Abstract
It is well known that perceiving another's body movements activates corresponding motor representations in an observer's brain. It is nevertheless true that in many situations simply imitating another's actions would not be an effective or appropriate response, as successful interaction often requires complementary rather than emulative movements. At what point does the automatic tendency to mirror another's actions become the inclination to carry out appropriate, complementary movements? In the present study, single-pulse transcranial magnetic stimulation (TMS) was used to explore corticospinal excitability in participants observing action sequences evoking imitative or complementary movements. TMS was delivered at five time points corresponding to different moments in time when key kinematic landmarks characterizing an observed action occurred. A variation in motor evoked potentials (MEPs) confirmed that the motor system flexibly shifts from imitative to complementary action tendencies. That shift appears to take place very precociously in time. Observers are attuned to advance movement information and can use it to anticipate a future course of action and to prepare for an appropriate, complementary action. Altogether, these findings represent a step forward in research concerning social action-perception coupling mechanisms providing important data to better understand the role of predictive simulation in social contexts.
Keywords: action observation, action prediction, complementary actions, transcranial magnetic stimulation, motor evoked potentials
INTRODUCTION
A large body of evidence suggests that observing others’ body movements activates motor representations in the observer's brain (e.g. Fadiga et al., 1995; Avenanti et al., 2007; Aglioti et al., 2008). It would seem from studies reported in the literature that activation of motor representations is imitative in nature. The motor system seems to simulate in a strictly congruent fashion action stimuli being observed. The representations of the muscles involved are the same as those used in the observed action and their activation is temporally and strictly coupled with the dynamics of the observed movement (Fadiga et al., 2005).
But the automatic tendency to ‘mirror’ is clearly not always the best response in real-life situations. When two or more individuals are involved in joint actions, complementary rather than imitative actions are, in fact, often more appropriate. If, e.g. an individual hands someone a mug by its handle, the receiver does not imitate the action but carries out a complementary one by choosing the appropriate grip aperture to grasp it. Mirroring the observed movement in that case would impede an effective interaction between those individuals. But how do humans resolve the dilemma between the automatic tendency to ‘mirror’ and the inclination to perform context related, complementary actions appropriate to social interaction?
Some studies, designed to assess cortical activity of the primary motor cortex (M1) during action observation, have shown that there is an anticipatory bias in the motor response to observed actions (Gangitano et al., 2001; Kilner et al., 2004; Borroni et al., 2005; Urgesi et al., 2006, 2010; Aglioti et al., 2008). Using motor-evoked potentials (MEP) induced by single-pulse transcranial magnetic stimulation (spTMS), M1 was found to be activated while static pictures of ongoing but incomplete human actions were being observed (Urgesi et al., 2006; Candidi et al., 2010). Just as importantly, motor facilitation has been found to be greater for images depicting hand actions in their initial-middle phases than for their final stages (Urgesi et al., 2006, 2010). The hypothesis that motor reactivity to implied actions reflects anticipatory simulation of future phases of an action is compatible with the theory that action perception relies on forward models predicting the future course of another's motor acts (Wilson and Knoblich, 2005; Gazzola and Keysers, 2009; Schippers and Keysers, 2011). In this perspective, motor simulation seems to be called into play to solve fundamental computational dilemmas posed by action perception in those cases when information is missing or ambiguous (Wilson and Knoblich, 2005; Schütz-Bosbach and Prinz, 2007; Aglioti and Pazzaglia, 2011; Avenanti and Urgesi, 2011). When the appropriate action is a complementary one, the initially observed motor act must be coded from the very beginning in terms of the subsequent steps required to fulfill the action goal.
This hypothesis has been substantiated by a series of studies reporting that observing a two-step action sequence characterized by an implicit, complementary request evokes a shift from emulation to reciprocity in the observer's corticospinal activity (Sartori et al., 2011b, 2012). Accordingly, differential motor facilitation has been reported when video clips evoking imitative and complementary gestures were being observed. These results provide compelling evidence that when an observed action evokes a non-identical complementary one, an interaction takes place between the automatic tendency to mirror and the inclination to prepare for a complementary action. On the basis of MEP recordings, it would seem that there is a pure matching mechanism at the beginning of an action sequence and a complementary one at the time the request for a complementary action becomes evident. It would seem then that any potential dilemma taking place between an observed action and a non-identical, complementary one is resolved flexibly in a two-step manner by the system itself. During the first step, the observed action is processed in order to predict its goal. During the second step, associations are made between the observed action and a possible, appropriate, non-identical movement needed to accomplish a complementary goal. MEPs have until now been recorded only at two different stages of an action sequence: during the first observed action and at the end of the complementary request. The stage during the action sequence when the functional shift takes places has yet to be identified. It was the aim of the present study to determine when the automatic tendency to mirror another's actions (emulation) turns into anticipatory simulation of complementary acts (reciprocity).
MEPs were, thus, recorded from two hand muscles (i.e. the first dorsal interosseous—FDI, and abductor digiti minimi—ADM) involved in different ways in whole-hand and precision grasps at five points in time while participants watched video clips. Half of the clips evoked complementary gestures in the observer (social condition), while the other half did not (non-social condition).
METHODS
Participants
Thirty participants (22 females and 8 males: age = 21 ± 5 years), all right handed according to a Standard Handedness Inventory (Briggs and Nebes, 1975) and with normal or corrected-to-normal vision, took part in the experiment. None had any contraindication to TMS (Wasserman, 1998; Rossi et al., 2009) nor experienced discomfort during the experiment. The experimental procedures outlined here were granted ethical approval (Ethics Committee of the University of Padova) in accordance with the principles of the 1964 Declaration of Helsinki and all the participants gave written informed consent.
Experimental stimuli
A model was commissioned to perform four action sequences which were filmed.
The model is seen grasping a sugar spoon and pouring sugar into three mugs located near to her on a table (a fourth mug is set apart from the other three and is sitting on the other side of the table in the foreground). When she finishes pouring sugar in the third mug she begins to move her hand as if she intended to pour sugar into a fourth one (Figure 1a).
The model is seen grasping a sugar spoon, pouring sugar into the same three mugs. Once she has poured sugar into the third mug her hand begins to return to its original position (Figure 1b).
The model is seen grasping a thermos, pouring coffee into three espresso coffee cups located near to her on the table (a fourth cup is set apart from the other three and is sitting on the other side of the table in the foreground). After pouring coffee in the third cup she moves her hand as if she intended to pour coffee into the fourth cup (Figure 1c).
The model is seen grasping the same thermos, pouring coffee into the three closeby coffee cups and then simply bringing her arm back to its original position (Figure 1d).
Fig. 1.
The frames extracted from the four video clips serving as stimuli for this experiment. Specifically, the frames showed when the model’s hand made contact with the object, the moment when the model finished pouring sugar(a,b)/coffee(c,d) into a third cup and started moving her hand, and the final phase of the action sequence.
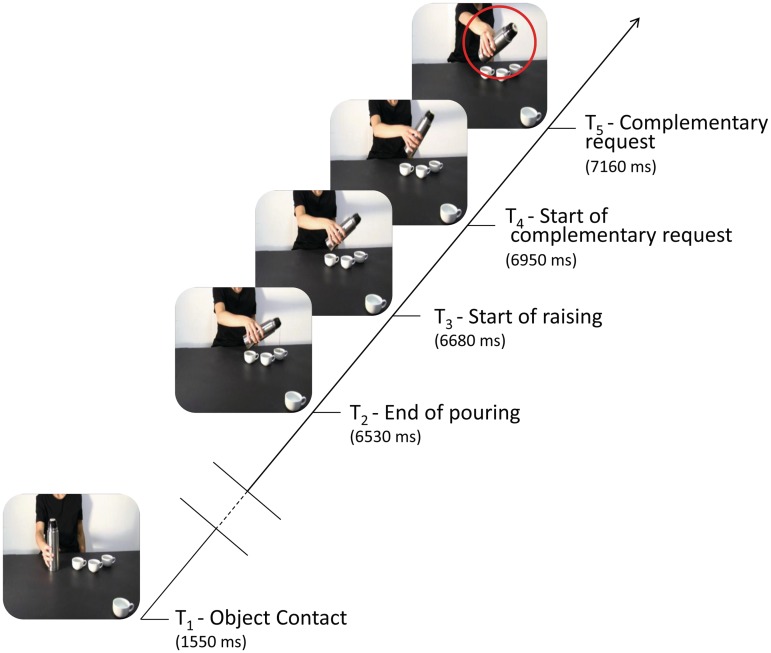
The model grasped the thermos in a natural way using a whole-hand grasp (WHG; i.e. the opposition of the thumb with the other fingers) and she grasped the sugar spoon using a precision grip (PG; i.e. the opposition of the thumb with the index finger). As outlined in Figure 2, at the beginning of each video clip the hand of the model was shown in a prone position resting on the table. About 890 ms later, the model started her reach-to-grasp movement and her fingers made contact with the first object at 1550 ms. About 4980 ms later, the model finished pouring sugar/coffee into a third cup and started moving her hand to perform another action ending at 7600 ms. Since the precise movement of reaching and grasping the sugar spoon was slower with respect to the time needed to grasp the thermos, the first contact in that case occurred at 1880 ms; the timing of all the other movements was the same in the two series. The animation effect was obtained by presenting series of single frames each lasting 30 ms (resolution 720 × 576 pixels, colour depth 24 bits, frame rate 30 fps) plus the first and last frames which lasted 500 and 1000 ms, respectively. The video clips underwent a post hoc kinematic analysis using a digitalization technique. Each movement could then be marked by manually assigning a marker to the model's wrist. All her movements were tracked frame by frame with the analysis being limited to her wrist trajectories in the attempt to determine a correspondence between kinematic events characterizing the double-step action phases and TMS stimulation.
Fig. 2.
A schematization of the sequence of events during each single trial is shown. The continuous oblique line represents the duration of the video clip presentation. The five time points when a single TMS pulse was delivered are represented by horizontal lines: T1 (the time the hand made contact with the object), T2 (when the model finished pouring), T3 (when the model raised her hand from the third cup/mug), T4 (the onset of the complementary request gesture) and T5 (the end of the complementary request gesture). The frames not shown in the figure (the time between the object contact and the completion of the pouring gesture) are represented by double oblique bars.
TMS stimulation and MEP recording
TMS was delivered using a 70-mm figure-of-eight coil connected to a Magstim BiStim2 stimulator (Magstim, Whitlan, Dyfed, Wales, UK). Pulses were delivered to the left primary motor cortex corresponding to the hand region. The coil was angled 45° relative to the interhemispheric fissure and perpendicularly to the central sulcus with the handle pointing laterally and caudally (Brasil-Neto et al., 1992). This orientation induced a posterior–anterior current in the brain tending to activate corticospinal neurons indirectly via excitatory synaptic inputs (Di Lazzaro et al., 1998). The coil was positioned at the optimal scalp position (OSP), defined as the position at which the stimulation of a slightly suprathreshold intensity consistently produced the highest level of MEP activity from both the abductor digiti minimi (ADM; the muscle serving little finger abduction) and the first dorsal interosseus (FDI; the muscle serving index finger flexion/extension) muscles. Held by a tripod, the coil was continually checked by the experimenters to maintain consistent positioning. Electromyographic (EMG) activity was recorded through pairs of 9 mm diameter Ag–AgCl surface electrodes. The active electrodes were placed over the belly of the right ADM and FDI muscles and the reference electrodes over the ipsilateral proximal interphalangeal joint (belly-tendon montage). The electrodes were connected to an isolated portable ExG input box linked to the main EMG amplifier for signal transmission via a twin fiber optic cable (Professional BrainAmp ExG MR, Brain Products, Munich, Germany). The ground electrode was attached to the participant's left wrist and connected to the common input of the ExG input box. The raw myographic signals were bandpass filtered (20 Hz–1 kHz), amplified prior to being digitized (5 kHz sampling rate) and stored in a computer for offline analysis. To prevent contamination of MEP measurements by background EMG activity, all trials in which EMG activity >100 µV was present in the 100 ms window before TMS pulses were delivered were eliminated. EMG data were recorded for another 200 ms after the TMS pulse. The resting motor threshold (rMT) was determined for each participant as the minimum stimulation intensity producing reliable MEP (≥50 μV peak-to-peak amplitude) in a relaxed muscle in 5 out of 10 consecutive trials. The stimulation intensity during the recording session was 110% of the rMT and ranged between 37% and 66% (mean 53.4%) of the maximum stimulator output intensity. MEP were recorded simultaneously from electrodes placed over the contralateral ADM and FDI muscles. The presentation of stimuli and the timing of TMS stimulation were managed by E-Prime V2.0 software (Psychology Software Tools Inc., Pittsburgh, PA, USA) running on a PC. Each participant's baseline corticospinal excitability was assessed by acquiring 10 MEP while he/she passively watched a white-coloured fixation cross on a black background on the computer screen. Another series of 10 MEP was recorded at the end of the experimental session. Possible variations in corticospinal excitability related to TMS per se were assessed by comparing the MEP amplitudes for the two resting periods and their average amplitude was calculated to set individual baselines for data normalization procedures.
Procedure
The experiments were carried out in a sound-attenuated Faraday room with each participant seated in a comfortable armchair with the right arm positioned on a full-arm support, the left arm relaxed with the hand resting on the legs, and the head positioned on a fixed head rest with an eye distance of about 80 cm away from the screen. The participants were asked to watch the visual stimuli presented on a 19” monitor (resolution 1280 × 1024 pixels, refresh frequency 75 Hz, background luminance of 0.5 cd/m2) set at eye level and to encourage them to maintain a good level of attention, they were told that they would be questioned about what they had seen.
TMS-induced MEP from the right ADM and the right FDI muscles were acquired once for each video presentation at one of five possible time points (Figure 2): the point the model's hand first contacted the sugar spoon or the thermos (T1), the point the model finished pouring sugar/coffee into the third cup/mug (T2), the point the model began to distance her hand from the cup/mug (T3), the point the model's wrist trajectory started to return to its original position in the non-social condition or to move towards the 4th cup/mug in the social condition (T4), and, finally, the point the model's arm was clearly returning to its original position or reaching towards the fourth cup/mug (T5).
Each video presentation was followed by a 10 s rest interval. During the first 5 s a message reminding the participants to keep their hands still and fully relaxed was presented. The message was replaced by a fixation cross for the remaining 5 s. All the participants watched each of the four video-clips five times, for a total of 20 trials, which were presented in a fully randomized order. Twenty-five MEPs were acquired per muscle during the presentation of the videos, resulting in a total of 200 MEP per participant (5 time points × 2 hand muscles × 5 repetitions × 4 video clips = 200). Baseline CS excitability was assessed prior to and following the video presentations.
The participants watched video clips showing four experimental conditions.
Social precision grip movement
In this video clip, the model used a precision grip to handle a sugar spoon to pour sugar into three mugs located close by to her. After she poured sugar into the third mug the model moved her arm as if she intended to pour sugar into a fourth mug on the other side of the table (Figure 1a). The mug needed, of course, a whole-hand grasping movement to be handled. In this experimental condition, the model's action (i.e. precision grip) did not match the movement an observer would need to perform to carry out an appropriate action (i.e. WHG).
Non-social precision grip movement
In this video clip the model went through the same action sequence as above (‘Social precision grip movement’). The only difference was that after pouring sugar in the third mug located nearby, the model's hand returned to its initial position even though a fourth mug was clearly in view (Figure 1b). In this case, the action sequence was not inferring any complementary action.
Social WHG movement
In this video clip the model used a WHG to handle a thermos. The model was seen pouring coffee into three espresso coffee cups located near to her hand. After coffee was poured into the third cup the model began to move her arm as if she intended to pour coffee into a fourth out-of-reach cup located on the other side of the table (Figure 1c). The espresso cup needed, of course, a precision grip movement to be handled. The model's gesture (i.e. WHG), thus, did not match with an act an observer would need to perform an appropriate action (i.e. precision grip).
Non-social WHG movement
In this video clip, the model went through the same action sequence as above (Social WHG movement). The only difference was that after pouring coffee in the third espresso coffee cup located nearby, the model's hand returned to its original position even though a fourth cup was clearly in view (Figure 1d). In this case, the action sequence was not inferring any complementary action.
Please note that from the observer's point of view the out-of-reach object was located in the foreground in the bottom right corner of the image.
In order to ascertain if the objects used in the video clips elicited the type of grasp targeted in the present study, a preliminary pilot experiment consisting in a reach-to-grasp test was carried out. Subjects with characteristics that were similar to those of the participants taking part in the main experiment were asked to grasp objects that were similar to those seen in the video clips and the response frequency was recorded. During the entirety of the trials, all the subjects grasped the espresso cup with a precision grip and the mug with a WHG.
While in previous studies (Sartori et al., 2011b, 2012) the control conditions during the experiment were obtained by keeping the model's movement unvaried and deleting the out-of-reach object from the scene, in the experiment performed in this study they were obtained by directing the model to bring her hand back to its initial position, despite the fact that a fourth object was visible. That control condition was created in order to detach the role of an intentional gesture from object affordances. Indeed, the two hypotheses that an object's features always potentiate actions that might be performed on it or that the mechanism works only when the affordances becomes salient evoking a readiness to act, are still being debated (Jeannerod, 1994; Craighero et al., 1998; Tucker and Ellis, 1998; Buccino et al., 2009).
Data analysis
Kinematic analysis of the model's action
In the attempt to determine a correspondence between kinematic events characterizing the action phases and TMS stimulation, each video clip underwent a post hoc kinematic analysis using a digitalization technique. Each section of the movement was marked and tracked frame by frame. In particular, a break detection algorithm (Castiello et al., 1993) was applied to determine a key kinematic landmark, i.e. the time point during the social trials (the actions calling for a complementary gesture) when the model's hand began to significantly move away from the trajectory used in the non-social trials (when the model's hand returned to its original position). It was found that that point became evident 240 ms after the model completed the first step of the action sequence (pouring sugar/coffee in the third cup/mug) [t(39) = 20.45, P < 0.001].
TMS stimulation and MEP recordings
Peak-to-peak amplitudes of the MEP from both the ADM and FDI muscles were measured and averaged separately for each condition. MEP amplitudes deviating more than 2 s.d. from the mean for each type of action and trials contaminated by muscular pre-activation were excluded as outliers (<2%). A paired sample t-test (two-tailed) was used to compare the amplitude of MEP from the ADM and FDI muscles in the two series of baseline trials carried out for each participant at the beginning and at the end of each experimental session. Ratios were then computed using the participant's individual mean MEP amplitude recorded during the pre- and post-testing sessions as baseline values (MEP ratio = MEPobtained/MEPbaseline). A mixed-design analysis of variance (ANOVA) was conducted on the MEP ratios with ‘condition’ (social, non-social), ‘time’ (T1, T2, T3, T4, T5) and ‘type of grasp’ (PG, WHG) as within-subjects factors, and ‘muscle’ (FDI, ADM) as between-subjects factors. Sphericity of the data was verified prior to performing statistical analysis (Mauchly's test, P > 0.05). Post hoc pairwise comparisons were carried out using t-tests and the Bonferroni adjustment for multiple comparisons was applied.
RESULTS
TMS per se induced no changes in CS excitability during our experiment. Mean raw MEP amplitudes recorded for each participant during pre- and post-sessions were not significantly different for either the ADM [t(29) = –0.34, P = 0.73] or the FDI [t(29) = –0.68, P = 0.50] muscles (Table 1). This suggests that TMS per se did not induce any alterations in corticospinal excitability during our experimental procedure. Mean MEP ratios from the ADM and the FDI muscles for each ‘condition’ (social, non-social), ‘time’ (T1, T2, T3, T4, T5), and ‘type of grasp’ (PG, WHG) are listed in Table 1. As FDI was recruited for both the PG and WHG, no MEP modulation was expected in terms of the type of grasp observed. The mixed-design ANOVA on normalized MEP amplitudes showed a significant main effect of condition [F(1,58) = 16.09, P < 0.001, 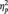 = 0.22] and time [F(4,232) = 2.77, P < 0.05,
= 0.22] and time [F(4,232) = 2.77, P < 0.05,  = 0.05], but not of type of grasp (P > 0.05). MEP amplitude was greater for the social than for the non-social condition and it was greater at T5 with respect to T1 (P > 0.05), T2 (P > 0.05) and T3 (P > 0.05). Two significant two-way interactions of ‘muscle by type of grasp’ [F(1,118) = 5.86, P < 0.05,
= 0.05], but not of type of grasp (P > 0.05). MEP amplitude was greater for the social than for the non-social condition and it was greater at T5 with respect to T1 (P > 0.05), T2 (P > 0.05) and T3 (P > 0.05). Two significant two-way interactions of ‘muscle by type of grasp’ [F(1,118) = 5.86, P < 0.05,  = 0.09] and ‘type of grasp by condition’ [F(1,58) = 10.30, P < 0.05,
= 0.09] and ‘type of grasp by condition’ [F(1,58) = 10.30, P < 0.05, 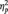 = 0.15], and a significant four-way interaction of ‘muscle by condition by type of grasp by time’ [F(4,472) = 2.43, P < 0.05,
= 0.15], and a significant four-way interaction of ‘muscle by condition by type of grasp by time’ [F(4,472) = 2.43, P < 0.05, 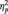 = 0.04] were also noted. The results obtained from the post hoc contrasts exploring the higher order interaction are listed as follows.
= 0.04] were also noted. The results obtained from the post hoc contrasts exploring the higher order interaction are listed as follows.
Table 1.
Normalized mean (±s.e.m.) peak to peak amplitude of MEPs recorded from the FDI and the ADM muscles during the four conditions for each type of observed grasp at each time point
| Social |
Non-social |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T1 | T2 | T3 | T4 | T5 | |
| Precision grip movement | ||||||||||
| FDI | 2.40 (±0.39) | 2.30 (±0.30) | 2.11 (±0.27) | 2.51 (±0.46) | 2.66 (±0.39) | 2.11 (±0.35) | 2.00 (±0.28) | 2.15 (±0.33) | 2.11 (±0.35) | 2.28 (±0.35) |
| ADM | 1.23 (±0.12) | 1.48 (±0.17) | 1.42 (± 0.12) | 1.76 (±0.18) | 1.99 (±0.23) | 1.19 (±0.12) | 1.20 (±0.09) | 1.31 (±0.12) | 1.41 (±0.14) | 1.27 (±0.11) |
| WHG movement | ||||||||||
| FDI | 2.20 (±0.51) | 2.04 (±0.31) | 2.08 (±0.26) | 2.28 (±0.31) | 2.72 (±0.44) | 2.07 (±0.30) | 2.23 (±0.36) | 2.10 (±0.27) | 2.08 (±0.26) | 2.43 (±0.44) |
| ADM | 1.66 (±0.18) | 1.78 (±0.20) | 1.74 (±0.17) | 1.39 (±0.15) | 1.37 (±0.10) | 1.43 (±0.15) | 1.56 (±0.15) | 1.60 (±0.13) | 1.68 (±0.17) | 1.82 (±0.21) |
When MEP are modulated in terms of a complementary action
When the social vs the non-social conditions were compared, the normalized MEP responses for the ADM muscle were greater when the participants were observing the model holding the sugar spoon and reaching towards the fourth mug needing a WHG (social condition; Figure 3) than when they observed the model simply holding the same sugar spoon with a PG as she returned her hand to its initial position (non-social condition). This effect only occurred at T4 and T5 (P < 0.05 and P < 0.001, respectively). Conversely, MEP responses at T4 and T5 were smaller (P's < 0.05) when the participants observed the model holding the thermos and reaching towards a fourth espresso coffee cup needing a PG (Figure 3) than when they observed the model simply holding the same thermos with a WHG as her hand returned to its original position. The fact that a statistically significant difference was found between the social and the non-social conditions only at T4 and T5 time points seems to suggest that the mere presence of a fourth cup had no priming effect per se. Only when the model's action was directed to reaching in the direction of the cup/mug did the ‘social effect’ arise.
Fig. 3.
The frames extracted from the four video clips across time points accompany the lines of the graph which represent the means of the ADM normalized MEP amplitudes. Social precision grip movements requiring a WHG (white) and social WHG movements requiring a PG (black) are illustrated. Bars represent the standard error of means.
The time-course of complementary activations
In terms of normalized MEP responses for the ADM muscle, no difference was noticed in the time points for the non-social conditions (P's > 0.05; Table 1). As was expected, statistical differences were found only for the social conditions. Specifically, normalized MEP responses were greater at the T4 time point with respect to T1 (P < 0.001), T2 (P < 0.05) and T3 (P < 0.05), and at the T5 time point with respect to T1 (P < 0.001), T2 (P < 0.05) and T3 (P < 0.05) when the participants were observing the model holding the sugar spoon reaching towards the mug evoking a WHG (Figure 3). Notably, MEP responses at both T4 and T5 time points were not significantly different (P = 0.36), suggesting that the participants had already acknowledged the goal of the action as soon as the model's arm began to evoke a complementary gesture. Conversely, normalized MEP responses were smaller at T4 with respect to T2 (P < 0.05) and T3 (P < 0.05) and at T5 with respect to T2 (P < 0.05) and T3 (P < 0.05) when the participants observed the model holding the thermos and reaching towards the espresso coffee cup evoking a PG (Figure 3). Again, MEP responses at both T4 and T5 time points were not significantly different (P = 0.87). Taken together, these results indicate that the switch from a symmetrical motor resonance to a complementary activation of the ADM muscle took place while the participants were observing the action sequence within the 270 ms time window.
MEP are modulated depending on the type of grasp being observed
With reference to the type of grasp, post hoc comparisons of the ADM muscle revealed statistically significant differences for both the social and non-social conditions depending on the point in time. In particular, MEP responses were smaller when the participants were observing the model holding the sugar spoon with a PG with respect to observing the model handling the thermos with a WHG at T1, T2 and T3 (P's < 0.05; Table 1) time points indicating perhaps that at an early stage of the action sequence participants were resonating with the model's action and ignoring the action goal. Instead, at T4 and T5 time points MEP responses were modified only during the social conditions. MEP response was greater (P's < 0.05) when the participants were observing the model's action evoking a WHG with regard to the fourth mug rather than a PG for the fourth espresso cup.
DISCUSSION
The principle aim of the present study was to investigate action observation evoking complementary actions in a social context. The present research is linked to and based on studies focusing on how intentions (social vs non-social) are inferred from action observation and how corticospinal excitability is modulated during action observation in social contexts. Our findings suggest that humans are able to anticipate the social intent of an observed action on the basis of precocious kinematic cues signalling the need for a complementary action. The findings outlined here take previous reports (Sartori et al., 2011b, 2012) a step further by identifying the precise moment, as far as MEPs are concerned, that the transition from emulation to reciprocity takes place.
How the motor system's tendency to match (emulate) observed actions is reconciled with the inclination to prepare for a non-identical response is not well known. Some studies have taken into consideration the task context and the relation between the model and the observer by comparing imitation and complementary action tasks (Newman-Nordlund et al., 2007; van Schie et al., 2008; Ocampo and Kritikos, 2010). In behavioural terms, these studies all agree that there are differences between preparing and executing complementary with respect to imitative actions (Ocampo and Kritikos, 2010; van Schie et al., 2008). In neural terms, a higher level of activation has been found during the preparation of complementary than that of imitative actions in key areas of the human mirror system, namely the inferior frontal gyrus and the inferior parietal lobe (Newman-Nordlund et al., 2007). Recent TMS studies have, moreover, indicated that corticospinal activation resulting from action observation does not necessarily possess an imitative bias but, depending on contextual factors (Sartori et al., 2011b, 2012), can also prime motor activation for complementary actions. These single-pulse TMS studies have demonstrated that observation of a two-step action sequence characterized by an implicit complementary request evokes a shift from emulation to reciprocity in the participant’s corticospinal activity. Findings from the present study confirm that a shift does occur and indicate exactly when it takes place.
Specifically, we noted that the precise moment the model's wrist started to move towards an isolated mug (the social condition) signalled a variation in MEP heralding a reciprocal action. On the contrary, the moment the model's wrist started to return to its original position (the non-social condition) signalled a variation in MEP heralding an emulative action. This suggests that humans are able to code an action as social or non-social even before the action becomes explicit. Indeed, the first task-dependent, measurable variation in wrist trajectory direction in the two conditions occurred 240 ms after the model completed the first of the two-step sequence, at a point in which it was difficult to visually perceive what course of action the model was taking. These results demonstrate that observers are attuned to advance movement information provided by subtle kinematic cues and can use it to anticipate a future course of action. In this experiment, participants were able to discriminate between an action driven by a social goal from one that was not simply by observing the first, almost imperceptible kinematic cue signalling the subsequent phase in a two-stage sequence. Modulation of corticospinal excitability appears then to be a reliable, ‘indirect’ measure of an automatic tendency to activate appropriate motor programs for an interaction context, regardless of whether an effective interaction will take place.
What mechanisms do humans utilize then to predict social intentions when they are observing others’ movements?
It has been hypothesized that one of the motor system's basic functions is to predict another’s actions (Blakemore and Frith, 2005; Wilson and Knoblich, 2005). Observing another’s actions seems to activate similar representations in the observer's motor system that may be used to generate predictions on the basis of internal simulations. In this perspective, the same predictive mechanisms used to anticipate sensory consequences of one's own movement may be employed to predict what others will do next (Wolpert and Flanagan, 2001). It is possible then that in the present study participants relied on simulation processes in their own motor systems to anticipate the model's social intention in that context. Indeed, observing another's action is not simply a post hoc reconstruction of visual input, but an intrinsically predictive activity. When we observe others’ actions, we automatically anticipate their future ones. At the most basic level, humans can predict how a movement will evolve simply by watching how it was begun. For example, by observing how a person throws a dart at a dartboard, an observer can predict where the dart will land (Knoblich and Flach, 2001). An observer can likewise anticipate the type of tennis or volleyball serve (Abernethy and Zawi, 2007; Abernethy et al., 2008), predict the success of a basketball shot (Aglioti et al., 2008), foresee if a player is about to launch a real or a mimic throw (Sebanz and Shiffrar, 2009), predict if an action heralds a competitive or cooperative interaction (Sartori et al., 2011a). In more complex situations, predictive coding allows us to understand others’ intentions, i.e. to predict what they will do next. The findings outlined here demonstrate that information deduced from an observed action might even help an observer make inferences about an agent's intentionality. An observer may be able to predict simply on the basis of subtle kinematic variations what the person observed is going to do next and thus to prepare a complementary response.
These findings have direct implications with regard to theories of action representation as they suggest that intention attribution is sensitive to kinematic constraints. Since different kinds of intentional actions have different motion signatures, observers can take advantage of precocious differences in kinematics to estimate intentions from action observation. By observing ‘body movements’, individuals seem to be able to predict the type of action to be performed (Dittrich, 1993; Vanrie and Verfaillie, 2004), the actor's identity (e.g. Loula et al., 2005), gender (e.g. Kozlowski and Cutting, 1977; Pollick et al., 2002; Troje, 2002; Brooks et al., 2008) and age (Montepare and Zebrowitz- McArthur, 1988). By observing day to day movements, observers seem to be able to perceive an actor's emotions (Pollick et al., 2001), expectations (Runeson and Frykholm, 1983) and even deceptive intentions (Grezes et al., 2004; Sebanz and Shiffrar, 2009). Our findings take these studies one step further by showing that advance information gained while an action sequence is being observed allows observers not only to mirror an observed action but to see behind the what and why of the action and how to interact appropriately.
Some of these effects could be ascribed simply to the motor coding of object affordances, i.e. if an objects’ features have the ability to potentiate the actions that might be performed on them, even in the absence of the explicit intention to act (Jeannerod, 1994; Craighero et al., 1998; Tucker and Ellis, 1998; Buccino et al., 2009). Our data demonstrate, instead, that there is a shift from symmetrical emulation to covert planning for a non-identical action only when the gesture evoking a response is within a social context. The presence of an object when the gesture does not evoke a reciprocal action elicits no shift. In conclusion, the present findings support the hypothesis that the mechanisms underlying action observation are flexible, rapid and highly responsive to the complex requests embedded in contexts characterized by a social dimension. They indicate that observing a two-step action sequence characterized by an implicit complementary request evokes an early changeover from emulation to reciprocity. Finally, they identify the precise moment when the emulation stage terminates and the complementary activation begins in an observer's corticospinal activity. Future research will undoubtedly uncover if these processes are serial or if some form of parallel processing exists.
FUNDING
Ministero dell’Educazione, Universitaè Ricerca (2008YTNXXZ) to U.C.
REFERENCES
- Abernethy B, Zawi K. Pickup of essential kinematics underpins expert perception of movement patterns. Journal of Motor Behaviour. 2007;39(5):353–67. doi: 10.3200/JMBR.39.5.353-368. [DOI] [PubMed] [Google Scholar]
- Abernethy B, Zawi K, Jackson RC. Expertise and attunement to kinematic constraints. Perception. 2008;37(6):931–48. doi: 10.1068/p5340. [DOI] [PubMed] [Google Scholar]
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nature Neuroscience. 2008;11:1109–16. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Aglioti SM, Pazzaglia M. Sounds and scents in (social) action. Trends in Cognitive Science. 2011;15(2):47–55. doi: 10.1016/j.tics.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Malavita A, Aglioti SM. Somatic and motor components of action simulation. Current Biology. 2007;17:2129–35. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Urgesi C. Understanding ‘what’ others do: mirror mechanisms play a crucial role in action perception. Social Cognitive and Affective Neuroscience. 2011;6(3):257–9. doi: 10.1093/scan/nsr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Frith C. The role of motor contagion in the prediction of action. Neuropsychologia. 2005;43(2):260–7. doi: 10.1016/j.neuropsychologia.2004.11.012. Review. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during observation of hand actions in humans. European Journal of Neuroscience. 2005;13:190–4. [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. Journal of Clinical Neurophysiolology. 1992;9:132–6. [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–8. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Brooks A, Schouten B, Troje NF, Verfaillie K, Blanke O, van der Zwan R. Correlated changes in perceptions of the gender and orientation of ambiguous biological motion figures. Current Biology. 2008;18(17):R728–9. doi: 10.1016/j.cub.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Candidi M, Leone-Fernandez B, Barber HA, Carreiras M, Aglioti SM. Hands on the future: facilitation of cortico-spinal hand-representation when reading the future tense of hand-related action verbs. European Journal of Neuroscience. 2010;32(4):677–83. doi: 10.1111/j.1460-9568.2010.07305.x. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennet KMB, Stelmach GE. Reach to grasp: the natural response to perturbation of object size. Experimental Brain Research. 1993;94:163–78. doi: 10.1007/BF00230479. [DOI] [PubMed] [Google Scholar]
- Craighero L, Fadiga L, Rizzolatti G, Umiltà C. Visuomotor Priming. Visual Cognition. 1998;5:109–125. [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, et al. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalography and Clinical Neurophysiology. 1998;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Dittrich WH. Action categories and the perception of biological motion. Perception. 1993;22(1):15–22. doi: 10.1068/p220015. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of others’ action. Current Opinion in Neurobiology. 2005;15:213–8. doi: 10.1016/j.conb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. Journal of Neurophysiology. 1995;73:2608–11. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12:1489–92. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cerebral Cortex. 2009;19:1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Frith CD, Passingham RE. Inferring false beliefs from the actions of oneself and others: an fMRI study. Neuroimage. 2004;21(2):744–50. doi: 10.1016/S1053-8119(03)00665-7. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The hand and the object: the role of posterior parietal cortex in forming motor representations. Canadian Journal of Physiology and Pharmacology. 1994;72:535–41. doi: 10.1139/y94-077. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore SJ, Sirigu A. Motor activation prior to observation of a predicted movement. Nature Neuroscience. 2004;7(12):1299–301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Knoblich G, Flach R. Predicting the effects of actions: interactions of perception and action. Psychological Science. 2001;12(6):467–72. doi: 10.1111/1467-9280.00387. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Cutting JE. Recognizing the sex of a walker from a dynamic point-light display. Perception and Psychophysics. 1977;21:575–80. [Google Scholar]
- Loula F, Prasad S, Harber K, Shiffrar M. Recognizing people from their movement. Journal of Experimental Psychology: Human Perception and Performance. 2005;31(1):210–20. doi: 10.1037/0096-1523.31.1.210. [DOI] [PubMed] [Google Scholar]
- Montepare JM, Zebrowitz-McArthur L. Impressions of people created by age-related qualities of their gaits. Journal of Personality and Social Psychology. 1988;55(4):547–56. doi: 10.1037//0022-3514.55.4.547. [DOI] [PubMed] [Google Scholar]
- Newman-Nordlund RD, van Schie HT, van Zuijlen AM, Bekkering H. The mirror neuron system is more activated during complementary compared with imitative action. Nature Neuroscience. 2007;10:817–8. doi: 10.1038/nn1911. [DOI] [PubMed] [Google Scholar]
- Ocampo B, Kritikos A. Placing actions in context: motor facilitation following observation of identical and non-identical manual acts. Experimental Brain Research. 2010;201:743–51. doi: 10.1007/s00221-009-2089-6. [DOI] [PubMed] [Google Scholar]
- Pollick FE, Lestou V, Ryu J, Cho SB. Estimating the efficiency of recognizing gender and affect from biological motion. Vision Research. 2002;42(20):2345–55. doi: 10.1016/s0042-6989(02)00196-7. [DOI] [PubMed] [Google Scholar]
- Pollick FE, Paterson HM, Bruderlin A, Sanford AJ. Perceiving affect from arm movement. Cognition. 2001;82(2):B51–61. doi: 10.1016/s0010-0277(01)00147-0. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeson S, Frykholm G. Kinematic specification of dynamics as an informational basis for person-and-action perception: Expectation, gender recognition, and deceptive intention. Journal of Experimental Psychology: General. 1983;112(4):585–615. [Google Scholar]
- Sartori L, Becchio C, Castiello U. Cues to intention: the role of movement information. Cognition. 2011a;119(2):242–52. doi: 10.1016/j.cognition.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Sartori L, Cavallo A, Bucchioni G, Castiello U. Corticospinal excitability is specifically modulated by the social dimension of observed actions. Experimental Brain Research. 2011b;211(3–4):557–68. doi: 10.1007/s00221-011-2650-y. [DOI] [PubMed] [Google Scholar]
- Sartori L, Cavallo A, Bucchioni G, Castiello U. From simulation to reciprocity: the case of complementary actions. Social Neuroscience. 2012;7:146–158. doi: 10.1080/17470919.2011.586579. [DOI] [PubMed] [Google Scholar]
- Schippers MB, Keysers C. Mapping the flow of information within the putative mirror neuron system during gesture observation. Neuroimage. 2011;57(1):37–44. doi: 10.1016/j.neuroimage.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Schütz-Bosbach S, Prinz W. Prospective coding in event representation. Cognitive Processing. 2007;8(2):93–102. doi: 10.1007/s10339-007-0167-x. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Shiffrar M. Detecting deception in a bluffing body: the role of expertise. Psychonomic Bulletin and Review. 2009;16(1):170–5. doi: 10.3758/PBR.16.1.170. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R. On the relations between seen objects and components of potential actions. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:830–46. doi: 10.1037//0096-1523.24.3.830. [DOI] [PubMed] [Google Scholar]
- Troje NF. Decomposing biological motion: a framework for analysis and synthesis of human gait patterns. Journal of Vision. 2002;2(5):371–87. doi: 10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Maieron M, Avenanti A, Tidoni E, Fabbro F, Aglioti SM. Simulating the future of actions in the human corticospinal system. Cerebral Cortex. 2010;20(11):2511–21. doi: 10.1093/cercor/bhp292. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Moro V, Candidi M, Aglioti SM. Mapping implied body actions in the human motor system. Journal of Neuroscience. 2006;26:7942–9. doi: 10.1523/JNEUROSCI.1289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie HT, van Waterschoot BM, Bekkering H. Understanding action beyond imitation: reversed compatibility effects of action observation in imitation and joint action. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:1493–1500. doi: 10.1037/a0011750. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Verfaillie K. Perception of biological motion: a stimulus set of human point-light actions. Behavior Research Methods, Instruments, and Computers. 2004;36(4):625–9. doi: 10.3758/bf03206542. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wilson M, Knoblich G. The case for motor involvement in perceiving conspecifics. Psychological Bulletin. 2005;131(3):460–73. doi: 10.1037/0033-2909.131.3.460. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Current Biology. 2001;11(18):R729–32. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]