They also had a significant increase in bladder capacity (P < 0.01) and improved QoL compared to placebo.46 This study using Dysport®, like the study by Reitz et al. using Botox®, showed both the effectiveness of BoNT and the ability to reduce the intake of anticholinergic medications. Within 6 weeks post-injection, those receiving Dysport® had a significant decrease of anticholinergic (tolterodine) intake compared to placebo (P = 0.003). This decrease in anticholinergic intake continued throughout the study.
The study by Kalsi et al. was unique in that it focused on individuals with NDO due to MS. They prospectively evaluated the impact of Botox® 300 U on bladder function and the QoL in 43 patients with MS with significant NDO despite anticholinergic medications. Post-injections there were significant clinical and urodynamic improvements (P < 0.0001). At 4 weeks post-Botox®, there was a 45% decrease in urinary frequency, 77% decrease in incontinence episodes, 78% decrease in micturition episodes associated with urgency, and 47% decrease in nocturia. Urodynamic studies showed an increased bladder capacity of 303% at 4 weeks, and 33.5% decrease in MDP during involuntary contraction. The mean duration of effect was 9.4 months.42
Deffontaines-Rufin et al.45 described results on BoNT/A in patients with NDO secondary to MS. Their study gave further insights into the use of BoNT in this patient population. Seventy-seven percent of the treated patients had clinical improvement or full success with a reduction of their urgency and incontinence. Significant urodynamic improvement after treatment was shown on different parameters: volume at first involuntary bladder contraction (P = 0.0000001), MCC (P = 0.0035), and MDP (P = 0.0000001). Forty-six percent of the patients were in the ‘full success’ group, and 31% of the patients had a partial improvement. However, BoNT/A failed to treat 23% of the patients suffering from MS. The investigators found that the duration of MS was a predictive factor of treatment failure (P = 0.015). As the neurological condition progresses, so does the severity of the urinary symptoms.47 Therefore, these authors conclude that injection therapy should be considered as soon as anticholinergic medications fail to control NDO in those with MS rather than waiting until potentially irreversible histological and neurological damage has occurred.45
Kuo reported on a prospective study involving the impact of BoNT on NDO not only in those with SCI but also in those with chronic cerebrovascular accident (CVA). Several concepts regarding the use of BoNT in those with a CVA were learned in this study. A lower dose of Botox®, specifically 200 U rather than 300 U, was used in order to minimize the need for clean intermittent catheterization (CIC) as a result of urinary retention caused by Botox®. This was important since intermittent catheterization can be more difficult for the elderly chronic CVA population.48 Twenty-four subjects were enrolled, 12 had CVA and 12 had SCI. After treatment, the volume of the first involuntary detrusor contraction and bladder capacity increased two-fold and the post-void residual volume four-fold for both patient groups after 1 month, decreasing slightly at 3 months. At 4 weeks, there was a significant improvement in complete continence and improvement in incontinence in the SCI group. Five achieved urinary continence after treatment, six had improvement, and one had treatment failure. At 3 months, a successful result was noted in 11 (91%) of the SCI group. However, at 4 weeks, only one patient with CVA had regained urinary continence, three had improvement, and six had failure. At 3 months, there was only a 50% successful outcome in the CVA group. The investigators point to several causes. Urinary incontinence in persons with CVA is due to DO with a weak sphincter. Therefore, when the bladder reaches its maximum capacity, even if it is larger, and a contraction occurs, a person with a CVA is more likely to have incontinence. There may also be cognitive issues affecting the sensation of bladder fullness, mobility issues, and greater technical difficulties in performing intermittent catheterization. In addition, the elderly are more likely to have DO combined with impaired bladder wall contractility (also known as detrusor hyperactivity with impaired contractility) making urinary retention and overflow incontinence more likely. These issues need to be taken into consideration prior to injection of BoNT/A in an elderly person with NDO due to a CVA. The authors also point out that especially with a lower dose BoNT/A, patients with SCI may be able to use suprapubic tapping as an alternative to intermittent catheterization.48
Recently, Cruz et al. reported their results of a pivotal phase III randomized, double-blind, placebo-controlled trial on the efficacy and safety of Botox® in patients (SCI and MS) with urinary incontinence due to NDO.18 Patients received 30 intradetrusor injections of 200 U, 300 U, or placebo. Improvements with the drug were present at the 2-week interval and further improved at the 6-week interval compared to placebo. The mean decrease in urinary incontinence (UI) episodes per week was significantly improved with 200 U and 300 U compared to placebo (−21.8, −19.4, and −13.2, respectively) (P < 0.01). Mean improvements in MCC, MDP, and QoL at 6 weeks were significantly better than placebo (P < 0.001). The median time before a patient asked for retreatment was 42 weeks. There were no differences noted in the efficacy or duration between the two doses of drug. However, those with the 300 U dose had more adverse events (AEs) (see Adverse reactions/complications section).
In summary, there have been a number of excellent review articles34,41,49,50 and clinical studies,17,18,37,38,42,43,45,46 confirming the effectiveness of BoNT in those with NDO. Results of a number of clinical studies using BoNT/A in adults with DO due to a neurogenic cause are shown in Table 2. These findings showed that there was a significant decrease in urinary incontinence of patients (19–89%), significant improvement in urodynamic parameters, specificity bladder capacity, and detrusor maximum pressure. The mean duration of action was 6–9 months. It was noted that there are specific considerations concerning the use of BoNT in those with NDO due to SCI, MS, and CVA.
Botulinum neurotoxin – injection techniques
A number of injection techniques have been discussed in the literature. However, currently it is not known what minimum effective dose, type of toxin, dilution volume, location, number, depth of injection instrumentation, and anesthetic technique will give the best results.34,41,49–51 An important reason is that studies frequently did not give all of these details, making comparisons between studies difficult. The variable most frequently described is dosage. Cruz et al. noted that there was a dose ceiling effect with a dose of 300 U for onabotulinumtoxinA both in clinical and urodynamic efficacy and duration of effect.18 Until recently, most studies used Botox® 300 U; however, evidence now suggests that 200 U is equally effective with fewer side effects.18 There is controversy over whether or not to inject the trigone. In most studies, injecting the trigone is avoided because of concerns of vesicoureteral reflex. However, more recently, studies have suggested that this is not a problem.51 It has not been established whether there is any difference by injecting the Botox® submucosally or deeper into the detrusor muscle. It is not known whether the toxin dilution volume makes a difference in its effectiveness.
With the recent FDA approval of Botox® (onabotulinumtoxinA), there are now standardized instructions from the manufacturer's (Allergan, Inc.) prescribing information, revised 11/2011, for Botox® (onabotulinumtoxinA).52
A summary of the manufacturer's (Allergan, Inc.) FDA-approved recommendations for Botox® (onabotulinumtoxinA) is that patients receiving Botox® need to be free of infection and off anticoagulant medications and receiving prophylactic antibiotics. Regarding antibiotics, patients receiving concomitant treatment of Botox® and aminoglycoside or other agents interfering with neuromuscular transmission (e.g. cuare-like agents) or muscle relaxants should be observed closely because the effect of Botox® may be potentiated.
The recommendation not to use aminoglycosides is based in part on a retrospective study involving observations in 10 children with infant botulism who experienced rapid deterioration following aminoglycoside treatment and rapid recovery following cessation of aminoglycoside therapy. There was no temporal deterioration in five children who received only penicillin or a semi-derivative of penicillin.53
If a 200 U vial of Botox® is used, the recommendations are to reconstitute the 200 U vial with 6 ml of 0.9% non-preserved saline solution and mix the vial gently. Draw 2 ml from the vial into each of three 10-ml syringes. Complete the reconstitution by adding 8 ml of 0.9% non-preserved saline solution into each of the 10-mL syringes, and mix gently. This will result in three 10-ml syringes each containing 10 ml (∼67 U in each), for a total of 200 U of reconstituted Botox®. Inject 1 ml (approximately 6.7 U) across 30 sites into the detrusor muscle approximately 1 cm apart. It is recommended that the needle be inserted approximately 2 mm into the detrusor using a flexible or rigid cystoscope, avoiding the trigone. The bladder should be instilled with enough saline to achieve adequate visualization for the injection, but to avoid over distention. No more than 360 U are recommended over a 3-month period. Patients are considered for re-injection when the clinical effect of the previous injection diminishes (median time to qualification for re-treatment in the double-blind, placebo-controlled clinical studies was 295–337 days (42–48 weeks) for Botox® 200 U), but no sooner than 12 weeks from the prior bladder injection. Detailed instructions on indications, dosage, and administration can be found at http://www.allergan.com/assets/pdf/botox_pi.pdf.52 In summary, there is now an FDA approved recommended injection protocol for Botox®. However further work is needed to determine if this is the “optimum BoNT protocol” in all patients in all clinical situations.
Long-term efficacy and tolerability
Many studies evaluate the effects on BoNT after a single injection. There are several investigators who reported on repeated, long-term use of BoNT. Reitz et al. evaluated 20 consecutive patients with NDO who had received at least five intradetrusor injections sessions of 300 U of onabotulinumtoxinA.54 Each session consisted of endoscopic injections into the detrusor muscle at 30 sites (10 U/per site sparing the trigone). They were followed both clinically and with urodynamic evaluations for at least four of the five injections. The results of 100 injections were analyzed. Clinical continence and urodynamic parameters improved significantly after the first injection and then remained constant after repeat injections. Clinically, there were no toxin-related side effects.
Karsenty et al. analyzed clinical parameters and urodynamic parameters after repeated injections in 17 patients with NDO.55 Their time points for evaluation were before the first injection, after the first injection, and after the last injection. The mean number of injections per patient was 5.4 (range 3–9). The mean number of incontinence episodes per day decreased from 2.6 at baseline to 0 after the first injection, and remained at 0 after the last injection. The maximal cystometric bladder capacity and residual volume increased significantly after the first and last injection compared to baseline. The maximal detrusor pressure decreased significantly after the first and last injection compared to baseline. They found significant and consistent improvement in both clinical and urodynamic parameters in patients that persisted after 4.5 injections.
Grosse et al. reported on 66 patients with NDO who had received repeated BoNT/A (Dyport® 750 U or Botox® 300 U) injections. This is one of only a few peer reviewed articles on the use of Dysport® (abobotulinumtoxinA) in those with NDO. They studied patients up to three injection sessions.39 The interval between subsequent injections (on an average 9–11 months) did not change between injections. Repeated injections of BoNT/A maintained statistically significant improvements in the clinical parameter, QoL, and urodynamic parameters. The anticholinergic use decreased substantially. Improvements in bladder wall compliance were noted after the second set of injections. No intolerance to either BoNT/A agent was noted. Four patients had transient muscular weakness up to 2 months in the trunk and/or lower extremities, all after Dysport® injections.
Kuo et al. evaluated the impact of repeated Botox® injections into the bladder in SCI individuals on urinary incontinence, renal function, MCC, and mean detrusor pressures.40 Individuals were injected every 6 months with Botox® 200 U for 2 years. They found that repeated injections reduced incontinence, increased bladder capacity (almost doubled in size from 207± 111 ml to 412 ± 33 ml.), and reduced intravesical pressure. Over a 2-year treatment period, there was no change in serum creatinine; however, overall, mean glomerular filtration rate (GFR) decreased significantly as measured by renal scans over the 2 years (decreased from 93.4 ± 20.4 to 83.5 ± 24 ml/min (P = 0.028)). However, this significant reduction in GFR was noted in patients who had a poorer response to Botox®, with bladder compliance that increased by <10 cmH2O (P = 0.002) and in patients with Pdet that decreased by less than 10 cmH2O after treatment (P = 0.036). However, they did not find any improvement in renal function. Regarding failure to see improvement in renal function, there are several explanations. The authors note the small group of patients (n = 38), with a 7-year mean duration of injury and relatively short follow-up (2 years). Because there was no placebo group, it is difficult to know whether the injection group would have maintained renal function much better than the control group. It is also possible that prior renal damage was manifesting itself, and the rate of decline in function would have actually have decreased in the Botox® treated group compared to the placebo group. Longer follow-up may have also showed an improvement in renal function.
Bladder histology post-injection
Questions have been raised on whether repeated injections of BoNT into the detrusor muscle may possibly cause atrophy of the bladder wall, inflammatory changes, dysplasia, or fibrotic changes. Several studies have addressed this issue.31,44,56,57
Apostolidis et al.56 prospectively obtained bladder biopsies using a flexible cystoscope in patients with intractable NDO or idiopathic DO after one or more injections of BoNT. Signs of chronic inflammation were found in 59.1% of baseline biopsies (65.6% of NDO vs. 50% of IDO). Post-injection inflammation was found in 67.6% of post-first biopsies and 86.4% after repeat injections. The two groups were comparable for degree of baseline inflammation, which did not change significantly after first injection and up to 16 weeks after a third injection. Mild fibrosis was found in 2.2% of biopsies examined, equally before and after treatment, but not after repeat injections. No dysplasia or hyperplasia was identified. Eosinophils were identified more frequently in biopsies taken after repeat injections compared with the post-first injection and baseline biopsies (χ2 = 8.23, P = 0.018). No difference existed between NDO and IDO bladders.
The authors concluded that BoNTA injections do not appear to be producing significant inflammatory changes, fibrosis, or dysplastic changes in human bladder urothelium/suburothelium after a single injection and in a limited number of repeat treatment biopsies. The presence of eosinophils might be treatment-related, because they were mostly found in post-treatment biopsies. It should be noted that the frequency of injections was higher than normally expected becasue BoNT/A frequently has a clinical effect for 6–9 months.
Comperat and co-workers57 evaluated bladder wall specimens of 45 patients with NDO with and without injection of botulinum toxin injection into the detrusor muscle that underwent partial or total cystectomy and urinary diversion because of failure of conservative or minimally invasive (Botox®) therapy. Both groups of those with NDO had general edema, fibrosis, and inflammatory infiltrations. However, when comparing specimens from those who had received BoNT/A with those who had not, there were no differences in inflammation or edema. Moreover, patients who had received BoNT/A had significantly less fibrosis of the bladder wall than those who had not received the injections (P < 0.00073). Although not significant, there was a trend towards less fibrosis and edema in responders compared to non-responders in those who had received botulinum toxin injections.
In another study, Haferkamp et al.31 evaluated 30 biopsies from 24 patients with NDO. Biopsies were performed and evaluated in two groups. Group 1: before BoNT/A injection, and Group 2: 3 or more months post-BoNT/A injection (six biopsies) and at the time the Botox® was wearing off (11 biopsies). A median of 70% of intrinsic axon terminals presented with signs of degeneration before injection, compared to 66% with signs of degeneration after injection. It was interesting to note that out of a total of 309 evaluated axon terminals, only three sprouting axons were found in the 3-month post-injection biopsies. There was minimal collagen deposition post-injection and no changes in the ultrastructure of the detrusor before and after BoNT/A injections of the same patient.
Similar results were noted in long-term studies. Giannantoni et al.44 performed a long-term study with multiple biopsies on patients over a 6-year period in which the patients had a mean of 4.5 injections. Repeated injections caused no adverse effect on urodynamic parameters. In fact, there was an improvement in bladder wall compliance. These findings were consistent with the short-term studies showing a decrease in fibrosis since a decrease in fibrosis would be expected to also cause an improvement in bladder wall compliance.
In summary, short- and long-term studies have shown no evidence that repeated BoNT/A injections cause any significant histological changes. Further evidence is needed to determine whether fibrosis may actually be inhibited by repeated BoNT injections.
Botulinum neurotoxin – clinical benefits
Here are a number of potential clinical benefits following the injection of BoNT/A. The following is a discussion of some of the more common benefits that have been discussed in the literature regarding the use on BONT/A for NDO.
Improvement in urinary incontinence
As discussed above, the major clinical benefit of BoNT is that it decreases the incidence of urinary incontinence in those with NDO who are not tolerating anticholinergics. This occurs by increasing the maximum cystometric (bladder) capacity and decreasing the MDP. This is accomplished by suppressing the uninhibited bladder contractions. Studies also suggest that this may occur by decreasing the afferent input from bladder wall distention and may prevent and over time decrease bladder wall fibrosis.
Reduction in autonomic dysreflexia
While there have been no randomized or placebo-controlled studies on the impact of BoNT on autonomic dysreflexia, a beneficial effect can be presumed. Linsenmeyer et al.10 documented that autonomic dysreflexia occurs not only during bladder distention but also with the onset of an uninhibited bladder contraction in those with SCI injuries at T6 and above. Schurch et al.17 reported in their original article that three patients with tetraplegia and severe AD experienced resolution of AD with 300 U of Botox® injected into the bladder wall.
Decrease in the use of anticholinergic medications
It has also been noted that BoNT/A allows individuals to decrease or even stop their anticholinergic medications. This effect has been found to be persistent.39
Decrease in urinary tract infection
It has been reported that BoNT/A injections have helped decrease the number of UTIs, specifically pyelonephritis, orchitis, and prostatitis.44,58,59 Gamé et al. recorded UTI (i.e. cystitis, pyelonephritis, orchitis, prostatitis) in 30 individuals with NDO for 6 months before BoNT injection (Botox® 300 U) and for 6 months after injection. Before BoNT injection, the mean number of symptomatic urinary infections over 6 months was 1.75 ± 1.87. After injection, the mean was 0.2 ± 0.41 (P = 0.003). It was also noted that the three individuals who did present with UTI showed less improvement in their urodynamic parameters (volume of the first uninhibited contraction, maximum bladder pressure and MCC) after injection than those who did not have UTIs.58
One reason for a decrease in pyelonephritis may be due to a decrease in the MDPs, which reduces vesicoureteral reflux of urine back up into the kidneys.44 Another reason for a decrease in UTIs may be improved blood flow and increased tissue oxygen levels after BoNT injections due to a decrease in the MDP and increase in the MCC. Since a variety of studies have shown that there is a decrease in MDP after BoNT/A detrusor injections, decreases in UTIs and possibly formation of fibrosis of the bladder wall may be due to a reduction of a relative ischemia of the bladder wall by lowering the mean detrusor pressure. One study evaluated bladder blood flow and tissue oxygen levels in canines with and without ligated bladder necks. In those with a ligated bladder neck (and higher bladder pressures during the contraction) there was a 58.4 ± 4.8% decrease in bladder blood flow during a bladder contraction compared to the pre-contraction blood flow. In animals with non-ligated bladder necks, the blood flow during the contraction actually increased 160.9 ± 11.3% of the precontraction blood flow. Moreover, during the bladder contractions, the tissue oxygen levels (pO2) dropped in both groups but had a statistically significant larger drop in the animals with the ligated bladder necks.60
Upper tract improvement
With significant improvements in the MCC and decreases in the MDP post-BoNT injections, it would be expected that improvements in upper tract drainage would be noted. Most studies have been relatively short-term studies focused on urinary incontinence, changes in urodynamic parameters, and QoL after BoNT injections. Giannantoni et al. did evaluate and discuss upper tract changes in their study. They prospectively evaluated 17 SCI patients receiving repeated Botox® injections over a 6-year period. Six of their 17 patients initially had bilateral renal pelvis dilatation and five had unilateral dilatation. An additional three had unilateral dilatation due to Grade 3 vesicoureteral reflux. At the end of six years, in addition to sustained improvement in clinical, urodynamic, and QoL changes, there were significant upper tract changes. Vesicoureteral reflux and renal pelvis dilatation resolved in all patients.44
Kuo et al. evaluated both bladder and renal function over a 2-year period, giving a total of four injections at intervals of 6 months to 33 patients with chronic SCI. All patients were satisfied, with a reduced incontinence grade, increased bladder capacity, and decreased intravesical pressure.40 However, a significant reduction in the GFR was noted in patients with bladder wall compliance that increased by <10 cmH2O, and in patients with Pdet decreased by <10 cmH2O after treatment. It is unclear if the decreased GFR was due to a decreased response to BoNT due to pre-existing poor bladder wall compliance, pre-existing renal disease that was manifesting itself over the 2-year period, or both. It is possible that a longer follow-up such as Giannantoni et al.44 study would have shown stabilization or improvement in renal function. This study does emphasize the importance of monitoring upper tract function, even in patients who clinically and urodynamically appear to be doing well.40
Further long-term studies are needed to evaluate the impact of BoNT on renal function. It is possible that those with certain urodynamic parameters will not improve despite Botox®. Upper tract monitoring in those with SCI is important despite clinical and urodynamic improvements post-BoNT injections.
QoL and patient perspective
Studies have shown that BoNT/A injections improve QoL in NDO regardless of whether this is due to SCI or MS. Schurch et al. reported results from their multi-center randomized double-blind, placebo-controlled study which was conducted in eight centers.61 The study included 53 individuals with SCI and six with MS with NDO and incontinence despite anticholinergics. Patients received either a 200 U Botox® dose or 300 U Botox® dose or placebo. Mean total and subscale I-QOL scores increased significantly from screening at all time points: 2, 6, 12, 18, and 24 weeks compared to placebo (P < 0.05) for the 300 U dose. With the 200 U dose, there was a significant increase compared to placebo at all time points for the total I-QoL score and avoidance limiting behavior subscale, and at weeks 2, 6, 18, but not at 24 weeks for the Psychosocial Impact and Social Embarrassment subscales (P < 0.05).
Kalsi evaluated the QoL in 43 patients with NDO due to MS who were refractory to anticholinergic medications. Botox® significantly reduced urgency, frequency, urinary incontinence, and nocturia.42 Despite 98% of the patients having to perform intermittent catheterization, there were sustained improvements in QoL scores in all categories. There was a highly significant decrease in combined Urogenital Distress Inventory-6 and Incontinence Impact Questionnaire-7 score. The mean duration of clinical effects was 8 months.
Giannantoni et al. prospectively evaluated clinical, urodynamic, and QoL issues in 17 individuals over 6 years. Patients received repeated injections of Botox® 300 U over this time. The mean interval between injections was 11 months. In addition to significant clinical and urodynamic improvements, this study showed that a sustained QoL could be achieved by repeated detrusor BoNT/A injections over a 6-year period.44
Hori et al.62 evaluated patients’ perspective of BoNT/A (Dysport® (abobotulinumtoxinA) 750 U and 1000 U) in 72 individuals with SCI who had urodynamically confirmed NDO and poor results with anticholinergic medications. Patients who had had one or more injections with BoNT/A were invited to participate in a 5-minute telephone questionnaire and rated their satisfaction on a scale of 1–10, with 10 being “very satisfied”. Of the 72 patients, 48 (67%) were still undergoing treatment, and 24 (33%) had discontinued repeat BoNT/A injections because they were ineffective, short-lived, or had caused transient generalized muscle weakness (on the 1000 U dose but not 750 U dose). The mean satisfaction score of all patients was 6.2. Of those who were still having BoNT/A, 43 (90%) reported that they would consider using BoNT/A as a long-term option. This study shows that once a person has initial success with BoNT/A, there is a high satisfaction rate and acceptance as a long-term management option.
In summary, a number of studies have confirmed that after the injection of BoNT, there is a significant improvement in the QoL in individuals with SCI or MS who have NDO. This was despite nearly all study patients having to perform CIC, suggesting that CIC has a less significant effect on patients’ QoL than relief from their troublesome bladder symptoms.
Costs analysis
There have only been a few studies concerning cost–benefit analysis of BoNT compared to other modalities. Wu et al. evaluated the cost effectiveness of BoNT/A injection compared to anticholinergic medications in those with refractory urge incontinence (not those with neurogenic bladder). It was conducted over a 2-year period with 3-month time windows. OnabotulinumtoxinA was found to be cost-effective compared to anticholinergic medications. Anticholinergics become cost-effective if compliance exceeds 75% and if the botulinum toxin A procedure cost exceeds $3875.63
Wefer et al. evaluated resource consumption before and after onabotulinumtoxinA use in Germany in patients with SCI. They found that prior to onabotulinumtoxinA, 68% reported UTI, 63% had incontinent episodes, and 58% used incontinence aids. These numbers decreased significantly (P < 0.05) after treatment to 28, 33, 28%, respectively. In patients using incontinence aids, mean costs per patient decreased from 2 euros to 1 euro per day, whereas the mean cost of drugs to treat UTIs per patient decreased from €163 to €80 per year, respectively.64
Kalsi et al. evaluated the resource utilization, health benefits, and cost-effectiveness of intra-detrusor injections of BoNT/A in patients with OAB, 101 patients (63 with NDO and 38 with IDO). They showed that intra-detrusor BoNT/A is an effective treatment for OAB that is very likely to be cost-effective in both idiopathic and neurogenic disease. In an intent-to-treat analysis, 82% of patients showed a 25% or greater improvement in at least two out of five parameters (urinary frequency, urgency, urgency incontinence episodes, MCC and MDP) 4 weeks after treatment. A 50% or greater improvement in the frequency of micturition, urgency or urgency incontinence was seen in 73% of patients at 4 weeks and 54% at 16 weeks. There were no significant differences between IDO and NDO patients meeting these endpoints. Therapy costs 826 pounds per patient, with a cost-effectiveness ratio of 617 pounds per patient-year with ≥25% clinical improvement.65
Adverse reactions/complications
Here are a number of potential adverse reactions or complications following the injection of BoNT/A. The following is a discussion of some of the more common concerns which have been discussed in the literature regarding the use on BONT/A for NDO.
Urinary tract infections
The most common adverse reactions ≥5% and greater than placebo listed in the Prescribing Information for Botox®, revised 8/11 for DO, are UTI and urinary retention.52 Schurch et al. noted in their prospective randomized single treatment placebo-controlled 6-month study that UTIs were the most common AE.38 This occurred in all three of the groups in this study, specifically placebo 3/21 (14.3%), 6/19 (31.6%) of the 200 U group, and 4/19 (21.1%) in the 300 U group. However, there was no statistically significant differences among the three groups (P = 0.455).38 In the study by Cruz et al.,18 the most common AE during the first 12 weeks was UTI. In the SCI population, the incidence was the same among all treatment groups including placebo. The overall UTI incidence for patients with SCI, for the placebo group, and onabotulinumtoxinA 200 U and 300 U groups was 50, 52.6, and 56.4%, respectively. In the MS population, it was higher in the onabotulinumtoxinA groups (highest in the 300 U group) compared to placebo. The incidences for the placebo group, onabotulinumtoxinA 200 U dose and 300 U dose were 32.0, 58.2, and 70.0%, respectively. As discussed below, there was a significant rise in urinary retention and the need to begin intermittent catheterization following onabotulinumtoxinA in the MS group, so the two findings may be related.
It should be noted that there was not a clear distinction between ‘symptomatic’ UTI and colonization. A UTI diagnosis was based on a laboratory finding of a positive urine culture. However, for an individual with neurogenic bladder dysfunction to be considered to have a UTI, three criteria have to be met. These criteria are bacteria in the urine, elevated WBCs in the urine, and new onset of symptoms.66
Kalsi et al. discussed symptomatic UTI post-Botox® injections in 43 patients with MS. They reported that seven (16%) of patients developed UTI (single or recurrent) after injection. However, three of these patients had a history of UTI prior to injection. Therefore, 4/43 (9%) of patients developed UTI as a consequence of their intervention. The reason for this is not known. However, a large number of patients had to start CIC post-injection. It is possible that with the resolution of symptoms, patients decreased the frequency of catheterization or allowed their bladders to become overdistended.42
Urinary retention
The second most common AE in the study by Cruz et al.18 was elevation in post-void residual urine (PVR) after onabotulinumtoxinA injections. In patients not using CIC at baseline, PVRs significantly increased following onabotulinumtoxinA injections in a dose-related manner over the 12 weeks. The percent of patients with a PVR >200 cc was highest in the 300 U group. The percent of patients with a PVR ≥200 cc in the placebo, onabotulinumtoxinA 200 U and 300 U groups was 2.7, 28.6, and 53.7%, respectively. Urinary retention primarily occurred in the patients with MS and again was the highest in the 300 U group. Overall, the incidence of initiating intermittent catheterization in the placebo, onabotulinumtoxinA 200 U, and 300 U groups was 12.2, 29.5, and 42%, respectively. See Table 2 for urinary retention data from other studies.
While not commented on, it is possible that the combination of intermittent catheterization and stopping/decreasing anticholinergic medications improved the QoL of a number of individuals with NDO who were having a large number of incontinent episodes and side effects from their anticholinergic medications.
Hematuria
Mild hematuria has been reported in several series. This was found to occur in 2–21% of patients and was self-limiting.38,48
Injection site pain
Schurch et al. entered 59 individuals with NDO into their study. They reported injection site pain, which lasted for less than 1 hour in duration, occurred in 1 patient (4.8%) in the placebo group and 2 patients (10.5%) in the 300 U BoNT/A group.38 This does not seem to be a major issue since it was not reported in a large phase 3 study.18
Immunology issues
Both commercially available Botox® and Dysport® use progenitor toxins because they are easy to use and more stable than the actual neurotoxin. A potential problem is that anti-progenitor toxin including anti-neurotoxin antibodies may be produced after several injections.34
Development of antibodies would cause injected BoNT to lose its effectiveness. Antibody formation and loss of effectiveness have been noted with frequent repeated BoNT injections into skeletal muscle. Formation of BoNT antibodies has been associated with various risk factors, including certain treatment parameters, patient characteristics, and immunological properties of the therapeutic BoNT preparations used. For the current formulation of Botox®, the rate of antibody-induced therapy failure is reportedly less than 1%. To determine the antigenicity of different BT preparations in more detail, prospective studies on large series of unbiased patients with sensitive and specific BT antibody tests are necessary.67
Despite the above concerns, there have no studies documenting the development of BoNT antibioties in those receiving BoNT/A for NDO. Perhaps this is because of the long interval between injections for NDO compared to shorter (3-month) intervals required for injections into skeletal striated muscle. In addition, larger doses are used to treat striated muscle spasticity.
Schurch et al. analyzed serum samples for antibodies in their 6-month study.38 All samples were negative for antibodies at baseline and week 24. Cruz et al. monitored for antibodies in 275 patients receiving onabotulinumtoxinA (Botox® Allergan, Inc.) in a randomized double-blind placebo-controlled study. Those receiving Botox® received either 200 or 300 U.18 No antibodies were detected. In summary, despite theoretical concerns, currently there are no documented cases of loss of effectiveness due to antibody formation in those receiving BoNT/A for NDO.
Autonomic dysreflexia
It was previously discussed that a long-term positive effect of BoNT injections into the bladder wall is the reduction of AD in those with SCI at T6 and above. However, AD can occur with any noxious stimuli. Therefore, instrumentation, bladder distention, or the injection itself have the potential to cause AD. Schurch et al.38 did not find any episodes of autonomic dysreflexia in the 59 subjects with NDO in their placebo-controlled 6-month study. Cruz et al.18 reported that two patients receiving onabotulinum toxin developed autonomic dysreflexia. It was not clear whether this occurred during the injection itself or later on. It is important when injecting BoNT to know how to evaluate and treat autonomic dysreflexia. Monitoring blood pressure during the procedure is essential, especially in those with injuries at T6 and above, as 40% of these individuals may have ‘silent autonomic dysreflexia’.10,68
Distal effects
In April 2009, the FDA announced the new safety label changes including a boxed warning, and a Medication Guide/Risk Evaluation and Mitigation Strategy highlighting the risk of the spread of the toxin effect from the area of injection causing symptoms similar to those of botulism.52 This was based on distal effect symptoms of children with cerebral palsy (CP) being treated for spasticity. A similar review by Health Canada identified 10 cases of serious reactions following injections, including five deaths, two which occurred in children with CP.69 The Black box warning reads: ‘WARNING: DISTANT SPREAD OF TOXIN EFFECT See full prescribing information for complete boxed warning’. The effects of Botox® and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms.52
O'Flaherty et al. conducted a prospective pre–post cohort study of all children undergoing BoNT/A injections in a single children's rehabilitation department over a 16-month period. They found that in the month following an injection episode, an AE occurred in 23% of injection episodes; 2% of these were serious (lower respiratory tract infections, worsening dysphagia, or generalized weakness). However, the rate of these events was slightly lower or no different from the rates of these events in the month before these injections.70
While there have been no life-threatening side effects from BoNT in those with NDO, there have been occasional reports of muscular weakness beyond the injection site. Wyendale et al. reported two individuals with SCI who developed muscle weakness post-BoNT injection. The first was a 57-year-old woman with T9 paraplegia who developed generalized muscle weakness after a dose of 1000 U (no problems with first dose of 500 U) of Dysport® (abobotulinumtoxinA), which made it hard to perform transfers for 3 months. In a second case, a 24-year-old man with tetraplegia received Botox® (onabotulinumtoxinA) and within 2 weeks developed generalized muscle weakness of his arms that affected his transfers and participation in sports. His strength did not return for 3 months, but he then regained his original strength.71
Hori et al. reported that 6% (4/72) of their patients had transient generalized muscle weakness, which was self-limiting. This occurred in patients receiving intradetrusor injections of Dysport® (abotulinumtoxinA) 1000 U doses, but none when the dose was reduced to 750 U.62
Grosse et al. reported that four patients had transient weakness in the trunk and/or lower extremities, all after Dysport® injections.39 In one of them the weakness lasted for 2 months after 1000 U/10 ml, for two the weakness lasted for 4 weeks after 750 U/10 ml – one of those had two previous Botox® injections 300 U/15 ml without problems. The fourth patient had weakness for 2 months after Dysport® 1000 U/10 ml, for 6 weeks after a second injection of Dysport® 750 U/10 ml, and again for 6 weeks after a third injection of Dysport® 750 U/5 ml. The fourth patient had received three injections because he was very satisfied with the treatment effects and had stated this condition only at the interview after the third injection. Del Popolo et al. noted that 5/199 patients receiving a high dose of intravesical Dysport® (1000 U) developed hyposthenia. The supralesional weakness was transient, disappearing 2–4 weeks post-injection.43 Cruz et al. reported that one patient who received a Botox® 300 U dose experienced generalized weakness. No patients had distal effects on the 200 U dose.18
Kalsi et al. reported one individual with MS developed bilateral leg weakness after intradetrusor Botox® injections. However, careful evaluation by a neurologist determined that this weakness was due to an exacerbation of MS.42 This emphasizes that weakness post-Botox® may be due to other causes and deserves further evaluation. For example, other potential causes of weakness in individuals with NDO include exacerbation of MS symptoms, syringomyelia in SCI, or new onset CVA.
Summary
BoNT may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. The FDA warning is for all botulinum toxin products. In those receiving BoNT, there have not been any life-threatening side effects; however, there have been a few cases of self-limiting generalized weakness. While self-limiting, this can be a problem in those who already have weakness, since they rely on the strength they have to perform transfers, propel their wheelchairs, etc. There have only been a few reported cases of weakness, so it is difficult to comment on what factors may have been responsible. It appears that weakness occurs with higher doses. More cases have been reported with abobotulinumtoxinA than with onabotulinumtoxinA. Further experience and research is needed to determine what factors, such as dosage, frequency of injection, type of BoNT used, injection technique, dilution, and type of bladder – smooth or trabeculated, are more likely to cause distal effects.
Future areas of research
There are a number of areas of future research, both in the areas of basic science and clinical work (see Table 3). These general areas can be categorized as structure and function, mechanisms of action, clinical and urodynamic studies, injection technique, potential beneficial and adverse effects.
Table 3.
Potential areas of BoNT future research
| Basic science |
| Impact of BoNT on the afferent system |
| Neurotoxin specificity at receptor sites |
| Improving duration |
| Immunology/antibody responses |
| Clinical |
| Degree of interchangeability within the same or different serotypes |
| Injection techniques |
| Dosages |
| Dilution |
| Site of injection |
The structure and function of botulinum toxin is known. However, it is not known if there is any degree of interchangeability. This is particularly true concerning the various serotypes. The majority of studies have been done with serotype A, onabotulinumtoxinA, and to a lesser extent abobotulinumtoxinA. There is not enough evidence to comment on the interchangeability of these two agents. Currently, it is not recommended that these be used interchangeably. There is no evidence on the interchangeability between the various serotypes. This information may be clinically useful if it is found that an individual responds to one serotype better than another or develops problems with one serotype but not another. Another question is if a combination of various serotypes would be better than a single serotype.
There are many questions regarding the mechanisms of action of BoNT. It is not known why there is a much longer duration of action when used in patients with NDO. There is strong evidence that BoNT affects the afferent system; however, this is incompletely understood. A better understanding of these mechanisms may allow BoNT that has an even longer duration of action. Once there is a better understanding of the role of the afferent system, work can be done to develop novel BoNTs whose neuronal specificity targets a selected group of neurons (e.g. nociceptive afferent neurons, autonomic nerve terminals).
With regard to the clinical use of BoNTs, it is evident in the literature that there is a need to standardize clinical protocols. Several review articles also address these issues.28,34,41,49,51,72–74 This will be helped with the FDA's recent approved protocol of Botox® (onabotulinumtoxinA). However, there continue to be a number of questions. One of the overall goals is to continue to improve the delivery of small toxin doses close to the affected neuromuscular junctions and afferent receptors. With this in mind, one question is whether there is a single optimum dose or different doses for different conditions. For example, do those with poor bladder wall compliance and bladder wall trabeculation need more or less BoNT injected? A recent phase 3 study showed that 200 U and 300 U doses are similar in effectiveness with regards to improvements in NDO and QoL.75 Of interest is if subsequent injections can be titrated down further. Regarding the technique itself, should the injection be delivered into the submucosal region, detrusor muscle, or both? Further work is needed on the optimum location of injection. Most studies and the current FDA recommendations are to avoid the trigone. There is concern that injections into this area may cause vesicoureteral reflux. However, in studies in which the trigone has been injected this problem has not been reported. It may be that the highly innervated trigone region is in fact a better location for BoNT injections than the rest of the detrusor wall.
Conclusions
Botulinum toxin has emerged as an excellent alternative for individuals with NDO who fail to tolerate anticholinergic medications. Its popularity has increased because of the literature, which has supported its effectiveness, safety, easy use and learning curve, reproducibility of results on repeated use, and recent FDA approval of Botox® (onabotulinumtoxinA). Further research is expected to continue to improve the effectiveness of BoNT in those with NDO.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21(2):167–78 [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Andersson KE, Birder L, Brubaker L, Cardoz L, Chapple C, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 2010;29(1):213–40 [DOI] [PubMed] [Google Scholar]
- 3.Linsenmeyer TA. Neurogenic bladder following spinal cord injury. In: Kirshblum S, Campagnolo DI. (eds.) Spinal cord medicine. 2nd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2011. p. 211–41 [Google Scholar]
- 4.Anderson KD, Borisoff JF, Johnson RD, Stiens SA, Elliott SL. The impact of spinal cord injury on sexual function: concerns of the general population. Spinal Cord 2007;45(5):328–37 [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 2008;101(11):1388–95 [DOI] [PubMed] [Google Scholar]
- 6.Consortium for Spinal Cord Medicine Bladder management for adults with spinal cord injury: a clinical practice guideline for health-care providers. J Spinal Cord Med 2006;29(5):527–73 [PMC free article] [PubMed] [Google Scholar]
- 7.Tempkin A, Sullivan G, Paldi J, Perkash I. Radioisotope renography in spinal cord injury. J Urol 1985;133(2):228–30 [DOI] [PubMed] [Google Scholar]
- 8.Nabi G, Cody JD, Ellis G, Hay-Smith J, Herbison GP. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults (Review). Cochrane Database Syst Rev 2009;18(4):CD003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarker YE, Goa KL, Fitton A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging 1995;6(3):243–62 [DOI] [PubMed] [Google Scholar]
- 10.Linsenmeyer TA, Campagnolo DI, Chou IH. Silent autonomic dysreflexia during voiding in men with spinal cord injuries. J Urol 1996;155(2):519–22 [PubMed] [Google Scholar]
- 11.Horstmann M, Schaefer T, Aguilar Y, Stenzl A, Sievert KD. Neurogenic bladder treatment by doubling the recommended antimuscarinic dosage. Neurourol Urodyn 2006;25(5):441–5 [DOI] [PubMed] [Google Scholar]
- 12.Manack A, Motsko SP, Haag-Molkenteller C, Dmochowski RR, Goehring EL, Jr, Nguyen-Khoa BA, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn 2011;30(3):395–401 [DOI] [PubMed] [Google Scholar]
- 13.Van Ermengem E. Ueber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus. Z Hyg Infektionskr 1897;26:1–56 [PubMed] [Google Scholar]
- 14.PDR (package insert) (Allergan Botox®) [accessed 2012 May 24]. Available from: https://hcp.botoxmedical.com/
- 15.Verheyden J, Blitzer A. Other noncosmetic uses of Botox®. Dis Mon 2002;48(3):357–66 [DOI] [PubMed] [Google Scholar]
- 16.Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD. Effects of botulinum A toxin on detrusor–sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139(5):919–22 [DOI] [PubMed] [Google Scholar]
- 17.Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000;164(3 Pt 1):692–7 [DOI] [PubMed] [Google Scholar]
- 18.Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity; a randomized, double blind, placebo-controlled trial. Eur Urol 2011;60(4):742–50 [DOI] [PubMed] [Google Scholar]
- 19.[Verified 2012 Feb 9]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm269509.htm
- 20.[accessed 2012 Feb 5]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm175013.htm
- 21.Albanese A. Terminology for preparations of botulinum neurotoxins: what a difference a name makes. JAMA 2011;305(1):89–90 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Guidelines on the use of international nonproprietary names (INNS) for pharmaceutical substances Geneva, Switzerland: World Health Organization; 1997. [accessed 2012 May 9]. Available from: http://whqlibdoc.who.int/hq/1997/WHO_PHARM_S_NOM_1570.pdf [Google Scholar]
- 23.Inoue K, Fujinaga Y, Watanabe T, Ohyama T, Tekeshi K, Moriishi K, et al. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun 1996;64(5):1589–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirai Y. Clostridium botulinum and botulinum neurotoxin [Japanese]. Brain Nerve 2011;63(7):755–61 [PubMed] [Google Scholar]
- 25.Meier T, Wallace BG. Formation of the neuromuscular junction: molecules and mechanisms. BioEssays 1998;20(10):819–29 [DOI] [PubMed] [Google Scholar]
- 26.Burnstock G. Neurotransmitters and tropic factors in the autonomic nervous system. J Physiol 1981;313:1–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chancellor MB, Fowler CJ, Apostolidis A, de Groat WC, Smith CP, Somogyi GT, et al. Drug insight: biological effects of botulinum toxin A in the lower urinary tract. Nat Clin Pract Urol 2008;5(6):319–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montecucco C, Molgó J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol 2005;5(3):274–9 [DOI] [PubMed] [Google Scholar]
- 29.Coelho A, Dinis P, Pinto R, Gorgal T, Silva C, Silva A, et al. Distribution of the high-affinity binding site and intra cellular target of botulinum toxin type A in the human bladder. Eur Urol 2010;57(5):884–90 [DOI] [PubMed] [Google Scholar]
- 30.de Paiva A, Meunier FA, Molgó J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci USA 1999;96(6):3200–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haferkamp A, Schurch B, Reitz A, Krengel U, Grosse J, Kramer G, et al. Lack of ultrastructural detrusor changes following endoscopic injection of botulinum toxin type A in overactive neurogenic bladder. Eur Urol 2004;46(6):784–91 [DOI] [PubMed] [Google Scholar]
- 32.Schulte-Baukloh H, Zurawski TH, Knispel HH, Miller K, Haferkamp A, Dolly JO. Persistence of the synaptosomal-associated protein-25 cleavage product after intradetrusor botulinum toxin A injections in patients with myelomeningocele showing an inadequate response to treatment. BJU Int 2007;100(5):1075–80 [DOI] [PubMed] [Google Scholar]
- 33.Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord injured rats. Urology 2005;66(1):208–12 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama T, Chancellor M, Oguma K, Yamamoto Y, Suzuki T, Kumon H, et al. Botulinum toxin type A for the treatment of lower urinary tract disorders. Int J Urol 2012;19(3):202–15 [DOI] [PubMed] [Google Scholar]
- 35.Conte A, Giannantoni A, Proietti S, Giovannozzi S, Fabbrini G, Rossi A, et al. Botulinum toxin A modulates afferent fibers in neurogenic detrusor overactivity. Eur J Neurol 2012;19(5):725–32 [DOI] [PubMed] [Google Scholar]
- 36.Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 2005;174(3):977–83 [DOI] [PubMed] [Google Scholar]
- 37.Reitz A, Stöhrer M, Kramer G, Del Popolo G, Chartier-Kastler E, Pannek J, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol 2004;45(4):510–15 [DOI] [PubMed] [Google Scholar]
- 38.Schurch B, de Sèze M, Denys P, Chartier-Kastler E, Haab F, Everaert K, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005;174(1):196–200 [DOI] [PubMed] [Google Scholar]
- 39.Grosse J, Kramer G, Stöhrer M. Success of repeat detrusor injections of botulinum A toxin in patients with severe neurogenic detrusor overactivity and incontinence. Eur Urol 2005;47(5):653–9 [DOI] [PubMed] [Google Scholar]
- 40.Kuo HC, Liu SH. Effect of repeated detrusor onabotulinumtoxinA injections on bladder and renal function in patients with chronic spinal cord injury. Neurourol Urodyn 2011;30(8):1541–5 [DOI] [PubMed] [Google Scholar]
- 41.Karsenty G, Denys P, Amarenco G, De Seze M, Game? X, Haab F, et al. Botulinum toxin A (Botox®) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic review of the literature. Eur Urol 2008;53(2):275–87 [DOI] [PubMed] [Google Scholar]
- 42.Kalsi V, Gonzales G, Popat R, Apostolidis A, Elneil S, Dasgupta P, et al. Botulinum injections for the treatment of bladder symptoms of multiple sclerosis. Ann Neurol 2007;62(5):452–57 [DOI] [PubMed] [Google Scholar]
- 43.Del Popolo G, Filocamo MT, Li Marzi V, Macchiarella A, Cecconi F, Lombardi G, et al. Neurogenic detrusor overactivity treated with English botulinum toxin A: 8-year experience of one single centre. Eur Urol 2008;53(5):1013–19 [DOI] [PubMed] [Google Scholar]
- 44.Giannantoni A, Mearini E, Del Zingaro M, Porena M. Six-year follow-up of botulinum toxin A intradetrusotial injections in patients with refractory neurogenic detrusor overactivity: clinical and urodynamic results. Eur Urol 2009;55(3):705–11 [DOI] [PubMed] [Google Scholar]
- 45.Deffontaines-Rufin S, Weil M, Verollet D, Peyrat L, Amarenco G. Botulinum toxin A for the treatment of neurogenic detrusor overactivity in multiple sclerosis patients. Int Braz J Urol 2011;37(5):642–8 [DOI] [PubMed] [Google Scholar]
- 46.Ehren I, Volz D, Farrelly E, Berglund L, Brundin L, Hultling C, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomised placebo-controlled, double-blinded study. Scand J Urol Nephrol 2007;41(4):335–40 [DOI] [PubMed] [Google Scholar]
- 47.DasGupta R, Fowler CJ. Sexual and urological dysfunction in multiple sclerosis: better understanding and improving therapies. Curr Opin Neurol 2002;15(3):271–9 [DOI] [PubMed] [Google Scholar]
- 48.Kuo HC. Therapeutic effects of suburothelial injection of botulinum A toxin for neurogenic detrusor overactivity due to chronic cerebrovascular accident and spinal cord lesions. Urology 2006;67(2):232–6 [DOI] [PubMed] [Google Scholar]
- 49.Nitti VW. Botulinum toxin for the treatment of idiopathic and neurogenic overactive bladder: state of the art. Rev Urol 2006;8(4):198–208 [PMC free article] [PubMed] [Google Scholar]
- 50.Mangera A, Andersson KE, Apostolidis A, Chapple C, Dasgupta P, Giannantoni A, et al. Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and Dysport (AbobotulinumtoxinA). Eur Urol 2011;60(4):784–95 [DOI] [PubMed] [Google Scholar]
- 51.Rapp DE, Lucioni A, Bales GT. Botulinum toxin injection: a review of injection principles and protocols. Int Braz J Urol 2007;33(2):132–41 [DOI] [PubMed] [Google Scholar]
- 52.Product information [accessed 2012 Feb 9]. Available from: http://www.allergan.com/assets/pdf/botox_pi.pdf
- 53.L'Hommedieu C, Stough R, Brown L, Kettrick R, Polin R. Potentiation of neuromuscular weakness in infant botulism by aminoglycosides. J Pediatr 1979;95(6):1065–70 [DOI] [PubMed] [Google Scholar]
- 54.Reitz A, Denys P, Fermanian C, Schurch B, Comperat E, Chartier-Kastler E. Do repeat intradetrusor botulinum toxin type A injections yield valuable results? Clinical and urodynamic results after five injections in patients with neurogenic detrusor overactivity. Eur Urol 2007;52(6):1729–35 [DOI] [PubMed] [Google Scholar]
- 55.Karsenty G, Reitz A, Lindemann G, Boy S, Schurch B. Persistence of therapeutic effect after repeated injections of botulinum toxin type A to treat incontinence due to neurogenic detrusor overactivity. Urology 2006;68(6):1193–7 [DOI] [PubMed] [Google Scholar]
- 56.Apostolidis A, Jacques TS, Freeman A, Kalsi V, Popat R, Gonzales G, et al. Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol 2008;53(6):1245–53 Epub 2008 Mar 7 [DOI] [PubMed] [Google Scholar]
- 57.Compeérat E, Reitz A, Delcourt A, Capron F, Denys P, Chartier-Kastler E. Histological features in the urinary bladder wall affected from neurogenic overactivity – a comparison of inflammation, oedema and fibrosis with and without injection of botulinum toxin type A. Eur Urol 2006;50(5):1058–64 [DOI] [PubMed] [Google Scholar]
- 58.Gamé X, Castel-Lacanal E, Bentaleb Y, Thiry-Escudié I, De Boissezon X, Malavaud B, et al. Botulinum toxin A detrusor injections in patients with neurogenic detrusor overactivity significantly decrease the incidence of urinary tract infections. Eur Urol 2008;53(3):613–18 [DOI] [PubMed] [Google Scholar]
- 59.Boy S, Seif C, Braun PM, Bremer J, Bugdorfer H, Chalaen R, et al. Retrospective analysis of treatment outcomes and medical care of patients with neurogenic detrusor overactivity (NDO) receiving Botox® therapy. Eur Urol Suppl 2008;7(3):212 [Google Scholar]
- 60.Azadzoi KM, Pontari M, Vlachiotis J, Siroky MB. Canine bladder blood flow and oxygenation: changes induced by filling, contraction and outlet obstruction. J Urol 1996;155(4):1459–65 [DOI] [PubMed] [Google Scholar]
- 61.Schurch B, Denys P, Kozma CM, Reese PR, Slaton T, Barron RL. Botulinum Toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur Urol 2007;52(3):850–8 [DOI] [PubMed] [Google Scholar]
- 62.Hori S, Patki P, Attar KH, Ismail S, Vasconcelos JC, Shah PJ. Patients’ perspective of botulinum toxin-A as a long-term treatment option for neurogenic detrusor overactivity secondary to spinal cord injury. BJU Int 2009;104(2):216–20 [DOI] [PubMed] [Google Scholar]
- 63.Wu JM, Siddiqui NY, Amundsen CL, Myers ER, Havrilesky LJ, Visco AG. Cost-effectiveness of botulinum toxin A versus anticholinergic medications for idiopathic urge incontinence. J Urol 2009;181(5):613–8 [DOI] [PubMed] [Google Scholar]
- 64.Wefer B, Ehlken B, Bremer J, Burgdörfer H, Domurath B, Hampel C, et al. Treatment outcomes and resource use of patients with neurogenic detrusor overactivity receiving botulinum toxin A (Botox®) therapy in Germany. World J Urol 2010;28(3):385–90 [DOI] [PubMed] [Google Scholar]
- 65.Kalsi V, Popat RB, Apostolidis A, Kavia R, Odeyemi IA, Dakin HA, et al. Cost-consequence analysis evaluating the use of botulinum neurotoxin-A in patients with detrusor overactivity based on clinical outcomes observed at a single UK centre. Eur Urol 2006;49(3):519–27 [DOI] [PubMed] [Google Scholar]
- 66.National Institute on Disability and Rehabilitation Research The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. January 27–29, 1992. J Am Paraplegia Soc 1992;15(3):194–204 [DOI] [PubMed] [Google Scholar]
- 67.Dressler D, Hallett M. Immunological aspects of Botox®, Dysport and Myobloc/NeuroBloc. Eur J Neurol 2006;13Suppl 1:11–15 [DOI] [PubMed] [Google Scholar]
- 68.Consortium for Spinal Cord Medicine Acute management of autonomic dysreflexia: adults with spinal cord injury presenting to health-care facilities. J Spinal Cord Med 2002;25Suppl 1:S67–88 [PubMed] [Google Scholar]
- 69.Narayanan UG. Botulinum toxin: does the black box warning justify change in practice? (Commentary). Dev Med Child Neurol 2011;53(2):101–2 [DOI] [PubMed] [Google Scholar]
- 70.O'Flaherty SJ, Janakan V, Morrow AM, Scheinberg AM, Waugh MC. Adverse events and health status following botulinum toxin A injections in children with cerebral palsy. Dev Med Child Neurol 2011;53(2):125–30 [DOI] [PubMed] [Google Scholar]
- 71.Wyndaele JJ, Van Dromme SA. Muscular weakness as side effect of botulinum toxin injection for neurogenic detrusor overactivity. Spinal Cord 2002;40(11):599–600 [DOI] [PubMed] [Google Scholar]
- 72.Smaldone MC, Ristau BT, Leng WW. Botulinum toxin therapy for neurogenic detrusor overactivity. Urol Clin N Am 2010;37(4):567–80 [DOI] [PubMed] [Google Scholar]
- 73.da Silva CM, Cruz F. Has botulinum toxin therapy come of age: what do we know, what do we need to know, and should we use it? Curr Opin Urol 2009;19(4):347–52 [DOI] [PubMed] [Google Scholar]
- 74.Chancellor MB. Ten years single surgeon experience with botulinum toxin in the urinary tract; clinical observations and research discovery. Int Urol Nephrol 2010;42(2):383–91 [DOI] [PubMed] [Google Scholar]
- 75.Ginsberg D, Gousse A, Keppenne V, Sievert K-D, Thompson C, Lam W, et al. Phase 3. Efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic overactivity. J Urol 2012;187:213–39 [DOI] [PubMed] [Google Scholar]


