Abstract
Objective
To evaluate, whether once-daily oral baclofen administration increases and/or sustains plasma insulin-like growth factor-1 (IGF-1) concentration in 11 men with chronic spinal cord injury (SCI) and IGF-1 deficiency (i.e. <250 ng/ml).
Design
Prospective, open-label, dose titration study. Baclofen was administered at 20 mg/day for 8 weeks; then increased to 40 mg/day for another 8 weeks. Plasma IGF-1 and self-reported side effects were measured at baseline and every other week for the duration of the study.
Results
The subjects were 43 ± 12 years old, had duration of injury of 20 ± 12 years; eight subjects had a complete motor injury, and eight had paraplegia. Nine of 11 subjects completed the 20 mg/day treatment and 5 subjects completed the 40 mg/day treatment. Plasma IGF-1 levels improved with each baclofen dose; however, only one subject increased from baseline and remained above the targeted physiological range of 250 ng/ml throughout treatment. A significant increase in IGF-1concentration was observed between baseline and week 2 (154 ± 63 vs. 217 ± 69 ng/ml; P < 0.05), weeks 8 and 10 (188 ± 95 vs. 228 ± 93 ng/ml; P < 0.05), and weeks 8 and 16 (188 ± 95 vs. 259 ± 92 ng/ml; P < 0.05). No serious side effects were observed at 20 mg/day; the 40 mg/day dose was less well tolerated.
Conclusion
Baclofen was not effective at sustaining plasma IGF-1 concentrations in the physiological range in men with chronic SCI.
Keywords: Baclofen, Gamma-aminobutyric acid, Insulin-like growth factor-1, Testosterone, Spinal cord injuries, Paraplegia, Tetraplegia, Spasticity
Introduction
Baclofen is a β p-chlorophenyl derivative of gamma-aminobutyric acid (GABA) that is routinely prescribed to reduce muscle spasticity in individuals with upper motor neuron disease, including spinal cord injury (SCI). Baclofen crosses the blood–brain barrier and binds to presynaptic GABAB receptors on the laminae I–IV of the spinal cord where primary sensory fibers terminate.1,2 After titration from low doses, standard and routine treatment may include a daily oral administration of 40–80 mg in single or multiple administrations with the higher doses, depending on the severity of muscle spasticity, individual tolerance, and presentation and/or tolerance of symptoms related to the central nervous system depression. Baclofen's actions on GABAB receptors also have the potential to mediate acute increases in the basal concentration of growth hormone (GH).3–5 Binding to GABAB receptors in the hypothalamus by baclofen induces the release of GH releasing factor by inhibiting central somatostatin release, which then permits pituitary release of GH.6–10 Because of a relatively short serum half-life and the pulsatile secretory pattern of GH, a single blood sample may not reflect daily release or integrity of hypothalamic-pituitary axis. To evaluate GH secretion, plasma insulin-like growth-factor (IGF)-1, often referred to as the “second messenger” of GH, has been used as a practical, albeit not totally representative, surrogate marker of integrated GH secretion.11,12 IGF-1 levels do not fluctuate widely during the day,13 as may GH concentration due to its pulsatile release.
In persons with SCI, GH responses to provocative stimulation have previously been shown to be blunted in individuals who have depressed plasma IGF-1 levels.14–16 These deficiencies may reflect a global dysregulation of the hypothalamic-pituitary axis in men with SCI, which is also characterized by an increased prevalence of hypogonadism and elevated levels of gonadotropins and prolactin, possibly suggestive of reduced central dopaminergic tone.17–19 Reduced integrity of pituitary GH secretion would be anticipated to worsen the losses of lean tissue mass (LTM) and bone mineral density (BMD) below the level of lesion, imparting a further risk of fragility fractures in persons with SCI.20–22 Muscle wasting reduces energy expenditure and may be associated with increased relative adiposity and, potentially, associated deleterious metabolic consequences.23,24 These accelerated adverse body compositional changes in persons with SCI could hamper functional independence and social integration,25 which may adversely affect quality of life26,27 and be associated with increased cost of care.26,27 Our study evaluated the safety and tolerability of a once-daily oral baclofen administration at two relatively low daily doses (e.g. 20 or 40 mg/day) on the efficacy of increasing and sustaining plasma IGF-1 concentration (>250 ng/ml) in men with chronic SCI who have been identified to have a relative deficiency of IGF-1.
Materials and methods
Study population
Subjects were recruited from the SCI Services of two metropolitan area hospitals. From a consecutive cohort of 60 men who otherwise met the entrance criteria of the study, 16 men with chronic SCI were screened and found to have low IGF-1 concentrations (i.e. <250 ng/ml); however, upon repeat testing at baseline, 5 of the 16 men were found to have IGF-1 levels that were in the normal range (≥250 ng/ml) and, as such, were removed from further analysis. Thus, 11 subjects of the 60 initially screened were enrolled in the study. Exclusion criteria included any or all of the following conditions: acute illness of any etiology; patients with chronic renal, liver, lung or cardiac disease; patients taking pain medications (narcotics), major tranquilizers or l-DOPA; and frequent alcohol consumption (>1 drink/day). The research study was approved by the respective Institutional Review Boards for each study site. Written informed consent was obtained from each subject prior to study participation.
Procedures
Baclofen was self-administered orally in the evening prior to sleep at 20 mg/day for an 8-week period and then, without interruption, at 40 mg/day for an additional 8-week period. At the end of this 16-week treatment period, the dose of drug was reduced to 20 mg/day for two additional weeks in an effort to minimize the potential occurrence of symptoms of drug withdrawal. These dose concentrations represent the low end of standard clinical use for treatment of spasticity in persons with SCI. At the end of week 18, participants were discontinued from drug treatment and were followed for the remaining 2 weeks of the study. Subjects reported at baseline and then every 2 weeks over the 20-week study period (i.e. bi-weekly intervals for follow-up a total of 11 visits) for the collection of venous blood samples. Plasma IGF-1, total testosterone (T), sex hormone-binding globulin (SHBG), and albumin (the latter two proteins were determined to permit the calculation of free testosterone concentration) were measured at baseline, and throughout the drug treatment, taper, and withdrawal periods. During each of the drug treatment periods (i.e. four sample collections during 8 weeks for each dose at 20 or 40 mg/day) were pooled to provide mean values of plasma IGF-1. Subjects completed a questionnaire to document self-reported side effects related to baclofen treatment at baseline and after each study visit. As an additional measure to monitor potential adverse side effects, a study physician completed a review of medical symptoms and history at baseline and at weeks 8, 16, and 20.
Analysis of blood samples
The plasma IGF-1 levels were performed during the screening phase by a commercial laboratory (Quest Diagnostics, Inc., Teterboro, NJ, USA) to facilitate the identification of potential subjects for participation in the study. All subsequent plasma IGF-1 values were determined in our core research laboratory facility in batch assay at the completion of the study. Blood work was performed at approximately 9:00 AM under fasting conditions at the completion of each treatment week. Samples were immediately centrifuged to separate the formed blood cell elements from sera/plasma and stored at −70°C until assays were batch processed in duplicate at the completion of the study. Plasma IGF-1, total T, albumin, and SHBG concentrations were drawn at baseline and bi-weekly during the study. Plasma IGF-1 values were determined by a commercial kit assay using a non-extraction immunoradiometric assay (IRMA; Diagnostic Systems Laboratories, Inc., Webster, TX, USA) with an intra-assay coefficient of variation (CV) of 7.0, 3.9, and 3.9% at 34, 143, and 374 ng/ml. The normal range for the IGF-1 assay kit for adult males older than 24 years is 100–600(±104) ng/ml. The median distribution for serum IGF-1 for the IGF-1 assay is 188–304 ng/ml; thus, IGF-1 values less than approximately 200 ng/ml may be considered below the median limit for an early adulthood. The authors’ selection of 250 ng/ml as the threshold value for IGF-1, below which individuals would have been qualified to receive baclofen treatment, was derived from the lower limit of the median distribution of “youthful” values for IGF-1 (i.e. 200 ng/ml) plus 1/2 of 1 standard deviation. For the purpose of this report, an IGF-1 concentrations <250 ng/ml is defined by the authors as an IGF-1 deficiency because of its reduced anabolic potential than that of more youthful levels. Serum total testosterone was determined by a radioimmunoassay (RIA; ICN Biomedical Institute, Costa Mesa, CA, USA) kit with an intra-assay CV of 10.0, 9.6, and 13% at 1.4, 4.6, and 8.0 nmol/l. SHBG was determined by IRMA (Diagnostic Systems Laboratories, Inc.) with an intra-assay CV of 3.7, 1.1, and 3.4% at 27, 92, and 119 nmol/l. Serum albumin was determined by autoanalyzer methodology (Beckman Ly 20 Synchron, Brea, CA, USA) in the James J. Peters VA Medical Center Clinical Laboratory. Serum free T concentrations were calculated using previously described methods from weekly measurements of total T, SHBG, and albumin.28
Statistical analyses
The primary outcome variable was the group mean change from baseline in plasma IGF-1 to baclofen treatment at each dose level. Secondary outcome variables included change in total and free T. The incidence of symptoms that were related to baclofen treatment is reported as a description of occurrence. Patient characteristics and hormonal data are expressed as mean ± standard deviation. Separate repeated measures analysis of variance (ANOVA) were performed to identify the possible change in concentration of plasma IGF-1 in treatment weeks from baseline. Particular emphasis was placed on the respective changes: Baseline (BL) – week 2; BL – week 8; BL – week 16; weeks 8–10; and weeks 8–16. Separate repeated measures ANOVA were performed to evaluate change in plasma IGF-1 concentrations between baseline and the pooled mean of all available samples during the respective treatment periods. Similarly, separate repeated measures ANOVA were performed to monitor changes in total and free T. Single degree of freedom univariate correlations were performed to determine if relationships were present among baseline plasma IGF-1 concentration and demographics (age, body mass index (BMI), and duration of injury (DOI)). An a priori level of significance was set at P ≤ 0.05. Statistical analyses were completed using Statview 5.0 (SAS Institute, Inc.).
Results
The demographic data for subjects are provided (Table 1). Eleven subjects were found at baseline to have levels of plasma IGF-1 that were below the lower limit of normal (range of levels: 51–246 ng/ml). These subjects had an average age of 43 ± 12 years and DOI of 20 ± 12 years. Of the 11 subjects who initiated 20 mg/day baclofen, 9 completed the 8 weeks of treatment, with 2 subjects discontinuing drug after having completed 2 weeks of administration. Upon titration to baclofen 40 mg/day, nine subjects initiated treatment and only five completed the 8-week treatment period. In total four subjects failed to tolerate the higher dose: two subjects were discontinued within 1 week, one subject completed 2 weeks, and the remaining subject completed 4 weeks of treatment.
Table 1.
Characteristics of the study subjects
| n | 11 |
|---|---|
| Age (years) | 43 ± 12 |
| Height (meters) | 1.79 ± 0.09 |
| Weight (kg) | 79.3 ± 12.6 |
| BMI (kg/m2) | 24.7 ± 3.3 |
| DOI (years) | 20 ± 12 |
| Paraplegia/tetraplegia (n) | 8/3 |
| Motor complete (n) | 8 |
Data are presented as group mean ± SD.
SD, standard deviation; BMI, body mass index; DOI, duration of injury.
Plasma IGF-1 levels were monitored on bi-weekly intervals for the entire study (Fig. 1). The mean baseline concentration of IGF-1 was 154 ± 63 and 188 ± 95 ng/ml at treatment week 8 (Fig. 1). A significant increase in IGF-1concentration was observed between baseline and week 2 (154 ± 63 vs. 217 ± 69; F(1) = 9.165, P < 0.05), from weeks 8 to 10 (188 ± 95 vs. 228 ± 93; F(1) = 13.390, P < 0.05), and from weeks 8 to 16 (188 ± 95 vs. 259 ± 92; F(1) = 15.480, P < 0.05). Not until, week 16 of treatment, did mean IGF-1concentration exceed our targeted threshold of 250 ng/ml. Of note, only one subject had IGF-1 concentrations that increased and were maintained above 250 ng/ml at all time points for baclofen administration 20 and 40 mg/day. The group mean elevation in IGF-1 levels across visits were largely achieved by the changes observed in this one subject (Table 2).
Figure 1.
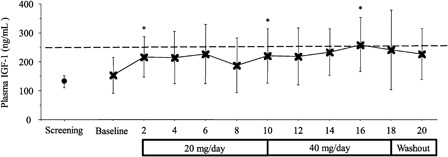
Plasma IGF-1 concentration across bi-weekly treatment observations. Time point data are reported as group mean (±SD). The mean change in plasma IGF-1 concentration from baseline to week 2 (*P < 0.05), weeks 8 to 10 (*P < 0.05), and weeks 8 to 16 (*P < 0.05) was significant.
Table 2.
Plasma IGF-1 concentrations (ng/ml) by subject
| Subject | Baseline | Week 8 | Week 16 |
|---|---|---|---|
| 1 | 193 | 122 | 201 |
| 2 | 189 | 183 | 229 |
| 3 | 79 | 130 | 229 |
| 4 | 246 | 180 | 214 |
| 5 | 189 | 401 | 423 |
| 6 | 51 | – | – |
| 7 | 71 | – | – |
| 8 | 145 | 281 | – |
| 9 | 157 | 108 | – |
| 10 | 157 | 137 | – |
| 11 | 214 | 151 | – |
| Group mean ± SD | 154 ± 63 | 188 ± 95 | 259 ± 92 |
Data are presented as individual concentration for the observation period. Group mean ± SD for each observation period are also provided.
In all combined observations on baclofen 20 mg/day, the change in pooled mean plasma IGF-1 concentration from baseline was significant (F(1) = 5.090, P < 0.05). Although the mean concentration for plasma IGF-1 was higher than 20 mg/day (206 ± 76), on 40 mg/day (220 ± 83), the concentration was not significantly compared to the baseline value (154 ± 63) (Fig. 2); of note, all five subjects had IGF-1 levels >200 ng/ml at 16 weeks, whereas only one had a level above this level at baseline. Univariate analyses revealed that plasma IGF-1 concentrations were not related to age, BMI, or DOI (Table 3). There were no significant changes in total or free T concentrations at either level of drug dose (data not shown).
Figure 2.

Plasma IGF-1 across treatment periods for all groups combined. Data for each period represent the pooled mean (±SD) of available observations for the respective treatment baclofen treatment period. The mean change in plasma IGF-1 concentration from baseline to 20 mg/day was significant (*P < 0.05). The change from baseline to 40 mg/day and between 20 and 40 mg/day was not significant.
Table 3.
Correlations with plasma IGF-1
| R | P value | |
|---|---|---|
| Age | −0.36 | 0.29 |
| BMI | 0.01 | 0.97 |
| DOI | −0.48 | 0.14 |
BMI, body mass index; DOI, duration of injury.
Over the course of the study, adverse events occurred that ranged from mild to moderate and were categorized as possibly or probably related to baclofen administration. The most commonly reported adverse side effects from subjects that completed baclofen 20 mg/day included symptoms of dry mouth, fatigue, and general weakness. Symptoms generally resolved within the initial 3 weeks of drug treatment. Two weeks after initiating baclofen 20 mg/day, one subject was withdrawn due to severe headaches that resolved shortly after discontinuing baclofen, and one subject sustained a bone fracture that was unrelated to administration of baclofen but necessitating termination of his participation in the study. At baclofen 40 mg/day, most subjects had a recurrence of dry mouth, fatigue, and general weakness, symptoms that usually resolved by week 10. Study termination at the higher dose level of baclofen appeared to be the result of intolerance to the cumulative effect of the occurrence of several relatively mild symptoms rather than any single, more severe symptom, with the exception of one subject who experienced a dramatic loss in muscle spasticity that reduced his ability to transfer, necessitating his study withdrawal to maintain independence of care. Of interest, five of the six subjects who discontinued treatment had the lowest observed IFG-1 values in our study cohort (i.e. <158 ng/ml) at baseline.
Discussion
Individuals with SCI are reported to have a blunted response of GH to provocative stimulation, and younger persons with SCI had lower plasma IGF-1 levels compared to able-bodied controls.14–16 Because of the likelihood that deficient GH production in those with SCI could worsen soft tissue body composition and possibly reduce bone integrity, our group has postulated a novel and, perhaps, practical manner to increase circulating GH–IGF-1 levels by treatment with a GABAB receptor agonist.29 In a previous report by our group, 10 healthy outpatient males with chronic SCI received escalating doses of baclofen from 5–10 to 20 mg/day for 4 weeks at each dosage.29 An increase in peak plasma IGF-I was noted with increasing doses of baclofen, with significant increases in plasma IGF-I at 2 weeks after administration of drug at doses of 10 and 20 mg/day. Only the dose of baclofen at 20 mg/day had an effect on circulating IGF-1 that appeared to be sustained for the entire 4-week treatment period.29 No subject was withdrawn due to adverse side effects in this prior work. Thus, a question may be raised from this earlier report as to whether the baclofen effect to raise plasma IGF-1 levels could be sustained over a longer length of time, and also whether a slightly higher dose of this agent could further raise plasma levels of IGF-1, as well as be tolerated. Doses of baclofen used in the SCI population to control muscle spasticity often begin at levels of 40–60 mg/day and may be escalated to as high as 80 mg/day, or higher, administered chronically, if required to control symptoms.
In our study herein, plasma IGF-1 concentrations increased significantly within 2 weeks of initiating 20 mg/day of baclofen, a finding that confirms our prior work;29 of note, continued administration of baclofen 20 mg/day appeared to result in the inability to sustain elevated levels at 8 weeks. When increasing the dose to 40 mg/day, a statistically significant increase was observed from weeks 8 to 10 in this same cohort. In the pooled plasma samples, a significant increase in plasma IGF-1 was observed in subjects while on baclofen 20 mg/day but not in those on baclofen 40 mg/day, possibly in part because of the diminished sample size due to the high drop-out rate. Baclofen treatment did not increase mean plasma IGF-1 concentrations into the normal range until week 16, and then because one subject had a value at 16 weeks well above the lower limit of normal; this subject was also the only one to have had sustained levels of plasma IGF-1 within the normal physiological range (i.e. >250 ng/ml) after initiating treatment with baclofen. Although it has been reported in an animal model that baclofen reduced T concentrations,30 there was not a statistically significant decrease observed in serum total or free T levels by baclofen administration in our study. To the contrary, the mean observations for each group for testosterone appeared to increase from baseline across visits, with the exception of week 8. However, due to the relatively small sample size and subject attrition rate, conjecture to the validity and possible implications of this observation with regard to the effect of baclofen on serum T levels appears not to be warranted.
At baseline, five of the six subjects who discontinued treatment had depressed plasma IGF-1 concentrations (<158 ng/ml) that corresponded to five of the six lowest observed IGF-1 values in our study cohort. Whether or not this was coincidence or suggestive of causality could not be determined by our study design. However, raising severely depressed hormone levels may have a disproportionate effect on body tissues/cellular biochemical processes compared to that of raising less extreme values.31 Of note, three of these subjects had the longest durations of injury of those in our study population, and they were 28, 30, and 40 years since injury, providing anecdotal evidence for a negative association between DOI and plasma IGF-1 concentrations.
The observation period of this study was too short to provide insight on the ability of baclofen to modulate the adverse secondary outcomes of SCI on muscle mass and sublesional bone. Although observations to support associations between “youthful” IGF-1 concentrations and BMD and LTM do not exist in the SCI literature, reports in able-bodied individuals support this concept.32 GH/IGF-1 levels progressively decline with advancing age, but appear to decline prematurely in those with SCI.13–16 In persons with SCI, there is an accelerated decline in BMD and LTM compared to that of age- and gender-matched contemporaries;33,34 chronic physical inactivity due to lower-extremity muscle paralysis and resulting degrees of immobilization, is largely responsible for this observed decline. However, it can be reasonably speculated in the SCI population that a combination of extreme physical inactivity, aging, and dysregulation of the GH/IGF-1 axis, as well as that of the pituitary-gonadal axis, resulting in depressed serum testosterone levels, may in combination contribute to the accelerated decline in the musculoskeletal tissue compartment.15,16,32,35–37 Longitudinal investigations in the future may conceivably be performed by collaborating with clinicians who regularly prescribe baclofen for its properties as an anti-spastic agent in the SCI population. Such an approach of clinical investigation of “tagging onto” prescribed therapy may well provide insight into this agent's ability to improve GH secretion and circulating IGF-1 concentrations at higher doses over far longer periods of time, which may perhaps be shown to reduce adverse changes in body composition over time since injury compared to those not on such therapy.
The study cohort was relatively small, and because of the fairly high drop-out rate on the higher dose of baclofen, the cohort was reduced to only five subjects, which severely limited our ability to draw firm conclusions from the higher dose of baclofen administered. Owing to a small sampling pool of eligible women, women were excluded from study participation. Thus, an effort to replicate this study in women with SCI may be of interest. Baclofen is primarily used for its anti-spasmodic properties. However, because our objective in this study was to determine its effect of two relatively low doses of baclofen on plasma IGF-1 concentrations in men with SCI, we are neither reporting, nor was an evaluation performed, on the expected dose-efficacy of this medication on muscle spasticity in our subjects. However, one subject withdrew from the study due to a complete loss of lower-extremity spasticity/muscle tone that prevented him from transferring independently.
Conclusion
Low-dose baclofen administrated to men with chronic SCI deficient in circulating IGF-1 appeared not to be effective at sustaining normal physiological plasma levels of IGF-1 (>250 ng/ml). However, mean levels of plasma IGF-1 were increased after initiating baclofen at doses of 20 or 40 mg/day, albeit only transiently. In our relatively small cohort, possibly because of the high drop-out rate, there was not sufficient data to determine any additional benefit of a somewhat higher dose baclofen (i.e. 40 mg/day) to raise plasma IGF-1 levels, but mean plasma IGF-1 levels were higher than baseline on baclofen 40 mg/day and four of five subjects had higher levels at study termination on the higher dose than those at baseline. However, even at this higher dose of baclofen, the increase above baseline in IGF-1 levels was not into or sustained at the normal physiological range. As anticipated, baclofen 40 mg/day dosage was associated with a greater proportion of subjects who terminated the study due to their inability to tolerate therapy. Serum total and free levels of T were not changed by the administration of baclofen. No serious side effects were noted at either baclofen dosage, albeit several mild symptoms seemingly reduced study participation to a greater extent on the higher than that on the lower dose of drug.
Acknowledgements
The authors thank the James J. Peters VA Medical Center, Bronx, NY, the Department of Veterans Affairs Rehabilitation Research & Development Service, and Kessler Institute for Rehabilitation, West Orange, NJ for their support. This work was funded by the Veteran Affairs Rehabilitation Research & Development National Center of Excellence for the Medical Consequences of Spinal Cord Injury (No.'s B4162-C & B9212-C).
References
- 1.Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature 1981;290(5802):149–52 [DOI] [PubMed] [Google Scholar]
- 2.Davidoff RA. Antispasticity drugs: mechanisms of action. Ann Neurol 1985;17(2):107–16 [DOI] [PubMed] [Google Scholar]
- 3.Volpi R, Scaglioni A, Marcato A, Caffarra P, Rossi G, Caffarri G, et al. Failure of the gamma-aminobutyric acid (GABA) derivative, baclofen, to stimulate growth hormone secretion in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1991;3(4):259–64 [PubMed] [Google Scholar]
- 4.Volpi R, Gerra G, Vourna S, Vescovi PP, Maestri D, Chiodera P, Coiro V. Failure of the gamma-aminobutyric acid (GABA) derivative, baclofen, to stimulate growth hormone secretion in heroin addicts. Life Sci 1992;51(4):247–51 [DOI] [PubMed] [Google Scholar]
- 5.Coiro V, Capretti L, Bianconi L, Castelli A, Cerri L, Roberti G, et al. Reduction of baclofen-, but not sodium valproate-induced growth hormone release in type I diabetic men. Horm Metab Res 1991;23(12):600–4 [DOI] [PubMed] [Google Scholar]
- 6.Monteleone P, Maj M, Iovino M, Steardo L. Evidence for a sex difference in the basal growth hormone response to GABAergic stimulation in humans. Acta Endocrinol (Copenh) 1988;119(3):353–7 [DOI] [PubMed] [Google Scholar]
- 7.Monteleone P, Maj M, Iovino M, Forziati D, Veltro F, Steardo L. Baclofen-induced growth hormone secretion is blunted in chronic schizophrenics: neuroendocrine evidence for a GABA disturbance in schizophrenia. Psychiatry Res 1988;26(1):1–9 [DOI] [PubMed] [Google Scholar]
- 8.Koulu M, Lammintausta R, Dahlstrom S. Stimulatory effect of acute baclofen administration on human growth hormone secretion. J Clin Endocrinol Metab 1979;48(6):1038–40 [DOI] [PubMed] [Google Scholar]
- 9.Chiodera P, Volpi R, Coiro V, Barilli L, Rossi G, Roti E. Naloxone does not alter the effect of gamma aminobutyric acid derivative, baclofen, on GH release in man. J Endocrinol Invest 1983;6(5):381–4 [DOI] [PubMed] [Google Scholar]
- 10.Murakami Y, Kato Y, Kabayama Y, Tojo K, Inoue T, Imura H. Involvement of growth hormone-releasing factor in growth hormone secretion induced by gamma-aminobutyric acid in conscious rats. Endocrinology 1985;117(2):787–9 [DOI] [PubMed] [Google Scholar]
- 11.Daughaday WH. A personal history of the origin of the somatomedin hypothesis and recent challenges to its validity. Perspect Biol Med 1989;32(2):194–211 [DOI] [PubMed] [Google Scholar]
- 12.Schwander JC, Hauri C, Zapf J, Froesch ER. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology 1983;113(1):297–305 [DOI] [PubMed] [Google Scholar]
- 13.Florini JR, Prinz PN, Vitiello MV, Hintz RL. Somatomedin-C levels in healthy young and old men: relationship to peak and 24-hour integrated levels of growth hormone. J Gerontol 1985;40(1):2–7 [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Spungen AM, Flanagan S, Zhong YG, Alexander LR, Tsitouras PD. Blunted growth hormone response to intravenous arginine in subjects with a spinal cord injury. Horm Metab Res 1994;26(3):152–6 [DOI] [PubMed] [Google Scholar]
- 15.Tsitouras PD, Zhong YG, Spungen AM, Bauman WA. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm Metab Res 1995;27(6):287–92 [DOI] [PubMed] [Google Scholar]
- 16.Shetty KR, Sutton CH, Mattson DE, Rudman D. Hyposomatomedinemia in quadriplegic men. Am J Med Sci 1993;305(2):95–100 [DOI] [PubMed] [Google Scholar]
- 17.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of hypogonadism in men with spinal cord injury. J Spinal Cord Med 2013, published online, DOI: 10.1179/2045772313Y.0000000122. [Google Scholar]
- 18.Wang YH, Huang TS, Lien IN. Hormone changes in men with spinal cord injuries. Am J Phys Med Rehabil 1992;71(6):328–32 [DOI] [PubMed] [Google Scholar]
- 19.Huang TS, Wang YH, Chiang HS, Lien YN. Pituitary-testicular and pituitary-thyroid axes in spinal cord-injured males. Metabolism 1993;42(4):516–21 [DOI] [PubMed] [Google Scholar]
- 20.Spungen AM, Bauman WA, Wang J, Pierson RN., Jr. Measurement of body fat in individuals with tetraplegia: a comparison of eight clinical methods. Paraplegia 1995;33(7):402–8 [DOI] [PubMed] [Google Scholar]
- 21.Stewart AF, Adler M, Byers CM, Segre GV, Broadus AE. Calcium homeostasis in immobilization: an example of resorptive hypercalciuria. N Engl J Med 1982;306(19):1136–40 [DOI] [PubMed] [Google Scholar]
- 22.Naftchi NE, Viau AT, Sell GH, Lowman EW. Mineral metabolism in spinal cord injury. Arch Phys Med Rehabil 1980;61(3):139–42 [PubMed] [Google Scholar]
- 23.Sedlock DA, Laventure SJ. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia 1990;28(7):448–54 [DOI] [PubMed] [Google Scholar]
- 24.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003;77(2):371–8 [DOI] [PubMed] [Google Scholar]
- 25.Spungen AM, Bauman WA, Wang J, Pierson RN., Jr. The relationship between total body potassium and resting energy expenditure in individuals with paraplegia. Arch Phys Med Rehabil 1993;74(9):965–8 [PubMed] [Google Scholar]
- 26.Bauman WA, Spungen AM. Body composition in aging: adverse changes in able-bodied persons and in those with spinal cord injury. Top Spinal Cord Inj Rehabil 2001;6(3):22–36 [Google Scholar]
- 27.Kocina P. Body composition of spinal cord injured adults. Sports Med 1997;23(1):48–60 [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84(10):3666–72 [DOI] [PubMed] [Google Scholar]
- 29.Bauman WA, Kirshblum SC, Morrison NG, Cirnigliaro CM, Zhang RL, Spungen AM. Effect of low-dose baclofen administration on plasma insulin-like growth factor-I in persons with spinal cord injury. J Clin Pharmacol 2006;46(4):476–82 [DOI] [PubMed] [Google Scholar]
- 30.Amikishieva AV. Testosterone and behavior: involvement of the hormone in psychotropic effects of baclofen. Bull Exp Biol Med 2007;143(2):259–63 [DOI] [PubMed] [Google Scholar]
- 31.Oppenheimer JH, Silva E, Schwartz HL, Surks MI. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by l-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest 1977;59(3):517–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldspink G. Age-related loss of muscle mass and strength. J Aging Res 2012;1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95:2398–407 [DOI] [PubMed] [Google Scholar]
- 34.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88:1310–5 [DOI] [PubMed] [Google Scholar]
- 35.Bauman WA, Zhang RL, Spungen AM. Provocative stimulation of growth hormone: a monozygotic twin study discordant for spinal cord injury. J Spinal Cord Med 2007;30(5):467–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann NY Acad Sci 2010;1211:66–84 [DOI] [PubMed] [Google Scholar]
- 37.Lang TF. The bone-muscle relationship in men and women. J Osteoporos 2011:70235. [DOI] [PMC free article] [PubMed] [Google Scholar]


