Abstract
Context/objective
To describe and compare substrate oxidation and partitioning during voluntary arm ergometry in individuals with paraplegia and non-disabled individuals over a wide range of exercise intensities.
Design
Cross-sectional study.
Setting
Clinical research facility.
Participants
Ten apparently healthy, sedentary men with paraplegia and seven healthy, non-disabled subjects.
Interventions
Rest and continuous progressive voluntary arm ergometry between 30 and 80% of peak aerobic capacity (VO2peak).
Outcome measures
Total energy expenditure and whole body rates of fat and carbohydrate oxidation.
Results
A maximal whole body fat oxidation (WBFO) rate of 0.13 ± 0.07 g/minute was reached at 41 ± 9% VO2peak for subjects with paraplegia, although carbohydrate became the predominant fuel source during exercise exceeding an intensity of 30–40% VO2peak. Both the maximal WBFO rate (0.06 ± 0.04 g/minute) and the intensity at which it occurred (13 ± 3% VO2peak) were significantly lower for the non-disabled subjects than those with paraplegia.
Conclusion
Sedentary individuals with paraplegia are more capable of oxidizing fat during voluntary arm ergometry than non-disabled individuals perhaps due to local adaptations of upper body skeletal muscle used for daily locomotion. However, carbohydrate is the predominant fuel source oxidized across a wide range of intensities during voluntary arm ergometry in those with paraplegia, while WBFO is limited and maximally achieved at low exercise intensities compared to that achieved by able-bodied individuals during leg ergometry. These findings may partially explain the diminished rates of fat loss imposed by acute bouts of physical activity in those with paraplegia.
Keywords: Spinal cord injuries, Paraplegia, Ergometry, Energy metabolism, Physical exertion, Carbohydrates, Fats
Introduction
Low fat oxidation at rest and metabolic inflexibility, the reduced ability to increase fat oxidation in the face of increased fat intake, are considered strong risk factors for weight gain.1,2 Persons with physical disability have a 1.2–3.9-fold higher prevalence of obesity than those without disability.3 An improved understanding of substrate oxidation at rest and during physical activity in those with spinal cord injury (SCI) is important in evaluating exercise as a therapeutic modality to help reduce the prevalence and severity of metabolic diseases such as obesity in this population. In non-disabled individuals, the influence of exercise intensity on substrate partitioning is well understood. As exercise intensity increases the contribution of fat to total energy expenditure (EE) decreases while that of carbohydrate (CHO) increases resulting in a crossover at ∼45–55% of peak oxygen consumption (VO2peak).4,5
Compared to non-disabled controls performing voluntary leg exercise, those with paraplegia and tetraplegia have markedly reduced mobilization, delivery, and limb uptake of free fatty acids (FFA) during electrically stimulated leg exercise that results in the reduced reliance on fat as a substrate.6 These effects of SCI on FFA kinetics may also partially explain the heavy reliance on CHO and the limited contribution of fat during voluntary upper body exercise in well-trained individuals with paraplegia and tetraplegia.7–10 However, little is known regarding these possible effects because plasma FFA kinetics have not been examined in individuals with SCI performing voluntary upper body exercise to date.
Substrate partitioning at rest and during exercise has not been examined in sedentary individuals with SCI, who likely represent a large proportion of this population. These individuals may have a pronounced inability to use fat during voluntary exercise due to their lack of training-induced adaptations to upper body skeletal muscle that improve oxidative capacity11 and vasomotor deficits that compromise oxygen delivery.12 Finally, the range of exercise intensities studied in those with SCI to date is narrow (55–75% VO2peak),7–10 likely beyond the crossover point for sedentary SCI individuals, and prone to inadequately reflect maximal rates of fat oxidation during exercise for this population.
The purpose of this study was to describe and compare substrate oxidation and partitioning during voluntary arm ergometry in apparently healthy, sedentary paraplegic, and non-disabled individuals over a wide range of exercise intensities. Data from non-disabled individuals performing the same voluntary arm ergometry were included to better appreciate the independent effects of paraplegia and exercise mode on substrate oxidation and partitioning. It was hypothesized that both maximal whole body fat oxidation (WBFO) and the intensity at which it would occur would be lower in SCI than non-disabled individuals performing the same exercise task.
Methods
Subjects
Ten apparently healthy, sedentary men aged 29–58 years with motor complete (American Spinal Injury Association A or B) paraplegia at T4–T12 were recruited from the University of Miami, Miller School of Medicine campus (Table 1). Additionally, seven healthy non-disabled subjects (six men and one woman) with the age of 22–40 years who were moderately active, but untrained in arm ergometry were recruited from the University of Miami, Coral Gables campus (Table 1). Subjects were included in the study if they were non-smokers, were diet and body mass stable, had no history of cardiovascular, lung, or renal, or metabolic diseases, and were not taking antibiotics, anticoagulants, or any medication that would interfere with oxygen delivery. The procedures and risks were thoroughly explained to the subjects and their written, voluntary, informed consent was obtained.
Table 1.
Subject characteristics and peak exercise capacity
| Group | Subject | Age (years) | Height (cm) | LOI | Years post-injury | BW (kg) | Wpeak (W) | VO2peak (l/minute) | VO2peak (ml/kg/minute) | HRpeak (bpm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Paraplegic | A | 52 | 170 | T5 | 24 | 77.1 | 75 | 1.5 | 19.6 | 184 |
| B | 47 | 170 | T4 | 2 | 122.5 | 50 | 1.1 | 8.7 | 150 | |
| C | 50 | 173 | T7/8 | 5 | 76.2 | 60 | 1.4 | 18.5 | 126 | |
| D | 50 | 175 | T10 | 32 | 83.9 | 45 | 1.3 | 15.4 | 125 | |
| E | 30 | 191 | T10 | 8 | 135.2 | 90 | 1.9 | 13.8 | 161 | |
| F | 42 | 195 | T6 | 18 | 77.1 | 60 | 1.3 | 16.3 | 151 | |
| G | 52 | 175 | T11 | 14 | 64.0 | 67.5 | 1.2 | 19.1 | 140 | |
| H | 29 | 188 | T12 | 13 | 65.8 | 45 | 1.2 | 17.6 | 171 | |
| I | 58 | 178 | T9 | 19 | 86.8 | 70 | 1.8 | 20.4 | 164 | |
| J | 41 | 178 | T10 | 16 | 93.5 | 90 | 1.9 | 20.6 | 165 | |
| Mean: | 45.1 | 179.3 | — | 15.1 | 88.2 | 65 | 1.45 | 17.0 | 154 | |
| SD: | 9.6 | 8.9 | — | 8.9 | 23.4 | 17 | 0.32 | 3.7 | 19 | |
| Non-disabled | Mean: | 30.3* | 177.8 | — | — | 76.8 | 118* | 2.10* | 27.3* | 168 |
| SD: | 5.6 | 8.7 | — | — | 11.0 | 21 | 0.64 | 5.7 | 8 |
Values are mean ± SD; n = 10 paraplegic and seven non-disabled.
LOI, level of injury; BW, body weight; Wpeak, peak work capacity; VO2peak, peak oxygen consumption; HRpeak, peak heart rate.
*Significantly different than paraplegic (P < 0.015).
Exercise capacity and substrate oxidation
All subjects were screened for exercise safety by completing both PAR-Q and health history questionnaires prior to exercise testing. Subjects were instructed to consume their habitual diet before each trial and to report to the laboratory after a 3–4-hour fast. The time of day of all trials was standardized across all subjects. VO2peak was assessed via continuous progressive voluntary arm exercise on an arm crank ergometer (Monark 881E, Vansbro, Sweden). After 10 minutes of rest, subjects began cycling at 0 W and the workload was increased by 15 W every 3 minutes. Subjects were instructed to maintain a cadence of 55–60 revolutions/minute set by a metronome. Subjects were considered to have reached VO2peak if they attained two of the following three criteria: a plateau in VO2 despite increase in workload, a respiratory exchange ratio (RER) value >1.10, and volitional exhaustion when a cadence of 50 revolutions/minute could not be maintained for more than 10 seconds. These criteria are standard for our laboratory13 and are similar to those used in previously published studies.14,15 Peak work capacity (Wpeak) was calculated as described previously to account for work performed in partially completed stages.16 Expired respiratory gases and heart rate (HR) were continuously collected at rest and during exercise, and analysed with an online open-circuit metabolic analyser integrated with electrocardiographic monitoring (Sensormedics VMax Encore 29C, Palm Springs, CA, USA). Blood pressure was assessed by auscultation at rest and during recovery.
At least 48 hours after the exercise capacity test, subjects reported to the laboratory to complete another continuous progressive voluntary arm ergometer test to evaluate whole body substrate oxidation at 20, 30, 40, 50, 60, 70, and 80% VO2peak. Diet and the time of day of these trials were controlled in the same manner as the exercise capacity test. Individual VO2 data from the exercise capacity test were averaged over the last minute of each stage and plotted against corresponding workloads to generate regression equations used to predict the desired workloads for each subject. After 10 minutes of rest, subjects began cycling at 0 W and the workload was increased by 5–15 W every 3 minutes until all seven work stages were completed. Previous work with moderately trained non-disabled subjects performing leg ergometry found no difference in WBFO rates determined from either 3- or 5-minute stages.17 Expired respiratory gases, HR, and blood pressure were assessed using the same methods described for the exercise capacity test.
Calculations
Substrate oxidation rates were derived from respiratory data averaged over the last minute of each 3-minute stage when RER was less than 1.0. The proportion of EE derived from CHO and fat and the rates of EE, whole body CHO and fat oxidation rates were calculated using stochiometric equations assuming that urinary nitrogen excretion was negligible due to the subjects being diet and body mass stable and not having renal diseases as previously described18:
 |
where VO2 is expressed in l/minute.
Statistics
All data are represented as mean ± SD. The significance of differences between paraplegic and non-disabled subjects in subject characteristics, maximal WBFO, and the intensity at which it occurred were assessed by unpaired t-tests. The significance of differences in the physiological responses and substrate oxidation across stages were assessed by two-way analysis of variance (group × stage) with repeated measures followed by post hoc analyses using the least significant difference test. Significance was set a priori at α < 0.05.
Results
Subject characteristics and Wpeak
Paraplegic subjects were significantly older than the non-disabled subjects and had significantly lower VO2peak and Wpeak values (Table 1; P < 0.015). Peak exercise capacity (17.0 ± 3.7 ml/kg/minute) was similar to previously published values for untrained SCI men19 and ∼40–60% lower than those of trained SCI subjects (27.8–40.2 ml/kg/minute).8–10
HR, respiratory measures, and EE
The seven prescribed workloads during the continual graded exercise test were targeted to elicit relative exercise intensities of 20, 30, 40, 50, 60, 70, and 80% VO2peak. However, most paraplegic subjects' resting VO2 represented ∼20% of VO2peak and the prescribed workloads ranging from 0 to 70 W elicited relative intensities of 30–80% VO2peak (Table 2). While most subjects completed six stages during the continuous progressive voluntary arm ergometer test, five that completed seven stages tended to have higher VO2peak values (18.6 ± 2.8 vs. 15.4 ± 4.0 ml/kg/minute, P= 0.11). There was a significant effect of stage in which HR, VO2, %VO2peak, VCO2, and EE increased with each stage, while RER increased from stage four and higher (P < 0.001). HR values at the end of the continuous progressive voluntary arm ergometer test (∼80% VO2peak) were 84–87% of HRpeak. RER values were greater than 1.0 at relative exercise intensities above 60% VO2peak. There were no significant differences in VO2 (stages 1–7) or calculated rates of WBFO (stages 1–4) during the last minute of each 3-minute stage (i.e. 30 seconds average from minute 2:00–2:30 vs. 30 seconds average from minute 2:30–3:00) indicating that subjects had reached a steady state in the last minute of each stage.
Table 2.
HR, respiratory measures, and TEE at rest and during continuous progressive voluntary arm ergometry for paraplegic subjects
| Variable | Rest | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 6 | Stage 7 |
|---|---|---|---|---|---|---|---|---|
| n | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 5 |
| Power output range (W) | — | 0–0 | 5–10 | 10–20 | 15–35 | 20–45 | 30–60 | 40–70 |
| Power output (W) | — | 0 ± 0 | 6 ± 2 | 14 ± 4 | 24 ± 7 | 32 ± 8 | 43 ± 11 | 56 ± 13 |
| HR (beats/minute) | 77 ± 8 | 84 ± 6 | 94 ± 7 | 97 ± 8 | 105 ± 11 | 113 ± 12 | 127 ± 17 | 134 ± 19 |
| VO2 (ml/kg/minute) | 3.4 ± 0.6 | 4.9 ± 1.0 | 6.9 ± 1.6 | 7.7 ± 1.5 | 9.6 ± 1.9 | 11.1 ± 2.0 | 13.4 ± 2.8 | 15.0 ± 3.1 |
| VO2peak (%) | 20.4 ± 4.3 | 29.7 ± 6.8 | 41.1 ± 8.8 | 46.3 ± 8.0 | 58.2 ± 13.1 | 67.6 ± 14.5 | 75.0 ± 13.1 | 81.2 ± 12.9 |
| VCO2 (ml/kg/minute) | 2.9 ± 0.6 | 4.2 ± 1.1 | 6.1 ± 1.9 | 7.1 ± 1.7 | 9.4 ± 2.1 | 11.6 ± 2.9 | 14.8 ± 4.1 | 16.6 ± 4.2 |
| RER | 0.87 ± 0.06 | 0.85 ± 0.07 | 0.88 ± 0.06 | 0.91 ± 0.06 | 0.97 ± 0.06 | 1.03 ± 0.09 | 1.09 ± 0.09 | 1.09 ± 0.06 |
| EE (kcal/minute) | 1.42 ± 0.32 | 2.03 ± 0.32 | 2.85 ± 0.47 | 3.26 ± 0.58 | 4.14 ± 0.80 | — | — | — |
Values are mean ± SD; n = 10 subjects. Significant main effect of intensity for all variables (RER stages 4–7 > all previous) (P < 0.001).
W, power output; VO2, oxygen consumption; VCO2, carbon dioxide production; RER, respiratory exchange ratio; EE, energy expenditure.
Substrate oxidation
The range of WBFO at intensities below 60% VO2peak where RER was less than 1.0 was 0.07–0.13 g/minute (2.39–4.87 µmol/kg/minute) for subjects with paraplegia and only 0.02–0.06 g/minute (0.88–2.59 µmol/kg/minute) for non-disabled subjects (Fig. 1A and B). Subjects with paraplegia had significantly higher values than non-disabled subjects for both maximal WBFO (0.13 ± 0.07 vs. 0.06 ± 0.04 g/minute, P< 0.05) and the intensity at which it occurred (41 ± 9 vs. 13 ± 3% VO2peak, P< 0.05). The range of whole body CHO oxidation at intensities below 60% VO2peak where RER was less than 1.0 was 0.16–0.87 g/minute (11.30–58.18 µmol/kg/minute) for subjects with paraplegia and 0.22–1.19 g/minute (16.21–86.30 µmol/kg/minute) for non-disabled subjects (Fig. 1A and B). There were significant main effects of stage for both whole body fat and CHO oxidation. There was clearly a crossover point for paraplegic subjects between 30 and 40% of VO2peak, beyond which CHO oxidation supported the majority of EE (Figs. 2A and 3A). Fat and CHO contributed nearly equally to EE up to 30% VO2peak for subjects with paraplegia, while CHO supported 56–85% of EE between 40 and 60% of VO2peak (Fig. 3A). Substrate oxidation of non-disabled subjects was predominated by CHO (Figs. 2B and 3B), which supported 64–97% of EE between rest and 49% of VO2peak (Fig. 3B).
Figure 1.
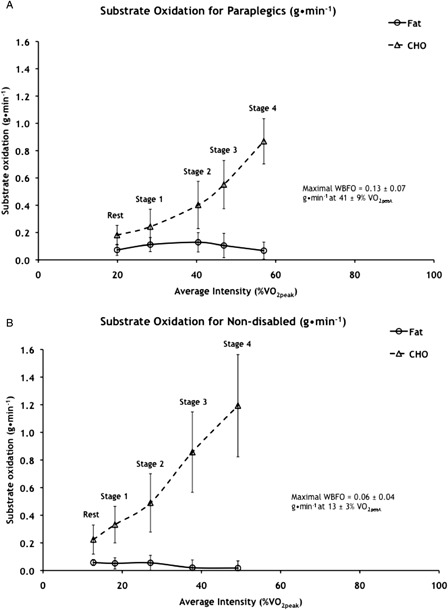
Whole body fat and carbohydrate oxidation expressed as g/minute for (A) paraplegic and (B) non-disabled subjects. Values are mean ± SD; n = 10 paraplegic and 7 non-disabled subjects. VO2peak, peak oxygen consumption. Significant main effect of stage for fat oxidation of paraplegic: stage 1 > rest, stage 4 (P < 0.008), stage 2 > rest, stages 3 and 4 (P < 0.044), and stage 4 < stages 1–3 (P < 0.008). Significant effect of stage for fat oxidation of non-disabled: rest, stages 1 and 2 > stages 3 and 4 (P < 0.009). Significant main effect of stage for carbohydrate oxidation of paraplegic and non-disabled: each stage > all previous stages (P < 0.008).
Figure 2.
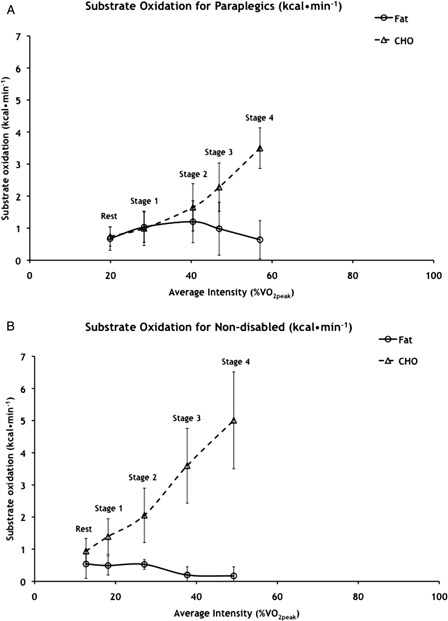
Whole body fat and carbohydrate oxidation expressed as kcal/minute for (A) paraplegic and (B) non-disabled subjects. Values are means ± SD; n = 10 paraplegic and 7 non-disabled subjects. CHO, carbohydrate; VO2peak, peak oxygen consumption. Significant main effect of stage for fat oxidation of paraplegic: stage 1 > rest, stage 4 (P < 0.013) and stage 2 > rest, stages 3 and 4 (P < 0.022). Significant main effect of stage for CHO oxidation of paraplegic: each stage > previous stage (P < 0.005). Significant effect of stage for fat oxidation of non-disabled: rest, stages 1 and 2 > stages 3 and 4 (P < 0.022). Significant effect of stage for CHO oxidation of non-disabled: each stage > previous stage (P < 0.005).
Figure 3.
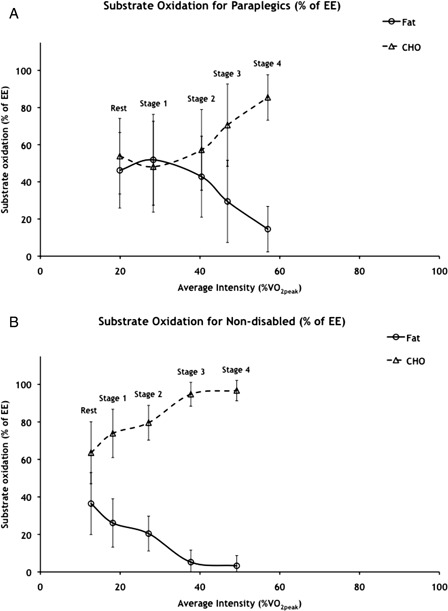
Whole body fat and carbohydrate oxidation expressed as percent of EE for (A) paraplegic and (B) non-disabled subjects. Values are means ± SD; n = 10 paraplegic and 7 non-disabled subjects. CHO, carbohydrate; VO2peak, peak oxygen consumption. Significant main effect of stage for fat oxidation for paraplegic: stage 1 > stages 2–4 (P < 0.048), stage 2 < stage 1 (P < 0.048), and stages 3 and 4 < all previous stages (P < 0.008). Significant main effect of stage for CHO oxidation of paraplegic: stage 1 < stages 2–4 (P < 0.048), stage 2 > stage 1 (P < 0.048), and stages 3 and 4 > all previous stages (P < 0.008) for carbohydrate. Significant effect of stage for fat oxidation of non-disabled: rest > stages 1–4 (P < 0.038), stages 3 and 4 < all previous stages (P < 0.015). Significant effect of stage for CHO oxidation of non-disabled: rest < stages 1–4 (P < 0.038), stages 3 and 4 > all previous stages (P < 0.015).
Discussion
Our results indicate that apparently healthy, sedentary individuals with paraplegia reach maximal WBFO at a relatively low intensity and crossover to heavily rely on CHO oxidation to support a majority of their energy needs during voluntary arm ergometry between 30 and 40% of VO2peak. The limited ability of individuals with SCI to increase EE and oxidize fat during voluntary exercise highlights the need for a multifaceted approach in the prevention and treatment of obesity and other metabolic diseases in this population.
SCI and substrate use during exercise
Substrate partitioning among non-disabled subjects performing treadmill exercise is characterized by a crossover at ∼45–55% of VO2peak, beyond which CHO supports the majority of EE.5 However, the subjects with paraplegia in this study reached an earlier crossover point between 30 and 40% of VO2peak while performing voluntary arm ergometry (Figs. 2A and 3A). This heavy reliance on CHO as a fuel source across a wide range of exercise intensities may be partly attributable to the impact of SCI on fat mobilization. In the transition from rest to involuntary electrically stimulated leg exercise, individuals with paraplegia and tetraplegia have marked decreases in plasma FFA mobilization and delivery to peripheral tissues resulting in little or no increase in active limb uptake of plasma FFA.6 These findings highlight the importance of reduced neural activity in motor centers and afferent nerves from working skeletal muscle in most individuals with SCI, and impaired release of catecholamines in those with high cervical injuries during involuntary electrically stimulated leg exercise. However, plasma FFA kinetics have not been examined in individuals with SCI performing voluntary upper body exercise as in this study and the influences of neural activity and catecholamine concentrations on WBFO during this type of exercise are not clearly understood.
There remains the possibility that vasomotor deficits in those with paraplegia may compromise oxygen delivery to active skeletal muscle and ultimately impair WBFO. Untrained paraplegic individuals with much lower arm ergometry VO2peak values than non-disabled controls were able to achieve equal peak cardiac outputs.12 The much lower resultant calculated peak (a − v) O2 difference values for the individuals with paraplegia was thought to be due to their compromised ability to regulate blood flow below their level of injury and deliver oxygen to active upper body skeletal muscle. Future work will have to more closely examine the relationship between level of SCI and active limb balances of oxygen and metabolites to better understand whether vasomotor deficits associated with SCI alter oxygen delivery and active muscle substrate use.
Exercise mode and substrate use during exercise
The minimal reliance on fat as a fuel observed in this study may be more a function of the mode of voluntary exercise performed rather than paraplegia per se. Maximal WBFO rates of non-disabled individuals performing treadmill exercise (0.46 ± 0.1 g/minute at 48 ± 1% VO2peak)5 and those of the paraplegic subjects from this study performing voluntary arm ergometry (0.13 ± 0.07 g/minute at 41 ± 9% VO2peak; Fig. 1A) differ widely. However, when the non-disabled subjects of this study performed the same continuous progressive voluntary arm ergometer test as the subjects with paraplegia, they only reached a maximal WBFO rate of 0.06 ± 0.04 g/minute at 13 ± 3% VO2peak.
Differences in substrate partitioning between upper and lower body exercise have primarily been examined in non-disabled individuals20 and are complicated by large differences in VO2peak between the two modes of exercise. Certainly, the smaller mass of musculature in the upper body and consequent 25–30% lower VO2peak achieved during upper vs. lower body exercise21 would translate to significantly lower maximal rates of whole body fat and CHO oxidation. Additionally, lower metabolic efficiency20 and lower oxygen extraction22 may explain the heavy reliance on CHO found in this study.
WBFO in paraplegic vs. non-disabled subjects
The paraplegic subjects of this study had WBFO values that were twice those of non-disabled subjects. One other study has shown that subjects with paraplegia (T3–L1) have similar or higher rates of WBFO than non-disabled subjects during 60 minutes of arm ergometry after consuming water or glucose, respectively.8 These authors suggested that the daily use of the upper body by those with SCI for locomotion and other common tasks may be sufficient to produce improvements in WBFO during exercise by inducing a shift towards Type I muscle fiber. While no longitudinal studies to date have examined training-induced adaptations in substrate oxidation in those with SCI, cross-sectional evidence does suggest that that long-term voluntary upper body exercise training may induce adaptations that increase WBFO during exercise in those with SCI. Examination of the anterior portion of the deltoid of trained and untrained individuals with SCI indicated that training resulted in higher levels of mitochondrial enzymes and greater capillary density.11 Additionally, trained subjects with SCI with similar levels of injury (C7–L1), but much higher VO2peak values than the current subjects (35.0–37.5 ml/kg/minute) have been shown to have maximal WBFO rates during voluntary arm ergometry (0.22–0.28 g/minute) that exceed those of this study (0.13 ± 0.07 g/minute).9,10 However, these studies only measured substrate oxidation at intensities ranging from 55 to 75% VO2peak, and given that maximal WBFO occurred at 41 ± 9% VO2peak in this study (Figs. 1A and 2A), they may have missed the true maximal WBFO rates of their subjects. Therefore, the greater ability of the paraplegic subjects to oxidize fat compared to the non-disabled subjects of this study may have been due to some degree of adaptation within the upper body skeletal muscle of the subjects with paraplegia induced by daily locomotion coupled with the unfamiliarity of arm ergometry exercise to the non-disabled and their likely lower metabolic efficiency.
Implications for the use of exercise as a therapeutic modality
Prescribing exercise for those with SCI that coincides with maximal rates of WBFO observed in our study (∼40% VO2peak) is likely impractical, as this intensity is below the training threshold needed to induce significant increases in VO2peak. Regardless of the chosen exercise intensity, individuals with SCI may lack the ability to oxidize significant amounts of fat during voluntary exercise. The addition of involuntary FES training of the lower body holds the promise of involving a greater volume of contracting musculature, thus maximizing fat oxidation and total EE during exercise. Exercise programs that promote maximal EE, regardless of substrate source, are likely to be the most beneficial in reducing the prevalence and severity of obesity and other related metabolic disorders in those with SCI. The limited ability of persons with SCI to increase EE during voluntary exercise highlights the necessity of a multifaceted approach in the prevention and treatment of obesity and other metabolic diseases in this population, in which exercise is accompanied by nutritional and behavioral interventions.
Study limitations
As with many studies of those with SCI, our subject pool was small and heterogeneous. A limitation of this study was that the subjects with paraplegia had significantly lower Wpeak and VO2peak values compared with the non-disabled subjects. This is a common limitation of studies that compare SCI and non-disabled subjects and others have reported similar differences in VO2peak between untrained paraplegic (1.12 l/minute, 17.5 ml/kg/minute) and non-disabled subjects (2.53 l/minute, 38.7 ml/kg/minute) performing voluntary arm ergometry.12 Matching these subjects on arm ergometry VO2peak requires the recruitment of highly trained subjects with paraplegia,14,23 significantly narrowing the scope and applicability of the findings. It also should be noted that the workloads of the paraplegic and non-disabled subjects were matched for relative intensity, the most important variable in the determination of substrate partitioning during exercise.4
Subjects were instructed to maintain their habitual food intake prior to each trial and reported to the laboratory after a 3–4-hour fast, but diet was not rigorously controlled. However, a recent study in individuals with SCI found no difference in CHO or fat oxidation rates during 60 minutes of moderate intensity exercise with or without pre-exercise glucose feeding.8
Conclusions
Use of their upper body on a daily basis may improve the ability of sedentary individuals with paraplegia to oxidize fat during exercise compared to non-disabled individuals. However, an early crossover from fat to CHO, low maximal rates of WBFO compared to what can be achieved by non-disabled individuals during leg ergometry, and a heavy reliance on CHO characterizes substrate oxidation during voluntary arm ergometry among those with paraplegia across a wide range of exercise intensities. This limited ability to oxidize fat may largely be due to the mode of voluntary exercise available to those with paraplegia rather than their injury per se. This highlights the need for the incorporation of other forms of exercise such as involuntary FES training of the lower body as part of a multifaceted approach in the prevention and treatment of obesity and other metabolic diseases in this population.
Acknowledgements
The authors wish to thank the participants for their time and efforts. Additionally, we would like to thank Jennifer Raeburn and Elizabeth Edwards for their assistance in the preparation of this manuscript.
References
- 1.Astrup A. The relevance of increased fat oxidation for body-weight management: metabolic inflexibility in the predisposition to weight gain. Obes Rev 2011;12(10):859–65 [DOI] [PubMed] [Google Scholar]
- 2.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259(5 Pt 1):E650–7 [DOI] [PubMed] [Google Scholar]
- 3.Liou TH, Pi-Sunyer FX, Laferrere B. Physical disability and obesity. Nutr Rev 2005;63(10):321–31 [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 1994;76(6):2253–61 [DOI] [PubMed] [Google Scholar]
- 5.Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol 2005;98(1):160–7 [DOI] [PubMed] [Google Scholar]
- 6.Kjaer M, Dela F, Sorensen FB, Secher NH, Bangsbo J, Mohr T, et al. Fatty acid kinetics and carbohydrate metabolism during electrical exercise in spinal cord-injured humans. Am J Physiol Regul Integr Comp Physiol 2001;281(5):R1492–8 [DOI] [PubMed] [Google Scholar]
- 7.Astorino TA, Harness ET. Substrate metabolism during exercise in the spinal cord injured. Eur J Appl Physiol 2009;106(2):187–93 [DOI] [PubMed] [Google Scholar]
- 8.Jung W, Yamasaki M. Effect of pre-exercise carbohydrate ingestion on substrate consumption in persons with spinal cord injury. Spinal Cord 2009;47(6):464–9 [DOI] [PubMed] [Google Scholar]
- 9.Knechtle B, Muller G, Knecht H. Optimal exercise intensities for fat metabolism in handbike cycling and cycling. Spinal Cord 2004;42(10):564–72 [DOI] [PubMed] [Google Scholar]
- 10.Knechtle B, Muller G, Willmann F, Eser P, Knecht H. Fat oxidation at different intensities in wheelchair racing. Spinal Cord 2004;42(1):24–8 [DOI] [PubMed] [Google Scholar]
- 11.Schantz P, Sjoberg B, Widebeck AM, Ekblom B. Skeletal muscle of trained and untrained paraplegics and tetraplegics. Acta Physiol Scand 1997;161(1):31–9 [DOI] [PubMed] [Google Scholar]
- 12.Jehl JL, Gandmontagne M, Pastene G, Eyssette M, Flandrois R, Coudert J. Cardiac output during exercise in paraplegic subjects. Eur J Appl Physiol Occup Physiol 1991;62(4):256–60 [DOI] [PubMed] [Google Scholar]
- 13.Jacobs PL, Nash MS, Rusinowski JW. Circuit training provides cardiorespiratory and strength benefits in persons with paraplegia. Med Sci Sports Exerc 2001;33(5):711–7 [DOI] [PubMed] [Google Scholar]
- 14.Hopman MT, Pistorius M, Kamerbeek IC, Binkhorst RA. Cardiac output in paraplegic subjects at high exercise intensities. Eur J Appl Physiol Occup Physiol 1993;66(6):531–5 [DOI] [PubMed] [Google Scholar]
- 15.Yasuda N, Ruby BC, Gaskill SE. Substrate oxidation during incremental arm and leg exercise in men and women matched for ventilatory threshold. J Sports Sci 2006;24(12):1281–9 [DOI] [PubMed] [Google Scholar]
- 16.Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc 1996;28:266–70 [DOI] [PubMed] [Google Scholar]
- 17.Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc 2002;34(1):92–7 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs KA, Casazza GA, Suh SH, Horning MA, Brooks GA. Fatty acid reesterification but not oxidation is increased by oral contraceptive use in women. J Appl Physiol 2005;98(5):1720–31 [DOI] [PubMed] [Google Scholar]
- 19.Nash MS, van de Ven I, van Elk N, Johnson BM. Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch Phys Med Rehabil 2007;88(1):70–5 [DOI] [PubMed] [Google Scholar]
- 20.Kang J, Robertson RJ, Goss FL, Dasilva SG, Suminski RR, Utter AC, et al. Metabolic efficiency during arm and leg exercise at the same relative intensities. Med Sci Sports Exerc 1997;29(3):377–82 [DOI] [PubMed] [Google Scholar]
- 21.Astrand PO, Saltin B. Maximal oxygen uptake and heart rate in various types of muscular activity. J Appl Physiol 1961;16:977–81 [DOI] [PubMed] [Google Scholar]
- 22.Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M, Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol 2005;289(5):R1448–58 [DOI] [PubMed] [Google Scholar]
- 23.Schneider DA, Sedlock DA, Gass E, Gass G. VO2peak and the gas-exchange anaerobic threshold during incremental arm cranking in able-bodied and paraplegic men. Eur J Appl Physiol Occup Physiol 1999;80(4):292–7 [DOI] [PubMed] [Google Scholar]


