Abstract
This electroencephalographic study investigated if negating one’s emotion results in paradoxical effects or leads to effective emotional downregulation. Healthy participants were asked to downregulate their emotions to happy and fearful faces by using negated emotional cue words (e.g. no fun, no fear). Cue words were congruent with the emotion depicted in the face and presented prior to each face. Stimuli were presented in blocks of happy and fearful faces. Blocks of passive stimulus viewing served as control condition. Active regulation reduced amplitudes of early event-related brain potentials (early posterior negativity, but not N170) and the late positive potential for fearful faces. A fronto-central negativity peaking at about 250 ms after target face onset showed larger amplitude modulations during downregulation of fearful and happy faces. Behaviorally, negating was more associated with reappraisal than with suppression. Our results suggest that in an emotional context, negation processing could be quite effective for emotional downregulation but that its effects depend on the type of the negated emotion (pleasant vs unpleasant). Results are discussed in the context of dual process models of cognition and emotion regulation.
Keywords: emotion regulation, event-related brain potentials, negation, reappraisal, suppression
INTRODUCTION
Emotion regulation is an important aspect of everyday life (Gross and John, 2003; Nezlek and Kuppens, 2008). Imagine the following situation: your boss wants to see you immediately at his office; he is very upset. What most people would do in this situation to regulate their emotions is (i) to reappraise the situation in a more positive light, (ii) to avoid showing any sign of fear (emotion suppression) or (iii) to instruct oneself verbally to not be afraid or anxious. In other words, those people employing the final strategy would try to calm themselves by verbally negating their emotions.
Effects of reappraisal and suppression have been studied intensely in the last few years. The results clearly support a number of conclusions: Reappraising a situation (or stimulus) in a way that its emotional significance becomes less intense decreases emotional reactivity, both on a physiological and subjective experiential level (e.g. Gross, 2002; Ochsner et al., 2002). Emotion suppression, in contrast, can have strikingly paradoxical effects on affective responding (e.g. Gross and Levenson, 1993, 1997), increasing physiological arousal (Wegner et al., 1990; Gross, 2002; Goldin et al., 2008) and the availability in memory of the item meant to be suppressed (Richards and Gross, 2000; Dillon et al., 2007).
Emotion negation is a strategy that superficially seems to resemble suppression. As illustrated above, attempts to suppress one’s emotions by instructing individuals ‘not to’ think of a specific event maintains or even increases the accessibility of the content intended to be suppressed in memory. Negating one’s own emotion could result in similar paradoxical effects because, the cognitive focus may still rest on the negated concept (e.g. Giora et al., 2005; Hasson and Glucksberg, 2006). On the other hand, however, negating might reframe the content of an emotion (e.g. no fear), thus having effects akin to reappraisal.
Previous research investigating negations in an emotional context provided mixed results lending support for either or one of the above mentioned speculations. Evidence for paradox effects comes from studies investigating negation in the context of inhibition of unwanted behaviors such as inhibition of unhealthy eating habits or of stereotypes. Results of these studies often revealed paradoxical effects pointing in the opposite direction of what was implied logically by the negation [e.g. increased snacking after processing of negated sentences like ‘If (situation) … , then I will not eat chocolate’] (e.g. Otis and Pelletier, 2008; Adriaanse et al., 2010; for different results see Gollwitzer and Schaal, 1998; Schweiger-Gallo et al., 2009). Other studies have used affective priming tasks and measured changes in reaction times to target words that were preceded by briefly presented negated and un-negated emotional prime words (e.g. Draine, 1997; Deutsch et al., 2006). In these studies, processing of negated emotional prime words had the same affective priming effects as their un-negated emotional counterparts. However, affective priming tasks including evaluative decisions are largely based on mechanisms of response interference (Gawronski et al., 2008), which are not specific to affect or emotion per se (e.g. Deutsch and Gawronski, 2009). In addition, some of the results from studies using complex behavioral regulation instructions revealing paradoxical effects can be accounted for by dual process models of negation processing. These models assume that complex statements containing negations are difficult to process and require additional cognitive processing resources before their meaning is extracted and fully understood (Kaup et al., 2007; Hasson and Glucksberg, 2006).
Thus, the above described results do not support the notion that emotion negation is generally ineffective in regulating emotions. In fact, a competing line of research casts doubt on the generality of such paradoxical effects. For instance, negated emotional prime words have been found to have a strong impact on evaluative priming in the Affect Misattribution Paradigm (Payne et al., 2005), which is not based on response interference (Deutsch et al., 2009). Likewise, processing of simple emotion words containing negations (e.g. no fear, no panic, no fun, no success) has been shown to increase or decrease affective responses like the defensive startle reflex, a measure of an individual’s approach and avoidance tendencies, in line with the emotional content of the be negated expression (Herbert et al., 2011). Although in the study by Deutsch et al. (2009), negation processing was quick and relatively automatic—independent of intentions but easily disrupted by a secondary task—participants in the study by Herbert et al. (2011) had at least 1800 ms to process the negations and reflect upon their meaning.
Together, these results suggest that effects of negation on emotion depend on the time available for negation processing, the type of instruction provided to the participants and also the valence of the emotion that is to be negated (i.e. pleasant or unpleasant). Furthermore, these studies suggest that in contrast to complex sentential negations, simple negated expressions could serve as effective cues for emotion regulation in at least some everyday situations. In these cases, one critical question that remains to be answered pertains to how negated emotional cues impact emotion processing in an active emotion regulation context, and what the underlying strategies or mechanisms might be.
Event-related brain potential (ERP) methodology could shed further light on these questions: with ERPs, a temporally fine-grained examination of both automatic and controlled cognitive processes can be achieved without requiring an overt response of the participant. Furthermore, in the context of active emotion regulation, negation paradigms based on ERP methods could differentiate immediate from cognitive elaborate regulation effects initiated by negated emotional cue words by determining changes in the cortical processing of emotional stimuli following these regulation cues.
Many studies in emotion research suggest that emotional stimuli (like pictures and faces eliciting fear or happiness) are preferentially processed by capturing the viewer’s attention. EEG–ERP studies consistently reveal an enhancement of early brain potentials during processing of emotional compared with neutral pictures and faces. Modulations of early ERP components such as the face-specific N170, and the EPN (early posterior negativity) are considered to reflect stimulus-driven processing related to increased structural encoding (N170) and facilitated capture of attentional resources (EPN) by stimuli of emotional relevance (e.g. Bentin and Deouell, 2000; Eimer, 2000; Junghofer et al., 2001; Sato et al., 2001; Schupp et al., 2006; Blau et al., 2007). Regarding later ERPs, the late positive potential (LPP) is indicative of a more sustained and cognitively elaborate stimulus processing and encoding (e.g. Paller et al., 1995; Kok, 1997). Its amplitude is modulated by the way a stimulus is appraised by the participant (e.g. Hajcak et al., 2006). In previous EEG–ERP emotion regulation studies, the LPP demonstrated particularly large effects when people were instructed to downregulate their emotions to emotional stimuli either by means of cognitive reappraisal (e.g. Hajcak and Nieuwenhuis, 2006; Moser et al., 2006; Krompinger et al., 2008) or by verbal reappraisal frames (Foti and Hajcak, 2008; MacNamara et al., 2009). Hajcak and Nieuwenhuis (2006), Moser et al. (2006) and Krompinger et al. (2008) recorded ERPs while participants had to down- or upregulate their responses to emotionally arousing unpleasant or pleasant pictures by self-generated reappraisal strategies. Foti and Hajcak (2008) and McNamara et al. (2009) recorded ERPs while participants processed unpleasant pictures whose contents were reframed by externally provided reappraisal frames consisting of either neutral or negative verbal descriptions. Across studies, self-generated reappraisal or reappraisal by neutral descriptions significantly attenuated amplitudes of the LPP to unpleasant pictures during downregulation compared with passive viewing. In sum, this line of research demonstrates that successful emotional downregulation decreases ERPs, particularly the LPP. Furthermore, they show that in addition to self-generated reappraisal, verbal descriptions provide a context for emotion regulation.
In line with these emotion regulation studies, the present study uses event-related brain potentials as outcome measures of emotion regulation. Building upon the previous emotion negation literature, the present study aimed to answer the following open questions: first, at which processing stages (early and automatic vs late and cognitively controlled) do verbal negations influence the processing of emotional stimuli during active emotional downregulation? Second, are these effects the same for unpleasant and pleasant stimuli? Third, is negation induced emotion regulation attributable to mechanisms associated with reappraisal or with suppression? To this end, we presented healthy participants with pictures of fearful and happy faces, instructing them to downregulate their emotions to these faces by using negated emotional cue words of unpleasant or pleasant emotional significance (e.g. no fear, no fun). Cue words were congruent with the emotion depicted in the face and presented prior to each face with durations sufficient to process the meaning of the cues. In addition, regulation strategies elicited by the cues were assessed via self-report measures.
METHODS
Subjects
In total, 24 adult native speakers of German (9 males, 15 females) recruited via an advertisements’ board at the University of Würzburg participated in the study. Participants were financially reimbursed for participation. Participants reported normal audition, normal or corrected to normal vision and were free from reported drug abuse, chronic somatic, neurological or psychiatric diseases and medication for any of these diseases. Three subjects (one female, two males) were excluded from further analysis due to high depression scores (BDI scrores ≥18) on the Beck Depression Inventory (Hautzinger et al., 1994). Thus, the final sample consisted of 21 subjects [8 males, 13 females, mean (s.d.) age 25.7 (3.6) years], who all showed low depression scores [M (s.d.) 3.28 (2.53)] on the BDI-Inventory, scored normally on the Spielberger State-Trait Anxiety Inventory (STAI, Laux et al., 1981) in terms of trait [M (s.d.) 35.5 (7.27)] and state anxiety [M (s.d.) 34.6 (7.11)] and reported more positive than negative affect [M (s.d.) 11.6 (2.7)] on the PANAS, Positive and Negative Affect Schedule mood assessment scales (Watson et al., 1988). The study was conducted in accordance with standard ethical guidelines as defined in the Declaration of Helsinki and participants gave written informed consent prior to their participation in the experiment.
Stimuli
Experimental stimuli consisted of 20 negated unpleasant and 20 negated pleasant emotional nouns, as well as 40 emotional faces (20 happy male or female faces and 20 fearful male or female faces). Faces were taken from the Radboud Faces Database (Langner et al., 2010). Unpleasant nouns described negative emotions, threat in particular (e.g. panic, fear). Pleasant nouns described positive emotions, happiness in particular (e.g. fun, joy). To ensure that negated nouns (e.g. no fear, no panic, no fun, no joy, etc.) were reliable emotion regulation cues, a pilot study was conducted. In total, 19 healthy, native speakers of German [12 females, 7 males, mean (s.d.) age 29.8 (9.8) years], who did not take part in the present experiment, were asked to freely associate whatever emotion words (nouns) came to their mind when confronted with one of the happy or fearful RADBOUD faces and were asked to write down at least 10 of their associations on a sheet of paper. Then, those nouns that were freely associated by at least three participants were selected and presented to another sample of N = 17 healthy native speakers of German [11 females, 6 males, mean (s.d.) 26.6 (2.1) years] in a negated version. Again, subjects were asked to write down their associations related to these negated emotional nouns. Results showed that across subjects negated unpleasant nouns were spontaneously associated with pleasant or calming associations, negated pleasant nouns with unpleasant associations (threat in particular).
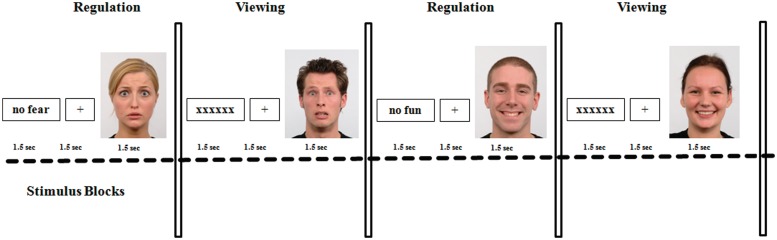
Stimuli (cues and faces) were presented in four different randomized blocks, each block containing 20 unpleasant or 20 pleasant stimulus pairs. Two blocks were regulation blocks and two blocks of passive stimulus viewing served as the control condition. Happy and fearful faces shown during regulation and passive viewing were presented in randomized order to avoid effects attributable to stimulus repetition. In each block, each face was preceded by a negated emotional cue word (regulation blocks) or meaningless letter strings (control condition). Negated unpleasant and pleasant cues did not differ in word-length (Munpleasant = 12.0, s.d.unpleasant = 3.0; Mpleasant = 12.25, s.d.pleasant = 3.4, P > 0.58). In each trial, cues were shown for 1.5 s and were followed by a fixation cross for 1.5 s. The fixation cross was presented prior to the onset of the face stimulus that was shown for 1.5 s (Figure 1 for an overview of the experimental set-up). In addition, each trial was separated by an intertrial-interval of about 2 s and stimulus blocks were separated by a pause of about 15 s. Cues were presented in black letters (font ‘Times’; size = 40) centered on a white background of a 19-inch computer monitor. Faces were presented in color, centered on the computer screen.
Fig. 1.
Brief overview of the experimental design (see text for detailed information).
Experimental runs were controlled by Presentation software (Neurobehavioral Systems Inc.).
Procedure
Participants were familiarized with the laboratory settings. The experiment was explained to them in general terms; they were questioned about their handedness, health, mood and anxiety. Electrodes for electroencephalographic (EEG) recordings were attached and participants were given detailed instructions. They were told that they would be shown a series of stimuli consisting of verbal cues and happy or fearful faces. They were asked to downregulate their emotions to each of these faces by using the negated emotional cue words presented prior to each of the faces. In the blocks of passive stimulus viewing, the participants were asked to simply view and attend to the faces without active regulation. They were asked to refrain from head and eye movements and to keep their eyes fixated on the screen throughout the entire stimulus presentation (including interstimulus-intervals). Participants then rated the task for task difficulty and indicated their regulation effort separately for the two regulation blocks on 9-point Likert scales. In addition, participants were asked for specific strategies they had used during regulation and rated the stimuli for subjective valence and arousal on a 9-point paper–pencil version of the Self-Assessment Manikin (Bradley and Lang, 1994), a pictoral rating scale frequently used in emotion research to assess dimensions of perceived stimulus pleasantness/unpleasantness and intensity. Finally, participants were debriefed in detail about the purpose of the experiment.
Data collection and reduction
Electrophysiological recordings
The EEG was recorded from 32 active electrodes using the actiCAP system (Brain Products GmBH). For all electrodes, impedance was kept <10 kΩ. The raw EEG was recorded continuously at a sampling rate of 500 Hz; FCz served as a reference. Off-line, raw EEG signals were digitally re-referenced to an average reference, filtered from 0.01 to 30 Hz and corrected for eye-movement artifacts using the ocular correction algorithm (Gratton et al., 1988) implemented in the Analyzer 2 software package (Brain Products GmBH). The EEG signals were corrected for additional artifacts using the Analyzer 2 semi-automatic artifact-correction module. Artifact-free EEG data were segmented for each processing condition (active regulation vs passive viewing) and valence category (positive vs negative) from 500 ms before until 1500 ms after onset of the target faces. The 100 ms interval before onset of the target face was used for baseline correction.
Peak latency1 and amplitudes2 were analyzed to determine effects of regulation on emotional face processing in terms of its speed and its strength. Electrodes and time-windows for peak and amplitude scoring of early (N170, EPN) and late (LPP) ERP components were determined via a semi-automated peak detection algorithm of the Analyzer 2 software package and by visual inspection of the grand mean waveforms. In line with previous literature on emotional face processing, these were analyzed at left and right occipital and parieto-occipital electrodes from 110 to 180 ms (N170), 200–400 ms (EPN) and 400–1000 ms (LPP) post-target face onset. Grand mean ERP waveforms revealed an additional fronto-central negativity potential in the time-window from 250 to 400 ms after target face onset, which was included in subsequent analyses. This N300-/N400-like brain potential was most pronounced over frontal and fronto-central midline electrodes for happy and fearful faces during active regulation compared with passive viewing trials.
Data reduction and statistical analysis
Amplitude and latency effects were statistically analyzed with repeated measures analysis of variance (ANOVA) for each ERP of interest (N170, EPN, N3/N4 and LPP). The corresponding ANOVA factors were ‘stimulus valence’ (happy or fearful), ‘condition’ (active regulation vs passive viewing) and ‘electrode location’. Electrodes included into the factor ‘electrode location’ were grouped as follows: N170 and EPN (PO9, PO10, P8, P7, O1 and O2); N3/N4 (FCz, FC1, FC2, C3, Cz, C4), LPP (P3, P4, Pz). In addition, for the N170 and EPN, the additional factor ‘hemisphere’ (right hemisphere electrodes O2, PO10, P8 vs left hemisphere electrodes O1, PO9, P7) was included in the ANOVAs to control for hemisphere differences.
When the assumption of sphericity was not met, P-values were adjusted according to Greenhouse and Geisser. Significant interaction effects were decomposed by single post hoc planned comparison tests.
RESULTS
Electrophysiological data
Active regulation vs passive viewing (happy vs fearful faces)
N170
Active regulation had no significant impact on N170 latency or amplitude. For both N170 latency and amplitude, ANOVAs revealed no significant main effects of the factor ‘condition’, nor any significant interactions of ‘condition’ with ‘stimulus valence’, ‘electrode location’ and/or ‘hemisphere’. Regarding ‘stimulus valence’, the interaction of ‘stimulus valence × hemisphere × electrode location’ showed a trend toward significance, F(2,40) = 2.85, P = 0.06, suggesting that fearful faces elicited larger N170 amplitudes than happy faces, regardless of condition, especially at right compared with left posterior sensors. No such effect was observed for peak latency, but N170 latency was generally faster at occipital electrodes [‘electrode location’: F(2,40) = 11.6, P = 0.001], possibly due to facilitated structural encoding of facial features in primary visual areas.
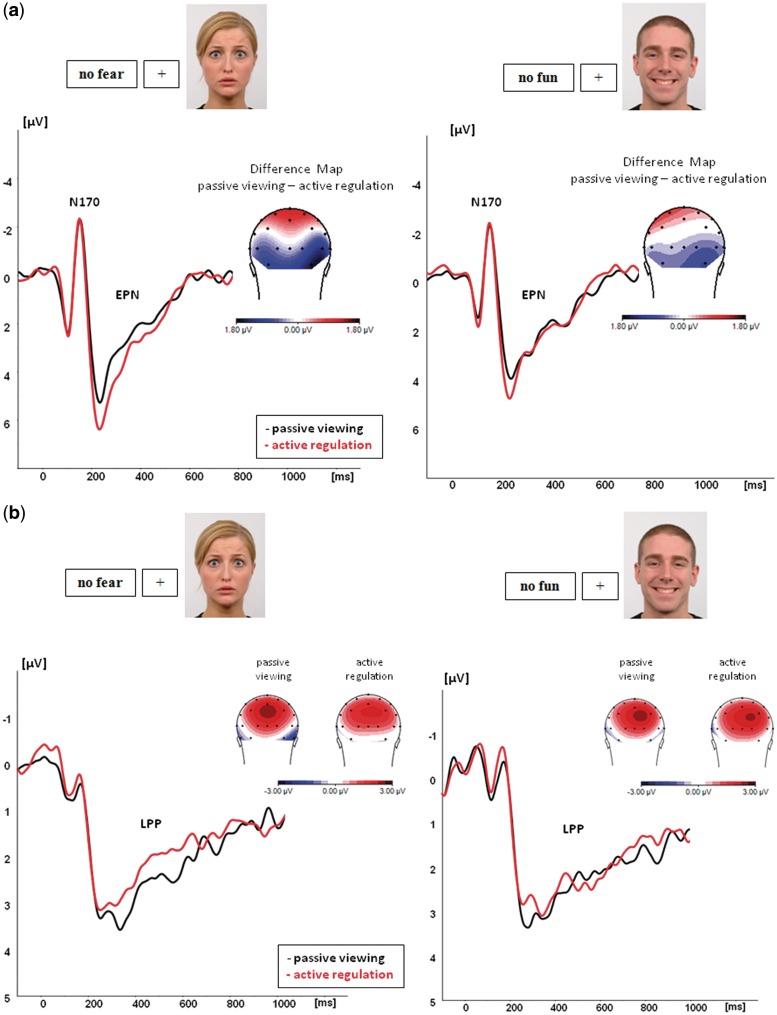
EPN
EPN amplitudes varied significantly as a function of ‘condition’, F(1,20) = 7.5, P = 0.01. EPN amplitudes were significantly reduced during active regulation compared with passive viewing. In addition, a significant main effect of ‘stimulus valence’, F(1,20) = 11.5, P = 0.005, as well as a significant interaction of ‘condition’ and ‘stimulus valence’ were observed, F(1,20) = 4.7, P = 0.04). Post hoc planned comparison tests revealed that active regulation attenuated EPN amplitudes for fearful faces in comparison with passive viewing of fearful, F(1,20) = 17.8, P = 0.001, and of happy faces, F(1,20) = 4.7, P = 0.04. This was also true in comparison with active regulation of happy faces, F(1,20) = 13.5; P = 0.001. These effects were most pronounced at parieto-occipital electrodes irrespective of hemisphere [condition × stimulus valence × electrode location: F(2,40) = 3.5, P = 0.04], albeit EPN amplitudes were generally more pronounced at right compared with left hemisphere electrodes (‘hemisphere’: F(1,20) = 6.2, P = 0.02). For happy faces, EPN amplitudes did not differ significantly across conditions, F(1,20) = 0.49, P = 0.49, nor did EPN amplitudes for fearful and happy faces differ during passive viewing, F(1,20) = 2.3, P = 0.12. In contrast to EPN amplitude, latency did not differ across conditions [F(1,20) = 0.57, P = 0.46] and stimulus valence [F(1,20) = 0.37, P = 0.55]. There were no significant interactions of ‘stimulus valence’ and ‘condition’, F(1,20) = 0.30, P = 0.87, nor any significant interactions between ‘stimulus valence’ and ‘condition’ with the factors ‘hemisphere’ and or ‘electrode location’ (all P > 0.3), but trended toward shorter latencies at right compared with left hemisphere electrodes, specifically right parieto-occipital electrodes [Electrode Location: F(2,40) = 7.67, P = 0.003; Hemisphere × Electrode Location: F(2,40) = 3.612, P = 0.04].
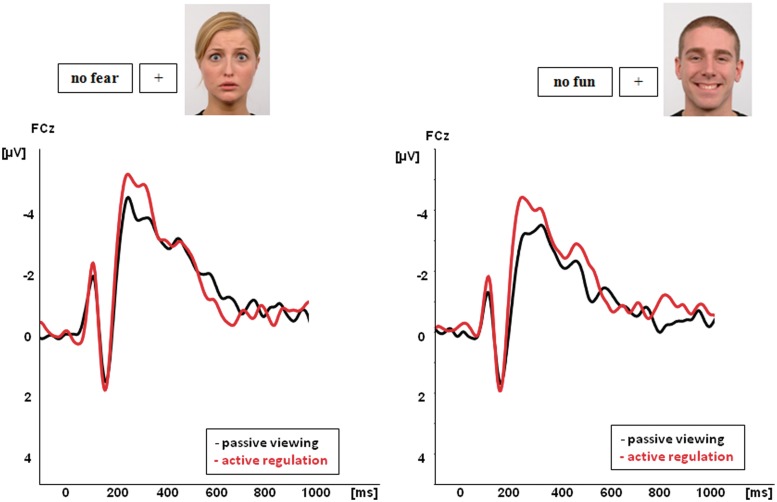
Frontal negativity
Amplitudes of the frontal negativity showed a main effect of ‘condition’, F(1,20) = 11.0, P = 0.003, with larger amplitudes during active regulation compared with passive viewing for both fearful and happy faces. There was no significant effect of ‘stimulus valence’, F(1,20) = 2.4, P = 0.13, and no significant interaction effects between ‘condition’ and ‘stimulus valence’, F(1,20) = 0.17, P = 0.89. In addition, no other significant interactions could be observed. For peak latency of this component the ANOVA did not produce any significant results.
LPP
Amplitudes of the LPP showed a significant interaction effect of ‘condition × stimulus valence’, F(1,20) = 4.5, P = 0.045. According to post hoc planned comparison tests, LPP amplitudes were significantly reduced for fearful faces during active regulation compared with passive viewing of fearful, F(1,20) = 4.54; P = 0.046, and of happy faces, F(1,20) = 4.59; P = 0.045. For happy faces, no such effect was observed, F(1,20) = 0.02, P = 0.8. In addition, LPP amplitudes did not differ for fearful and happy faces during passive viewing [F(1,20) = 2.3, P = 0.14]. Finally, analysis of latency data of the LPP did not reveal any significant effects. The effects are illustrated in Figure 2A–C.
Fig. 2.
ERP results: active regulation compared with passive viewing of fearful and happy faces Significant EPN and LPP effects [collapsed across parieto-occipital (EPN) and parietal electrodes (LPP)] are shown in (A) (EPN) and (B) (LPP). Effects for fearful faces (left panel), effects for happy faces (right panel). Topographic voltage maps display the topographic distribution and size of the effects. Effects of the fronto-central negativity are displayed in (C). Effects for happy faces: right panel. Effects for fearful faces (left panel).
Behavioral data
Task difficulty, regulation success and strategies
All participants rated the task as moderately demanding [M (s.d) 5.3 (1.4)], reporting successful downregulation overall but with relative difficulty in downregulating the happy faces vs the fearful faces. Regarding strategy use, active regulation by means of negation cues was associated mostly with attempts to reappraise the meaning of the negated cues (Table 1), i.e. participants elaborated the meaning of the negated cue and then reappraised the face in line with its meaning during regulation. Some subjects replied to have additionally rehearsed the cues. Two subjects reported trying to regulate their emotions exclusively by suppressing their emotional reactions or by attempts to avoid thinking about the negated item. Results of the strategy use are summarized in Table 1.
Table 1.
Description of strategies used during emotion regulation
| Strategies used during active regulation |
|---|
| Reappraisal |
| I tried to reframe the content of the cues in the direction implied by the negation. |
| I tried to reappraise the meaning of the cues in a more positive or negative way. |
| Suppression |
| I tried to avoid thinking of the content expressed by the cues (thought suppression). |
| I tried not to express my feelings elicited by the cues (expressive suppression). |
| Other |
| I rehearsed the cues to increase their significance for downregulation. |
| I tried to think of something completely different (attention deployment). |
| I tried to show the opposite expression shown in the faces. |
| Total number of subjects usinga: |
| Reappraisal: 18 |
| Suppression (only): 2 |
| Other (only): 1 |
| Reappraisal and rehearsal: 5 |
| Suppression and rehearsal: 1 |
aNumber of subjects using a particular strategy or a combination of strategies.
Ratings
Rating data assessed immediately after the regulation experiment showed the following results: Negated unpleasant cues [M (s.d.) 5.19 (1.28)] were rated as significantly more positive in valence compared with negated pleasant cues [M (s.d.) 3.37 (1.5)], F(1,20) = 17.5, P = 0.001. Regarding arousal, no significant difference between negated unpleasant [M (s.d.) 3.87 (1.5)] and pleasant [M (s.d.) 3.57 (1.39)] cues was observed, F(1,20) = 2.11, P = 0.17. Both cue types were rated as moderately arousing.
DISCUSSION
The present study investigated emotion negation as a strategy for emotional downregulation. Building upon previous research and using ERPs as outcome measures, the general aim of our study was to elucidate whether emotion regulation by negated cue words results in paradoxical effects enhancing cortical processing of emotional targets (happy and fearful faces) or leads to effective emotional downregulation; this latter result would be expressed in reduced amplitudes of early and/or late ERP components to fearful or happy faces. Additionally, we examined whether negation effects could be accounted for by specific regulation strategies of reappraisal or suppression.
Instructing individuals to downregulate their emotions by negated unpleasant cues reduced amplitudes of the LPP for fearful faces. Furthermore, downregulation by means of negated unpleasant cues attenuated amplitudes of the EPN to fearful faces but did not change amplitudes of the face specific N170 potential. Previous EEG–ERP studies have demonstrated that successful emotional downregulation induced via either a self-generated reappraisal or by sentences reframing the content of a stimulus in a less emotional way decreases event-related brain potentials, particularly the LPP to unpleasant stimuli (Hajcak and Nieuwenhuis, 2006; Moser et al., 2006; Foti and Hajcak, 2008; MacNamara et al., 2009). Temporally earlier reframing effects in the P2 time-window have also occasionally been reported (Dillon, 2006). In line with these findings, our ERP results suggest that negating modulates emotion processing at later stages (LPP) of stimulus processing, but also at earlier processing stages (EPN).
The EPN is sensitive to changes in stimulus-driven attention capture (e.g. Junghofer et al., 2006; Schupp et al., 2006) and the LPP is modulated by changes in stimulus appraisal and depth of stimulus encoding (e.g. Kok, 1997; Hajcak et al., 2006), whereas the N170 indicates automatic decoding of structural stimulus features from faces (e.g. Bentin and Deouell, 2000; Eimer, 2000; Blau et al., 2007). More specifically, our ERP results imply that negating downregulates emotional processing by decreasing early attention capture and encoding of emotional faces while leaving very automatic processing of emotional faces unaffected. These ERP effects were specific for the downregulation of fearful faces by negated unpleasant cues and were not observed during downregulation of one’s emotions to happy faces by negated pleasant cues.
The observed ERP patterns do not support the notion that processing of negated emotional cues, as well as negating as an emotion regulation strategy trigger paradoxical processing effects enhancing emotional stimulus processing akin to suppression. Previous research (e.g. Draine, 1997; Hasson and Glucksberg, 2006; Deutsch et al., 2006), as well as dual-process models of information processing including negation (e.g. Kaup et al., 2007; Strack and Deutsch, 2004) predict negating to result in paradoxical processing effects when processing time for negation processing is severely limited, or taxed by another task or process. In the present study, participants had enough time to process and reflect upon the meaning of the negated cues (i.e. the Stimulus-Onset Asynchrony between cue and target was 3 s) and all participants judged the task as generally moderately demanding. Moreover, none of the participants reported that processing of negated cues redirected their attention back to the emotional content of the negated word during emotion regulation trials. In contrast, most of the participants replied to have regulated their emotion during face processing by reframing or reappraising the meaning of the cues in the direction implied by the negation. Some subjects also reported to have rehearsed the cues in addition to reframing or suppressing their content. However, only two of the subjects reported that processing of negated cues encouraged them to use exclusively suppression-like emotion regulation strategies (Table 1). Our results, therefore, suggest that attempts to regulate one’s emotion by means of negated emotional cues spontaneously triggers emotion regulation strategies that are more associated with reappraisal than with suppression.
Even the specific finding of generally larger amplitudes of an N3/N4-like frontal negativity potential during active regulation compared with passive viewing of both, happy and fearful faces argues against paradoxical suppression-like effects. Previous research using emotional faces (and/or pictures) as targets often report negativity potentials in the same time frame and with the same fronto-central topography as observed in the present study for primed faces compared with unprimed control faces when primes and targets are not of the same category (e.g. Schweinberger et al., 1995), or when participants have strong expectations about the following context (Hamm et al., 2002). The observed fronto-central negativity signaling larger effects for faces during active regulation compared with passive viewing might thus be attributable to experimental differences and specific attributes of our task, i.e. comparing primed regulation conditions with unprimed viewing conditions. In addition, regulation conditions might induce expectancies in subjects to a larger extent than passive viewing of the faces. Finally, although more speculatively, the reported fronto-central effects might reflect enhanced activation of frontal control networks during active regulation.
The observation that reframing happy faces by use of negated pleasant cues did not change cortical processing of happy faces during downregulation in the same way that was observed for negated unpleasant cues and fearful faces reinforces a previous finding that processing of negated pleasant cues leads to an enhancement of defensive responses such as the startle reflex (Herbert et al., 2011). Thus, reframing one’s pleasant emotions by negating them appears to be less effective for emotional and behavioral downregulation, perhaps because negated pleasant cues are experienced as negative in valence that makes downregulation more difficult. Although this speculation needs further investigation, our rating data lend some support for this explanation. Participants rated negated pleasant cues as significantly more negative in valence compared with negated unpleasant cues and experienced downregulation by negated pleasant cues as more difficult than by negated unpleasant cues. It is also unlikely that effects of negation during active regulation resulted from general differences in cortical processing of happy and fearful faces. Although fearful faces elicited somewhat larger N170 amplitudes than happy faces at right posterior electrodes amplitudes of the EPN and LPP for which differential effects of negating were observed, did not differ significantly for fearful and happy faces during passive viewing. Likewise, ERP latencies did not differ between happy and fearful faces across conditions nor did regulation by means of negation change the speed of emotional face processing.
The results of the present study pave the way for a new look at negating as a strategy for emotion regulation. They shed light on some of the mechanisms underlying negation processing in the context of an active emotion regulation account using ERP methodology and negated emotional cues of both positive and negative valence. Given that our study is the first, probing negation processing as a strategy for active emotion regulation future studies are needed to replicate our results or extend them to domains other than happiness and fear (e.g. anger, disgust, surprise, etc.). Nevertheless, our results allow for a number of conclusions: They suggest that in a negative stimulus context, negating one’s emotion by using negated unpleasant cues might be an effective strategy for emotional downregulation. It reduces cortical processing associated with stimulus-driven attention capture (EPN) and depth of stimulus encoding (LPP) to unpleasant stimuli and spontaneously triggers emotion regulation strategies that are more associated with reappraisal than with suppression. Inclusion of further control stimuli (e.g. neutral stimuli) and of biomarkers other than EEG (e.g. heart rate or skin conductance) could further help determine the strength and specificity of negation effects across different levels of responding.
FUNDING
Research was supported by the German Research Foundation (DFG).
Conflict of Interest
None declared.
Acknowledgments
We thank Alexandra Dittmann for help with data collection and analysis.
Footnotes
1Since the EPN is defined as a negative deflection in the ERP waveform, the onset of the EPN was analyzed instead of the peak latency.
2ERP amplitudes were determined at each electrode as the average amplitude (in microvolts) across the whole of the relevant time window.
REFERENCES
- Adriaanse MA, Van Oosten JMF, De Ridder DTD, De Wit JBF, Evers C. Planning what not to eat: ironic effects of implementation intentions negating unhealthy habits. Personality and Social Psychology Bulletin. 2011;37:69–81. doi: 10.1177/0146167210390523. [DOI] [PubMed] [Google Scholar]
- Bentin S, Deouell LY. Face detection and face identification: ERP evidence for separate mechanisms. Cognitive Neuropsychology. 2000;17:35–55. doi: 10.1080/026432900380472. [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behavioral and Brain Functions. 2007;23:3–7. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Deutsch R, Gawronski B. When the method makes a difference: Antagonistic effects on “automatic evaluations” as a function of task characteristics of the measure. Journal of Experimental Social Psychology. 2009;45:101–14. [Google Scholar]
- Deutsch R, Gawronski B, Strack F. At the boundaries of automaticity: negation as reflective operation. Journal of Personality and Social Psychology. 2006;91:385–405. doi: 10.1037/0022-3514.91.3.385. [DOI] [PubMed] [Google Scholar]
- Deutsch R, Kordts-Freudinger R, Gawronski B, Strack F. Fast and fragile: a new look at the automaticity of negation processing. Experimental Psychology. 2009;56:434–46. doi: 10.1027/1618-3169.56.6.434. [DOI] [PubMed] [Google Scholar]
- Dillon DG. Voluntary emotion regulation: physiological correlates and mnemonic consequences. 2006. ProQuest. PhD Dissertation, Duke University. [Google Scholar]
- Dillon DG, Ritchey M, Johnson BD, LaBar KS. Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion. 2007;7:354–65. doi: 10.1037/1528-3542.7.2.354. [DOI] [PubMed] [Google Scholar]
- Draine SC. Analytic limitations of unconscious language processing. 1997. University of Washington, Seattle: Unpublished doctoral dissertation. [Google Scholar]
- Eimer E. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clinical Neurophysiology. 2000;111:694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–88. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Gawronski B, Deutsch R, LeBel EP, Peters KR. Response interference as a mechanism underlying implicit measures: some traps and gaps in the assessment of mental associations with experimental paradigms. European Journal of Psychological Assessment. 2008;24:218–25. [Google Scholar]
- Giora R, Balaban N, Fein O, Alkabets I. Negation as positivity in disguise. In: Colston HL, Katz A, editors. Figurative Language Comprehension: Social and Cultural Influences. Hillsdale, NJ: Erlbaum; 2005. pp. 233–58. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer PM, Schaal B. Metacognition in action: the importance of implementation intentions. Personality and Social Psychology Review. 1998;2:124–36. doi: 10.1207/s15327957pspr0202_5. [DOI] [PubMed] [Google Scholar]
- Gratton D, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1988;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–86. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis ST. Reappraisal modulates the electrocortical response to negative pictures. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:291–7. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–22. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hamm JP, Johnson BW, Kirk IJ. Comparison of the N300 and N400 ERPs to picture stimuli in congruent and incongruent contexts. Clinical Neurophysiology. 2002;113:1339–50. doi: 10.1016/s1388-2457(02)00161-x. [DOI] [PubMed] [Google Scholar]
- Hasson U, Glucksberg S. Does understanding negation entail affirmation? An examination of negated metaphors. Journal of Pragmatics. 2006;38:1015–32. [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Beck-Depressions-Inventar (BDI) Bern, Switzerland: Huber; 1994. [Google Scholar]
- Herbert C, Deutsch R, Sütterlin S, Kübler A, Pauli P. Negation as a means for emotion regulation? Startle reflex modulation during processing of negated emotional words. Cognitive Affective and Behavioral Neuroscience. 2011;11:199–206. doi: 10.3758/s13415-011-0026-1. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Sabatinelli D, Bradley MM, Schupp HT, Elbert TR, Lang PJ. Fleeting images: Rapid affect discrimination in the visual cortex. NeuroReport. 2001;17:225–9. doi: 10.1097/01.wnr.0000198437.59883.bb. [DOI] [PubMed] [Google Scholar]
- Kaup B, Zwaan RA, Lüdtke J. The experiential view of language comprehension: how is negated text information represented? In: Schmalhofer F, Perfetti CA, editors. Higher Level Language Processes in the Brain: Inference and Comprehension Processes. Mahwah, NJ: Erlbaum; 2007. pp. 255–88. [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biological Psychology. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8:132–7. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition & Emotion. 2010;24:1377–88. [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: Neural activity elicited by emotional stimuli and preceding descriptions. Emotion. 2009;9:531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional regulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–6. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Kuppens P. Regulating positive and negative emotions in daily life. Journal of Personality. 2008;76:561–80. doi: 10.1111/j.1467-6494.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Otis N, Pelletier LG. Women’s regulation styles for eating behaviors and outcomes: the mediating role of approach and avoidance food planning. Motivation and Emotion. 2008;32:55–67. [Google Scholar]
- Paller KA, Kutas M, McIsaac HK. Monitoring conscious recollection via the electrical activity of the brain. Psychological Science. 1995;6:107–11. [Google Scholar]
- Payne BK, Cheng CM, Govorun O, Stewart BD. An inkblot for attitudes: affect misattribution as implicit measurement. Journal of Personality and Social Psychology. 2005;89:277–93. doi: 10.1037/0022-3514.89.3.277. [DOI] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: the cognitive costs of keeping one's cool. Journal of Personality and Social Psychology. 2000;79:410–24. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Matsumura M. Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. NeuroReport. 2001;12:709–14. doi: 10.1097/00001756-200103260-00019. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghofer M. Emotion and attention: Event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schweiger Gallo I, Keil A, McCulloch KC, Rockstroh B, Gollwitzer PM. Strategic automation of emotion regulation. Journal of Personality and Social Psychology. 2009;96:11–31. doi: 10.1037/a0013460. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pfütze EM, Sommer W. Repetition priming and associative priming of face recognition: Evidence from event-related potentials. Journal of Experimental Psychology - Learning, Memory, and Cognition. 1995;21:722–36. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review. 2004;8:220–47. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- Wegner DM, Shortt JW, Blake AW, Page MS. The suppression of exciting thoughts. Journal of Personality and Social Psychology. 1990;58:409–18. doi: 10.1037//0022-3514.58.3.409. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;47:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]