Abstract
The ability to read emotions in the face of another person is an important social skill that can be impaired in subjects with traumatic brain injury (TBI). To determine the brain regions that modulate facial emotion recognition, we conducted a whole-brain analysis using a well-validated facial emotion recognition task and voxel-based lesion symptom mapping (VLSM) in a large sample of patients with focal penetrating TBIs (pTBIs). Our results revealed that individuals with pTBI performed significantly worse than normal controls in recognizing unpleasant emotions. VLSM mapping results showed that impairment in facial emotion recognition was due to damage in a bilateral fronto-temporo-limbic network, including medial prefrontal cortex (PFC), anterior cingulate cortex, left insula and temporal areas. Beside those common areas, damage to the bilateral and anterior regions of PFC led to impairment in recognizing unpleasant emotions, whereas bilateral posterior PFC and left temporal areas led to impairment in recognizing pleasant emotions. Our findings add empirical evidence that the ability to read pleasant and unpleasant emotions in other people's faces is a complex process involving not only a common network that includes bilateral fronto-temporo-limbic lobes, but also other regions depending on emotional valence.
Keywords: facial emotion recognition, voxel-based lesion symptom mapping, labeling task, basic emotions, traumatic brain injury
INTRODUCTION
The ability to interpret and recognize emotions of other people is one of the most ancient and important human social skills. Recognizing facial expressions allows us to detect another person's emotional state and to appropriately engage in social interactions (Frank and Stennett, 2001; Grossmann and Johnson, 2007). Fear, disgust, anger, happiness, surprise and sadness are the six basic emotions, which can be universally recognized through others’ facial expressions (Izard, 1971; Ekman, 1992). Facial emotion perception is defined as ‘any higher-level visual processing of faces’ (Kanwisher et al., 1997), which involves both perceptual processing and recognition of a stimulus’ emotional meaning (Adolphs, 2002). Facial emotion recognition combines the use of current visual sensory input with the retrievable memory to recognize emotions, an ability that develops during the neonatal stages. Finally, the ability to name an emotion requires both conceptual and lexical knowledge, each of which is necessary to assign an explicit verbal name to the emotion (Adolphs et al., 2000).
The ability to read emotions in other people's faces can be impaired in a number of neurological and injured populations. Given the importance of emotion recognition abilities and the great interest in understanding the brain regions that support these skills, many studies have been carried out in patients with schizophrenia (Blonder et al., 1991; Bora et al., 2008), Parkinson's disease (Borod and Caron, 1980; Borod et al., 1990), multiple sclerosis (Calder et al., 2000), depression (Derntl et al., 2011), dementia syndromes (Kessels et al., 2007; Miller et al., 2012), cerebro-vascular accident or tumor (Calder et al., 1996; Borod et al., 1998), human immunodeficiency virus (Clark et al., 2010), Huntington's disease (Ille et al., 2011), epilepsy (Carver, 2001) and traumatic brain injury (TBI) (Green et al., 2004). Several studies have shown that TBI can lead to an impairment in the recognition of emotions signaled by a face (Adolphs et al., 1994; McDonald and Flanagan, 2004) while retaining the ability to recognize other types of information (e.g. identity or gender of a person). Hence, the deficit can lead to problems in social daily life, including social withdrawal and an inability to maintain meaningful relationships (Hoofien et al., 2001). Importantly, it has been noted that the nature of difficulties in emotion recognition may vary depending on the emotional valence of the stimuli (Jackson and Moffat, 1987; Beck et al., 1996; Hopkins et al., 2002; Hornak et al., 2003; McDonald and Flanagan, 2004; Alves et al., 2009). For example, Croker and McDonald (2005) assessed emotion labelling in a group of individuals with TBI and a group of healthy individuals and demonstrated that both groups were more accurate in discriminating pleasant compared to unpleasant expressions of emotions. Moreover, Williams and Wood (2010) showed that the differences in accuracy between pleasant and unpleasant expressions of emotions were greater in TBI group than in normal control group.
Converging evidence indicate the importance of the prefrontal cortex (PFC) in regulating emotional responses, and the left temporal and left inferior frontal cortex in the lexical and semantic analysis of faces (Nakamura et al., 1999; Hariri et al., 2000; Narumoto et al., 2000; Gorno-Tempini et al., 2001; Derntl et al., 2011), suggesting that fronto-temporal regions are involved in tasks requiring explicit identification of emotions as opposed to passive viewing of emotional faces. Neuroimaging studies demonstrated that the temporal lobe and amygdala are activated when recognizing static emotional facial expression (Morris et al., 1996; Sprengelmeyer et al., 1998; Phan et al., 2002; Habel et al., 2007) in addition to frontal lobe regions, including the orbitofrontal cortex (OFC; Vuilleumier et al., 2001; Goodkind et al., 2011), the ventro medial prefrontal cortex (vmPFC; Narumoto et al., 2000) and the inferior frontal gyri (George et al., 1993; Sprengelmeyer, et al., 1998). Consistent with these neuroimaging studies, patient research reported deficits in facial emotion recognition following temporal lobe (Anderson and Phelps, 2000; Adolphs, 2002; Rosen et al., 2006) and frontal lobe damage (Hornak et al., 1996; Blair and Cipolotti, 2000; Marinkovic et al., 2000; Beer et al., 2003; Mah et al., 2004; Heberlein et al., 2008; Miller et al., 2012). Despite over two decades of study and general agreement about the brain regions involved in emotion, conflicting findings are often produced by studies using different methods, stimuli, tasks and techniques. Some studies found an asymmetrical network in recognition of emotions with the right hemisphere specialized for processing unpleasant emotions and the left hemisphere for pleasant emotions (Silberman and Weingartner, 1986; Davidson, 1992; Drevets and Raichle, 1998; Davidson and Irwin, 2000; Eslinger et al., 2002; Decety and Lamm, 2006; Decety, 2010). Recent meta-analyses of neuroimaging studies on face emotion recognition found evidence for a more symmetrical view where pleasant and unpleasant emotions are implemented in both hemispheres (Wager et al., 2003; Fusar-Poli et al., 2009) consistent with findings from lesion studies (Adolphs, et al., 2000; Heberlein et al., 2008; Philippi et al., 2009; Miller et al., 2012; Tsuchida and Fellows, 2012).
This uncertainty concerning emotions, their network, processing and interpretation led us to investigate facial emotion recognition in control and penetrating TBI (pTBI) subjects and identify the underlying brain network supporting the recognition of pleasant and unpleasant facial emotions using a lesion analysis approach that complements functional neuroimaging and electrophysiological studies. Our study utilized a well-validated facial emotion recognition task and voxel-based lesion symptom mapping (VLSM) in a large sample of patients with focal pTBI. The purpose of this article was 2-fold: first, to investigate the role of affective valence of stimuli on facial emotion recognition in pTBI; and second, to clarify the participation of different neural networks in facial emotion recognition. In particular, we were interested in investigating the common and distinctive brain networks that encode pleasant and unpleasant emotions.
MATERIALS AND METHODS
Subjects
Subjects were drawn from Phase III of the W.F. Caveness Vietnam Head Injury Study (VHIS) registry, which is a prospective, long-term follow-up study of veterans with mostly focal pTBI (Raymont et al., 2011). The VHIS has been organized in three phases: Phase I (1967–70) included 1221 male American veterans who survived penetrating brain injuries suffered in Vietnam. For Phase II (1981–84), 520 veterans who had participated in Phase I participated in an extensive follow-up clinical study at Walter Reed Army Medical Center. At that time, 85 Vietnam veterans without head injuries were recruited as healthy controls. For Phase III (2003–06), 199 brain-injured and 55 non-brain injured veterans participated in the study located at the National Naval Medical Center, Bethesda, MD. Since not all subjects completed the entire cognitive battery, our population consisted of a sample of 233 male military veterans, divided into an experimental pTBI lesion group (LG = 180) and a normal control group (CG = 53) who served in Vietnam but did not sustain brain injuries. The advantages of studying the VHIS population include their uniformity of age, gender, and education, the availability of pre-injury intelligence scores, and their shared history of being soldiers, trained for war who experienced trauma. Pre-injury general intelligence was assessed with the Armed Forces Qualification Test (AFQT-7A), which was administered to individuals upon entry into the military (Department of Defense, 1960). This test has been extensively standardized within the US military and correlates highly with WAIS IQ scores (Gorno-Tempini et al., 2001). All subjects gave their written informed consent, which was approved by the Institutional Review Board at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Neuropsychological measures
All subjects underwent a 5–7 day neuropsychological assessment of several areas of cognitive functioning, including memory, language, perception, general intelligence and social cognition. For our experimental measure, we employed the Facial Expression of Emotion: Stimuli and Tests (FEEST) to assess the recognition and naming of basic emotions conveyed by facial expression (Ekman and Friesen, 1976). The FEEST measures emotion recognition ability that requires access to both conceptual knowledge of emotions and lexical knowledge necessary to name emotions (Adolphs et al., 1994, 1995). Subjects were shown, in random order, black-and-white slides of faces expressing basic emotions and asked to name the emotion shown using one of the six labels (anger, disgust, fear, happiness, surprise and sadness). Responses were recorded via computer keyboard. The subjects could take as long as they wished to respond. Performance feedback was not provided. For each correct answer, 1 point was given, up to a possible total score of 120 points. The FEEST provides a raw score for each of the six emotions in addition to the grand total score. We then calculated two additional scores based on the emotional valence of the stimuli (Adolphs, 2002): pleasant (i.e. happiness and surprise) and unpleasant (i.e. sadness, anger, fear and disgust) emotions. The reliability and validity of the FEEST have been demonstrated by a number of studies, including TBI samples (Ahem and Schwartz, 1979; Hornak et al., 1996; Adolphs et al., 2001; Wager, et al., 2003; Szekely et al., 2011). A more detailed discussion of the psychometric properties of the FEEST and how it has been developed can be found in the FEEST user's software manual v2.1 (Young et al., 2002).
As our control measures, we administered the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997) for post-injury general intelligence, Beck Depression Inventory-II (Beck et al., 1996) for post injury depression symptoms, Token Test (McNeil and Prescott, 1994) for basic verbal comprehension, Visual Object and Space Perception Battery (VOSP; Warrington and James, 1991) for object and space perception, the Boston Naming Test (Kaplan et al., 2001) for object naming, and the Morphed faces test (Ekman and Friesen, 1976) for assessing the perception and discrimination of pleasant and unpleasant facial expressions. The Morphed faces test is considered as a perceptual task (Hariri et al., 2000), which presents pairs of faces expressing the basic emotions and asks subjects to simply decide whether the emotional expressions of the two faces are the same or different. Naming of the emotions is not required.
Data analysis
Behavioral analysis
Behavioral data analyses were carried out using SPSS 14.0 (SPSS Inc., Chicago, IL, USA) with alpha set to P < 0.05 (two-tailed). First, we compared demographical and neuropsychological control measures between LG and CG using independent samples’ t-tests. Second, we investigated the facial emotion recognition performance on the FEEST using a mixed 2 × 2 analysis of variance (ANOVA) with valence (pleasant vs unpleasant) as a within-subjects factor and group (LG vs CG) as a between-subjects factor. In planned follow-up analyses, independent t-tests were performed to explore the contributions of specific emotions found in the main effects of the ANOVA.
Computed tomography acquisition and analysis
Axial computed tomography (CT) scans were acquired without contrast in helical mode on a General Electric Medical Systems Light Speed Plus CT scanner at the Bethesda Naval Hospital. (Note that the pTBI patients could not be imaged with a magnetic resonance scanner because of the retained metal fragments in their brain.) Structural neuroimaging data were reconstructed with an in-plane voxel measuring 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm and a 1 mm slice interval. Lesion location and volume from CT images were determined using the interactive analysis of brain lesions (ABLe) software implemented in MEDx v3.44 (Medical Numerics) (Makale et al., 2002; Solomon et al., 2007) with enhancements to include the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002). Lesion volume was calculated by manually tracing the lesions in all relevant slices of the CT image in native space, summing the traced areas and multiplying by slice thickness. As in many other lesion analysis studies (Heberlein et al., 2004, 2008; Krueger et al., 2009; Schwartz et al., 2009; Koenigs et al., 2010; Anderson et al., 2011; Geva et al., 2011; Leopold et al., 2011; Tsuchida and Fellows, 2012), the lesion tracing was performed by a neuropsychiatrist experienced in imaging analysis (V.R.) and then reviewed by an observer who was blind to the results of the clinical evaluation and neuropsychological testing (J.G.), enabling a consensus to be reached regarding the boundaries of each patient's lesion. We combined the CT images with processing procedures common to the neuroimaging community, such as alignment to Montreal Neurological Institute (MNI) space and false-discovery rate (FDR) corrections, based on previous VLSM studies (Kimberg and Farah, 1993; Kimberg et al., 2007; Glascher et al., 2009, 2010; Walker et al., 2011; Tsuchida and Fellows, 2012). The CT image of each individual's brain was normalized to a CT template brain image in MNI space. The spatial normalization was performed with the automated image registration (AIR) algorithm (Woods et al., 1993), using a 12-parameter affine fit. Note that both the patient's brain and the MNI template brain are first skull-stripped in order to maximize the efficacy of the AIR registration from native space to MNI space. In addition, voxels inside the traced lesion were not included in the spatial normalization procedure.
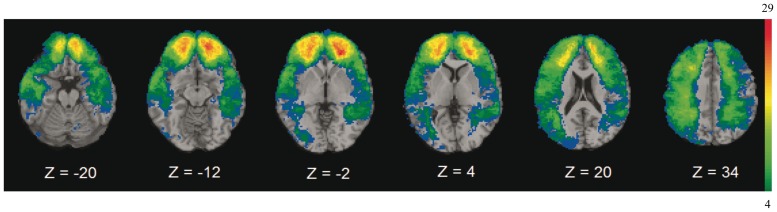
First, we looked at the distribution of lesions in our sample. A lesion density overlap map was created by overlaying the individual normalized lesion maps of all pTBI subjects to show how many patients had lesions at each voxel (Figure 1). We restricted all analyses to a minimum overlap of four patients in a given voxel to assure sufficient statistical power (Glascher et al., 2009). Since the power of a voxel analysis largely reflects the regional variations of vulnerability to brain injury, maximal power was observed in prefrontal-temporo areas, regions that are linked with face emotion recognition (Adolphs, 2002).
Fig. 1.
Lesion density map for pTBI patients. Color indicates the number of overlapping lesions at each voxel. Red indicates more subjects and blue fewer. We restricted all analyses to a minimum overlap of four patients in a given voxel. In each slice, the right hemisphere is on the reader’s left.
Second, VLSM analyses were applied to the whole brain for total pleasant and unpleasant emotion scores. This method allowed us to perform a t-test within LG comparing face emotion recognition scores between the group of subjects having a lesion at that voxel and those that did not. On each voxel, a t-test was performed to find any significant positive association of lesioned voxels and emotion recognition. A P-value was determined for each voxel, based on its t-value and degrees of freedom. Multiple comparison correction was performed using the FDR method (q = 0.01), where the P-values were ordered from smallest to largest and a P-value threshold was determined. Having q = 0.01 means that on average we allowed 1% of suprathreshold voxels to be false positives. Brain regions where a significant lesion–deficit relationship (i.e. association between deficits in facial emotion recognition and a lesion at each voxel) was found were mapped. Gyri and Talairach coordinates were obtained by applying the AAL atlas (Tzourio-Mazoyer et al., 2002).
Finally, the overlap between the pleasant and unpleasant emotion lesion maps was investigated using the percentage of AAL structures. To examine the areas exclusive to a single factor, all the significant lesion–deficit areas in common among the binomial categorization were first removed. Afterward, a conjunction analysis was applied between the two emotion categories; this produced three maps: the first shows common areas shared between pleasant and unpleasant emotions, and the second and third show unique lesion–deficit areas for each emotion condition. A summary of the findings is displayed in a single map by using different colors for the three lesion–deficit maps that highlight both the common network in green and the unique lesion areas for unpleasant emotions in yellow and for pleasant emotions in blue.
RESULTS
Behavioral results
The LG and CG did not differ significantly on demographic measures (i.e. age, education, handedness) and neuropsychological control measures (i.e. pre-injury intelligence, verbal comprehension, perception, language, depression and discrimination of faces) (Table 1). However, the LG had a lower post-injury intelligence score than that of the CG event though their scores were still in the normal range.
Table 1.
Mean ± standard deviations and statistics of demographic characteristics and neuropsychological tests of pTBI lesion group (LG) and non-head-injured control group (CG)
| Group | LG = 180 | CG = 53 | Statistics |
|
|---|---|---|---|---|
| t-value | P-value | |||
| Age (years) | 58.3 ± 3.0 | 58.9 ± 3.4 | 1.30 | 0.197 |
| Education (years) | 14.8 ± 2.5 | 15.2 ± 2.5 | 0.85 | 0.369 |
| Handedness (R:A:L) | 150:4:26 | 42:3:8 | χ2 = 1.7 | 0.426 |
| Pre-injury intelligence (AFQT) | 62.0 ± 24.5 | 66.9 ± 21.8 | 1.07 | 0.284 |
| Post-injury intelligence (WAIS-III) | 103.4 ± 14.4 | 110.4 ± 12.4 | 3.20 | 0.022 |
| BDI-II | 9.2 ± 9.1 | 11.4 ± 9.7 | 1.54 | 0.125 |
| TT | 97.6 ± 5.9 | 98.8 ± 1.6 | 1.48 | 0.140 |
| VOSP | 19.8 ± 0.6 | 19.7 ± 1.5 | −0.73 | 0.465 |
| Boston naming | 53.7 ± 7.5 | 55.9 ± 3.6 | 1.86 | 0.063 |
| Morphed faces | 38.1 ± 7.5 | 37.5 ± 7.3 | −0.52 | 0.604 |
| Morphed unpleasant | 39.9 ± 8.3 | 39.3 ± 7.8 | −0.44 | 0.661 |
| Morphed pleasant | 34.6 ± 8.6 | 33.4 ± 7.9 | −0.94 | 0.350 |
| FEEST unpleasant emotions | 15.14 ± 3.8 | 16.77 ± 2.4 | 2.90 | 0.000 |
| FEEST pleasant emotions | 18.23 ± 1.9 | 18.76 ± 1.3 | 1.89 | 0.059 |
Age: years at the time of FEEST administration; Handedness: R, right-handed; A, ambidextrous; L, left-handed; Pre-injury Intelligence: AFQT; Post-injury Intelligence: WAIS-III, Wechsler Adult Intelligence Scale-III for cognitive intellectual ability; BDI-II, Beck Depression Inventory-II for post-injury depression symptoms; TT: Token test for basic verbal comprehension; VOSP: visual object and space perception for object and space perception; Boston naming: for object naming; Morphed faces (percentage): for basic discrimination of facial expression; Morphed unpleasant emotions: anger, sadness, disgust and fear; Morphed pleasant emotions: happiness and surprise; FEEST: Facial Expression of Emotion Stimuli Test for emotion recognition and naming ability; FEEST unpleasant emotions: anger, sadness, disgust and fear; FEEST pleasant emotions: happiness and surprise.
For the facial emotion recognition performance on the FEEST, the two-way ANOVA revealed a significant main effect of valence [F(1,231) = 94.0, P < 0.001], a significant main effect of group [F(1,231) = 9.1, P < 0.003] and a significant interaction effect of Valence × Group [F(1,231) = 4.4, P < 0.038]. Follow-up analyses revealed that the LG compared to the CG had significantly lower scores for unpleasant emotions [t(231) = 15.8, P < 0.008, Bonferroni correction] but not for pleasant emotions [t(231) = 2.4, P = 0.118, Bonferroni correction].
Lesion results
First, we looked at the distribution of brain lesions in our sample by analyzing the overlap of the spatially normalized lesion images (Figure 1). Because not every voxel contains a lesion across subjects, statistical power is often lacking in VLSM analyses. We tolerated low power in order to be able to test lesion locations over much of the brain. For our VLSM analysis, we considered brain regions where at least four subjects had overlapping lesions in a given voxel. If fewer than four injured veterans had a lesion in a given voxel, then that voxel was excluded from our analyses. The maximum overlap of 29 subjects occurred in fronto-temporal regions; areas that previous studies have shown to be involved in tasks requiring explicit identification and recognition of emotions (Hariri, et al., 2000; Derntl et al., 2011).
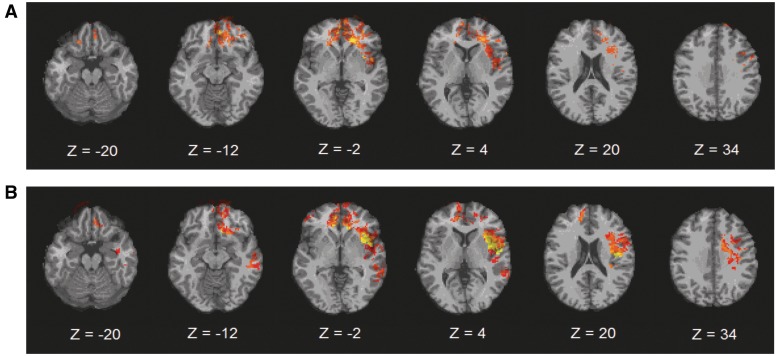
Second, we investigated brain lesions that affected facial emotion recognition, by VLSM analyses applied to the whole brain for both pleasant and unpleasant emotions. Lesions that affected recognition of unpleasant emotions were found in a fronto-temporo-limbic network, including the bilateral inferior frontal gyrus (IFG), bilateral medial frontal gyrus (MFG), bilateral middle frontal gyrus (MidFG), bilateral superior frontal gyrus (SFG) and left precentral gyrus (PCG) in the frontal lobe; bilateral anterior cingulate gyrus (ACG) and left insula in the limbic lobe; and left superior temporal gyrus (STG) in the temporal lobe (Figure 2A).
Fig. 2.
Voxel-based lesion-symptom mapping of emotional valence scores. (A) Top row, the VLSM analysis results, comparing voxel-by-voxel the index scores in facial recognition of unpleasant emotions of pTBI patients with a lesion against those without a lesion in that voxel. (B) Second row, VLSM analysis results for pleasant emotions. All colored regions in the slices show significant correlation with deficits in facial emotion recognition [q(FDR) = 0.01, corrected for multiple comparisons]. Bright color (yellow) indicates stronger correlation, darker colors (red) indicate less. In each slice, the right hemisphere is on the reader’s left.
Lesions that affected recognition of pleasant emotions were found in a fronto-temporo-limbic network that included left IFG, bilateral MFG, bilateral MidFG, bilateral SFG and left PCG in the frontal lobe; bilateral ACG and left insula in the limbic lobe; left STG, along with left middle temporal gyrus (MidTG) and left inferior temporal gyrus (ITG) in the temporal lobe (Figure 2B). (Note that MNI coordinates of the peak lesion–deficit relationship for the unpleasant and pleasant emotion scores are listed in Table 2.)
Table 2.
Description of the MNI coordinates of the peak lesion–deficit relationships for FEEST unpleasant and pleasant emotions total scores
| Region | X | Y | Z |
|---|---|---|---|
| Unpleasant emotions | |||
| Right superior frontal gyrus | 22 | 8 | 64 |
| Left superior frontal gyrus | −18 | 52 | 28 |
| Left middle frontal gyrus | −24 | −8 | 54 |
| Right middle frontal gyrus | 27 | 37 | −20 |
| Left medial frontal gyrus | −8 | 50 | −14 |
| Right inferior frontal gyrus | 20 | 36 | −20 |
| Left inferior frontal gyrus | −62 | 18 | 4 |
| Left precentral gyrus | −45 | −3 | 26 |
| Left insula | −46 | −10 | 16 |
| Right anterior cingulate gyrus | 11 | 46 | −2 |
| Left anterior cingulate gyrus | −8 | 47 | 0 |
| Left superior temporal gyrus | −48 | 8 | −2 |
| Pleasant emotions | |||
| Left superior frontal gyrus | −24 | 10 | 48 |
| Right superior frontal gyrus | 34 | 70 | 2 |
| Right middle frontal gyrus | 52 | 46 | −6 |
| Left inferior frontal gyrus | −14 | 38 | −20 |
| Left precentral gyrus | −37 | −4 | 42 |
| Left insula | −41 | 18 | 5 |
| Right anterior cingulate gyrus | 13 | 45 | −2 |
| Left anterior cingulate gyrus | −16 | 29 | −11 |
| Left superior temporal gyrus | −48 | −12 | 1 |
| Left middle temporal gyrus | −66 | −29 | 1 |
| Left inferior temporal gyrus | −53 | −26 | −14 |
X, Y and Z, MNI coordinates in a 3-dimensional human brain. (X specifies the point in the left/right direction of the brain; Y specifies the posterior/anterior direction; Z specifies the inferior/superior direction.)
To further investigate the common areas that mediate pleasant and unpleasant emotions, we examined with a conjunction analysis the overlap between the location of voxels significantly associated with pleasant and unpleasant face emotion recognition. Pleasant and unpleasant emotions shared 48% of lesion areas in a fronto-temporo-limbic network (left IFG, bilateral MFG, bilateral MidFG, left SFG and left PCG in the frontal lobe; bilateral ACG and left insula in the limbic lobe; left STG in the temporal lobe) (see green areas, Figure 3). We next examined the unique voxels significantly associated with pleasant or unpleasant emotions. The lesion-areas exclusive to unpleasant emotions (see yellow areas, Figure 3) were distributed in the frontal lobe (bilateral IFG, MFG, MidFG and SFG), whereas unique lesion areas for pleasant emotions (see blue areas, Figure 3) were found in the fronto-temporal network (bilateral SFG, MFG, MidFG, left IFG and left PCG in the frontal lobe; left MidTG and ITG in the temporal lobe).
Fig. 3.
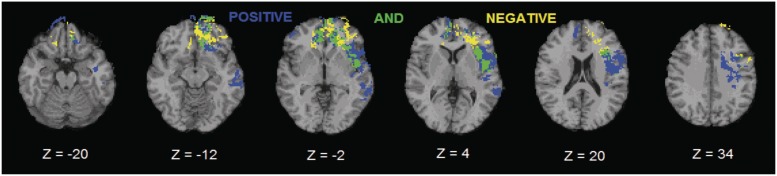
Conjunction analysis between pleasant and unpleasant emotions. Green shows brain structures that overlap between pleasant and unpleasant emotion conditions. Yellow shows lesion–deficit areas unique for unpleasant emotions. Blue shows lesion–deficit areas unique for pleasant emotions. In each slice, the right hemisphere is on the reader’s left.
DISCUSSION
The goal of this study was to investigate the role of affective valence of stimuli in facial emotion recognition and the neural network involved in this skill. We used both behavioral and neuroimaging data to assess performance in pTBI associated with specific neural areas related to facial emotion recognition. The behavioral results revealed that patients with pTBI had difficulties with facial recognition of unpleasant emotions. The VLSM results showed that recognition of both pleasant and unpleasant emotions is impaired due to damage to a bilateral fronto-temporo-limbic network. Besides a common network, unique regions responsible for recognizing each emotional valence were identified: damage to anterior and bilateral PFC lesions resulted in deficits in recognizing unpleasant emotions, whereas damage to posterior and bilateral PFC and left temporal areas resulted in impaired recognition of pleasant emotions.
Our LG was significantly impaired in facial emotion recognition compared to the CG, consistent with previous research (Heller et al., 1998; Hall et al., 2004; Henry et al., 2009). Importantly, our results could not be accounted for by a general impairment in facial information processing since all pTBIs were able to discriminate faces normally as indicated by their performance on the Morphed Faces Test, nor to demographic measure or to a general impairment in intelligence, verbal comprehension, perception, language or depression, indicating a specific impairment in recognizing and naming the emotional content in the faces.
For the facial emotion recognition performance on the FEEST, both groups were more accurate when recognizing pleasant emotions, consistent with previous research (Hall et al., 2004; Croker and McDonald, 2005). Moreover, the LG performed significantly worse than the CG in the recognition of unpleasant emotions, providing some support for separate networks for these two categories of emotions. It has been proposed that negative emotions are mediated by discrete pathways involving vmPFC and OFC, all structures that are vulnerable to damage following TBI (Hornak, et al., 1996; Blair et al., 1999; Narumoto, et al., 2000; Vuilleumier, et al., 2001; Adolphs, 2002; Hornak, et al., 2003; Heberlein, et al., 2008; Tsuchida and Fellows, 2012). In addition, unpleasant emotions may require more elaborate processing as they share a number of features; for example furrowed brows appear in both anger and sadness (Ekman and Friesen, 1976). Therefore, if brain injury affects emotional processing, those with pTBI might find unpleasant emotions more difficult to interpret.
With a VLSM whole-brain analysis looking at each voxel independently, we were able to localize neural regions responsible for facial emotion recognition deficits without an a priori division of patients into lesion subgroups (e.g. vmPFC vs dorsolateral PFC). In our pTBI population, damage to the bilateral PFC, limbic lobe and left temporal regions resulted in impaired naming of both pleasant and unpleasant emotions from faces. Overall, we found limited support for the hemispheric asymmetry theories (Ahem and Schwartz, 1979; Silberman and Weingartner, 1986; Adolphs et al., 1996; Sprengelmeyer, et al., 1998; Wiedemann et al., 1999; Davidson and Irwin, 2000; Adolphs, 2002), instead our results are more consistent with recent results from neuroimaging analyses suggesting a symmetrical involvement of the right and left hemispheres in recognizing both pleasant and unpleasant emotions (Sprengelmeyer et al., 1996; Murphy et al., 2003; Wager et al., 2003; Benedetti et al., 2009; Fusar-Poli et al., 2009; Miller et al., 2012).
Given that recognition of pleasant and unpleasant emotions shared almost 50% of brain regions, a conjunction analysis allowed us to detect a common fronto-temporo-limbic network that seems to play a reliable and important role in the complex process of facial emotion recognition. Adolphs (2003) reported that the limbic system, bilateral PFC and anterior temporal lobe might be crucial for recognizing and naming facial emotional stimuli. Moreover, previous studies have described the role of the PFC in regulating emotional responses, while the left temporal lobe may be more involved in the semantic analysis of faces or in the lexical knowledge of emotion (Nakamura et al., 1999; Hariri, et al., 2000; Narumoto et al., 2000; Derntl, et al., 2011) and in the retrieval of information from long-term memory (Fletcher and Henson, 2001; Henson et al., 2002).
Specifically, we found that damage to the medial PFC (mPFC), ACC and insula was responsible for the complex process of facial emotion recognition independent of emotional valence as recently suggested (Hoofien et al., 2001; Phan et al., 2002). The ACC and insula are known to be involved in a form of explicit attention that serves to regulate both cognitive and emotional processing, particularly in cognitively demanding tasks (Whalen et al., 1998; Bush et al., 2000) and these regions are closely interconnected with the mPFC (Hoofien et al., 2001).
Besides the common network involved in perception, recognition and naming facial emotions independent of the emotional valence (Adolphs, 2002; Derntl et al., 2011), our conjunction analysis allowed us to isolate overlapping areas along with unique regions responsible for recognizing each valence of emotion. Anterior and bilateral PFC lesions corresponded with decreased performance in recognizing unpleasant emotions, while bilateral and posterior PFC and left temporal areas appear important for recognizing pleasant emotions. A number of studies have observed the main role of anterior PFC in unpleasant rather than pleasant emotions. For example, one study applied transcranial magnetic stimulation (TMS) to the mPFC, temporarily disrupting its functioning and found longer reaction times in response to angry facial expressions but not in response to happy facial expressions (Harmer et al., 2001). Blair and colleagues (1999) reported increased activation in the orbitofrontal and anterior cingulate cortex when subjects viewed angry facial expressions. Heberlein et al., (2008) found lower scores in recognition of fear after vmPFC damage. Furthermore, given that the experience, expression and recognition of emotions are associated with ‘the generation of a simulation’ of that emotion (Adolphs, 2002), the main involvement of PFC in negative emotions is underscored by the critical and causal role of vmPFC in mediating negative affect (Koenigs et al., 2008). In addition, recent literature suggests that babies are able to recognize pleasant emotions within the first few months of life; but the recognition of unpleasant emotions, like fear, disgust and anger, appear later on in the first/second year of life (LaBarbera et al., 1976; Nelson and De Haan, 1996; Nelson, 2001). The recognition of unpleasant facial emotions and the knowledge of the meaning of the emotions’ name are acquired over development and improve with age (Szekely et al., 2011). Therefore, it is possible that the emotion-labelling required larger engagement of higher neocortical regions, which are less, well developed in younger children, and most often affected by pTBI.
The ability to name an emotion from a face is a complex process with many variables, including perceiving, recognizing and naming an emotion, each of which may be impaired in pTBI patients. Our patients had preserved abilities to recognize and name objects, and discriminate emotional faces; however, it is not clear whether their impairments in facial emotion recognition involve a lack of access to conceptual or lexical emotional knowledge. Since these two kinds of knowledge draw on neuroanatomically separable systems (Adolphs et al., 2000), further work should be performed to determine this distinction. Future VLSM research should include tests, such as the Benton and Cambridge Face Perception batteries, which determine impairments in basic face processing skills in order to clarify whether the temporal lobes are more involved with face perception or facial emotion recognition. Moreover, going beyond the six basic emotions by investigating possible relationships between lesion location and recognition of more complex and social emotions may be fruitful for narrowing down the context dependence of facial emotion recognition processes. Finally, since our findings do not reveal the entire network activated in the complex process of facial emotion recognition but only surveyed regions that were damaged in our patients and that were correlated with lower performance in the specific task we used, it is a reminder that convergent evidence from healthy volunteers undergoing functional neuroimaging, diffusion tensor imaging and reversible non-invasive brain functioning disruption through TMS is necessary to fully understand how facial emotion recognition processes are implemented in the brain.
In conclusion, we used a large sample of patients with pTBI and employed both a well-validated task and VLSM as a powerful statistical tool to identify specific brain regions associated with deficits in facial emotion recognition. Our study provides neuroanatomical details and additional empirical evidence indicating that the ability to read and name emotions in other people's face is a complex process involving a bilateral fronto-temporo-limbic network. Unpleasant and pleasant emotions shared almost 50% of the same areas in this common network but they also depend on unique brain regions responsible for emotional valence recognition; bilateral anterior areas of PFC play an important role in recognition of unpleasant emotions while bilateral posterior PFC and left temporal areas seem to be more involved in pleasant emotions.
FUNDING
The work was supported by the US National Institute of Health, National Institute of Neurological Disorders and Stroke Intramural research program, and a project grant from the United States Army Medical Research and Material Command administrated by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: A 30-year post-injury follow-up study, Grant DAMD17-01-1-0675). Olga Dal Monte was supported with funding from the Center for Neuroscience and Regenerative Medicine via the Henry M. Jackson Foundation. Note that the views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, nor the US Government.
Conflict of Interest
None declared.
Acknowledgments
The authors are grateful to all the Vietnam veterans who participated in this study. Without their long-term commitment to improving the health care of veterans, this study could not have been completed. We thank the National Naval Medical Center for their support and provision of their facilities as well as S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding and G. Tasick for their invaluable help with the testing of participants and organization of this study. For further information about the Vietnam Head Injury Study, please contact Dr. Grafman at: jgrafman@kesslerfoundation.org
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. The Journal of Neuroscience. 2000;20:2683–90. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel T, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. The Journal of Neuroscience. 1996;16:7678–87. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Jansari A, Tranel D. Hemispheric perception of emotional valence from facial expressions. Neuropsychology. 2001;15:516–24. [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. The Journal of Neuroscience. 1995;15:5879–91. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahem GL, Schwartz GE. Differential lateralization for positive versus negative emotion. Neuropsychologia. 1979;17:693–8. doi: 10.1016/0028-3932(79)90045-9. [DOI] [PubMed] [Google Scholar]
- Alves NT, Aznar-Casanova JA, Fukusima SS. Patterns of brain asymmetry in the perception of positive and negative facial expression. Laterality. 2009;14:256–72. doi: 10.1080/13576500802362927. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Expression without recognition: contributions of the human amygdala to emotional communication. Psychological Science. 2000;11:106–11. doi: 10.1111/1467-9280.00224. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Davidson PSR, Mason WP, Gao F, Binns MA, Winocur G. Right frontal lobe mediation of recollection and familiarity based verbal recognition memory: evidence from patients with tumor resections. Journal of Cognitive Neuroscience. 2011;23:3804–16. doi: 10.1162/jocn_a_00050. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research. 2009;114:154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal: a case of ‘acquired sociopathy’. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Bowers D, Heilman KM. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–27. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- Bora E, Gökçen S, Veznedaroglu B. Empathic abilities in people with schizophrenia. Psychiatry Research. 2008;160:23–9. doi: 10.1016/j.psychres.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Borod JC, Caron HS. Facedness and emotion related to lateral dominance, sex and expression type. Neuropsychologia. 1980;18:237–42. doi: 10.1016/0028-3932(80)90070-6. [DOI] [PubMed] [Google Scholar]
- Borod JC, Cicero BA, Obler LK, et al. Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology. 1998;12:446–58. doi: 10.1037//0894-4105.12.3.446. [DOI] [PubMed] [Google Scholar]
- Borod JC, Welkowitz J, Alpert M, et al. Parameters of emotional processing in neuropsychiatric disorders: conceptual issues and a battery of tests. Journal of Communication Disorders. 1990;23:247–71. doi: 10.1016/0021-9924(90)90003-h. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young W. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial emotion recognition after bilateral amygdala damage: differential impairment of fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- Carver CS. Affect and the functional bases of behavior: on the dimensional structure of affective experience. Personality and Social Psychology Review. 2001;5:345–56. [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT. Facial emotion recognition impairments in individuals with HIV. Journal of International Neuropsychological Society. 2010;16:1127–37. doi: 10.1017/S1355617710001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker V, McDonald S. Recognition of emotion from facial expression following traumatic brain injury. Brain Injury. 2005;19:787–99. doi: 10.1080/02699050500110033. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–51. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. Functional MRI in the study of emotion. In: Moonen CTW, Bandettini PA, editors. Functional MRI. New York: Springer-Verlag; 2000. pp. 487–99. [Google Scholar]
- Decety J. The neurodevelopment of empathy in humans. Developmental Neuroscience. 2010;32:257–67. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. The Scientific World Journal. 2006;6:1146–63. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Seidel EM, Eickhoff SB, et al. Neural correlates of social approach and withdrawal in patients with major depression. Social Neuroscience. 2011;6:482–501. doi: 10.1080/17470919.2011.579800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interaction between emotion and cognition. Cognition and Emotion. 1998;12:353–85. [Google Scholar]
- Ekman P. Are there basic emotions? Psychological Review. 1992;99:550–3. doi: 10.1037/0033-295x.99.3.550. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Eslinger PJ, Parkinson K, Shamay SG. Empathy and social-emotional factors in recovery from stroke. Current Opinion in Neurology. 2002;15:91–7. doi: 10.1097/00019052-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–81. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Frank MG, Stennett J. The forced-choice paradigm and the perception of facial expressions of emotion. Journal of Personality and Social Psychology. 2001;80:75–85. doi: 10.1037//0022-3514.80.1.75. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry Neroscience. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- George MS, Ketter TA, Gill DS, et al. Brain regions involved in recognizing facial emotion or identity: an oxygen-15 PET study. Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:384–94. doi: 10.1176/jnp.5.4.384. [DOI] [PubMed] [Google Scholar]
- Geva S, Jones PS, Crinion JT, Price JC, Baron JC, Warburton AE. The neural correlates of inner speech defined by voxel-based lesion–symptom mapping. Brain. 2011;134:3071–82. doi: 10.1093/brain/awr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Rudrauf D, Colom R, et al. Distributed neural system for general intelligence revealed by lesion mapping. Proceedings of the National Academy of Sciences. 2010;107:4705–9. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Tranel D, Paul LK, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–91. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, et al. Tracking emotional valence: the role of the orbitofrontal cortex. Human Brain Mapping. 2011;33:753–762. doi: 10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, et al. Explicit and incidental facial expression processing: an fMRI study. NeuroImage. 2001;14:465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Green REA, Turnerb GR, Thompsonb WF. Deficits in facial emotion perception in adults with recent traumatic brain injury. Neuropsychologia. 2004;42:133–41. doi: 10.1016/j.neuropsychologia.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25:909–19. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Habel U, Windischberger C, Derntl B, et al. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45:2369–77. doi: 10.1016/j.neuropsychologia.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Hall J, Harris JM, Spremgelmeyer R, et al. Social cognition and face processing in schizophrenia. The British Journal of Psychiatry. 2004;185:169–70. doi: 10.1192/bjp.185.2.169. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Thilo KV, Rothwell JC, Goodwin GM. Transcranial magnetic stimulation of medial-frontal cortex impairs the processing of angry facial expressions. Nature Neuroscience. 2001;4:17–8. doi: 10.1038/82854. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Adolphs R, Tranel D, Damasio H. Cortical regions for judgments of emotions and personality traits from point-light walkers. Journal of Cognitive Neuroscience. 2004;16:1143–58. doi: 10.1162/0898929041920423. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. Journal of Cognitive Neuroscience. 2008;20:721–33. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Miller GA. Lateralization in emotionand emotional disorders. Current Directions in Psychological Science. 1998;7:26–32. [Google Scholar]
- Henry JD, Phillips LH, Beatty WW, et al. Evidence for deficits in facial affect recognition and theory of mind in multiple sclerosis. Journal of the International Neuropsychological Society. 2009;15:277–85. doi: 10.1017/S1355617709090195. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10± 20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Injury. 2001;15:189–209. doi: 10.1080/026990501300005659. [DOI] [PubMed] [Google Scholar]
- Hopkins M, Dywan J, Segalowitz J. Altered electrodermal response to facial expression after closed head injury. Brain Injury. 2002;16:245–57. doi: 10.1080/02699050110103346. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–61. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Ille R, Schaüfer A, Scharmuüller W, et al. Emotion recognition and experience in Huntington disease: a voxel-based morphometry study. Journal of Psychiatry Neuroscience. 2011;36:383–90. doi: 10.1503/jpn.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE. The Face of Emotion. New York: Appleton-Century-Crofts; 1971. [Google Scholar]
- Jackson H, Moffat N. Impaired emotional recognition following severe head injury. Cortex. 1987;23:293–300. doi: 10.1016/s0010-9452(87)80039-4. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Baltimore, MD: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- Kessels RPC, Gerritsen L, Montagne B, Ackl N, Diehl J, Danek A. Recognition of facial expressions of different emotional intensities in patients with frontotemporal lobar degeneration. Behavioural Neurology. 2007;18:31–6. doi: 10.1155/2007/868431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion–symptom mapping. Journal of Cognitive Neuroscience. 2007;19:1067–80. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: the role of working memory in complex, organized behavior. Journal of Experimental Psychology: General. 1993;122:411–28. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Holliday J, Solomon J, Grafman J. Left dorsomedial frontal brain damage is associated with insomnia. Journal of Neuroscience. 2010;30:16041–3. doi: 10.1523/JNEUROSCI.3745-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal of Neuroscience. 2008;28:12341–8. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, McCabe K, et al. The neural bases of key competencies of emotional intelligence. Proceedings of the National Academy of Sciences. 2009;106:22486–91. doi: 10.1073/pnas.0912568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarbera JD, Izard CE, Vietze P, Parisi SA. Fourand six-month-old infants visual responses to joy, anger, and neutral expressions. Child Development. 1976;47:535–8. [PubMed] [Google Scholar]
- Leopold A, Krueger F, Dal Monte O, et al. Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr071. doi: 10.1093/scan/nsr071 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry. 2004;161:1247–55. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behavior Research Methods, Instruments, and Computers. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Trebon P, Chauvel P, Halgren E. Localised face processing by the human prefrontal cortex: face-selective intracerebral potentials and post-lesion deficits. Cognitive Neuropsychology. 2000;17:187–99. doi: 10.1080/026432900380562. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S. Social perception deficits after traumatic brain injury: interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. 2004;18:572–9. doi: 10.1037/0894-4105.18.3.572. [DOI] [PubMed] [Google Scholar]
- McNeil MM, Prescott TE. Revised Token Test. Los Angeles, CA: Western Psychological Services; 1994. [Google Scholar]
- Miller LA, Hsieh S, Lah S, Savage S, Hodges JR, Piguet O. One size does not fit all: face emotion processing impairments in semantic dementia, behavioural-variant frontotemporal dementia and Alzheimer's disease are mediated by distinct cognitive deficits. Behavioural Neurology. 2012;25:53–60. doi: 10.3233/BEN-2012-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, KLawrence D. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, et al. Activation of the right inferior frontal cortex during assessment of facial emotion. Journal of Neurophysiology. 1999;82:1610–4. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Yamada H, Iidaka T, et al. Brain regions involved in verbal or nonverbal aspects of facial emotion recognition. NeuroReport. 2000;11:2571–6. doi: 10.1097/00001756-200008030-00044. [DOI] [PubMed] [Google Scholar]
- Nelson AC, De Haan M. Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Developmental Psychobiology. 1996;29:577–95. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Philippi CR, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience. 2009;29:15089–99. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Krueger F, Grafman J. ‘Studying injured minds’ – the Vietnam head injury study and 40 years of brain injury research. Frontiers in Neurotrauma. 2011;2:1–15. doi: 10.3389/fneur.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44:365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–27. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman EK, Weingartner H. Hemispheric lateralization of functions related to emotion. Brain and Cognition. 1986;5:322–53. doi: 10.1016/0278-2626(86)90035-7. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Computer Methods and Programs Biomedicine. 2007;86:245–54. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceeding of the Royal Society of London. 1998;265:1927–31. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Calder A, et al. Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain. 1996;119:1647–65. doi: 10.1093/brain/119.5.1647. [DOI] [PubMed] [Google Scholar]
- Szekely E, Tiemeier H, Arends LR, et al. Recognition of facial expressions of emotions by 3-year-olds. Emotion. 2011;11:425–35. doi: 10.1037/a0022587. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expression of emotion. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhr370. doi: 10.1093/cercor/bhr370 (in press) [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, et al. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain & Language. 2011;117:110–22. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, James M. Visual Object and Space Perception Battery. Oxford, UK: Pearson Assessment; 1991. [Google Scholar]
- Wechsler DA. Wechsler Memory Scale III. New York: Psychological Corporation; 1997. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotion in patients with panic disorders. Archives of General Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- Williams C, Wood RL. Impairment in the recognition of emotion across different media following traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2010;32:113–22. doi: 10.1080/13803390902806543. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Young AW, Perrett D, Calder A, Sprengelmeyer R, Ekman P. Facial Expressions of Emotion: Stimuli and Tests. Thurston, UK: Thames Valley Test Company; 2002. [Google Scholar]