Abstract
Individuals with schizophrenia are more prone to violent behaviors than the general population. It is increasingly recognized that processing of emotionally valenced stimuli is impaired in schizophrenia, a deficit that may play a role in aggressive behavior. Our goal was to establish whether patients with a history of violence would show more severe deficits in processing emotionally valenced inputs than non-violent patients. Using event-related potentials, we measured how early during processing of emotional valence, evidence of aberrant function was observed. A total of 42 schizophrenia patients (21 with history of violence; 21 without) and 28 healthy controls were tested. Participants performed an inhibitory control task, making speeded responses to pictorial stimuli. Pictures occasionally repeated twice and participants withheld responses to these repeats. Valenced pictures from the International Affective Picture System were presented. Results in controls showed modulations during the earliest phases of sensory processing (<100 ms) for negatively valenced pictures. A cascade of modulations ensued, involving sensory and perceptual processing stages. In contrast, neither schizophrenia group showed early differentiation. Non-violent patients showed earliest modulations beginning ∼150 ms. For violent patients, however, earliest modulations were further delayed and highly attenuated. The current study reveals sensory–perceptual processing dysfunction for negatively valenced inputs, which is particularly pronounced in aggressive patients.
Keywords: Schizophrenia, violence, amygdala, emotion, event-related potential
INTRODUCTION
Violent behavior in patients with schizophrenia, while relatively uncommon, has prevalence rates ranging from 15.3% to 19.1% (Swanson et al., 2004, 2006). The presence of these behaviors is a significant public health issue in that they often result in prolonged hospitalization, can greatly interfere with reintegration into the community and are associated with higher rates of criminal conviction (Hodgins et al., 2005). Understanding the underlying neurobiology that predisposes a subset of patients to violent behaviors is therefore of primary importance. Multiple risk factors of a clinical, interpersonal and social–environmental nature have been identified (Swanson et al., 2004, 2006; Fazel et al., 2009a, b). It should be noted, however, that patients with schizophrenia show significant deficits in basic affective processing (Kayton and Koh, 1975), and that such deficits have been identified as an important precursor to violence in the general population (Davidson et al., 2000). We focus here on disturbances in the processing of affectively valenced inputs in patients with schizophrenia and their potential relationship to violent behaviors in this population.
Disturbances in the processing of affective information in patients with schizophrenia include impaired recognition of facial affect (Morris et al., 2009; Leitman et al., 2010), and deficits in their abilities to interpret the emotional prosodic content of speech (Ross et al., 2001; Leitman et al., 2005, 2007). One consistent finding is a negativity bias, whereby patients show a strong inclination to misidentify neutral stimuli as negatively valenced (Edwards et al., 2001; Kohler et al., 2003; Premkumar et al., 2008; Tsoi et al., 2008). Such inabilities to accurately identify emotional valence, accompanied by a bias to interpret situations in a negative light, could heighten the risk of violence in schizophrenia. To our knowledge, possible links between affective deficits and violent behaviors has yet to be investigated in schizophrenia. Using high-density neurophysiological recordings, we tested how early during the processing of emotionally valenced stimuli, evidence of aberrant processing would be observed in patients relative to healthy controls, and in turn, whether more severe deficits would be evident in patients with a history of violent behaviors.
Event-related potentials (ERPs) provide temporally precise measures of information processing well suited to probe for modulation in response to affectively valenced inputs, allowing for the dissociation of early sensory–perceptual from later higher order cognitive processing stages. Studies in healthy populations have reported modulation of very early (60–90 ms) and later (500–800 ms) ERP components, pointing to valence sensitivity during both processing stages. Direct recordings from the amygdala in humans have shown fear-specific modulations as early as 140 ms after stimulation (Krolak-Salmon et al., 2004; Pourtois et al., 2010). Scalp-recorded valence-related ERP modulations have been reported at just 90 ms (Pourtois et al., 2004). In a clever experiment, pairings of facial stimuli were presented, one to each hemifield, with neutral–fearful and neutral–happy pairings used to test whether the affectively valenced stimuli initiated an automatic shift of spatial attention to that side, which would then facilitate processing of the following target stimulus at that same location. Enhanced visual-evoked potentials (VEPs) for targets presented at locations cued by fearful faces was indeed found, indicating rapid automatic orientation of attention toward fear stimuli. Most important, the fearful face-cue itself evoked enhanced amplitude at 90 ms (during the C1 component of the VEP). Eldar et al. (2010), using a similar design, also reported modulation of the C1 component to threat faces in anxious relative to non-anxious participants. Source analyses have localized C1 generators to early retinotopic regions, especially V1 (Clark and Hillyard, 1996; Foxe and Simpson, 2002; Kelly et al., 2008), suggesting that this enhanced processing of negatively valenced stimuli occurs during the initial phase of activity in primary visual cortex. It is highly unlikely that extraction of affective information is achieved in striate cortex; the more parsimonious explanation is that affective modulations of visual processing are driven by coincident processing within limbic structures such as the amygdala, as theorized by LeDoux (e.g. Quirk et al., 1997). LeDoux’s seminal work on fear conditioning proposed the existence of dual pathways for processing of threat-related stimuli: a ‘fast’ subcortical pathway rapidly transmitting low-resolution features of the stimulus to limbic structures, and a ‘slower’ cortical pathway providing more detailed sensory–perceptual processing. Animal studies have identified direct thalamo-amygdala transmission with extremely fast amygdalar response latencies (∼20 ms) (Quirk et al., 1997; LeDoux, 2000). In turn, anatomical studies have shown direct projections from the amygdala to V1 (Amaral et al., 2003). Based on this evidence, striate cortex modulation seen in humans to valenced inputs most likely results from modulatory inputs provided via the faster thalamo-amygdalar circuit.
There is reason to predict deficits in this circuit in schizophrenia. Neuroimaging studies show altered recruitment of limbic structures during emotional paradigms (Gur et al., 2007; Hall et al., 2008; Rauch et al., 2010; Satterthwaite et al., 2010). Also, electrophysiological evidence indicates less differentiation of emotional stimuli (90–300 ms) in schizophrenia (Rockstroh et al., 2006). There has been little effort to probe the neurobiology underlying violent behaviors in schizophrenia, although neuroimaging studies have reported aberrant responses in limbic and prefrontal areas (Dolan and Fullam, 2009). For example, reduced functional connectivity between amygdala and ventral prefrontal regions has been shown, with lower connectivity associated with higher levels of self-rated aggression (Hoptman et al., 2010). To date, there are no electrophysiological studies to our knowledge that test for a possible role of affective processing deficits in the expression of violent behaviors in schizophrenia. Here, we recorded high-density ERPs to probe the spatio-temporal dynamics of cortical emotional processing in violent and non-violent patients. In line with previous work, we expected to find emotional processing deficits in schizophrenia (Rockstroh et al., 2006). Our primary goal, however, was to assess whether these deficits would be more severe in violent relative to non-violent patients, and to establish when during neural processing such differences might arise.
MATERIALS AND METHODS
Participants
A total of 21 patients with a history of violence (VS), 21 without a history of violence (NVS) and 28 healthy controls (HCs) participated. All participants (aged 18–60 years) had no significant medical or neurological illnesses. We excluded participants with any drug or alcohol abuse in the last 6 months or any lifetime history of drug or alcohol dependence. Controls were administered the Diagnostic and Statistical Manual—non-patient version to confirm absence of any psychiatric disorder. Patients recruited from the Rockland Psychiatric Center (inpatient and outpatient units) received a SCID diagnosis of schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). The Life History of Aggression (LHA; Coccaro et al., 1997) was used to assess aggression. It was completed on the basis of chart review, staff and patient interviews and official arrest records (‘Record of Arrest and Prosecution’). For inclusion as a violent participant, the patient had to have a confirmed episode of physical assault directed at another person within the past year, a score of ≥20 on the LHA scale, and a score of ≥3 on the ‘Physical Aggression Against People’ scale (PA-people). For inclusion as a non-violent participant, the patient could not present with an episode of physical aggression over the past year, or any lifetime episode of severe physical aggression, had to have a score of ≤15 on the LHA and a score ≤2 on the PA-people. Psychiatric symptom severity was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). To measure sustained visual attention, we administrated part A of the Trail Making Test (TMT) of the Halstead–Reitan Neuropsychological Test Battery (Reitan and Wolfson, 1993). Written consent was required from all participants according to a protocol approved by the institutional review board at NKI and compliant with the tenets of the Declaration of Helsinki.
Stimuli and task
We used 478 pictures from the International Affective Picture System (IAPS; Lang et al., 2008), a set of normative photographs that includes content across a wide range of semantic categories. The 148 negative pictures depicted attack scenes, mutilated bodies and disgusting objects (valence: 2.56; arousal: 5.6). The 158 neutral pictures depicted people, landscapes, abstract pattern and objects (valence: 5.2; arousal: 3.5). The 172 positive pictures depicted babies/toddlers, family gatherings and prestige objects (valence: 7.4; arousal: 4.8). Images were selected to ascertain that neutral, positive and negative images did not significantly differ in luminance, contrast and spatial frequency. For the spatial frequency of images, we used a discrete wavelet analysis approach previously developed by Delplanque and colleagues for the analysis of the IAPS battery (Delplanque et al., 2007). This approach allows for the analysis of both small (high spatial frequencies) and large details (low spatial frequencies) separately (Delplanque et al., 2007). A band-specific analysis is important, as it is known that low spatial frequencies are particularly relevant for the processing of emotional content (Carretie et al., 2007). Analysis of Variance (ANOVA) was used to test mean values of luminance, contrast and mean energy of spatial frequency bands. Emotionally neutral, positive and negative stimuli were randomly presented with a probability of 0.45, 0.275 and 0.275, respectively. Images were presented centrally every 1000 ms for 800 ms with an interstimulus interval of 200 ms. Images subtended 8.6° horizontally by 6.5° vertically. The emotional valence of the images was incidental to task performance. To ensure sustained attention to the IAPS stimuli, participants performed a go/nogo task, responding quickly and accurately to every presentation, while withholding responses to the second instance of any stimulus repeated twice in a row. The probability of go and nogo trials was 0.85 and 0.15, respectively.
Procedure
Participants were seated in a dimly lit, sound attenuated, electrically shielded room, 115 cm from a monitor. Central fixation was required throughout each block (180 trials). Participants completed one mandatory practice block before the main experiment began. If needed, additional practice blocks were allowed. Fourteen experimental blocks were run, each lasting 3.5 min. Participants took mandatory breaks between blocks to prevent fatigue.
ERP recordings and analysis
ERPs were acquired from a 72-channel montage at a digitization rate of 512 Hz with a pass-band of 0.05–100 Hz using the BioSemi Amplifier System. Data were referenced to the nasion electrode-site. Epochs of 900 ms, including a 100 ms prestimulus baseline, were analyzed. Trials with eye movements and blinks were rejected based on vertical and horizontal EOG records. An automatic artifact rejection criterion of ±70 µV was used at all other scalp sites. All analyses were conducted on individual subject averages that were not digitally filtered but group data were subsequently low-pass filtered at 45 Hz for purpose of illustration.
Primary analysis strategy
Guided by previous work identifying ERP components modulated by valenced input (Olofsson et al., 2008), data were explored for the following components of interest: P1, N1, the Early Sustained Positivity (ESP) and the Late Sustained Positivity (LSP). Additionally, in light of previous recordings demonstrating very early valence-related modulation of visual processing (Pourtois et al., 2004), we also explored potential effects during the earliest stages of visual processing. In this regard, a distinct positive component peaking at 35 ms was identified in grand averaged waveforms (hereafter referred to as P35), and was therefore submitted to statistical testing. These five components were defined by examining the group-averaged data, collapsed across conditions and groups (i.e. independent of any possible between-groups or between-conditions effects). Amplitudes for P35, P1, N1, ESP and LSP were measured by defining time windows centered on the latency of peak amplitude. For the P35, peak latency was 35 ms and a time window of ±5 ms was chosen based on the fast frequency of this component. For P1, peak latency was 105 ms, and again a time window of ±5 ms was used. For N1, peak latency was 185 ms; time window was set as ±15 ms. For ESP and LSP, time-windows were set as 300–400 ms and 600–800 ms, respectively. Scalp regions-of-interest (ROI) were computed by averaging across frontal (FP1/FPz/FP2/AF7/AF3/AFz/AF4/AF8/F7/F5/F3/F1/Fz/F2/F4/F6/F8), central (FC5/FC3/FC1/FCz/FC2/FC4/FC6/C5/C3/C1/Cz/C2/C4/C6), parietal (CP5/CP3/CP1/CPz/CP2/CP4/CP6/P5/P3/P1/Pz/P2/P4/P6), temporal (FT8/FT8/T7/T8/TP7/TP8) and occipital (PO7/PO3/POz/PO4/PO8/O1/Oz/O2) sites. Affect-related modulations for each of the aforementioned five components were tested by computing the difference wave for negatively valenced stimuli (ERPnegative − ERPneutral) and positively valenced stimuli (ERPpositive − ERPneutral) and testing them in two separate sets of analyses. In particular, the principal statistical analysis investigated the valence effect in each group and compared the valence effects across the three study groups. Random regression hierarchical linear modeling (HLM; Bryk et al., 1992; Gibbons et al., 1998) was the primary statistical approach; this method (in contrast to the traditional ANCOVA analysis) makes allowance for heterogeneity among treatment groups and takes into account the time-dependent correlation structure of the observations (sampling points). In the HLM model, repeated assessments of the difference wave within each specified time window served as the dependent variable. The two independent variables were ‘treatment group’ and ‘time’ (sampling point relative to stimulus onset). Treatment group served as the between-subject factor, and Time (ms) as the within-subject, random effect factor. Age, education and gender served as fixed-effect covariates and were used for adjustment for potential confounding. Interaction between treatment group and time was included in the model. A first-order autoregressive moving average correlation matrix allowing for heterogeneity among treatment groups was specified in the HLM model. Test of the least squares mean (LSM) effect indicated whether there was a statistically significant valence effect in a given group. In order to compare the valence effect between groups, we formulated three pairwise contrasts (HC vs NVS, HC vs VS and NVS vs VS) in the HLM model. Analyses were repeated for each of the five ROIs. The Hochberg procedure was used to adjust for multiple testing of the valence effect within-group. For pairwise group comparisons, the Tukey–Kramer adjustment was used to correct for the three pairwise contrasts of valence effect. To characterize statistical effect sizes for the valence effect across components, we computed Cohen’s d (Cohen, 1988) based on the above mentioned time windows at each of the five ROIs. In particular, Cohen’s d was defined as the LSM estimate for the difference waveform (negative–neutral or, alternatively, positive–neutral) divided by the pooled within-group s.d. estimate derived from the primary HLM model. Thus, the closer the Cohen’s d approaches zero, the smaller the valence effect. We consider absolute values of Cohen’s d between 0.20 and 0.39 as small, between 0.40 and 0.69 as medium, and from 0.70 as large effect sizes. For behavioral results, we analyzed accuracy and reaction time (RTs) for ‘go’ trials using ANOVA with group as between-subject and stimulus valence as within-subject factors.
Source localization
Inverse source modeling using brain electric source analysis (BESA 5.1.8, MEGIS Software, Gräfelfing, Germany; Scherg and Von Cramon, 1991) was employed to localize neural generators of valence sensitive ERP components. Source modeling is based on the premise that an observable ERP deflection is related to a change in local activity within a circumscribed brain region (Scherg and Picton, 1985). Our main purpose in performing source analysis was to estimate sources for early VEP components that showed valence sensitive modulations, in particular the P35. We reasoned that if generators of the P35 modulation were localized to the amygdala, this would align with converging evidence suggesting a role for the amygdala in enhancing early perceptual processing. We modeled sources only in HC participants, since significant P35 modulation was only seen in this group. We refrained from source localizing late components (ESP and LSP) as these components likely reflect post-perceptual processing across widely distributed cortical networks. BESA employs a least squares fitting algorithm, defining location and orientation of dipoles for which the maximal amount of variance is explained (Scherg and Picton, 1991; Simpson et al., 1995). We used a data-driven stepwise approach, with each segment of the epoch that encompassed an ERP deflection successively fitted with a pair of symmetric sources. For the purpose of modeling, an idealized four-shell ellipsoidal head model with a radius of 90 mm and scalp and skull thicknesses of 6 and 7 mm, respectively, assumed.
Topographic maps
Scalp maps represent interpolated potential distributions, derived from 72-scalp measurements. Maps are displayed on the 3D reconstruction of a rendered scalp surface (derived from an anatomical MRI) as implemented in BESA.
Statistical cluster plots
An exploratory analysis was also performed to fully explore the richness of these high-density data, serving mainly as a hypothesis generation tool for future research. This represents a method for testing the entire data matrix for putative effects (Murray et al., 2001; Molholm et al., 2002) and involves the derivation of cluster plots by calculating pointwise, paired, two-tailed t-tests between the ERP responses to a given pair of experimental conditions. This method provides a metric for both estimating the onset of differential responses between conditions and for assessing significant conditional effects across the entire epoch. Periods of significant difference are only plotted if an α criterion of 0.05 is exceeded and then only if this criterion is exceeded for at least 11 consecutive data points (∼20 ms). The temporal cluster criterion is a means of obviating the false-positive rates inherent in the multiple comparisons made using this method, and makes allowance for possible temporal auto-correlation (Guthrie and Buchwald, 1991; Foxe and Simpson, 2002).
RESULTS
Demographic and clinical characteristics
Table 1 presents demographic, neuropsychological and clinical characteristics of the groups. There was a significant difference in education, inpatient vs outpatient status and a marginal difference in age; hence these variables were introduced as covariates. In order to characterize further the groups and in particular the two schizophrenic groups with regard to measures of attention, we investigated attention in two different ways. We looked at the PANSS Poor Attention item, as a clinical measure of attention. There were no differences between the VS and NVS on this measure. We also used a measure of sustained visual attention, Part A of the TMT. There was a significant difference among the three groups (F2,67 = 22.7, P < 0.001). Pairwise comparisons showed a significant difference between the HC and the VS (P = 0.001) and between the HC and NVS (P < 0.001). There was also a significant differences between the two schizophrenic groups with the VS showing a better performance on that test—as indicated by a faster response (P < 0.001). There were no differences between the two patient groups on the PANSS Total score, antipsychotic medications (typical vs atypical), antipsychotic dosage (chlorpromazine equivalents), or other psychotropic agents used.
Table 1.
Demographic, neuropsychological and clinical characteristics
| HC (n = 28) | NVS (n = 21) | VS (n = 21) | f/chi valuea | P-value | |
|---|---|---|---|---|---|
| Gender: male/female | 22/6 | 17/4 | 17/4 | 0.06 | 0.97 |
| Ethnicity AA/non-AA | 12/16 | 8/13 | 5/16 | 1.97 | 0.37 |
| Inpatient (in/outpatient) | – | 10/11 | 16/5 | 27.7 | 0.0001*** |
| Age (SD) | 39.7 (10.0) | 41.0 (8.6) | 35.5 (11.0) | 1.82 | 0.17 |
| Education in years (s.d.) | 14.7 (1.7) | 13.1 (1.9) | 11.7 (2.1) | 14.7 | 0.0001*** |
| PANSS | – | 75.6 (16.6) | 78.9 (15.8) | 0.39 | 0.54 |
| PANSS poor attention (s.d.) | – | 1.9 (1.3) | 2.2 (1.1) | 0.73 | 0.4 |
| Trail making test Part A, LS mean (s.d.) | 28 (3.8) | 66.4 (4.3) | 46.3 (3.9) | 22.7 | 0.001** |
| Medication dose (CPZ equivalents) | – | 1094.8 (737.6) | 1165.5 (751.0) | 0.09 | 0.76 |
aANOVA for continuous and chi-square test for categorical variables.
*P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
AA = African American; s.d. = standard deviation; CPZ = chlorpromazine.
Behavioral results
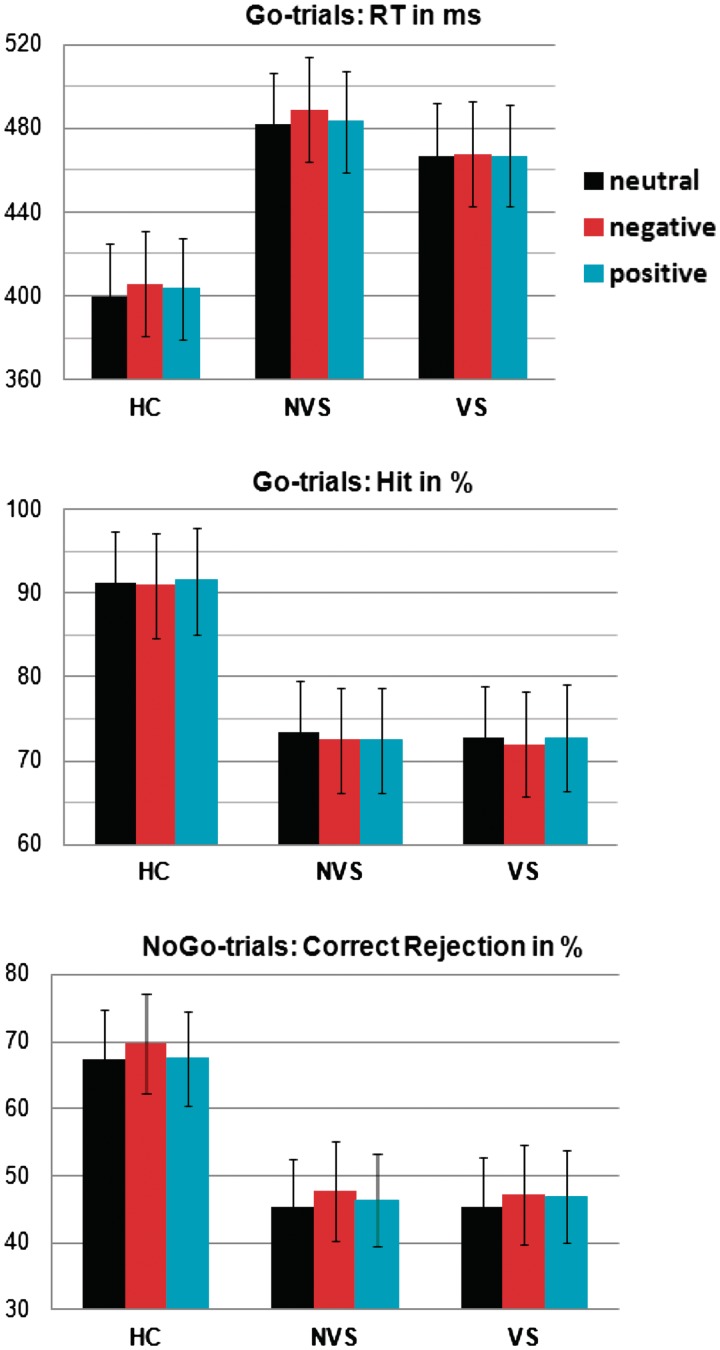
Figure 1 shows RT, hit and correct rejection rates in HC, NVS and VS. The ANOVA for RT revealed a Group effect (F2,67 = 11.74, P < 0.0001) and a Valence effect (F2,134 = 5.32, P = 0.006). For hit rates, a Group effect was found (F2,67 = 16.38, P < 0.0001). A Group effect was also found for correct rejection rates (F2,67 = 12.9, P < 0.0001). Protected analyses revealed increased RT and reduced accuracy in NVS and VS relative to HC (P < 0.002), and no differences between NVS and VS (P > 0.27). Analyses comparing RT across levels of Valence revealed increased RT for negative vs neutral stimuli in HC and NVS, but not in VS (HC: t27 = −4.2, P < 0.0001; NVS: t20 = −2.25, P = 0.035; VS: P = 0.81).
Fig. 1.
Mean RT, hit and correct rejection rates in HC, NVS and VS. Both groups of patients with schizophrenia showed increased RT and reduced accuracy. HC and NVS also showed increased RT for negative compared to neutral valenced stimuli.
Electrophysiological results
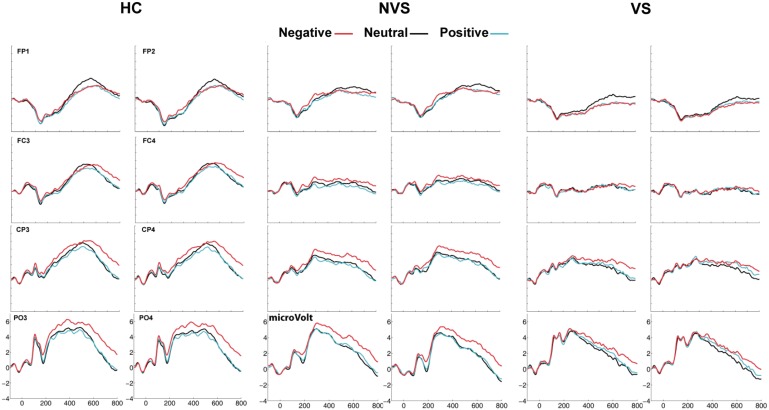
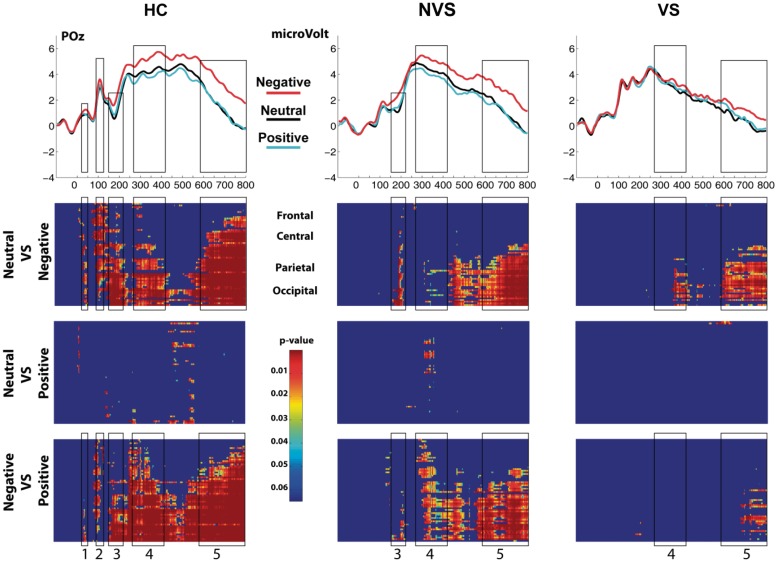
Figure 2 shows representative electrodes over fronto-central, centro-parietal and parieto-occipital scalp. Stimulus valence is marked by color. Enhanced activation evoked by negative stimuli (red trace) was strongest over parieto-occipital scalp regions. The top row of Figure 3 shows the ERP at POz. The black rectangles capture the five ERP components for which HC revealed robust affect-related modulations. The bottom three rows of Figure 3 show statistical cluster plots, which revealed the topography and onset of the differential activations. Table 2 summarizes the statistical results testing differential activation evoked by negative stimuli (ERPnegative − ERPneutral) for each of the ERP components. The differential activation evoked by positive stimuli (ERPpositive − ERPneutral) yielded very few statistically significant effects and are therefore not reported.
Fig. 2.
Overview of ERPs in HC, NVS and VS at representative frontal, central and parieto-occipital electrode sites for neutral, negative and positive valenced stimuli. HC showed early differential ERP responses to negative valenced stimuli, which were delayed and attenuated in NVS and VS.
Fig. 3.
ERPs at electrode side POz and statistical cluster plots capturing the five main timeframes of differential ERP responses to affective stimuli (1: 20–50 ms; 2: 90–135 ms; 3: 150–225 ms; 4: 260–425 ms; 5: 580–800 ms). Color values indicate the result of point-wise t-tests evaluating the Neutral vs Negative trials, Neutral vs Positive trials and Negative vs Positive trials across a 900-ms time epoch (x-axis) and electrode positions (y-axis) for the entire 64-electrode montage (see ‘Materials and Methods’ section for details of electrode locations). For clarity, only P < 0.05 is color coded and only then when 11 consecutive data points exceeded this criterion.
Table 2.
Differential activation evoked by negative vs neutral emotional pictures (ERPnegative − ERPneutral) for the three different groups
| LSMs for valence effect (negative–neutral difference)a in each group: P-values (Cohen d) |
Group difference LSM for valence effect: P-values |
|||||
|---|---|---|---|---|---|---|
| Scalp region | HC (n = 28) | NVS (n = 21) | VS (n = 21) | HC-NVS | HC-VS | NVS-VS |
| P35 | ||||||
| Front | 0.17 (0.31) | 0.55 (−0.14) | 0.26 (0.31) | 0.17 | 0.97 | 0.21 |
| Cent | 0.016* (0.51) | 0.32 (−0.21) | 0.47 (0.17) | 0.02* | 0.33 | 0.23 |
| Pari | 0.007* (0.55) | 0.32 (−0.21) | 0.46 (0.17) | 0.01* | 0.25 | 0.23 |
| Temp | 0.006* (0.64) | 0.12 (−0.37) | 0.97 (0.01) | 0.003* | 0.11 | 0.28 |
| Occi | 0.004* (0.56) | 0.76 (−0.06) | 0.67 (0.09) | 0.02b | 0.13 | 0.61 |
| P100 | ||||||
| Front | <0.0001*** (0.96) | 0.27 (0.27) | 0.61 (0.13) | 0.04b | 0.005* | 0.26 |
| Cent | <0.0001*** (1.06) | 0.12 (0.34) | 0.99 (0.0001) | 0.02* | 0.002* | 0.29 |
| Pari | <0.001** (0.92) | 0.14 (0.3) | 0.41 (0.18) | 0.02b | 0.02* | 0.69 |
| Temp | <0.0001** (1.01) | 0.33 (0.21) | 0.64 (0.11) | 0.01* | 0.01* | 0.76 |
| Occi | <0.0002** (0.69) | 0.07 (0.34) | 0.45 (0.15) | 0.18 | 0.06 | 0.49 |
| N100 | ||||||
| Front | 0.005* (0.45) | 0.0004** (0.59) | 0.87 (−0.03) | 0.45 | 0.07 | 0.01* |
| Cent | 0.0002** (0.54) | 0.0001*** (0.65) | 0.89 (0.04) | 0.53 | 0.02* | 0.004* |
| Pari | 0.0001*** (0.63) | 0.0001*** (0.71) | 0.36 (0.14) | 0.64 | 0.02* | 0.006* |
| Temp | 0.0001*** (0.81) | 0.0001*** (0.74) | 0.31 (0.19) | 0.99 | 0.01* | 0.01* |
| Occi | 0.0001*** (0.74) | 0.0001*** (0.77) | 0.22 (0.15) | 0.86 | 0.006* | 0.002* |
| ESP | ||||||
| Front | 0.18 (0.11) | 0.15 (0.13) | 0.61 (0.05) | 0.89 | 0.66 | 0.56 |
| Cent | 0.01* (0.21) | 0.008* (0.24) | 0.03* (0.21) | 0.79 | 0.96 | 0.77 |
| Pari | 0.0001*** (0.34) | 0.0002** (0.35) | 0.002** (0.31) | 0.91 | 0.83 | 0.74 |
| Temp | 0.002* (0.26) | 0.0004** (0.32) | 0.001** (0.32) | 0.57 | 0.65 | 0.95 |
| Occi | 0.0001*** (0.43) | 0.0001*** (0.37) | 0.006** (0.27) | 0.61 | 0.24 | 0.45 |
| LSP | ||||||
| Front | 0.03* (0.15) | 0.24 (−0.08) | 0.41 (−0.06) | 0.02b | 0.04b | 0.98 |
| Cent | 0.0001*** (0.41) | 0.009* (0.18) | 0.01* (0.18) | 0.02b | 0.06 | 0.77 |
| Pari | 0.0001*** (0.53) | 0.0001*** (0.42) | 0.0001*** (0.34) | 0.25 | 0.08 | 0.45 |
| Temp | 0.0001*** (0.35) | 0.0001*** (0.27) | 0.008** (0.21) | 0.39 | 0.13 | 0.43 |
| Occi | 0.0001*** (0.51) | 0.0001*** (0.51) | 0.0006** (0.27) | 0.95 | 0.03b | 0.02b |
aHLM.
bEffect becomes marginally significant after adjustment for multiple comparisons.
*P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
P35
The LSM analysis (Table 2) indicated that a significant differential response to negative stimuli was only evident in HC (P ≤ 0.01), with a medium effect size of ≥0.51. Both groups, NVS and VS failed to show valence modulations for the P35. The analyses suggested a P35 deficit for negative stimuli in NVS and VS.
P100
The LSM analysis indicated a significant differential response only in HC (P ≤ 0.001), with a medium effect size of ≥0.69. Both groups, NVS and VS failed to show valence modulations for the P100. The analyses suggested a P100 deficit for negative stimuli in NVS and VS.
N100
The LSM analysis indicated a highly significant differential response to negative stimuli in HC and NVS (HC: P ≤ 0.005; NVS: P ≤ 0.0004), but not in VS (P ≥ 0.22). Pairwise LSM comparisons revealed difference between HC and VS (P ≤ 0.02) and between NVS and VS (P ≤ 0.01). The analyses suggested a N100 deficit for negative stimuli present only in VS.
Early sustained positivity
The LSM analysis indicated a significant differential response to negative stimuli for all groups (HC: P ≤ 0.01; NVS: P ≤ 0.008; VS: P ≤ 0.03). The pairwise LSM comparison yielded no significant differences between groups, suggesting a comparable ESP response to negatively valenced stimuli across groups.
Late sustained positivity
The LSM analysis indicated a significant differential response to negative stimuli for all groups (HC: P ≤ 0.03; NVS: P ≤ 0.009; VS: P ≤ 0.01). The pairwise LSM comparison yielded significant results between all three groups. However, these effects proved not significant after correcting for multiple comparisons. The analyses suggested a largely comparable LSP response to negatively valenced stimuli across groups.
Statistical cluster plot
The bottom part of Figure 3 shows the cluster plots, which test for ERP differences between Negative vs Neutral stimuli (top), Positive vs Neutral stimuli (middle) and Negative vs Positive stimuli (bottom) across all electrodes (y-axis) and sample-points (x-axis). The analysis supports and extends the results discussed above; showing five robust clusters of differential ERP responses for negative stimuli in HC. Two early cluster of activation indexed with 1 and 2 in HC (see Figure 3 bottom, left panel) are essentially absent in both patient groups. Furthermore, NVS showed a robust cluster of activation for the Neutral vs Negative comparison indexed with 3. This cluster was largely absent in VS. In general, emerging modulations in patient groups were strongly attenuated, particularly in VS.
Topographic and source localization analysis
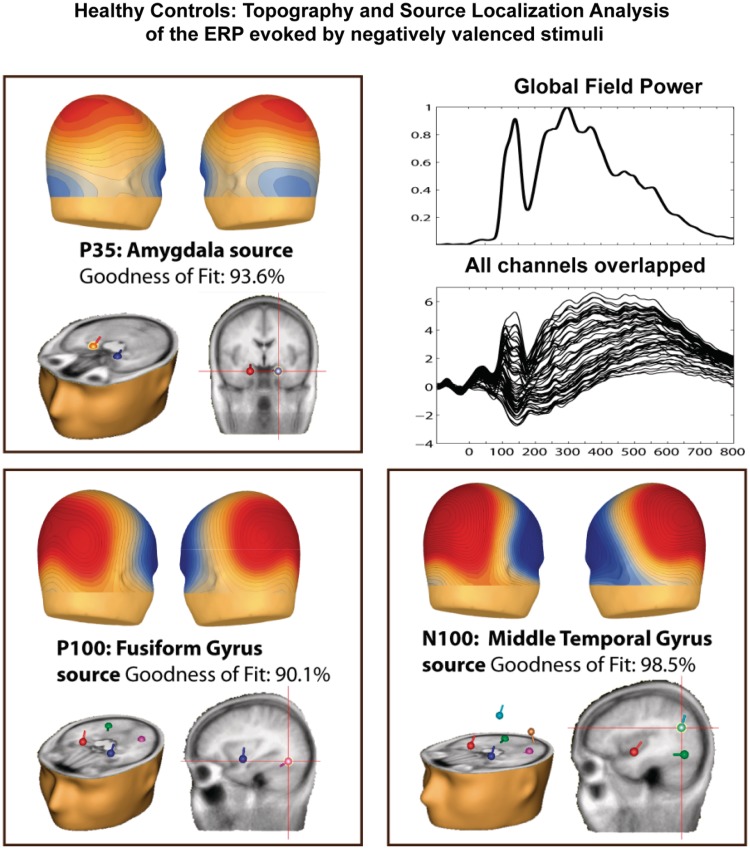
Figure 4 shows scalp voltage maps and estimated dipole source locations for the P35, P100 and N100 component in HC. The P35 topography was characterized by a broad centro-parietal distribution, which is consisted with a deep cortical source projecting widely across scalp regions. Indeed, source modeling of the P35 produced dipoles situated bilaterally in the amygdala. The model produced a goodness-of-fit (GOF) value of 93.6%. The P100 topography was characterized by the typical bilateral parieto-occipital distribution. Source modeling of the P100 produced dipoles situated bilaterally in the fusiform gyri, with a GOF of 90.1%. The N100 topography was also characterized by a parieto-occipital focus, with a broader and more anterior distribution compared to the P100. Source modeling of the N100 produced dipoles situated bilaterally in the middle temporal gyri, with a GOF of 98.5%. For our source analysis, we assumed bilateral symmetrical sources, as all components were largely symmetrically distributed across hemispheres.
Fig. 4.
Scalp voltage maps, and estimated dipole source locations of activity during the first three time frames (1:20–50 ms; 2:90–135 ms; 3:150–225 ms) for which affect-related modulations in HC were found.
DISCUSSION
We found abnormal processing of negatively valenced affective stimuli in patients with schizophrenia, both in those with and without a history of violence. In contrast, positive affective stimuli exerted only minimal ERP effects in all three groups. In line with our hypothesis, the most pronounced impairments were observed in the violent patients. High-density ERP data revealed a remarkable delay of the initial differential ERP response to affective stimuli in both patient groups. The initial affect-related ERP modulation was observed at just 35 ms in HC, with a second robust effect evident during the P1 VEP component (100 ms). Neither of these effects reached significance in either schizophrenia group. Three additional dissociable phases of differential processing were evident (∼150–225 ms, ∼270–425 ms and ∼570 ms onwards). It was during these later phases of processing that patients first exhibited sensitivity to the valenced content of the pictorial stimuli, and it was then that clear differences between the patient groups became evident, with greater diminutions in the violent group. Interestingly, healthy control participants showed a characteristically slowed response to negatively valenced stimuli relative to neutral stimuli (e.g. Leppanen and Hietanen, 2004) and this same slowing pattern was also observed in patients without a history of violence. In contrast, this slowing was not observed in the cohort with a history of violence, paralleling the general lack of sensitivity to negative valence observed in their ERPs.
Comparing healthy controls and schizophrenia patients
A robust ERP modulation was observed at just 35 ms in HC. The earliest valence-related effects reported in the extant literature have typically been observed at slightly later latencies between 60 and 110 ms (Krolak-Salmon et al., 2004; Pourtois et al., 2004, 2010; Eldar et al., 2010). The prevailing interpretation for these early modulations is that they reflect enhanced perceptual processing of emotionally salient stimuli, and that this is likely driven by modulatory inputs to early visual cortex via direct projections from the amygdala.
The role of the amygdala in enhancing perceptual processing for stimuli with emotional content is well supported by neuroimaging (Vuilleumier et al., 2001), anatomical (Satterthwaite et al., 2010), neurophysiological (Rotshtein et al., 2010) and neuropsychological data (Anderson and Phelps, 2001). Of particular relevance is a study in epilepsy patients with varying degrees of amygdala pathology (Rotshtein et al., 2010). Scalp-recorded VEP modulations to fearful faces were diminished in these patients relative to a control group of patients with lesions outside the amygdala. In the current data, source localization procedures pointed to bilateral amygdalae as the major generators of the early 35 ms modulation. The absence of this early modulation in both patient groups might point to dysfunction of limbic structures (which propagates to primary and secondary visual cortex), leading to abnormal cortical ERP responses to emotional stimuli. The subsequent component, the P1, reflects early sensory processing in extrastriate visual cortex (Clark and Hillyard, 1996). It modulated strongly to valence stimuli in HC, but not at all in either patient group. Thus this stage of sensory processing of valenced stimuli is impaired in the patients with schizophrenia, whether they are violent or not. This valence-specific deficit in the P1 modulation is in line with the robust finding of a P1 amplitude reduction in patients with schizophrenia (Yeap et al., 2008). The following component, the N1, demonstrated sensitivity to valence in both the HC and NVS, but not in the VS. The N1 response has been associated with basic object-recognition processes within the lateral occipital complex (Murray et al., 2002; Foxe et al., 2005). Our data indicate dysfunction during this basic object-processing timeframe for emotional content only in VS. This is, to our knowledge, the first report of an electrophysiological probe that distinguishes patients with schizophrenia depending on their history of violence. We will discuss the implication of this aggression-related N1 finding for interpersonal and social skills of patients in greater detail below.
Previous neuroimaging work with schizophrenia patients has also implicated dysfunctions of the frontal–limbic circuit in emotional tasks (Holt et al., 2006; Gur et al., 2007; Hall et al., 2008; Rauch et al., 2010; Satterthwaite et al., 2010). The low temporal resolution of the hemodynamic signal, however, has precluded conclusions about the timing of such dysfunction, making it impossible to determine whether deficits in affective processing stem from dysfunction in early sensory–perceptual registration of emotional stimuli or, whether they result from impairment in higher order cognitive processes. Our findings point to the former as the root cause, and the likely antecedent to a cascade of further reductions in responsivity during later processing timeframes that reflect cognitive mechanisms.
It should be noted that this is the first study we are aware of to report affect-related modulation as early as 35 ms in HC. While source analysis suggests a good fit within amygdalae, consistent with current models of early amygdalar activation to emotionally valenced inputs (Quirk et al., 1997), these results are not fully consistent with the previous EEG/MEG literature where similarly early modulations were not observed (Krolak-Salmon et al., 2004; Pourtois et al., 2004, 2010; Olofsson et al., 2008; Eldar et al., 2010). A number of features of the current study may have bolstered statistical power to detect these relatively small effects. They include the considerably larger cohort of HC used (N = 28), and the fact that individual ERPs were derived from a substantially larger number of trials than is typical (273–619 per condition). Clearly though, this early valence-related processing modulation will bear replication and further exploration in future studies.
Comparing non-violent and violent schizophrenia patients
When the two groups of patients were compared, striking differences in their responsivity to valenced inputs were evident. While both groups showed delayed onset of response sensitivity to valenced inputs, this was particularly pronounced in VS. NVS showed emergence of a first robust modulation during the third phase of processing at ∼150 ms, but this modulation was virtually absent in VS. Instead, the first robust modulation in VS emerged at ∼260 ms during phase-4 processing. Even when modulations did emerge in VS, they were considerably attenuated relative to NVS. A possible explanation for the ERP differences between VS and NVS is that VS have a more severe deficit in their ability to sustain attention. However, an attention-based explanation would also predict a more pronounced performance deficit in VS, which is not supported by our behavioral data. That is, VS and NVS do not differ in their ability to inhibit/execute responses to nogo/go stimuli. The fact that VS and NVS do not differ in task accuracy, but VS are about 10 ms faster in their mean RT, is also not supportive of the attention-related explanation. We therefore believe that our findings support a more severe deficits in the processing of affectively valenced stimuli in VS. This is the first time, to our knowledge, that electrophysiological measures have linked processing impairments for affective inputs to violence in schizophrenia.
These deficits in emotional processing may lead to violence. Inability to process the emotional context of a given situation accurately and quickly may lead to incorrect appraisal of social cues. The prominent lack of differentiation between negative and neutral stimuli in the violent group may underlie a more severe bias to misidentify neutral cues as negatively valenced, which has been reported more generally in schizophrenia (Kohler et al., 2003; Premkumar et al., 2008; Tsoi et al., 2008). If a patient misinterprets or overinterprets neutral situations as threatening, he is more likely to react with violence.
Advantages/limitations
Whereas in our study, the interstimulus interval was relatively short, since the stimulus sequence was randomly presented, there is no reason to believe that an overlapping offset response would confound the evoked activity differently between negative, positive and neutral stimuli. In fact, there was no such differentiation during the entire baseline period in any of the three groups as a function of stimulus valence. Furthermore, the reported ERP differences cannot be explained in terms of differences in physical properties between images, as stimuli were selected such that no significant differences in luminance, contrast or spatial frequency occurred.
It should be noted that our study removes an important confound in the neurophysiological assessment of aggression in schizophrenia. Comorbid substance abuse is present in many patients with a history of violence (e.g. Swanson et al., 2006; Fazel et al., 2009a, b), but may in itself account for neurophysiological abnormalities reported in this population. Here, we carefully excluded patients with comorbid substance abuse in order to remove this confound. Future studies will be required to explore these more complex interactions between schizophrenia, substance abuse and violence.
CONCLUSION
Fast effective processing of affective content is essential to the navigation of our interpersonal and social environment. We found differences in the early emotional processing of stimuli in patients with schizophrenia. These deficits may be linked to the compromised interpersonal and social skills present in schizophrenia. Our finding of a more pronounced deficit in violent patients might be related to the etiology of the violence: the non-violent patients are able to use compensatory neural mechanisms for this problem, while the violent patients are not able to do so.
CONFLICT OF INTEREST
None declared.
Acknowledgments
Sincere thanks go to Eileen Zenz and Melissa Brady for help with recruitment, clinical interviewing and neuropsychological testing, Karen Nolan (PhD) and Constance Shope (PhD) for clinical interviewing and Emma Jane Forde, Ryan Bell, Kristen Morie for EEG data collection. Dr Krakowski takes full responsibility for the integrity of the data and attests that all authors had full access to all the data in this study. Primary support for this work was provided by an RO1 grant from the U.S. National Institute of Mental Health (MH74767). Additional support was derived from MH85322.
REFERENCES
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park: Sage Publications; 1992. [Google Scholar]
- Carretié L, Hinojosa JA, López-Martín S, Tapia M. An electrophysiological study on the interaction between emotional content and spatial frequency of visual stimuli. Neuropsychologia. 2007;45(6):1187–95. doi: 10.1016/j.neuropsychologia.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Research. 1997;73(3):147–57. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation-a possible prelude to violence. Science. 2000;289(5479):591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Delplanque S, N'diaye K, Scherer K, Grandjean D. Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. Journal of Neuroscience Methods. 2007;165(1):144–50. doi: 10.1016/j.jneumeth.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Fullam RS. Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent responses to emotional faces in violent patients with schizophrenia. Biological Psychiatry. 2009;66(6):570–7. doi: 10.1016/j.biopsych.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophrenia Research. 2001;48(2–3):235–53. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology. 2010;85(2):252–7. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Fazel S, Gulati G, Linsell L, Geddes JR, Grann M. Schizophrenia and violence: systematic review and meta-analysis. PLoS Medicine. 2009a;6(8):e1000120. doi: 10.1371/journal.pmed.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Langström N, Hjern A, Grann M, Lichtenstein P. Schizophrenia, substance abuse, and violent crime. Journal of the American Medical Association. 2009b;301(19):2016–23. doi: 10.1001/jama.2009.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining ‘early’ visual processing. Experimental Brain Research. 2002;142(1):139–50. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cerebral Cortex. 2005;15(12):1914–27. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Waternaux C, Davis JM. Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacology Bulletin. 1988;24(3):438–43. [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Archives of General Psychiatry. 2007;64(12):1356–66. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28(2):240–4. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW. Overactivation of fear systems to neutral faces in schizophrenia. Biological Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Hodgins S, Tiihonen J, Ross D. The consequences of conduct disorder for males who develop schizophrenia: associations with criminality, aggressive behavior, substance use, and psychiatric services. Schizophrenia Research. 2005;78(2–3):323–35. doi: 10.1016/j.schres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia Research. 2006;82(2–3):153–62. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophrenia Bulletin. 2010;36(5):1020–8. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kayton L, Koh SD. Hypohedonia in schizophrenia. Journal of Nervous & Mental Disease. 1975;161(6):412–20. doi: 10.1097/00005053-197512000-00005. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral Cortex. 2008;18(11):2629–36. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. American Journal of Psychiatry. 2003;160(10):1768–74. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Hénaff MA, Vighetto A, Bertrand O, Mauguière F. Early amygdala reaction to fear spreading in occipital, temporal, and frontal cortex: a depth electrode ERP study in human. Neuron. 2004;42(4):665–76. doi: 10.1016/s0896-6273(04)00264-8. [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Taylor PJ, et al. Neural and behavioural responses to threat in men with a history of serious violence and schizophrenia or antisocial personality disorder. Schizophrenia Research. 2009;110(1–3):47–58. doi: 10.1016/j.schres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- LeDoux J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon & Schuster; 1996. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biological Psychiatry. 2005;58(1):56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Hoptman MJ, Foxe JJ, et al. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. American Journal of Psychiatry. 2007;164(3):474–82. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Loughead J, et al. Ventrolateral prefrontal cortex and the effects of task demand context on facial affect appraisal in schizophrenia. Social Cognitive and Affective Neuroscience. 2010;6:66–73. doi: 10.1093/scan/nsq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Loughead J, et al. Ventrolateral prefrontal cortex and the effects of task demand context on facial affect appraisal in schizophrenia. Soc Cogn Affect Neurosci. 2011;6:66–73. doi: 10.1093/scan/nsq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Hietanen JK. Positive facial expressions are recognized faster than negative facial expressions, but why? Psychology Research. 2004;69(1–2):22–9. doi: 10.1007/s00426-003-0157-2. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Research Cognitive Brain Research. 2002;14(1):115–28. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Current Opinion in Psychiatry. 2009;22(2):140–6. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- Murray MM, Foxe JJ, Higgins BA, Javitt DC, Schroeder CE. Visuo-spatial neural response interactions in early cortical processing during a simple reaction time task: a high-density electrical mapping study. Neuropsychologia. 2001;39:828–44. doi: 10.1016/s0028-3932(01)00004-5. [DOI] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgins BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. Journal of Neuroscience. 2002;22(12):5055–73. doi: 10.1523/JNEUROSCI.22-12-05055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14(6):619–33. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Temporal precedence of emotion over attention modulations in the lateral amygdala: intracranial ERP evidence from a patient with temporal lobe epilepsy. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:83–93. doi: 10.3758/CABN.10.1.83. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Cooke MA, Fannon D, et al. Misattribution bias of threat-related facial expressions is related to a longer duration of illness and poor executive function in schizophrenia and schizoaffective disorder. European Psychiatry. 2008;23(1):14–9. doi: 10.1016/j.eurpsy.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biological Psychology. 2008;77(3):247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19(3):613–24. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rauch AV, Reker M, Ohrmann P, et al. Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Research. 2010;182(3):200–6. doi: 10.1016/j.pscychresns.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery; Theory and Clinical Interpretation. Tucson, AZ: Neuropsychological Press; 1993. [Google Scholar]
- Rockstroh B, Junghöfer M, Elbert T, Buodo G, Miller GA. Electromagnetic brain activity evoked by affective stimuli in schizophrenia. Psychophysiology. 2006;43(5):431–9. doi: 10.1111/j.1469-8986.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- Ross ED, Orbelo DM, Cartwright J, et al. Affective-prosodic deficits in schizophrenia: comparison to patients with brain damage and relation to schizophrenic symptoms [corrected] Journal of Neurology, Neurosurgery & Psychiatry. 2001;70(5):597–604. doi: 10.1136/jnnp.70.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshtein P, Richardson MP, Winston JS, et al. Amygdala damage affects event-related potentials for fearful faces at specific time windows. Human Brain Mapping. 2010;31(7):1089–105. doi: 10.1002/hbm.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, et al. Association of enhanced limbic response to threat with decreased cortical facial recognition memory response in schizophrenia. American Journal of Psychiatry. 2010;167:418–26. doi: 10.1176/appi.ajp.2009.09060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroencephalography and Clinical Neurophysiology. 1985;62(1):32–44. doi: 10.1016/0168-5597(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Scherg M, Picton TW. Separation and identification of event-related potential components by brain electric source analysis. Electroencephalography and Clinical Neurophysiology. 1991;42:24–37. [PubMed] [Google Scholar]
- Simpson GV, Pflieger ME, Foxe JJ, et al. Dynamic neuroimaging of brain function. Journal of Clinical Neurophysiology. 1995;12:432–49. doi: 10.1097/00004691-199509010-00003. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Swartz MS, Elbogen EB. Effectiveness of atypical antipsychotic medications in reducing violent behavior among persons with schizophrenia in community-based treatment. Schizophrenia Bulletin. 2004;30(1):320. doi: 10.1093/oxfordjournals.schbul.a007065. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Archives of General Psychiatry. 2006;63(5):490–9. doi: 10.1001/archpsyc.63.5.490. [DOI] [PubMed] [Google Scholar]
- Tsoi DT, Lee KH, Khokhar WA, et al. Is facial emotion recognition impairment in schizophrenia identical for different emotions? A signal detection analysis. Schizophrenia Research. 2008;99(1–3):263–9. doi: 10.1016/j.schres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30(3):829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, et al. Visual sensory processing deficits in Schizophrenia and their relationship to disease state. European Archives of Psychiatry and Clinical Neuroscience. 2008;258(5):305–16. doi: 10.1007/s00406-008-0802-2. [DOI] [PubMed] [Google Scholar]