Abstract
Objective
Decentralized autonomic cardiovascular regulation may lead to increased prevalence of heart rate (HR) and blood pressure (BP) abnormalities in veterans with SCI. In addition, comorbid medical conditions and prescription medication use may increase HR and BP abnormalities. These abnormalities include bradycardia, and tachycardia, hypotension, hypertension as well as autonomic dysreflexia and orthostatic hypotension; the prevalence of which is unknown.
Design
HR and BP data were measured during a routine annual physical examination in 64 veterans with SCI. Measurements of HR and BP were recorded in the seated and supine positions to document the influence of body position and to document intra-subject variability in these assessments.
Results
All subjects were chronically injured (20 ± 14 years), 33 subjects were tetraplegic (T: C3–C8), nine had high paraplegia (HP: T1–T6), and 22 had low paraplegia (LP: T7–L2). Regardless of position, the prevalence of bradycardia was increased in the T group, whereas prevalence of tachycardia was increased in the HP and LP groups. Systolic hypotension was more common in the T and HP groups than the LP group and positional effects were most evident in the T group. Systolic hypertension was comparable in the T and HP groups but was twice as prevalent in the LP group. Increased prevalence of individuals with three or more medical conditions and prescribed three or more medications which might influence HR and BP was observed.
Conclusion
Decentralized autonomic regulation, comorbid medical conditions, and prescription medication use in veterans with SCI result in HR and BP abnormalities; our data suggest that these abnormalities vary depending on the level of injury and orthostatic positioning.
Keywords: Spinal cord injuries, Bradycardia, Tachycardia, Hypotension, Hypertension, Tetraplegia, Paraplegia, Autonomic function, Cardiovascular disease, Veterans
Introduction
Current estimates indicate that there are 25 000 veterans with spinal cord injury (SCI),1 and the clinical care and management of these individuals is reported to be one of the most costly endeavors to the veterans health administration.2 Although improvements in post-injury acute and subacute care have contributed to extended life expectancies, longevity in persons with SCI remains below that of the general population.3 An increased incidence of age-associated chronic illnesses in individuals with SCI is reported4 and accelerated cardiovascular aging has been proposed.5 As such, health care providers are confronted with the challenge of managing the secondary medical consequences of SCI with chronic conditions, which are prevalent in an aging population, including management of cardiovascular disease (CVD). Due to the lack of specific clinical guidelines for the management of CVD in the SCI population, guidelines that are followed in the general population are used; however, these treatments may be less efficacious, or may worsen cardiovascular homeostasis, in persons with SCI due to autonomic dysfunction.
The co-occurrence of an autonomic lesion at or about the site of motor and sensory loss may contribute to complete or partial decentralized cardiovascular control resulting in a multitude of regulatory changes post-SCI. Although the American Spinal Injury Association has developed a classification scale (AIS) to document remaining motor and sensory function following SCI,6,7 the degree of autonomic impairment is not considered within these classifications.8,9 That said, it is reported that decentralized autonomic cardiovascular control following SCI may alter heart rate (HR) and blood pressure (BP), and these cardiovascular alterations may relate to the level of SCI documented with the AIS classification,10,11 and may reflect orthostatic positioning.11,12 In fact, a recent review of the relevant literature reported lesion-dependent changes in cardiovascular function and suggested that orthostatic positioning significantly influenced BP outcomes in persons with tetraplegia.13
Prior to identifying the prevalence of HR and BP abnormalities (i.e. resting values that fall outside the expected normal range) in persons with SCI, operative definitions must be considered. The International Standards to document Remaining Autonomic Function after Spinal Cord Injury (ISAFSCI) initially established guidelines for the assessment of HR and BP abnormalities in 2009,9 which was updated in 2012, but the thresholds remained consistent.14,15 Specifically, bradycardia is defined as a HR ≤60 beats/minute (bpm) and tachycardia as a HR ≥100 bpm.14,15 Hypotension is defined as a systolic BP (SBP) ≤90 mmHg and a diastolic BP (DBP) ≤60 mmHg; hypertension is defined as SBP ≥140 and DBP ≥90 mmHg.14,15 While these definitions comply with standards established in the non-SCI population, due to decentralized cardiovascular control, they may not be appropriate for use in the SCI population. In addition, relatively recent evidence has emerged which associates adverse outcomes in the general population with other HR16,17 and BP thresholds.18–21 Beyond the clinical consequences of alterations in HR and BP, persons with SCI may experience loss of independence and life quality related to the inability to adequately maintain cardiovascular homeostasis; however, until we gain a better understanding of the prevalence of these abnormalities, the development and testing of effective treatment strategies will not be a priority. Therefore, we measured HR and BP during routine annual physical examinations in a sample of veterans with SCI from an urban Veterans Affairs Medical Center. Similar to a recent report,13 we hypothesized that level of SCI (i.e. the higher the lesion level the greater the prevalence of abnormal HR and BP recordings) and orthostatic positioning (i.e. increased prevalence of abnormal HR and BP recordings in the seated versus the supine position) would influence the prevalence of HR and BP abnormalities. In addition, we hypothesized that intra-subject variability would be increased in those with a higher cord lesion.
Methods
Patients with SCI were approached and asked to participate in this study during their annual physical examination. Eligible veterans with SCI who were injured for less than 1 year or had a current illness or infection were excluded from study participation. If a patient agreed to participate, the study coordinator reviewed the procedures with the patient and obtained informed consent in one of the clinic rooms. Prior to initiating data collection procedures for HR and BP assessments subjects were asked questions related to their personal characteristics (age, injury level, completeness, and duration of injury, etc.), medical history, and prescribed medications; this subject-reported information was then verified via medical chart review and corrected if needed by a study investigator (M.G.). Individuals with SCI were categorized by the neurological level of lesion with relevance to sympathetic cardiac innervation; specifically, tetraplegia (T: C3–C8), high paraplegia (HP: T1–T6), or low paraplegia (LP: T7–L2). Subjects were then transferred to a clinic bed for instrumentation and a period of quiet rest. All of the study procedures were approved by the IRB and were performed at the local Veterans Affairs Medical Center.
Heart rate and blood pressure data collection
Subjects were transferred to a clinic bed where they remained in the supine position while three electrodes were placed on their chests for HR assessments and a cuff was placed around their right upper arm for BP assessments (Dynamap Pro 300; GE Healthcare, Buckinghamshire, UK). Two electrocardiogram electrodes were placed in the left and right subclavicular space and one was placed in the V5 position; which was used to record HR (IVY 101R; Biomedical Systems Inc., Branford, CT, USA). After a 20-minute period of quiet rest, HR and BP were assessed and recorded once per minute for 5 minutes while the subject remained in the supine position. Subjects were then transferred to the seated position in their wheelchair, and after a period of acclimatization (∼5 minutes), a second period of HR and BP assessment was recorded for 5 minutes (one time per minute). HR and BP data were recorded at minutes 0, 1, 2, 3, 4, and 5 for six recordings, in most cases, in the seated and supine positions.
Operative definitions
The definitions for HR, SBP, and DBP abnormality thresholds are presented (Table 1). These definitions were taken primarily from the ISAFSCI guidelines published by an international consortium of scientists and clinicians;9,14,22 however, we are proposing modifications to these definitions.
Table 1.
Definitions of abnormal HR and BP
| HR (bpm) | SBP (mmHg) | DBP (mmHg) |
|---|---|---|
| ≤50* | ≤90 | ≤60 |
| ≤60 | Male ≤110* | ≥90 |
| ≥80*† | Females: ≤100* | |
| ≥100 | ≥140 |
*Proposed modification to abnormality threshold.
†Proposed modification to ISAFSCI threshold definitions.
Data analysis
Group demographic and HR and BP data are presented as mean ± standard deviation; statistical significance was set at the 0.05 alpha level. Data on medical comorbid conditions and prescription medication use are presented as the affirmative number and percent per categorical level of lesion. The proportion of observations of HR and BP within the defined categories was calculated as the number of observations/total observations within each group (T, HP, LP) and positional category (seated, supine). The observed recordings for HR, SBP, and DBP were averaged across the available number of scores within each subject. This subject data averaging occurred for the seated scores, the supine scores, and all scores combined, which were then analysed by group using Chi-square analyses. Average HR and BP data were categorized as defined (Table 1). Finally, the standard error of measurement (SEM) and associated 95% confidence interval was calculated from all observations in each subject to evaluate the degree of measurement error across multiple recordings of HR and BP during a single clinical visit as previously reported for physiological data.23,24
Results
Seventy-two veterans with SCI signed consent to participate in the study: two withdrew prior to data collection and six were lost due to scheduling issues; the remaining 64 subjects (89%) completed all study procedures (Table 2). The study population consisted of 63 men, all had chronic injuries (duration of injury range 1–54 years) and 23 were classified with complete injuries (AIS A); the other 42 were classified as having incomplete lesions (AIS B, C, and D). There were no significant effects of AIS classification on the prevalence of HR and BP abnormalities in this study cohort. It should be noted that the only female participant was a 62-year-old with incomplete tetraplegia, who had normal HR and BP recordings.
Table 2.
Subject characteristics
| Total | T | HP | LP | |
|---|---|---|---|---|
| n = 64 | n = 33 | n = 9 | n = 22 | |
| Age (years) | 58 ± 12 | 61 ± 10* | 48 ± 20† | 57 ± 10 |
| Age range (years) | 22–83 | 37–79 | 22–83 | 32–73 |
| Height (cm) | 179 ± 8 | 179 ± 9 | 178 ± 7 | 178 ± 6 |
| Weight (kg) | 88 ± 21 | 90 ± 21* | 72 ± 13† | 92 ± 23 |
| BMI (kg/m2) | 27.7 ± 6.5 | 27.9 ± 6.3 | 23.1 ± 4.0† | 29.1 ± 7.1 |
| Duration of injury (years) | 20 ± 14 | 20 ± 14 | 18 ± 18 | 20 ± 12 |
| Level of lesion | C3–L1 | C3–C8 | T1–T5 | T7–L1 |
| Completeness of lesion n (%) | 23 (36) | 9 (27) | 6 (67) | 8 (36) |
BMI = body mass index.
* versus HP.
† versus LP.
Results from the medical history questionnaire are presented for the total study population and by group (Table 3). The most commonly diagnosed comorbid conditions were hypercholesterolemia (44%; 18 subjects), hypertension (42%; 27 subjects), and diabetes mellitus (28%; 18 subjects) and the most commonly prescribed class of medication were anti-hypertensive agents (67%), followed by anti-spasmodic medications (41%), and medication for chronic pain (19%).
Table 3.
Medical history
| Total | T | HP | LP | |
|---|---|---|---|---|
| Current smokers - n (%) | 17 (27) | 9 (27) | 1 (11) | 7 (32) |
| Comorbid conditions | ||||
| None - n (%) | 7 (11) | 2 (6) | 2 (22) | 3 (14) |
| One - n (%) | 18 (28) | 11 (33) | 2 (22) | 5 (23) |
| Two -n(%) | 20 (31) | 10 (30) | 4 (44) | 6 (27) |
| Three or more - n (%) | 19 (30) | 10 (30) | 1 (11) | 8 (36) |
| Prescription medications | ||||
| None - n (%) | 7 (11) | 6 (18) | 1 (11) | 0 |
| One - n (%) | 7 (11) | 4 (12) | 1 (11) | 2 (9) |
| Two - n (%) | 19 (30) | 8 (24) | 2 (22) | 9 (41) |
| Three or more - n (%) | 31 (48) | 15 (45) | 5 (55) | 11 (50) |
We observed and recorded 699 HR and BP values among the 64 study subjects: 342 were in the seated position and 357 were in the supine position. Of the total observations, 345 were in individuals with tetraplegia, 173 in those with HP, and 181 in those with LP. The prevalence of HR and BP abnormalities is presented by group affiliation and orthostatic position (Table 4).
Table 4.
Prevalence data for HR and BP abnormalities
| Tetraplegia (%) |
High paraplegia (%) |
Low paraplegia (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Seated | Supine | Total | Seated | Supine | Total | Seated | Supine | |
| n = 345 | n = 167 | n = 178 | n = 173 | n = 88 | n = 85 | n = 181 | n = 87 | n = 94 | |
| Heart rate | |||||||||
| ≤50 bpm | 7.25 | 3.59 | 10.67 | 0.00 | 0.00 | 0.00 | 1.10 | 1.15 | 1.06 |
| 51-60 bpm | 18.26 | 16.17 | 20.22 | 1.16 | 0.00 | 2.35 | 9.39 | 5.75 | 12.77 |
| 80-99 bpm | 17.97 | 23.35 | 12.92 | 42.77 | 45.45 | 40.00 | 45.30 | 47.13 | 43.62 |
| ≥100 bpm | 0.00 | 0.00 | 0.00 | 15.61 | 19.32 | 11.76 | 2.21 | 4.60 | 0.00 |
| Systolic BP | |||||||||
| ≤90 mmHg | 7.25 | 11.98 | 2.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 91-110 mmHg | 20.58 | 22.75 | 18.54 | 26.59 | 27.27 | 25.88 | 4.42 | 1.15 | 7.45 |
| ≥140 mmHg | 22.90 | 17.96 | 27.53 | 18.50 | 20.45 | 16.47 | 46.41 | 58.62 | 35.11 |
| Diastolic BP | |||||||||
| ≤60 mmHg | 31.30 | 32.93 | 29.78 | 23.70 | 15.91 | 31.76 | 3.87 | 0.00 | 7.45 |
| ≥90 mmHg | 6.09 | 1.20 | 10.67 | 6.94 | 6.82 | 7.06 | 9.94 | 13.79 | 6.38 |
Heart rate
On average, HR was higher in the HP group compared to the T group in both the seated and supine positions (Table 5). In addition, HR was higher while seated in the HP compared to the LP group. Regardless of orthostatic position or group affiliation, more than half of all HR observations (57%) were within the normal range; however, more than a quarter (27%) of all the HR observations were ≥80 bpm (Fig. 1A). For the HR data, the omnibus 3 × 5 χ2 analyses (df = 6) were all statistically significant: seated χ2 = 17.32 (P = 0.03), supine χ2 = 16.91 (P = 0.03), and overall χ2 = 17.90 (P = 0.02). Examination of the categorical distribution for all HR analyses indicates that the subjects with T were disproportionally represented as having a HR ≤60 bpm, whereas those with HP and LP were disproportionally represented as having a HR ≥80 bpm.
Table 5.
Group mean data
| T | HP | LP | |
|---|---|---|---|
| HR (bpm) | |||
| Seated | 69 ± 11*** | 87 ± 17 | 76 ± 15 |
| Supine | 65 ± 10***† | 81 ± 14 | 73 ± 13 |
| SBP (mmHg) | |||
| Seated | 116 ± 24††† | 123 ± 16†† | 144 ± 16 |
| Supine | 126 ± 25 | 121 ± 14 | 135 ± 16 |
| DBP (mmHg) | |||
| Seated | 66 ± 13†† | 69 ± 11† | 82 ± 26 |
| Supine | 69 ± 14 | 68 ± 11 | 73 ± 8 |
†††P < 0.0001 versus LP; ††P < 0.01 versus LP; †P < 0.05 versus LP; *** P < 0.0001 versus HP
Figure 1.
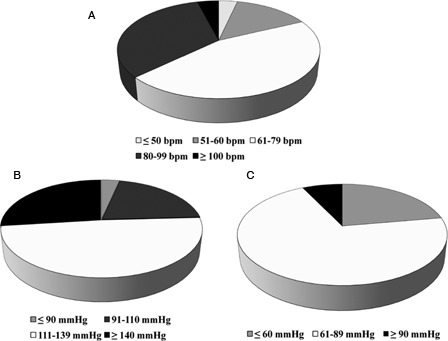
(A) Distribution of HR data among veterans with SCI. (B) Distribution of SBP data among veterans with SCI. (C) Distribution of DBP data among veterans with SCI.
Systolic blood pressure
Seated SBP was higher in the LP group compared to the T and HP groups; however, supine SBP did not differ among the three study groups (Table 5). Regardless of orthostatic position or group affiliation, more than half of all SBP values (53%) were within the normotensive range, and about a quarter of the SBP observations were ≥140 mmHg (Fig. 1B). For the SBP data, the 3 × 4 omnibus χ2 (df = 5) was significant for the seated (χ2 = 16.6, P = 0.011) and the overall scores (χ2 = 12.7, P = 0.047), but not for the supine condition (χ2 = 7.1, P = 0.32). Examination of the categorization distribution for seated SBP indicates that subjects in the T and HP groups were disproportionally represented as hypotensive (SBP ≤110 mmHg), whereas subjects in the LP group were disproportionally represented in the hypertensive category (SBP ≥140 mmHg). This pattern was similar for the overall analysis.
Diastolic blood pressure
Seated DBP was higher in the LP group compared to the T and HP groups; however, supine DBP did not differ among the three study groups (Table 5). Regardless of orthostatic position or group affiliation, two-thirds of all DBP observations (69%) were within the normotensive range, and less than 10% of the total DBP observations were ≥90 mmHg (Fig. 1C). For the DBP data, none of the 3 × 3 χ2 analyses (df = 4) were significant (seated χ2 = 7.60, P = 0.11; supine χ2 = 7.41, P = 0.12; overall χ2 = 8.15, P = 0.09). These results suggest that distribution of DBP across SCI groups was proportional to the total number of subjects within each group.
The SEM of the individual HR, SBP, and DBP measurements for supine and seated positions (i.e. approximately six measurements per subject per position) is presented among the study groups. The SEM for HR was comparable among the three groups (Fig. 2). Examination of the degree of overlap (or lack thereof) of the 95% CIs indicates that the SEM for seated SBP was significantly increased in the HP compared to the LP group and the SEM for seated DBP and supine SBP and DBP, was higher in the T group than HP and LP (Figs. 3 and 4). The finding of significantly increased SEM for the BP observations in the T and HP group likely reflects heterogeneity of intact cardiovascular sympathetic innervation, which stems from the upper thoracic spinal cord (i.e. T1–T5). Therefore, due to relatively increased measurement error in the T and HP groups, a BP single measurement may not accurately reflect resting BP in these individuals.
Figure 2.
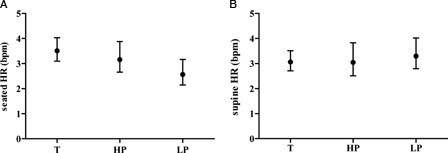
SEM data for HR data in the seated (A) and supine (B) positions among veterans with tetraplegia [T], high paraplegia [HP] and low paraplegia [LP].
Figure 3.
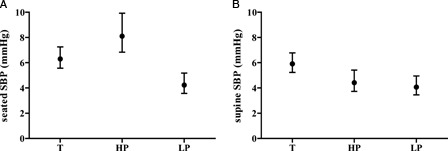
SEM data for SBP data in the seated (A) and supine (B) positions among veterans with tetraplegia [T], high paraplegia [HP] and low paraplegia [LP].
Figure 4.
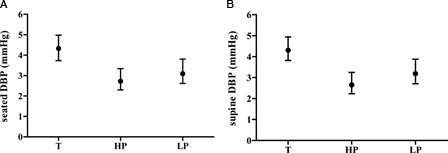
SEM data for DBP data in the seated (A) and supine (B) positions among veterans with tetraplegia [T], high paraplegia [HP] and low paraplegia [LP].
Discussion
The findings of this study suggest: (1) that the prevalence of HR and BP abnormalities differs based on the level of SCI and orthostatic position; (2) a relatively high percentage of veterans with SCI with multiple comorbid conditions which might contribute to HR and BP abnormalities; (3) an increased percentage of individuals, regardless of the level of lesion, prescribed three or more medications with known effects on HR and BP; and (4) an increased intra-subject variability in BP recordings during a single clinic visit in individuals with T and HP, regardless of orthostatic position. Thus, when assessing these vital signs in this population, close attention must be made to accurately document these factors prior to diagnosis and treatment.
Bradycardia is reported in persons with tetraplegia,25 and we found that one-quarter of all HR observations in the T group were ≤60 bpm. Increased prevalence of bradycardia in the T group presumably reflects decentralized sympathetic cardiac control and a relative increase in vagal tone to the heart. By this rationale, we would have expected increased prevalence of bradycardia in the HP group due to partial decentralized sympathetic cardiac control. However, we found a very low prevalence of bradycardia in the HP group (∼1%), whereas the prevalence of a HR ≥80 bpm was substantially increased. Although this finding was unexpected, our previous data suggest significantly elevated daytime HR in individuals with lesions between T2 and T5.26 Present clinical guidelines for the SCI population suggest that a resting HR ≥100 bpm should be considered tachycardia.9 Our data suggest that the prevalence rate of a HR ≥100 bpm was increased six-fold in the HP compared to the LP group. There is substantial evidence of an association between persistently elevated HR and accelerated progression of arterial stiffness (AS) in the general population,17,27,28 and several investigators suggest a threshold for tachycardia between 79 and 84 bpm.28–33 While we do not understand the potential consequence of increased resting HR on CVD and mortality in the SCI population; we and others have reported increased AS in individuals with SCI compared to non-SCI controls.34–36
The present clinical guideline for diagnosis of hypotension is a BP of ≤90/60 mmHg; our data suggest that hypotension was evident in 7% of the total BP observations in the T group (0% in HP and LP groups). However, in 1978 the World Health Organization (WHO) defined hypotension as a SBP ≤110 mmHg in males and ≤100 mmHg in females, without regard to diastolic pressure.37 Compared to the prevalence of a BP ≤90/60 mmHg, the prevalence of hypotension by the WHO criteria was significantly increased in the T and HP groups. Importantly, several investigators report significant adverse effects on mood,21 quality of life,38 and cognitive function39–41 in asymptomatic non-SCI individuals with a SBP below the WHO hypotensive thresholds. Further, we recently reported significant deficits in memory and attention and processing speed in males with SCI with a SBP ≤110 mmHg compared to a normotensive SCI cohort.42 These data suggest that although the prevalence of a BP ≤90/60 mmHg was low, a significant number of individuals with SCI may be at increased risk for adverse changes in mood, quality of life, and cognitive function as a result of relatively reduced BP. In the HP group the incidence of a DBP ≤60 mmHg was doubled, while SBP was relatively maintained, in the supine compared to the seated position; this finding suggests increased supine pulse pressure (SBP–DBP), which may facilitate early vascular aging as reported in the general population.43,44
The prevalence of hypertension in veterans with SCI has been reported to be 22% based on a retrospective review in 7959 patients with paraplegia and tetraplegia.45 In a prospective examination of BP in persons with SCI, Lee et al.46 reported hypertension in 45% of the study population. Although the proportion of hypertensive individuals with paraplegia was increased compared to those with tetraplegia,45,46 these authors did not distinguish between HP and LP. However, our data suggest that the prevalence of hypertension is increased in persons with lesions ≤T7 compared to those with injuries above this level. This is particularly true for SBP. In addition, we note differential orthostatic effects among the groups on systolic hypertension: the prevalence of a SBP ≥140 mmHg was lower in the supine compared to the seated position in the LP group, whereas the incidence of a SBP ≥140 mmHg was increased by 53% compared to seated hypertension in the T group. Therefore, these data suggest that not only does level of lesion play an important role in determining the prevalence of hypertension, but orthostatic position must also be considered when making this diagnosis in persons with SCI.
The influence of comorbid conditions and prescription medication use cannot be ignored when examining the prevalence of HR and BP abnormalities in the SCI population. Because nearly one-third of the entire study group was diagnosed with three or more comorbid conditions and nearly half of the study population was on three or more prescription medications with known effects on HR and BP. However, including information on the influence of comorbid conditions and prescription medication use on the prevalence of HR and BP abnormalities is beyond the scope of this study. The reader is directed to a sister manuscript published in this issue of the journal which reports the findings of a retrospective chart review of the prevalence of HR and BP abnormalities in veterans with SCI. In addition, a manuscript (still in preparation) will describe the influence of comorbid conditions and prescription medication use on the observed prevalence of HR and BP abnormalities in greater detail. It should be noted that because the HP group was younger and had a significantly reduced body mass, the prevalence of comorbid conditions may have been reduced compared to the LP and T groups. However, it is also noted that the percentage of individuals in the HP group on three or more prescription medications with effects of HR and BP was not reduced, which may be attributed to a small sample size.
Study limitations
The relatively small number of subjects, particularly in the HP group and the exclusively veteran sample limits extrapolation to the general SCI population. In addition, there was only one woman included in the study; therefore, the results cannot be extrapolated to women with SCI. Finally, the influence of completeness of lesion or AIS classification on the prevalence of HR and BP abnormalities could not be adequately tested in this small sample of subjects; but this should be studied in greater detail in a future investigation.
Conclusion
Our findings suggest that the prevalence of HR and BP abnormalities relate to the level of SCI and orthostatic positioning, particularly in those with tetraplegia. A second important finding was a relatively high percentage of veterans with SCI with multiple comorbid conditions and who are prescribed three or more medications with known HR and BP effects. Lastly, we note increased intra-subject variability in BP values recorded during a single clinic visit among individuals with a lesion ≥T5. Therefore, the accurate and reliable documentation of HR and BP in the medical record should consider these related factors. It must be appreciated that due to increased prevalence of HR and BP abnormalities in the SCI population guidelines established in the general population for the treatment of CVD may not be appropriate, which may result in reduced medication safety and perhaps efficacy. However, before we can tailor strategies for treatment of age-associated diseases in the SCI population, we must gain a better appreciation of the prevalence of HR and BP abnormalities.
Disclosure
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated and, if applicable, we certify that all financial and material support for this research (e.g., NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Acknowledgements
This research was supported by the Veterans Affairs Rehabilitation Research and Development Service (Grant no. B6999R).
References
- 1.SCI_QUERI Spinal Cord Injury Fact Sheet. [VA Queri]. April 2012. Available from: http://www.queri.research.va.gov/about/factsheets/sci_factsheet.pdf
- 2.Yu W, Ravelo A, Wagner TH, Phibbs CS, Bhandari A, Chen S, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev 2003;60Suppl 3:146S–67 [DOI] [PubMed] [Google Scholar]
- 3.Spinal cord injury facts and figures at a glance, University of Alabama, 2012. J Spinal Cord Med 2012;35(6):480–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005;43(7):408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groah SL, Charlifue S, Tate D, Jensen MP, Molton IR, Forchheimer M, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil 2012;91(1):80–93 [DOI] [PubMed] [Google Scholar]
- 6.Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003;26Suppl 1:S50–6 [DOI] [PubMed] [Google Scholar]
- 7.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev 2007;44(1):103–12 [DOI] [PubMed] [Google Scholar]
- 9.Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord 2009;47(1):36–43 [DOI] [PubMed] [Google Scholar]
- 10.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001;39(6):310–7 [DOI] [PubMed] [Google Scholar]
- 11.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006;23(12):1713–25 [DOI] [PubMed] [Google Scholar]
- 12.Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 2006;44(6):341–51 [DOI] [PubMed] [Google Scholar]
- 13.West CR, Mills P, Krassioukov AV. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord 2012;50(7):484–92 [DOI] [PubMed] [Google Scholar]
- 14.Krassioukov A. Introducing the revised International Standards on documentation of remaining autonomic function after SCI (ISAFSCI). J Spinal Cord Med 2012;35(4):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krassioukov A, Biering-Sorensen F, Donovan W, Kennelly M, Kirshblum S, Krogh K, et al. International standards to document remaining autonomic function after spinal cord injury. J Spinal Cord Med 2012;35(4):201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palatini P. Elevated heart rate: a ‘new’ cardiovascular risk factor? Prog Cardiovasc Dis 2009;52(1):1–5 [DOI] [PubMed] [Google Scholar]
- 17.Palatini P, Parati G. Persistently elevated heart rate accelerates the progression of arterial stiffness. J Hypertens 2010;28(4):653–6 [DOI] [PubMed] [Google Scholar]
- 18.Barrett-Connor E, Palinkas LA. Low blood pressure and depression in older men: a population based study. BMJ 1994;308(6926):446–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duschek S, Matthias E, Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behav Med 2005;30(4):149–58 [DOI] [PubMed] [Google Scholar]
- 20.Elias MF, Robbins MA, Schultz NR, Jr, Pierce TW. Is blood pressure an important variable in research on aging and neuropsychological test performance? J Gerontol 1990;45(4):P128–35 [DOI] [PubMed] [Google Scholar]
- 21.Pilgrim JA, Stansfeld S, Marmot M. Low blood pressure, low mood? BMJ 1992;304(6819):75–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med 2012;22(1):39–45 [DOI] [PubMed] [Google Scholar]
- 23.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005;19(1):231–40 [DOI] [PubMed] [Google Scholar]
- 24.Cumming G. Inference by eye: reading the overlap of independent confidence intervals. Stat Med 2009;28(2):205–20 [DOI] [PubMed] [Google Scholar]
- 25.Dixit S. Bradycardia associated with high cervical spinal cord injury. Surg Neurol 1995;43(5):514. [DOI] [PubMed] [Google Scholar]
- 26.Rosado-Rivera D, Radulovic M, Handrakis JP, Cirnigliaro CM, Jensen AM, Kirshblum S, et al. Comparison of 24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J Spinal Cord Med 2011;34(4):395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palatini P. Elevated heart rate as a predictor of increased cardiovascular morbidity. J Hypertens Suppl 1999;17(3):S3–10 [PubMed] [Google Scholar]
- 28.Palatini P, Casiglia E, Julius S, Pessina AC. High heart rate: a risk factor for cardiovascular death in elderly men. Arch Intern Med 1999;159(6):585–92 [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Hashimoto H, Tanaka H, Matsumoto C, Odaira M, Yamada J, et al. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: a prospective study. J Hypertens 2010;28(4):687–94 [DOI] [PubMed] [Google Scholar]
- 30.Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 1980;112(6):736–49 [DOI] [PubMed] [Google Scholar]
- 31.Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J 1993;125(4):1148–54 [DOI] [PubMed] [Google Scholar]
- 32.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J 1991;121(1 Pt 1):172–7 [DOI] [PubMed] [Google Scholar]
- 33.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J 1985;109(4):876–85 [DOI] [PubMed] [Google Scholar]
- 34.Wecht JM, Weir JP, DeMeersman RE, Spungen AM, Bauman WA. Arterial stiffness in persons with paraplegia. J Spinal Cord Med 2004;27(3):255–9 [DOI] [PubMed] [Google Scholar]
- 35.Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med 2009;32(1):72–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips AA, Cote AT, Bredin SS, Krassioukov AV, Warburton DE. Aortic stiffness increased in spinal cord injury when matched for physical activity. Med Sci Sports Exerc 2012;44(11):2065–70 [DOI] [PubMed] [Google Scholar]
- 37.Arterial Hypertension Report of a WHO expert committee. World Health Organ Tech Rep Ser 1978;(628):7–56 [PubMed] [Google Scholar]
- 38.Rosengren A, Tibblin G, Wilhelmsen L. Low systolic blood pressure and self perceived wellbeing in middle aged men. BMJ 1993;306(6872):243–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duschek S, Hadjamu M, Schandry R. Dissociation between cortical activation and cognitive performance under pharmacological blood pressure elevation in chronic hypotension. Biol Psychol 2007;75(3):277–85 [DOI] [PubMed] [Google Scholar]
- 40.Duschek S, Hadjamu M, Schandry R. Enhancement of cerebral blood flow and cognitive performance following pharmacological blood pressure elevation in chronic hypotension. Psychophysiology 2007;44(1):145–53 [DOI] [PubMed] [Google Scholar]
- 41.Duschek S, Heiss H, Buechner B, Werner N, Schandry R, Reyes del Paso GA. Hemodynamic determinants of chronic hypotension and their modification through vasopressor application. J Physiol Sci 2009;59(2):105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jegede AB, Rosado-Rivera D, Bauman WA, Cardozo CP, Sano M, Moyer JM, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res 2009;20(1):3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotsis V, Stabouli S, Karafillis I, Nilsson P. Early vascular aging and the role of central blood pressure. J Hypertens 2011;29(10):1847–53 [DOI] [PubMed] [Google Scholar]
- 44.Kotsis V, Stabouli S, Karafillis I, Papakatsika S, Rizos Z, Miyakis S, et al. Arterial stiffness and 24h ambulatory blood pressure monitoring in young healthy volunteers: the early vascular ageing Aristotle University Thessaloniki Study (EVA-ARIS Study). Atherosclerosis 2011;219(1):194–9 [DOI] [PubMed] [Google Scholar]
- 45.Weaver FM, Collins EG, Kurichi J, Miskevics S, Smith B, Rajan S, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil 2007;86(1):22–9 [DOI] [PubMed] [Google Scholar]
- 46.Lee MY, Myers J, Abella J, Froelicher VF, Perkash I, Kiratli BJ. Homocysteine and hypertension in persons with spinal cord injury. Spinal Cord 2006;44(8):474–9 [DOI] [PubMed] [Google Scholar]


