Abstract
Context
Meningeal abnormalities such as dural ectasia are seen in Marfan syndrome, but spinal meningeal cysts are rarely seen. These cysts usually asymptomatic and often found incidentally on magnetic resonance imaging, large cysts may cause neurological deficits and pain secondary to nerve root compression.
Design
Case reports.
Findings
Two patients with Marfan syndrome presented with urinary symptoms secondary to dural ectasia and sacral cysts. Patient 1 had a history of low back pain, erectile dysfunction, and occasional urinary incontinence and groin pain with recent symptom worsening. He underwent L5 partial laminectomy and S1-S2 laminectomy with sacral cyst decompression. Nine weeks later, he underwent drainage of a sacral pseudomeningocele. Pain and urinary symptoms resolved, and he remains neurologically normal 2 years after surgery. Patient 2 presented after a fall on his tailbone, complaining of low back pain and difficulty urinating. Physical therapy was implemented, but after 4 weeks, urinary retention had not improved. He then underwent resection of the sacral cyst and S1-S3 laminectomy. Pain and paresthesias resolved and bowel function returned to normal. Other than needing intermittent self-catheterization, all other neurologic findings were normal 30 months after surgery.
Conclusion/clinical relevance
Surgical goals for sacral cysts include resection as well as closure of the dura, which can be challenging due to thinning from ectasia. Neurosurgical intervention in Marfan syndrome is associated with a high risk of dural tears and osseous complications, and should be performed only when symptoms are severe.
Keywords: Cerebrospinal fluid leak, Ectasia, Dura mater, Marfan syndrome, Sacral perineural cyst, Urinary dysfunction, Spine surgery, Laminectomy
Introduction
Marfan syndrome is a connective tissue disorder with a diverse set of clinical findings including aortic dilation and aneurysm, bony abnormalities, and ocular disease. In addition, patients with Marfan syndrome may commonly show dural ectasia, implying a progressive dural weakness. There have also been reports suggesting an association between connective tissue disorders such as Marfan syndrome and the appearance of lumbosacral spinal cysts. Although dural ectasia itself may be asymptomatic in many cases, cerebrospinal fluid (CSF) leaks and significant arachnoidal cysts often lead to severe symptoms such as postural headache and neurological deficits secondary to lumbosacral nerve root compression.
We report two patients with Marfan syndrome presenting with neurological impairments due to dural ectasia and sacral meningeal cysts. One patient presented with a progressive history of impairment over several years, and the other patient had suffered recent trauma after which his symptoms became manifest.
Patient 1
A 34-year-old man with Marfan syndrome presented with a 5-year history of weakness and low back pain, a 1-year history of erectile dysfunction, and sharp groin pain. He described his pain as being constant, sharp, and throbbing, and mainly located in the mid- to lower-back but radiating to his hips and groin. The patient stated the pain was worse when driving a car, sleeping, walking longer distances, and lifting. The pain and weakness had worsened in the past 3 months and now included generalized weakness, loss of balance, dizziness, numbness, and heat and cold intolerance. The patient denied any current bowel or bladder changes, except for one episode of bowel and bladder incontinence which resolved. Chiropractic intervention and pain medications had provided only mild relief. The patient's history was significant for dural ectasia and migraines and surgical repair of an ascending aortic aneurysm and replacement of the aortic valve 2 years prior to this evaluation.
Neurological examination was normal. Magnetic resonance imaging (MRI) revealed a large sacral cyst at S1-S2 extending to the base of the sacrum as well as bony erosion. Computerized tomography (CT) of the lumbar spine revealed diffuse lumbar dural ectasia with posterior scalloping of the lumbar vertebral bodies. CT also showed severe chronic dilatation of the sacral spinal canal with marked posterior scalloping of the upper sacrum and enlargement of S1 and S2 ventral neuroforamina. Differential considerations included diffuse sacral dural ectasia or a multilocular sacral arachnoid cyst.
The patient underwent L5 partial laminectomy and S1-S2 laminectomy with exploration and decompression of the large sacral cyst. Incision of the cyst was followed by copious drainage of CSF. The thecal sac and related thecal space were extremely dilated in this region, and arachnoid material was prominent. The intrathecal space was explored and no additional cyst was noted. The dura was reapproximated with 4–0 nylon in a simple interrupted fashion. FloSeal® (Baxter International, Deerfield, IL, USA) and cottonoids were utilized to obtain hemostasis. The intrathecal space was copiously irrigated with normal saline prior to final closure and clear fluid was noted. Once hemostasis was achieved, DuraGen® (Integra Neurosciences, Plainsboro, NJ, USA) (a dural substitute) and EVICEL® (Ethicon Biosurgery, Somerville, NJ, USA) (a tissue sealant) were applied in two layers to the repaired thecal sac. Bipolar electrocautery was used to obtain additional hemostasis. Once retractors were removed, a 10F round Blake drain was instituted and secured with 3–0 nylon prior to closure. The patient began to ambulate on postoperative day 3, and a fluid collection at the incision site was noticed and monitored closely. The patient left the hospital on postoperative day 4 against medical advice.
Nine weeks later, the patient complained of severe pain at the incision site and was found to have a large posterior soft tissue pseudomeningocele with septations suggestive of blood products. During surgical drainage of the pseudomeningocele, a small dural opening was found at a site of previous attempted patching; this opening was oversewn, repaired with a muscle graft patch, and covered with DuraGen® (Integra Neurosciences, Plainsboro, NJ, USA) and EVICEL® (Ethicon Biosurgery, Somerville, NJ, USA). A lumbar drain was used for the first 4 postoperative days, and the patient recovered uneventfully. His pain and urinary symptoms resolved over the next 2 months. He remains neurologically normal 2 years after surgery.
Patient 2
A 47-year-old man with Marfan syndrome presented to an outside facility after a fall on his tailbone 2 days earlier. The patient complained of low back pain and difficulty urinating, though he could ambulate without difficulty. The urinary retention was treated using a straight catheter; 900 ml of urine was returned. Neurological examination revealed decreased rectal tone, decreased perianal sensation to light touch and pinprick, and pinpoint tenderness at the mid-to-low sacral regions. The patient was transferred to our facility 3 days after his fall.
Neurological examination revealed decreased perianal sensation to light touch and pinprick, decreased rectal tone, and no bulbocavernosus reflex. The patient continued to have urinary retention, and, other than perianal numbness, he denied any peripheral numbness or tingling. No specific testing for bowel and bladder function was performed at either facility. The patient's history was significant for aortic and mitral valve repairs 4 years previously.
CT revealed a large lobulated soft tissue density sacral canal mass of the sacrum causing osseous remodeling and erosion of the posterior vertebrae. There was involvement of the S1-S3 sacral foramen, and a small portion of the mass extended through the right S2 foramen. Differential considerations included peripheral nerve sheath tumor (Fig. 1A and B). MRI revealed an expansile mass within the sacral spinal canal, extending from the midportion of S1 through S4 with involvement of the left ventral S1 neural foramen. Also seen were chronic erosion and remodeling of the sacral canal. Differential considerations included a low-grade nerve sheath tumor such as schwannoma or a large complex arachnoid cyst with prior hemorrhage. An MRI 3 days later revealed a well-circumscribed heterogeneous mass within the sacrum that did not demonstrate significant contrast enhancement. Also seen were persistent findings of bone remodeling and scalloping of the sacrum. The nerve roots at the cauda equina appeared clumped and extended into the superior aspect of the mass (Fig. 2A and B). CT-guided biopsy revealed blood, fragments of fibroadipose tissue, and blood vessels. Cytology demonstrated only very few atypical cells.
Figure 1.
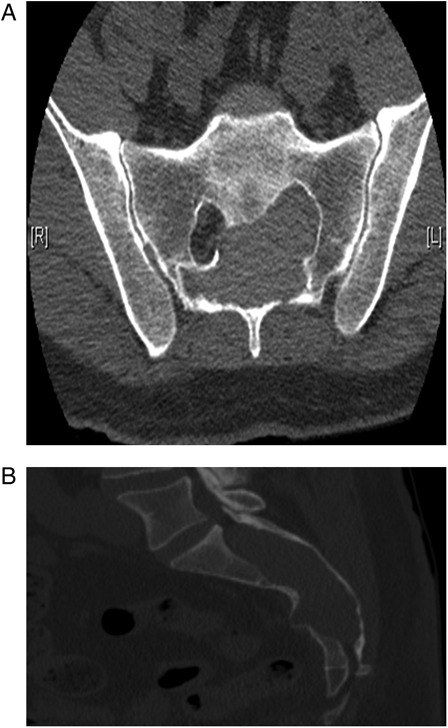
(A and B) Axial and sagittal CT scan showed a large lobulated mass in the sacral spinal canal, osseous remodeling of the posterior vertebrae, and involvement of the left S1-3 foramina. A small portion of the mass extended through the right S2 foramen.
Figure 2.
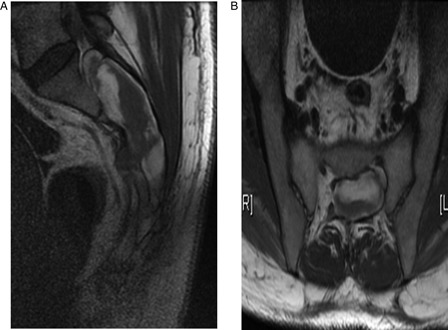
(A and B) Sagittal (A) and axial (B) MRI revealed hemorrhage within the thecal sac and bony erosion of the sacrum at S1-S4, as well as clumping of the nerve roots in the cephalad portion of the lesion.
Because the lesion was not compressing the spinal cord, only physical therapy was implemented. The patient was discharged after 12 days still requiring intermittent self-catheterization. MRI 4 weeks later showed no significant change of the sacral mass. Because his urinary retention still had not improved, the patient requested surgical exploration of the lesion. Ten weeks after his first hospital stay, the patient underwent decompression and resection of a sacral Tarlov-type expansile cyst and an organized subdural hematoma and decompression of sacral nerve roots with S1-3 laminectomy. The dura was closed primarily and followed by a secondary duraplasty using DuraGen® (Integra Neurosciences, Plainsboro, NJ, USA) and EVICEL® (Ethicon Biosurgery, Somerville, NJ, USA), and a lumbar drain was placed. Cyst contents revealed CSF, blood, and blood-breakdown products. The patient's pain and paresthesias resolved and his bowel function returned to normal. Other than the need for intermittent self-catheterization, all other neurologic findings are normal 30 months after surgery.
Discussion
Since its first description by Antoine Marfan in 1896, the congenital disorder that later came to bear his name has been of persistent clinical interest. Marfan syndrome is characterized by typical pathological features, most prominently involving the cardiovascular, skeletal, and ocular systems, and is generally categorized as a connective tissue disorder.1–6 Although the exact criteria for establishing the diagnosis are regularly revised, there is consensus that thinning of the aortic wall and aortic root aneurysms, arachnodactyly, elongation of long bones, and hypermobility of joints, as well as ectopia lentis, are key features of the syndrome. In addition, the pulmonary system, the myocardium, the mitral valve, and the skin may also be affected.1–3,5,6 Typically, Marfan symptoms do not manifest before adolescence or even adulthood,7,8 and phenotypes tend to be more pronounced in older patients.9
Dural ectasia, although very common in Marfan syndrome and other specific disorders like Ehlers-Danlos syndrome, neurofibromatosis, or ankylosing spondylitis,10,11 is often asymptomatic12,13 and is an incidental finding in many cases, although newer studies suggest a higher incidence of symptoms.14 Patients with severe dural ectasia, as well as lumbosacral meningoceles, however, may present with a diverse set of symptoms. Postural headaches that drastically worsen in the upright position and are relieved when the patient lies down are common. These headaches can be associated with nausea, dizziness, or blurred vision. In children, adolescents, and young adults, these headaches are often the only apparent symptom of dural ectasia and are generally attributed to intracranial hypotension secondary to CSF leaks, causing traction on pain-sensitive structures like cranial nerves, dura, and meninges.15–24 Pain in the lower back, the anogenital region and the legs, as well as muscular weakness and paresthesia of the lower extremities have been reported as being associated with dural ectasia and arachnoid cysts,13,14 typically indicating lumbosacral nerve root compression.10,25
A potential cause for the formation of dural ectasia and arachnoidal cysts in Marfan syndrome and other connective tissue disorders might be a progressive dural weakness due to hydrostatically induced higher intrathecal pressures and the propagation of CSF pulsations.26 This increasing dural dilation is thought to potentially increase the risk for CSF leakage upon even minor trauma and subsequent spontaneous intracranial hypotension.12,20,22,27 However, it remains unclear whether spontaneous CSF leakage without prior minor trauma might be increased in Marfan patients.22,28,29
Spinal meningeal cysts are uncommon, usually asymptomatic, and invariably found incidentally on MRI. Large cysts may cause mass effect and symptoms relating to compression of local structures such as nerve roots.30 Although meningeal abnormalities such as dural ectasia are seen in patients with Marfan syndrome, spinal meningeal cysts are rarely seen in these patients.31 Voermans et al.7 described three patients with Marfan syndrome and radicular symptoms. All of these patients had kyphoscoliosis and sacral cysts, but none underwent surgical correction of those lesions. Di Lazzaro et al.25 reported a patient with Marfan syndrome and a ventrally located arachnoid cyst at T12; no information other than a description of the lesion was presented.
Surgical indications for dural ectasia and sacral cysts are not well defined. One of our patients had symptoms for 5 years before surgery was performed after he began to have urinary dysfunction. Our other patient had an acute hemorrhage into the cyst following trauma, with development of urinary retention. The treatment goals for both of these patients were reversal of the bladder symptoms. Judicious use of surgery resulted in stabilization and improvement of those bladder issues.
Conclusion
We report two patients with Marfan syndrome and development of urinary symptoms secondary to sacral meningeal cysts. Surgical goals in the treatment of these lesions include resection of the cyst as well as closure of the dura, which can be challenging because the dura is often thinned out by ectasia. One patient required reoperation due to development of a pseudomeningocele at the surgical site. Neurosurgical intervention in the Marfan patient is associated with a high risk of dural tears and osseous complications. Spine surgery in these patients is therefore considered with caution,7,29,32,33 and should be primarily performed upon the occurrence of more severe symptoms.
References
- 1.Jondeau G, Michel JB, Boileau C. The translational science of Marfan syndrome. Heart 2011;97(15):1206–14 [DOI] [PubMed] [Google Scholar]
- 2.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47(7):476–85 [DOI] [PubMed] [Google Scholar]
- 3.Kiotsekoglou A, Sutherland GR, Moggridge JC, Nassiri DK, Camm AJ, Child AH. The unravelling of primary myocardial impairment in Marfan syndrome by modern echocardiography. Heart 2009;95(19):1561–6 [DOI] [PubMed] [Google Scholar]
- 4.von Kodolitsch Y, Rybczynski M, Detter C, Robinson PN. Diagnosis and management of Marfan syndrome. Future Cardiol 2008;4(1):85–96 [DOI] [PubMed] [Google Scholar]
- 5.von Kodolitsch Y, Robinson PN. Marfan syndrome: an update of genetics, medical and surgical management. Heart 2007;93(6):755–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Coster P, De Pauw G, Martens L, De Paepe A. Craniofacial structure in Marfan syndrome: a cephalometric study. Am J Med Genet A 2004;131(3):240–8 [DOI] [PubMed] [Google Scholar]
- 7.Voermans NC, Hosman AJ, van Alfen N, Bartels RH, de Kleuver M, op den Akker JW, et al. Radicular dysfunction due to spinal deformities in Marfan syndrome at older age: three case reports. Eur J Med Genet 2010;53(1):35–9 [DOI] [PubMed] [Google Scholar]
- 8.Voermans N, Timmermans J, van Alfen N, Pillen S, op den Akker J, Lammens M, et al. Neuromuscular features in Marfan syndrome. Clin Genet 2009;76(1):25–37 [DOI] [PubMed] [Google Scholar]
- 9.Chow K, Pyeritz RE, Litt HI. Abdominal visceral findings in patients with Marfan syndrome. Genet Med 2007;9(4):208–12 [DOI] [PubMed] [Google Scholar]
- 10.Ho NC, Hadley DW, Jain PK, Francomano CA. Case 47: dural ectasia associated with Marfan syndrome. Radiology 2002;223(3):767–71 [DOI] [PubMed] [Google Scholar]
- 11.Ahn NU, Sponseller PD, Ahn UM, Nallamshetty L, Rose PS, Buchowski JM, et al. Dural ectasia in the Marfan syndrome: MR and CT findings and criteria. Genet Med 2000;2(3):173–9 [DOI] [PubMed] [Google Scholar]
- 12.Fattori R, Nienaber CA, Descovich B, Ambrosetto P, Reggiani LB, Pepe G, et al. Importance of dural ectasia in phenotypic assessment of Marfan's syndrome. Lancet 1999;354(9182):910–3 [DOI] [PubMed] [Google Scholar]
- 13.Pyeritz RE, Fishman EK, Bernhardt BA, Siegelman SS. Dural ectasia is a common feature of the Marfan syndrome. Am J Hum Genet 1988;43(5):726–32 [PMC free article] [PubMed] [Google Scholar]
- 14.Foran JR, Pyeritz RE, Dietz HC, Sponseller PD. Characterization of the symptoms associated with dural ectasia in the Marfan patient. Am J Med Genet A 2005;134A(1):58–65 [DOI] [PubMed] [Google Scholar]
- 15.Voermans NC, Dijk KG, Bos MM, Geus-Oei LF, Verrips A, Lindert EJ. Postural headache in marfan syndrome associated with spinal cysts and liquor hypotension. Neuropediatrics 2009;40(4):201–4 [DOI] [PubMed] [Google Scholar]
- 16.Cheuret E, Edouard T, Mejdoubi M, Acar P, Pienkowski C, Cances C, et al. Intracranial hypotension in a girl with Marfan syndrome: case report and review of the literature. Childs Nerv Syst 2008;24(4):509–13 [DOI] [PubMed] [Google Scholar]
- 17.Puget S, Kondageski C, Wray A, Boddaert N, Roujeau T, Di Rocco F, et al. Chiari-like tonsillar herniation associated with intracranial hypotension in Marfan syndrome. Case report. J Neurosurg 2007;106(1 Suppl.):48–52 [DOI] [PubMed] [Google Scholar]
- 18.Rosser T, Finkel J, Vezina G, Majd M. Postural headache in a child with Marfan syndrome: case report and review of the literature. J Child Neurol 2005;20(2):153–5 [DOI] [PubMed] [Google Scholar]
- 19.Milledge JT, Ades LC, Cooper MG, Jaumees A, Onikul E. Severe spontaneous intracranial hypotension and Marfan syndrome in an adolescent. J Paediatr Child Health 2005;41(1–2):68–71 [DOI] [PubMed] [Google Scholar]
- 20.Ferrante E, Citterio A, Savino A, Santalucia P. Postural headache in a patient with Marfan's syndrome. Cephalalgia 2003;23(7):552–5 [DOI] [PubMed] [Google Scholar]
- 21.Mokri B. Headaches caused by decreased intracranial pressure: diagnosis and management. Curr Opin Neurol 2003;16(3):319–26 [DOI] [PubMed] [Google Scholar]
- 22.Mokri B, Maher CO, Sencakova D. Spontaneous CSF leaks: underlying disorder of connective tissue. Neurology 2002;58(5):814–6 [DOI] [PubMed] [Google Scholar]
- 23.Waguri N, Tomita M, Hayatsu K, Okamoto K, Shimoji K. Epidural blood patch for treatment of spontaneous intracranial hypotension. Acta Anaesthesiol Scand 2002;46(6):747–50 [DOI] [PubMed] [Google Scholar]
- 24.Chung SJ, Kim JS, Lee MC. Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology 2000;55(9):1321–7 [DOI] [PubMed] [Google Scholar]
- 25.Di Lazzaro V, Pilato F, Dileone M, Minicuci G, Profice P, Colosimo C, et al. Extradural arachnoid cyst with lumbosacral cord and root compression in marfan syndrome. Arch Neurol 2007;64(2):284–5 [DOI] [PubMed] [Google Scholar]
- 26.Sheikhzadeh S, Rybczynski M, Habermann CR, Bernhardt AM, Arslan-Kirchner M, Keyser B, et al. Dural ectasia in individuals with Marfan-like features but exclusion of mutations in the genes FBN1, TGFBR1 and TGFBR2. Clin Genet 2011;79(6):568–74 [DOI] [PubMed] [Google Scholar]
- 27.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks: a review. Neurosurg Focus 2000;9(1):e8. [DOI] [PubMed] [Google Scholar]
- 28.Schrijver I, Schievink WI, Godfrey M, Meyer FB, Francke U. Spontaneous spinal cerebrospinal fluid leaks and minor skeletal features of Marfan syndrome: a microfibrillopathy. J Neurosurg 2002;96(3):483–9 [DOI] [PubMed] [Google Scholar]
- 29.Schievink WI, Gordon OK, Tourje J. Connective tissue disorders with spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: a prospective study. Neurosurgery 2004;54(1):65–70 [DOI] [PubMed] [Google Scholar]
- 30.Tureyen K, Senol N, Sahin B, Karahan N. Spinal extradural arachnoid cyst. Spine J 2009;9(8):e10–5 [DOI] [PubMed] [Google Scholar]
- 31.Hoshino Y, Edakuni H, Shimada H, Hayashi S, Machida M, Shimano S, et al. Sacral arachnoid cyst associated with marfan syndrome. Intern Med 2005;44(3):271–3 [DOI] [PubMed] [Google Scholar]
- 32.Jones KB, Erkula G, Sponseller PD, Dormans JP. Spine deformity correction in Marfan syndrome. Spine 2002;27(18):2003–12 [DOI] [PubMed] [Google Scholar]
- 33.Zenner J, Hitzl W, Meier O, Auffarth A, Koller H. Surgical outcomes of scoliosis surgery in Marfan syndrome. J Spinal Disord Tech. 2012. Mar 6. [Epub ahead of print], DOI 10.1097/BSD.0b013e31824de6f1. [DOI] [PubMed] [Google Scholar]


