Abstract
Is a short visuomotor associative training sufficient to reverse the visuomotor tuning of mirror neurons in adult humans? We tested the effects of associative training on corticospinal modulation during action observation in the 100–320 ms interval after action onset. In two separate experiments, the acceleration of transcranial magnetic stimulation (TMS)-induced movements was recorded before and after training participants to respond to observed acts with an opposite or similar behavior. Before training, TMS-induced accelerations mirrored the observed action at 250 and 320 ms. After training, responses at 250 ms were unchanged and still mirrored the stimuli, without any effect of training direction. Only at 320 ms, we observed training-dependent changes in evoked responses. A control experiment with non-biological rotational movements as visual stimuli indicated that spatial stimulus–response compatibility is not sufficient to account for the results of the two main experiments. We show that the effects of a short visuomotor associative training are not pervasive on the automatic mirror responses. ‘Early’ (250 ms) responses were not influenced by training. Conversely only ‘late’ (320 ms) responses changed according to the training direction. This biphasic time course indicates that two distinct mechanisms produce the automatic mirror responses and the newly learned visuomotor associations.
Keywords: action observation, transcranial magnetic stimulation, mirror neurons, associative sequence learning, action selection, visual motor training
INTRODUCTION
The motor system of primates responds to the sight of others’ movements with stimulus-specific motor programs that replicate congruently the observed behavior (Cattaneo and Rizzolatti, 2009). The neural substrate of such motor resonance resides probably in parieto-frontal mirror neurons. These are one among the many classes of parieto-frontal sensorimotor neurons that couple sensory information about the world around us with automatic goal-directed motor behavior (Rizzolatti et al., 1998). Their peculiar properties are those of firing during both the perception and the execution of motor acts. Transcranial magnetic stimulation (TMS) studies have been conducted in humans measuring the output of the primary motor cortex (M1) on the assumption that it is a measure of mirror neuron activity in the parieto-premotor mirror circuit, as suggested by studies combining single-pulse TMS over M1 with repetitive transcranial magnetic stimulation (rTMS) over the premotor cortex (Avenanti et al., 2007) or using dual-coil TMS (Koch et al., 2010; Catmur et al., 2011).
It has been claimed that mirror neurons are a product of visuomotor-associative learning [associative sequence learning (ASL) hypothesis] occurring during visually guided behavior that necessarily couples the experiences of executing an action and seeing it similarly to a Pavlovian conditioned response (Heyes, 2010). The main experimental evidence in favor of the ASL theory is that by changing sensory motor contingencies, by training subjects to respond to seen actions with symmetrically opposite behavior, it is possible to transform in the short-term mirror neurons in ‘counter-mirror neurons’ (Catmur et al., 2007, 2011). Counter-mirror neurons have reversed their tuning and code new associations of incongruent observed and executed behaviors. Such empirical data, however, imply that visuomotor parieto-frontal circuits are acutely re-tunable by arbitrary rules. Conversely, in our view, their visuomotor matching properties are more hard wired in the central nervous system of typically developed adult humans, and their stimulus/response curves can be shifted by recent experience but only to a limited extent (Cattaneo et al., 2010) without reversing it.
The effects of counter-mirror training on the observers’ responses to action observation (Catmur et al., 2007, 2011) have been tested by stimulating M1 with single TMS pulses. In Catmur et al. (2007), corticospinal excitability was tested at intervals of 0, 320 and 640 ms from movement onset before and after the associative training. In Catmur et al. (2011), corticospinal excitability was tested before the training at the 200, 250 and 300 ms intervals from movement onset. However, after training, the authors tested only the 300 ms inter-stimulus interval (ISI) because of the interest in the effect of premotor stimulation over mirror motor facilitation, which was found at the 300 ms ISI only. The chronometry of the mirror effect to action observation is not entirely clear according to the available literature. Many studies used ongoing actions as visual stimuli, although the analyses were time locked to certain phases of the movement. In such studies, the timing of cortical activation is not clear because when watching continuous stimuli, the participants can in principle predict the forthcoming action. In these experiments, imitative responses were between 80 and 200 ms after informative visual cues (Borroni et al., 2005; Cattaneo et al., 2009). One neuromagnetic imaging study recorded activity time locked to object–hand interaction during an ongoing reach and grasp movement and found M1 to be active ∼40 ms from hand–object interaction, but also in this case, the averaging trigger was not the onset of movement but its final phase (Nishitani and Hari, 2000). In that study, however, some indirect information on the timing of mirror responses can be inferred by comparing the latency of the occipital and motor peaks during imitation, which are separated by ∼200 ms. The aforementioned articles, regardless of the use of continuous or of event-related paradigms, are based on motor events that are predictable by the observer. It has been shown that, during ongoing movements, predictory elements are strongly represented in the observer's motor system (Gangitano et al., 2004; Urgesi et al., 2010).
Only a few studies used event-related techniques time locked to the onset of unpredictable movement. These showed motor modulation as early as 90–100 ms after visual or auditory presentation of action stimuli (Lepage et al., 2010); however, this motor modulation was not muscle specific. Unpredictable movements were also used in an event-related paradigm by Catmur et al. (2011) who found in baseline conditions a muscle-specific mirror effect as early as 200 ms from movement onset. When processing categories of visual information other than upper limb movements, automatic motor responses are known to occur in an early time interval, between 100 and 200 ms from stimulus presentation. This has been shown for a variety of visual stimuli, such as the presentation of manipulable objects (Prabhu et al., 2007; Buccino et al., 2009), the vision of articulatory lip movements (Sato et al., 2010) or the visual presentation of Arabic numerals (Sato et al., 2007). Taken together, the data from the literature indicate that when investigating ‘automatic’ visuomotor-imitative responses, the excitability of the motor cortex should be tested in an early time window, approximately between 100 and 300 ms from stimulus onset.
A partial time course of the mirror effect in such early interval has been already described (Catmur et al., 2011). However, on that occasion, the authors tested the 200, 250 and 300 ms intervals from the onset of observed movement only before the counter-imitative training session but tested only the 300 ms interval after the training session. Here, we filled this information gap by investigating the chronometry of motor modulation following action observation, before and after associative behavioral trainings in the 100–320 ms interval after visual stimulation. As an instantaneous index of the transient changes in the cortical representation of movements, we recorded the kinematics of TMS-evoked movements rather than the muscular electrical activity of motor-evoked potentials (MEPs) because it better represents the cognitive process we wanted to measure. Kinematics measure the actual movement, whereas single muscular contractions may not be univocally associated with a single movement (Brochier et al., 2004; Weiss and Flanders, 2004) or with any movement at all, as for example in fixation of an articulation. More importantly, the motor cortex and, even more, the premotor cortex of primates represent motor acts and their end point rather than single muscles (for example see Rizzolatti et al., 1988; Kakei et al., 1999, 2001)
We performed three different experiments. In the first (counter imitative) experiment, participants were presented with two opposite biological actions, to which they trained with an associative counter-imitative protocol. The second (imitative) experiment was identical to the first one, but the associative training was imitative. In a control experiment (spatial compatibility), participants were presented with spatially oriented non-biological events to which they had to associate spatially non-compatible motor responses. The latter experiment was done to understand whether only spatial features could account for all the results of the counter-imitative experiment.
MATERIALS AND METHODS
Participants
Sixteen volunteers (10 women, age: 23–45 years, one left handed) took part in the counter-imitative experiment. Ten volunteers (7 women, age: 21–28 years) took part in the imitative experiment, and 16 volunteers (9 women, age: 21–36 years) participated in the spatial-compatibility experiment. None of the participants took part in more than one experiment. The experiment was approved by the local Ethical Committee and was conducted in compliance with the revised Helsinki declaration (World Medical Association General Assembly, 2008). All participants gave written informed consent to the experiment and were screened for contraindications to TMS (Rossi et al., 2009).
General design
In all three experiments, we recorded the acceleration of the participants’ wrist (W-Acc) evoked by TMS applied to the motor cortex. This measure was used as the dependent variable throughout the experiments. W-Acc was recorded in an event-related design, whereas participants watched short video clips, consisting in a moving hand in the counter-imitative and imitative experiments and in a moving arrow in the spatial-compatibility experiment (see later). Importantly, TMS was applied at four different time intervals (ISIs) from the onset of movement in the movie. Participants underwent a first TMS session in which event-related W-Acc was recorded. They then performed a behavioral associative training session, the rules of which changed in the three experiments and finally underwent another TMS session identical to the first one. In each experiment, the within-subject independent factors that were experimentally manipulated were three: (i) being tested before or after the behavioral training, (ii) the type of video clip and (iii) the ISI.
Video stimuli
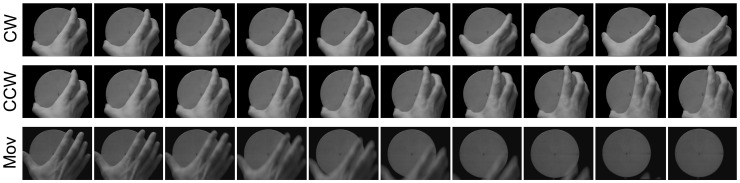
Three types of movies were presented in the counter-imitative and in the imitative training, as schematized in Figure 1. They showed a right hand in egocentric perspective turning a round lid in the clockwise direction (CW trials), in the counterclockwise direction (CCW trials) or simply moving down from the lid (Mov trials). The frame rate of the movies was 33 Hz. The first three frames were the same in all movies, showing a still hand. The onset of the movement happened in the fourth frame of each video, i.e. at 100 ms from the onset of the movie. The movements in all trials lasted for nine frames, i.e. 300 ms. In CW and CCW trials, the final wrist rotation was of 18° with respect to the starting position. The timing of TMS was calculated from the onset of the fourth frame, i.e. when the first information on the movement direction was available.
Fig. 1.
Frame-by-frame representation of the movements presented to the participants in the (A) counter-imitative and imitative experiments. Every clip showed a right hand in egocentric perspective turning a lid clockwise (CW trials), counterclockwise (CCW trials) or simply moving down from the lid (Mov trials). In 9% of trials, a red dot appeared on the screen, to which participants had to respond as fast as possible with a left-hand button press.
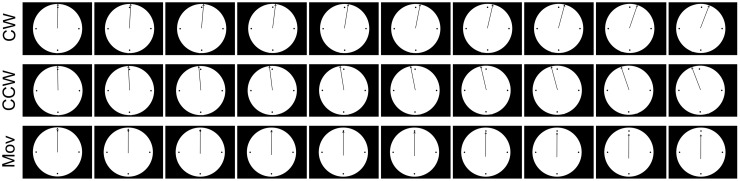
In the spatial-compatibility experiment, three different types of movies were presented. They showed a white circle with one single black vertical arrow in the middle (resembling the hand of a clock) and are schematized in Figure 2. Three different movie types showed the arrow turning clockwise, counterclockwise or falling down vertically. The movies were designed to match the three conditions (CW, CCW and Mov trials) of the first two experiments. Their frame rate was 33 Hz, the first three frames showed the still vertical arrow and the onset of the movement was at the fourth frame. Also, here the timing of TMS was calculated from the onset of the fourth frame. Also, the rotational degree (18°) and movement time (300 ms) were matched to the previous movies. A frame-by-frame representation of the single frames in which movement occurred in both the biological and non-biological movies is provided in Figures 1 and 2.
Fig. 2.
Frame-by-frame representation of the movements presented to the participants in the spatial-compatibility experiment.
It should be noted that both the hand movies and the arrow movies were designed, so that the movement direction could not be guessed from the initial three static frames because they were indistinguishable between conditions. All stimuli were presented on a Liquid Crystal Display (LCD) screen, with a visual angle of 14° in width by means of the Cogent 2000, developed by the Cogent 2000 team at the Functional Imaging Laboratories (FIL) and the Institute of Cognitive Neuroscience (ICN) and Cogent Graphics developed by John Romaya at the LON at the Wellcome Department of Imaging Neuroscience.
TMS sessions
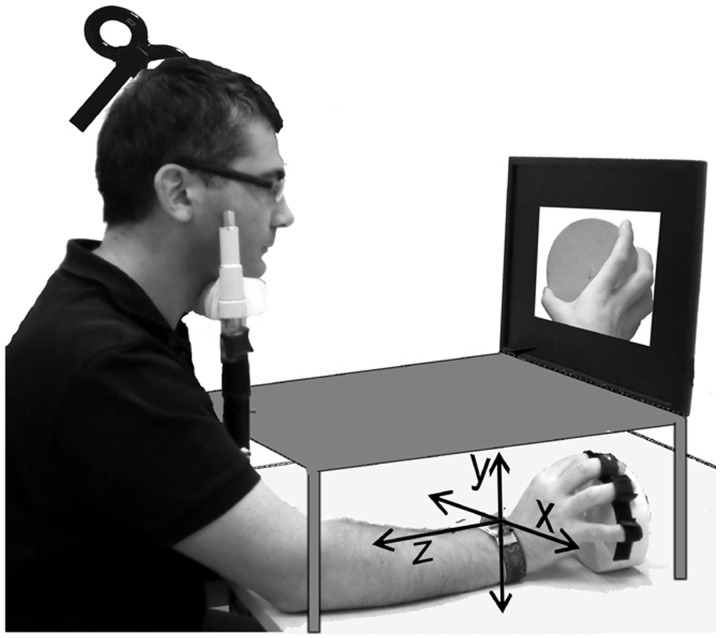
In all three experiments, participants were sitting comfortably with their head on a chin rest, which assured head stability. Figure 3 shows a typical setup. The participants’ fingers were fixed on the rim of a round box similar to the one observed in the video. The elbow and the box were placed above two different supports. The forearm was not supported so as to reduce friction of a rotational movement of the forearm/wrist. The vision of the own hand was occluded to participants throughout the experiment by means of an opaque shield. Each of the two TMS sessions, before and after training, consisted of 740 event-related trials. Each trial started with a fixation cross presented for 2000 ms followed by the movie (1500 ms) and by a blank screen of 1000 ms. TMS was delivered time locked to clip presentation at different ISIs (see later). Every 74 stimuli participants could take a short pause from TMS, therefore the session could be divided in 10 blocks of 74 trials. The 740 trials consisted of 672 movies (224 CW, 224 CCW and 224 Mov or the equivalent stimuli in the spatial-compatibility experiment) plus 68 still frames of a black background with a red dot in the center, all presented in a random order. The task was to completely relax the right arm and hand, watch carefully the movies and whenever the red dot appeared, to press a key as fast as possible with their left hand. If after 1500 ms from the presentation of the red dot participants did not respond, a ‘Is anybody out there?’ sign was presented in the center of the screen, to alert them. Single-biphasic stimuli were delivered with a Magpro unit (Magventure, Denmark) connected to an MCB65 figure of eight coil. Resting motor threshold was visually assessed as the intensity capable of evoking twitches of the thumb or index fingers in 5 of 10 consecutive trials. This technique is known to over-estimate the threshold assessed with electromyograph (EMG) by a factor between 2% (Conforto et al., 2004) and 15% (Hanajima et al., 2007). Participants were then stimulated with an intensity equal to 130% of threshold. The TMS site in the actual experiment was detected visually as the site evoked the most powerful visible wrist turning on the x-axis in the hand at rest.
Fig. 3.
Schematization of the experimental setup showing orientation of the x, y and z axes.
TMS was delivered at four different ISIs corresponding to 100, 150, 250 and 320 ms from the onset of movements in the video clips. The Cogent software sent a TTL signal from the PCs parallel port to an analog/digital input/output device, the CED 1401 (Cambridge Electronic Design, UK) 50 ms before the video onset. It was then the 1401 unit controlled by the Signal software (Cambridge Electronic Design, UK) that, through its digital output triggered the magnetic stimulator at different ISIs in a random order. On the presentation of the red dot, no TMS pulse was delivered.
Recording and interpretation of TMS-evoked accelerations
The accelerations were recorded by means of a two-axis accelerometer (Model DE-ACCM6G buffered 6G) fixed to the participants’ right wrist. The x-axis recorded the horizontal acceleration, whereas the y-axis recorded the acceleration on the vertical axis. Variations over the x-axis were informative of initial clockwise wrist rotations (positive accelerations) and counterclockwise rotations (negative accelerations). The accelerometer output was sampled at 1000 Hz by the CED Micro 1401 analog-to-digital converter and stored for offline analysis with the Signal software.
Comparing the two-axis system to the movement shown in stimulus movies (compare Figures 1 and 3), it is clear that the x-axis is the main component of the movement that distinguished CW from CCW movements, whereas CW and CCW trials were undistinguishable on the y-axis component. Therefore, the x-axis accelerations were considered to be informative of imitative motor responses, whereas the y-axis was not. The movement shown in Mov stimuli was orthogonal to the x-axis on which wrist rotation occurred, and, therefore, motor responses mimicking it (y-axis in Figure 3) could not be read on the x-axis. The acceleration associated with Mov trials provided therefore a neutral condition serving as baseline that was subtracted from accelerations of CW and CCW trials (see later, ‘Data processing’ section).
Preliminary evaluation of the TMS-evoked acceleration
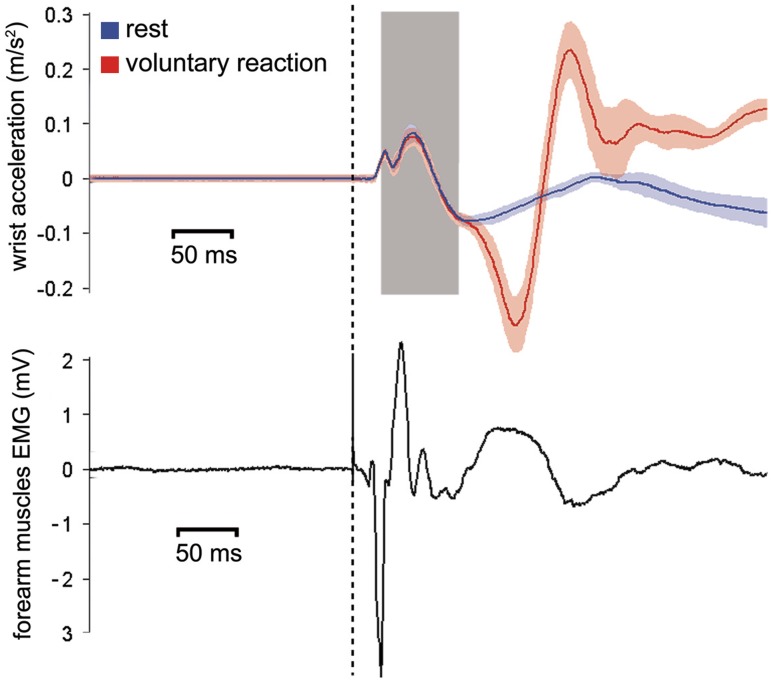
The mechanical twitch following synchronous depolarization of muscle fibers such as the one evoked by TMS occurs a few milliseconds after the electrical events spreading in the sarcolemma, which are macroscopically reflected by MEPs. This delay can vary according to muscular and joint properties (Barrett et al., 2009). The acceleration of body parts in response to TMS of M1 has already been used as an index of motor cortex excitability but limitedly to the thumb (Stefan et al., 2005, 2008) and index finger (Ingham et al., 2011) movements. TMS-evoked W-Acc has not been described before now in experimental settings. Therefore, we evaluated empirically in six new participants (three men and three women, mean age: 22 years) the timing of appearance of TMS-evoked acceleration. This was done mainly to establish the time interval after TMS in which acceleration was informative of evoked movements but could not yet be contaminated by voluntary reactions to the stimulus. Therefore, we asked participants in half of the trials to stay still in the other half react to the TMS pulse as fast as possible with a wrist movement. We recorded the electromyographic activity of the right forearm flexor group (including therefore the pronator teres muscle and the main actor of wrist pronation) and the wrist acceleration with the methods described earlier. The data from a representative subject are shown in Figure 4. We found that significant changes in W-Acc started between 4 and 7 ms from the onset of the MEP (recorded by EMG) and that changes to the W-Acc course related to voluntary activity appeared between 102 and 126 ms after stimulation. We deducted that the time window between 30 and 90 ms from stimulation could be safely considered as a genuine expression of TMS-evoked wrist movement.
Fig. 4.
Plot from one representative participant, taken from the pilot evaluation, of the mean x-axis W-Acc (upper panel) and forearm EMG recordings (lower panel) obtained from 25 consecutive ‘passive’ trials and 25 consecutive ‘active’ trials. The dashed vertical line represents the time of TMS. The onset latency of the MEP is of 17 ms. Initial deflection of accelerations at 22 ms. Initial deviation of the acceleration trace due to voluntary interference is at 112 ms. The colored shadings represent the s.e.m. of acceleration signals. The gray shading represents the 30–90 ms interval that we subsequently chose to consider for analysis in the main experiment.
Data processing
In all three experiments, the raw W-Acc recordings from each trial were pre-processed separately for each of the two axes by applying the following consecutive steps.
Baseline correction
It was made to adjust the baseline shifts due to the initial position of the hand, because the accelerometer senses also the gravitational acceleration and, therefore, emits different outputs according to the initial tilt of the still hand. For each trial, the mean value of the signal in the 100 ms preceding the magnetic stimulus was subtracted from the subsequent recording.
Averaging in the 30–90 ms window
Following the results of the preliminary evaluation of the TMS-evoked acceleration (Figure 4), we averaged the acceleration values between 30 and 90 ms from the magnetic stimulus. In this way, each single trial of the CW and CCW conditions corresponded finally to one single value for each of the x and y axes. Such value was used as dependent variable in subsequent statistical analyses.
Normalization of CW and CCW trials to Mov trials
The W-Acc from Mov trials (or downward arrow movements in the spatial-compatibility experiment) was considered as a neutral condition for the purpose of investigating mirror or counter-mirror responses. We averaged the acceleration values of neutral trials separately for each block of 74 trials, for each of the four ISIs and separately for the pre-training and the post-training session in the 30–90 ms time window. These average control data were then subtracted from the individual trials with CW and CCW stimuli (or the equivalent rightward and leftward arrow rotations in the spatial-compatibility experiment), thus obtaining a value representing the variation of the acceleration in CW or CCW trials with respect to the neutral ones.
Training session
A wooden manipulandum similar to the lid presented in the counter-imitative and imitative movies was used. The lid of the manipulandum could be turned clockwise or counterclockwise up to 15° from each side of the midline and thanks to a pair of springs it returned in the start position. When the extreme lateral position was reached, a 3.5 V circuit was closed, sending a TTL signal to the computers’ parallel port, providing information about the direction in which the lid had been turned, to give subjects a feedback on their performance (see later). A potentiometer (10 KOhm linear) within the manipulandum signaled the angle of the lid. The potentiometer’ signal was sampled by the CED Micro 1401 analog to digital converter and recorded with the Spike software (Cambridge Electronic Design, UK). In the training session, only CW and CCW movies were presented (or the equivalent stimuli of the spatial-compatibility experiment). Participants underwent 864 training trials (432 CW and 432 CCW), divided in 12 consecutive blocks, resulting in 72 randomly presented trials per block. The participants’ task was to turn the lid with their right hand, in the same direction (imitative experiment) or in the opposite direction (counter-imitative experiment) to the one shown in the movies. Participants were encouraged to turn the lid as fast as they could during the whole session. For each trial, as soon as the lid was completely turned in the correct direction, feedback was provided by displaying the time of end contact of the lid, therefore corresponding to the end-of-movement time. If the lid had been turned in the wrong direction, the text ‘Wrong!’ appeared in the center of the screen. If no response was produced, then a ‘no response’ message was displayed. The potentiometer's signal was used offline to detect the moment of movement onset and subsequently to calculate the subjects’ real reaction time (RT, rather than the displayed end-of-movement time). In the spatial-compatibility experiment, the training task was analogous to that of the counter-imitative experiment. Participants had to turn the manipulandum as fast as possible in the direction opposite to the one in which the arrow moved.
Statistical analysis on W-Acc
The single mean acceleration values obtained as described earlier (see ‘Data processing’ section) were averaged within each subject. In this way, each participant in each experiment was characterized by two sets (one for each axis) of 16 mean acceleration values corresponding to the two movie types, for each of the four ISIs, pre- and post-training. These values were then used as a dependent variable in two (one for each axis) repeated measures analysis of variance (ANOVA) (2 × 2 × 4) with TRAINING (pre-training and post-training), DIRECTION (clockwise and counterclockwise) and ISI (100, 150, 250 and 320 ms) as within-subject factors in each of the three experiments. All variables were tested for normality of the distribution by means of the Kolmogorov–Smirnov test (all variables with P > 0.18). The ANOVAs were also tested for sphericity in all three experiments, and the three-way interactions were found to be spherical for both the x-axis (all P values >0.22). Three-way interactions were further explored by means of four separate two-factor ANOVAs (one for each ISI).
One additional unplanned post hoc analysis was carried out with the scope of directly comparing the results of the counter-imitative experiment with those of the spatial-compatibility experiment. We performed four separate ANOVAs for each of the four ISIs, having one between-subject factor: EXPERIMENT (two levels: counter imitative or spatial compatibility) and two within-subject factors: TRAINING (two levels: pre-training or post-training) and DIRECTION (two levels: CW or CCW).
Statistical analysis on the training session
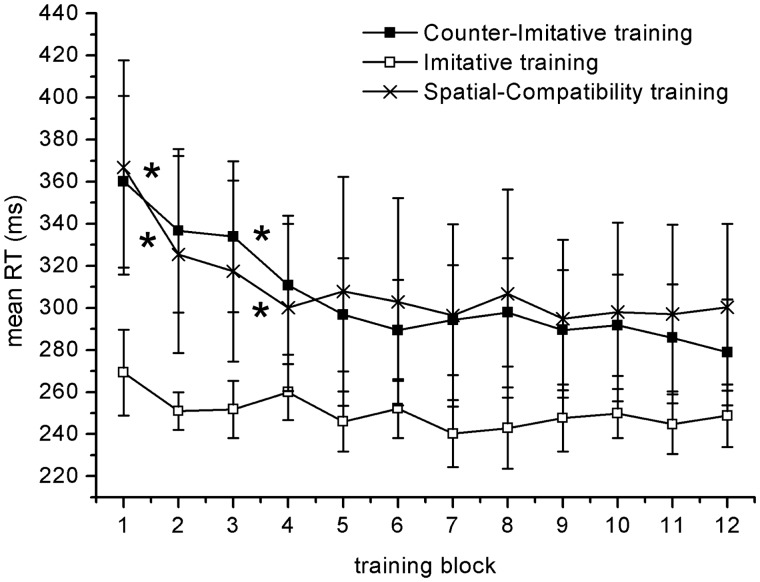
During training, the potentiometer signaled instantaneously the angle of the training lid and therefore the participant's responses in each trial. We decided to consider as errors all trials that started in the wrong direction, even if in some trials the trajectory was then corrected in the appropriate direction. To detect these responses, the derivative of the potentiometer's output was calculated, and the signal was low-pass filtered at 12 Hz. Deviations outside a threshold of rotational velocity of ±40°/s were considered as response onset and subsequently categorized in terms of latency and correctness of the response. Responses faster than 90 ms were excluded. For each participant and for each of the 12 training blocks, we calculated the individual median RT of correct responses and the proportion of errors. Subsequently, two mixed-design ANOVAs were performed separately on RTs and accuracy, with BLOCK as the within-subject variable and EXPERIMENT as the between-subject variable. Possible main effects of BLOCK were subsequently analyzed by means of pairwise t-tests on the data from each pair of consecutive blocks, to quantify the improvement in the performance (Figure 5).
Fig. 5.
Time course of the mean reaction times recorded during the behavioral trainings of the two main experiments and the control experiment. Asterisks indicate significant differences between consecutive blocks in paired-sample t-tests. Error bars represent 95% CI.
RESULTS
None of the participants reported undesired effects of TMS. Attendance to the visual stimuli was testified by the low rate of red dot trials in which participants were slower than 1500 ms, consisting of 2.9% for the counter-imitative experiment, 2.7% for the imitative experiment and 3.0% for the spatial-compatibility experiment. The average time interval between the end of the training and the beginning of the post-training TMS session was of 232 s (s.d.: 21 s) overall for the three experiments.
Counter-imitative experiment
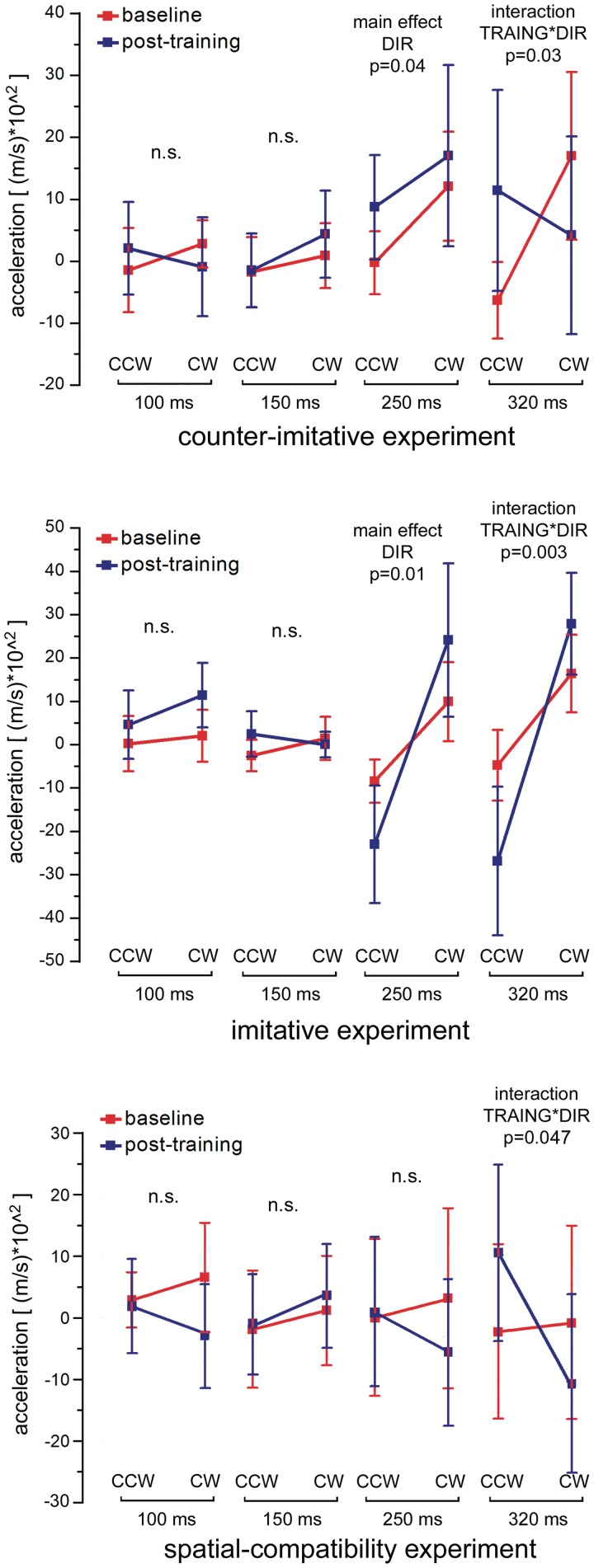
The ANOVA results on x-axis W-Acc are shown in Table 1. The most important result on the x-axis data was a significant three-way TRAINING × DIRECTION × ISI interaction. The single cells of this three-way interaction are reported in Table 2. As illustrated in Figure 6, to analyze this interaction, the data were analyzed separately for each of the four ISIs, therefore producing four distinct ANOVAs with two factors, TRAINING and DIRECTION. No significant results were observed at 100 or 150 ms. At 250 ms, we found only a main effect of DIRECTION [F(1,15) = 5.11, P = 0.039], showing that at this ISI the training did not influence the subjects’ responses, which were simulative both before and after training. At 320 ms, we did not find main effects, but we did find a significant TRAINING × DIRECTION interaction [F(1,15) = 6.10, P = 0.026], resulting from a clear mirror effect before the training (paired samples t-test between CW and CCW trials: P = 0.01) which was disrupted by training (paired samples t-test: P = 0.3). The ANOVA on y-axis accelerations did not produce significant results (all P values >0.21).
Table 1.
Results of the ANOVAs on x-axis accelerations in the counter-imitative and imitative experiments
| Effect | Counter imitative |
Imitative |
||||
|---|---|---|---|---|---|---|
| DF | F | P | DF | F | P | |
| TRAINING | 1 | 2.998 | 0.104 | 1 | 0.06 | 0.812 |
| DIRECTION | 1 | 5.261 | 0.037 | 1 | 10.45 | 0.010 |
| ISI | 3 | 1.889 | 0.145 | 3 | 0.76 | 0.526 |
| TRAINING × DIRECTION | 1 | 5.494 | 0.033 | 1 | 6.64 | 0.030 |
| TRAINING × ISI | 3 | 0.421 | 0.739 | 3 | 1.16 | 0.342 |
| DIRECTION × ISI | 3 | 2.093 | 0.114 | 3 | 8.83 | <0.0001 |
| TRAINING × DIRECTION × ISI | 3 | 3.776 | 0.017 | 3 | 3.94 | 0.019 |
Significant values are in bold italic.
Table 2.
Mean values (95% CI) of acceleration (102 m/s) on the x-axis for counter-imitative and imitative experiments
| Training | Direction | ISI | Counter imitative | Imitative |
|---|---|---|---|---|
| Mean (−95% to +95%) | Mean (−95% to +95%) | |||
| Pre-training | CCW | 100 | −1.4 (−8.8 to 6) | 0.2 (−9.1 to 9.6) |
| Pre-training | CCW | 150 | −1.8 (−7.9 to 4.4) | −2.6 (−7.9 to 2.8) |
| Pre-training | CCW | 250 | −0.2 (−5.8 to 5.3) | −8.4 (−15.7 to −1.2) |
| Pre-training | CCW | 320 | −6.3 (−13 to 0.4) | −4.8 (−16.7 to 7.1) |
| Pre-training | CW | 100 | 2.8 (−1.3 to 7) | 2 (−6.7 to 10.8) |
| Pre-training | CW | 150 | 0.9 (−4.8 to 6.6) | 1.4 (−5.9 to 8.7) |
| Pre-training | CW | 250 | 12.1 (2.5 to 21.7) | 9.9 (−3.4 to 23.2) |
| Pre-training | CW | 320 | 17 (2.3 to 31.7) | 16.4 (3.3 to 29.4) |
| Post-training | CCW | 100 | 2.1 (−6 to 10.2) | 4.6 (−6.9 to 16.2) |
| Post-training | CCW | 150 | −1.5 (−8 to 5) | 2.4 (−5.2 to 10.1) |
| Post-training | CCW | 250 | 8.7 (−0.4 to 17.9) | −23 (−42.8 to −3.2) |
| Post-training | CCW | 320 | 11.4 (−6.2 to 29.1) | −26.9 (−51.9 to −1.8) |
| Post-training | CW | 100 | −0.9 (−9.6 to 7.8) | 11.4 (0.6 to 22.2) |
| Post-training | CW | 150 | 4.4 (−3.3 to 12) | 0 (−4.3 to 4.3) |
| Post-training | CW | 250 | 17 (1.1 to 32.9) | 24.2 (−1.7 to 50) |
| Post-training | CW | 320 | 4.2 (−13.2 to 21.5) | 27.9 (10.7 to 45) |
Fig. 6.
Results of the counter-imitative (upper panel), imitative (middle panel) and spatial-compatibility (lower panel) experiments. The plot represents the mean of TMS-evoked W-Acc values measured on the x-axis of the accelerometer (±95% CI) in CCW and CW trials for all four ISIs, before training and after training. Error bars indicate 95% CI. The results of the two-way ANOVAs on single ISIs made to explore the three-way interaction are shown above each ISI; n.s. = non-significant.
Imitative experiment
The ANOVA results on x-axis W-Acc values are presented in Table 1. A significant three-way interaction was found, which is detailed in Table 2 and illustrated in Figure 6. The interaction was further investigated by dividing the data into four separate ANOVAs for each of the ISIs. At 100 and 150 ms, no significant effects were found. At 250 ms, a significant main effect of DIRECTION [F(1,9) = 10.41, P = 0.01] was found and at 320 both a main effect of DIRECTION [F(1,9) = 9.78, P = 0.012] and, more importantly, a significant TRAINING × DIRECTION interaction [F(1,9) = 15.6, P = 0.003] were found. No significant effect was found in the analysis on y-axis accelerations.
Spatial-compatibility experiment
The results of the ANOVA performed on x-axis W-Acc values are reported in Table 3. The analysis did not show a three-way interaction but only a two-way TRAINING × DIRECTION interaction was present, due to the fact that overall W-Acc values were modulated in a spatially compatible way before training (mean value for leftward trials: −6.5 × 102 m/s2. Mean value for rightward trials: 9.5 × 102 m/s2. Paired samples t-test, P = 0.03) and this difference was not present after training (mean value for leftward trials: 3.1 × 102 m/s2. Mean value for rightward trials: −3.7 × 102 m/s2. Paired samples t-test, P = 0.11). Even in the absence of a significant three-way interaction, we analyzed the data with four two-way ANOVAs, to compare the results with those of the other two experiments. The single values of this analysis are reported in Supplementary Table S2 and are illustrated in the lower panel of Figure 6.
Table 3.
Results of the ANOVAs on x-axis accelerations in the spatial compatibility experiment
| Effect | DF | F | P |
|---|---|---|---|
| TRAINING | 1 | 0.679 | 0.423 |
| DIRECTION | 1 | 4.331 | 0.055 |
| ISI | 3 | 0.448 | 0.72 |
| TRAINING × DIRECTION | 1 | 5.405 | 0.035 |
| TRAINING × ISI | 3 | 0.929 | 0.435 |
| DIRECTION × ISI | 3 | 1.295 | 0.288 |
| TRAINING × DIRECTION × ISI | 3 | 2.082 | 0.116 |
Significant values are in bold italic.
The post hoc direct comparison between the counter-imitative experiment and the spatial-compatibility experiment are reported in Supplementary Table S3. In summary, the ANOVAs produced no significant results at the 100 and 150 ms ISIs. At the 250 ms ISI, we found significant EXPERIMENT × DIRECTION interaction [F(1,30) = 7.51, P = 0.01]. This interaction was interestingly explained by the fact that, as described earlier, in the counter-imitative experiment a significant difference (P = 0.03) between CW and CCW directions (irrespective of training) was present, but in the spatial-compatibility experiment no such difference was observed (P = 0.15). At the 320 ms ISI, we found a DIRECTION × TRAINING interaction [F(1,30) = 10.77, P = 0.003] indicating that, unlike at the 250 ms ISI, at the 320 ms ISI the data from the two experiments were not significantly different.
Training sessions
The RTs in the 12 consecutive blocks of the three experiments are shown in Figure 5. The analysis showed a main effect of the factor EXPERIMENT [F(2,39) = 4.13, P = 0.02], with the imitative training [95% confidence interval (CI): 250.38 ± 10.4 ms] faster than the counter imitative (95% CI: 305.48 ± 26.38 ms), (P = 0.005) and faster than spatial compatibility (95% CI: 309.52 ± 33.90 ms) (P = 0.01). A main effect of BLOCK [F(11,429) = 13.804, P < 0.0001] and an interaction EXPERIMENT × BLOCK were found [F(22,429) = 1.99, P = 0.005]. The 36 cells representing this interaction are reported in Supplementary Table S1. Figure 5 shows also the pairs of consecutive blocks in which a significant difference was found by means of a pairwise t-test. We showed that a significant improvement was present between the first and second and between the third and fourth blocks in both the counter-imitative and the spatial-compatibility trainings. On the contrary, no significant difference between consecutive blocks was found in the imitative training. The analysis of the error rates revealed only a main effect of the factor EXPERIMENT with a higher proportion of errors in the counter-imitative (95% CI: 0.19 ± 0.04) training than the spatial-compatibility training (95% CI: 0.11 ± 0.02), (P = 0.002). No significant differences were found between the imitative training (95% CI: 0.14 ± 0.04) and the spatial-compatibility training, whereas a trend toward significance was found between the imitative and the counter-imitative trainings (P = 0.071)
DISCUSSION
The results of the two main experiments (the imitative and the counter-imitative one) show that in non-trained individuals (i.e. in the pre-training session) a clear and repeatable modulation of the motor system is evident at 250 and 320 ms after the first visual information on observed movement is available. This modulation occurs in the same direction as the observed movement, i.e. it is a mirror modulation. In the post-training session of both experiments, this mirror effect was unchanged at the 250 ms interval. On the contrary, a clear interaction of the training with the evoked responses was observed in the 320 ms interval. This modulation occurred in the direction of the trained visuomotor rule, i.e. subjects’ responses were even more mirror-like after the imitative training and were less mirror-like after the counter-imitative training. The present results show that the chronometry of brain activations to action observation distinguishes two different phases. In one early (after 150 ms and before 250 ms) interval, the motor cortex contains a mirror-like motor representation that is not modulated in the short term by an associative training. In one late phase (after 250 ms but before 320 ms), the motor cortex contains a motor program that is representative of the recent visuomotor training.
If, as hypothesized by the ASL theory, one single mechanism produces automatic mirror responses and trained counter-mirror responses, we would expect the same effect of training on each ISI. In other words, let us hypothesize that the subjects’ responses are described by a function (named f-mirror) having as argument the time from movement onset and the features of observed movement.
The ASL theory predicts that the effects of training on the function is that of multiplying it by a constant value, the polarity of which depends on the training rule, that is a negative value with counter-imitative training and a positive (or null if a ceiling effect is present) when imitative training is performed.
In our specific experimental set, we would expect an effect of training at all ISIs where a mirror effect was present before training. The present data, however, do not fit these predictions. On the contrary, we find a biphasic time course, with no effect of training on an early ISI (250 ms) irrespective of the direction of training. We propose a different model to explain the present data, assuming the presence of two different mechanisms interacting. The first mechanism is the one producing overlearned automatic mirror responses, which produces fast visuomotor associations, within 250 ms from stimulus onset. The second one mediates the responses compatible with the newly learned visuomotor arbitrary associations and seems to be slower than the first one, given the same visual stimuli, i.e. it becomes apparent only at 320 ms from stimulus onset. Our hypothesis is represented, therefore, by two distinct functions, named f-mirror and f-executive with the following temporal constraints: f-mirror produces null results with ISI < 250 and f-executive produces null results for ISI < 320.
Before training, K = 0 and, therefore, only f-mirror produces behavior. After training, K is either a positive or negative constant and, therefore, also the f-executive function contributes to the observer's response. It is important to stress that this model predicts correctly the data from both main experiments (imitative and counter imitative). The statistical implications of the model are indeed that a main effect of DIRECTION is to be expected at 250 ms, irrespective of training, whereas a TRAINING × DIRECTION interaction is to be found at 320 ms, irrespective of the training direction. As shown in Figure 6, our results fit exactly this prediction.
The present data replicate entirely the ones by Catmur et al. We employ a training of the same type and duration, and we obtain data that are compatible with their finding at the late ISI. However, testing an early ISI (250 ms) after training produces additional results that are at odds with the interpretation of the data of Catmur et al. Nevertheless, it should be noted that our data do not argue against an associative origin of the tuning properties of mirror neurons. We clearly demonstrate that a brief visuomotor training is not sufficient to reverse the tuning of mirror neurons, but the present data are fully compatible with an ontogenesis of mirror neurons based on visuomotor associations requiring a much longer history of congruent-visuomotor experience. In such account, which has been systematically theorized elsewhere (Keysers and Perrett, 2004; Del Giudice et al., 2009), Hebbian Learning is at the basis of mirror neuron development, and participants train their mirror neurons over the lifespan by having great interest in looking at their own actions and such cumulative learning of neurons would be much stronger, and persistent, in the face of a short counter-imitation training.
The ISIs that were chosen in the present experiment represent well-defined time points in the course of visual processing along the dorsal stream. The first ISI, 100 ms, represents an ‘early visual’ time, when the visual information is still confined to the early extrastriate visual cortex (see for example Amassian et al., 1989; Silvanto et al., 2005). According to our initial hypothesis, no modulation of the motor system was expected in this early interval. The two ISIs of 150 and 250 ms sample the time window in which visual information is likely to be processed in a parieto-frontal system along the dorsal visual stream as shown for different categories of visual information, such as biological movement (Catmur et al., 2011), object geometry (Bernier et al., 2009) or spatial information (Fierro et al., 2001; Naranjo et al., 2007). It should be noted that neural responses to action observation have been found in two studies as early as 90 ms, albeit in both studies activity was not specific to the type of observed acts (van Schie et al., 2008; Lepage et al., 2010). Interestingly, in one of these studies, van Schie et al. postulate that the early onset of the lateralized neural activity and the fact that the evoked component was insensitive to the correctness of the observed action suggest the operation of a fast and automatic form of motor resonance that may precede higher levels of action understanding. The last ISI, the 320 ms interval, on the contrary was chosen on the basis of the original description of counter-mirror training (Catmur et al., 2007), in which the intervals between stimulus onset and TMS of 0, 320 and 640 ms were tested. The anatomical structures of the two different neural systems described herein can be speculated on the basis of the activation time course. The most likely substrate of the fast motor resonance process is a temporal–parietal–ventral premotor route (Rizzolatti and Matelli, 2003). The arbitrary association route is probably residing in the prefrontal cortex, in its dorsolateral portion, that is thought to play a role in maintaining representations of stimulus–response associations (Ridderinkhof et al., 2010) and in the selection of the responses under conflict (Mansouri et al., 2009), in the right inferior frontal gyrus which has been found activated during the inhibition of prepotent responses (Aron et al., 2004) or in the anterior middle prefrontal cortex, that seems to be specifically involved in the inhibition of imitative responses (Brass et al., 2005). It should be noted that, compared with Catmur et al. (2007), we used a continuous movement rather than an implicit one. This means that the 250 ms TMS and the 320 ms TMS occur at different stages of the movement. This datum, however, accounts only for changes in the time course of the responses within each training block but is unlikely to justify the differences that are observed between training blocks at the different ISIs.
The parallel dual route model is essentially contained in the prevailing theories of action control (Ridderinkhof et al., 2010), which postulate that the appearance of a stimulus activates the correct response by a deliberate route and also captivates activation of other responses by a more direct processing route. The two processes necessarily converge at the level of response activation or even of response production. We cannot speculate at this point whether the executive system actively inhibits the fast visuomotor responses or whether a simple competition between the two processes occurs more distally, i.e. at the level of the motor cortex.
There are several possible ways to control for a purely spatial-compatibility effect in action observation. One is to produce a mismatch between proprioceptive information from one's own hand and the visual information on the observed hand's perspective (Catmur et al., 2007). Another way, which relies purely on the visual modality, is to test participants with flipped or rotated versions of the observed hand (as for example in Brass et al., 2001). The control we adopted here was to test subjects with non-biological movements matching the biological ones (compare Figures 1 and 2). The data that resulted were considerably variable between individuals and only a two-way interaction (TRAINING × DIRECTION) was found. The training had an effect on the observers’ responses in the post-training session, but the three-way ANOVA did not show any specific ISI at which this occurred. We conducted anyway two further post hoc analyses to better understand the information provided by the spatial-compatibility experiment. First, we analyzed separately each of the four time points and found significant results only at the 320 ms interval as a two-way interaction (Figure 6). Second, we compared directly the results of the counter-imitative training and of the spatial-compatibility experiment within each of the four ISIs (Supplementary Table S3). The significant EXPERIMENT × DIRECTION interaction at the 250 ms ISI showed that the early responses recorded when observing a biological effector unlikely to be due to a spatial-compatibility effect. At 320 ms, only a DIRECTION × TRAINING was found, corroborating our hypothesis that the responses recorded at the late ISI are the product of short-term associative visuomotor learning, independently of whether the visual stimulus is a hand or an arrow. The spatial-compatibility experiment would, therefore, suggest that the results of the counter-imitative and imitative experiments would not be due to a spatial-compatibility effect between stimulus and response but that the identity of the stimulus as a hand or as a non-biological entity does matter in producing the observers’ responses, especially at the relevant early 250 ms interval.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
This study was partially supported by the Provincia Autonoma di Trento and Fondazione Cassa di Risparmio di Trento e Rovereto.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by the Provincia Autonoma di Trento and Fondazione Cassa di Risparmio di Trento e Rovereto.
REFERENCES
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalography & Clinical Neurophysiology. 1989;74:458–62. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Current Biology. 2007;17:2129–35. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong's Review of Medical Physiology. 23rd edn. New York: McGraw-Hill Medical; 2009. [Google Scholar]
- Bernier PM, Burle B, Hasbroucq T, Blouin J. Spatio-temporal dynamics of reach-related neural activity for visual and somatosensory targets. Neuroimage. 2009;47:1767–77. doi: 10.1016/j.neuroimage.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Research. 2005;1065:115–24. doi: 10.1016/j.brainres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Prinz W. Movement observation affects movement execution in a simple response task. Acta Psychologica. 2001;106:3–22. doi: 10.1016/s0001-6918(00)00024-x. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, von Cramon DY. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005;43:89–98. doi: 10.1016/j.neuropsychologia.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Brochier T, Spinks RL, Umilta MA, Lemon RN. Patterns of muscle activity underlying object-specific grasp by the macaque monkey. Journal of Neurophysiology. 2004;92:1770–82. doi: 10.1152/jn.00976.2003. [DOI] [PubMed] [Google Scholar]
- Buccino G, Sato M, Cattaneo L, Roda F, Riggio L. Broken affordances, broken objects: a TMS study. Neuropsychologia. 2009;47:3074–8. doi: 10.1016/j.neuropsychologia.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Current Biology. 2007;17:1527–31. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Catmur C, Mars RB, Rushworth MF, Heyes C. Making mirrors: premotor cortex stimulation enhances mirror and counter-mirror motor facilitation. Journal of Cognition Neuroscience. 2011;23:2352–62. doi: 10.1162/jocn.2010.21590. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Barchiesi G, Tabarelli D, Arfeller C, Sato M, Glenberg AM. One's motor performance predictably modulates the understanding of others’ actions through adaptation of premotor visuo-motor neurons. Social Cognitive and Affective Neuroscience. 2011;6:301–10. doi: 10.1093/scan/nsq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. Representation of goal and movements without overt motor behavior in the human motor cortex: a transcranial magnetic stimulation study. Journal of Neuroscience. 2009;29:11134–8. doi: 10.1523/JNEUROSCI.2605-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Rizzolatti G. The mirror neuron system. Archives of Neurology. 2009;66:557–60. doi: 10.1001/archneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Z’Graggen WJ, Kohl AS, Rosler KM, Kaelin-Lang A. Impact of coil position and electrophysiological monitoring on determination of motor thresholds to transcranial magnetic stimulation. Clinical Neurophysiology. 2004;115:812–9. doi: 10.1016/j.clinph.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Manera V, Keysers C. Programmed to learn? The ontogeny of mirror neurons. Developmental Science. 2009;12:350–63. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Piazza A, Oliveri M, Bisiach E. Timing of right parietal and frontal cortex activity in visuo-spatial perception: a TMS study in normal individuals. Neuroreport. 2001;12:2605–7. doi: 10.1097/00001756-200108080-00062. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. The European Journal of Neuroscience. 2004;20:2193–202. doi: 10.1111/j.1460-9568.2004.03655.x. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Wang R, Nakatani-Enomoto S, et al. Comparison of different methods for estimating motor threshold with transcranial magnetic stimulation. Clinical Neurophysiology. 2007;118:2120–2. doi: 10.1016/j.clinph.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Heyes C. Where do mirror neurons come from? Neuroscience and Biobehavioral Reviews. 2010;34:575–83. doi: 10.1016/j.neubiorev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Ingham D, Tucker KJ, Tsao H, Hodges PW. The effect of pain on training-induced plasticity of the corticomotor system. European Journal of Pain. 2011;15:1028–34. doi: 10.1016/j.ejpain.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285:2136–9. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Direction of action is represented in the ventral premotor cortex. Nature Neuroscience. 2001;4:1020–5. doi: 10.1038/nn726. [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends in Cognitive Science. 2004;8:501–7. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Koch G, Versace V, Bonni S, Lupo F, Gerfo EL, Oliveri M, Caltagirone C. Resonance of cortico-cortical connections of the motor system with the observation of goal directed grasping movements. Neuropsychologia. 2010;48:3513–20. doi: 10.1016/j.neuropsychologia.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Tremblay S, Theoret H. Early non-specific modulation of corticospinal excitability during action observation. European Journal of Neuroscience. 2010;31:931–7. doi: 10.1111/j.1460-9568.2010.07121.x. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nature Reviews Neuroscience. 2009;10:141–52. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- Naranjo JR, Brovelli A, Longo R, Budai R, Kristeva R, Battaglini PP. EEG dynamics of the frontoparietal network during reaching preparation in humans. Neuroimage. 2007;34:1673–82. doi: 10.1016/j.neuroimage.2006.07.049. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:913–8. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu G, Voss M, Brochier T, Cattaneo L, Haggard P, Lemon R. Excitability of human motor cortex inputs prior to grasp. Journal of Physiology. 2007;581:189–201. doi: 10.1113/jphysiol.2006.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Forstmann BU, Wylie SA, Burle B, Van den Wildenberg WPM. Neurocognitive mechanisms of action control: resisting the call of the Sirens. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;2:174–92. doi: 10.1002/wcs.99. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Experimental Brain Research. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalography & Clinical Neurophysiology. 1998;106:283–96. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Experimental Brain Research. 2003;153:146–57. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Buccino G, Gentilucci M, Cattaneo L. On the tip of the tongue: modulation of the primary motor cortex during audiovisual speech perception. Speech Communication. 2010;5:2533–41. [Google Scholar]
- Sato M, Cattaneo L, Rizzolatti G, Gallese V. Numbers within our hands: modulation of corticospinal excitability of hand muscles during numerical judgment. Journal of Cognitive Neuroscience. 2007;19:684–93. doi: 10.1162/jocn.2007.19.4.684. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cerebral Cortex. 2005;15:1736–41. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Stefan K, Classen J, Celnik P, Cohen LG. Concurrent action observation modulates practice-induced motor memory formation. European Journal of Neuroscience. 2008;27:730–8. doi: 10.1111/j.1460-9568.2008.06035.x. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, et al. Formation of a motor memory by action observation. Journal of Neuroscience. 2005;25:9339–46. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Maieron M, Avenanti A, Tidoni E, Fabbro F, Aglioti SM. Simulating the future of actions in the human corticospinal system. Cerebral Cortex. 2010;20:2511–21. doi: 10.1093/cercor/bhp292. [DOI] [PubMed] [Google Scholar]
- van Schie HT, Koelewijn T, Jensen O, Oostenveld R, Maris E, Bekkering H. Evidence for fast, low-level motor resonance to action observation: an MEG study. Social Neuroscience. 2008;3:213–28. doi: 10.1080/17470910701414364. [DOI] [PubMed] [Google Scholar]
- Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. Journal of Neurophysiology. 2004;92:523–35. doi: 10.1152/jn.01265.2003. [DOI] [PubMed] [Google Scholar]
- World Medical Association General Assembly. Declaration of Helsinki. Ethical principles for medical research involving human subjects. World Medical Journal. 2008;54:122–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.