Abstract
Individuals with Autism Spectrum Conditions (ASC) have difficulties in social interaction and communication, which is reflected in hypoactivation of brain regions engaged in social processing, such as medial prefrontal cortex (mPFC), amygdala and insula. Resting state studies in ASC have identified reduced connectivity of the default mode network (DMN), which includes mPFC, suggesting that other resting state networks incorporating ‘social’ brain regions may also be abnormal. Using Seed-based Connectivity and Group Independent Component Analysis (ICA) approaches, we looked at resting functional connectivity in ASC between specific ‘social’ brain regions, as well as within and between whole networks incorporating these regions. We found reduced functional connectivity within the DMN in individuals with ASC, using both ICA and seed-based approaches. Two further networks identified by ICA, the salience network, incorporating the insula and a medial temporal lobe network, incorporating the amygdala, showed reduced inter-network connectivity. This was underlined by reduced seed-based connectivity between the insula and amygdala. The results demonstrate significantly reduced functional connectivity within and between resting state networks incorporating ‘social’ brain regions. This reduced connectivity may result in difficulties in communication and integration of information across these networks, which could contribute to the impaired processing of social signals in ASC.
Keywords: autism, resting state, fMRI
INTRODUCTION
Individuals with Autism Spectrum Conditions (ASC) are characterised by their difficulties in social interaction and communication, unusually repetitive patterns of behaviour, and extremely narrow interests. Their difficulties in social interaction and communication include abnormal eye contact, difficulties in maintaining a conversation, difficulties in reading emotions, gestures, and mental states and difficulties with the pragmatics of language (Baron-Cohen, 1995; Frith, 2001). Previous studies that have examined the neurobiological basis of social impairments in ASC have found reduced activity across several brain regions during a range of social tasks. A recent meta-analysis by Di Martino et al. (2009) identified these regions of hypoactivation during social tasks as comprising medial prefrontal cortex (mPFC), amygdala, insular cortex, angular gyrus/temporoparietal junction (TPJ) and posterior cingulate cortex (PCC).
While some of these regions, such as mPFC, TPJ and the amygdala, have been studied extensively in ASC, others like the insula and PCC have only more recently been associated with ASC (Silani et al., 2008; Di Martino et al., 2009). Amygdala hypoactivation has been related to atypical emotional processing in individuals with ASC (Baron-Cohen et al., 1999; Ashwin et al., 2007), while a number of studies have identified reduced activation in mPFC and TPJ during theory-of-mind, social attention and gaze perception tasks (Castelli et al., 2002; Pelphrey et al., 2005). Although the role of PCC in ASC remains elusive and understudied, hypoactivation of the insula, which plays a key role in interoceptive processing and monitoring bodily states of arousal (Craig, 2002), has been linked to reduced emotional awareness of the self and others in both typical controls and ASC (Silani et al., 2008).
Interestingly, the areas of mPFC and TPJ implicated in ASC show remarkable overlap with the default mode network, one of the key ‘resting state’ brain networks comprising regions whose activity is highly correlated at rest (Shulman et al., 1997; Raichle et al., 2001; Buckner et al., 2008). As the brain regions subserving the default mode network (mPFC, PCC, angular gyrus/TPJ) display abnormal activation in ASC during specific cognitive tasks, some studies have considered whether abnormalities are also apparent in the intrinsic activity or connectivity of these regions at rest. A key advantage of the resting state over task-based measures is the absence of confounds associated with underlying differences in task performance, or differences in the way in which the task is executed. Resting state studies of ASC identified reduced functional connectivity or reduced activity at rest within regions of the default mode network (Cherkassky et al., 2006; Kennedy et al., 2006; Kennedy and Courchesne, 2008b; Monk et al., 2009; Assaf et al., 2010), with the most consistent finding involving reduced activation of, or connectivity with, mPFC.
Although the most widely studied resting state network, the default mode is only one of many networks that show consistent, highly correlated activity at rest. Methodological advances, such as the application of independent component analyses (ICA) to resting state data, have enabled the identification of dozens of functionally relevant resting state networks (Beckmann et al., 2005; Damoiseaux et al., 2006; Smith et al., 2009; Allen et al., 2011). The presence of these networks is highly reliable across individuals, and connectivity within and between these networks has been shown to differ in certain neuropsychiatric conditions (Greicius et al., 2004; Jafri et al., 2008), as well as within the healthy ageing population (Damoiseaux et al., 2008; Allen et al., 2011). Importantly, it is becoming increasingly clear that these ‘resting’ networks also reflect ‘functional’ networks, i.e. sets of brain regions that are engaged during specific cognitive or mental processes (Smith et al., 2009). Furthermore, behavioural measures relating to the function a network subserves are correlated with the resting functional connectivity of that network (Seeley et al., 2007).
In light of these observations, an interesting question is to what extent task-based differences in specific brain regions in ASC are reflected in different connectivity patterns of their respective brain networks at rest. While the previously observed reduced connectivity within the default mode network in ASC suggests this may be true for regions like mPFC and TPJ, other resting state networks comprising areas implicated in ASC, such as the salience network incorporating the insula (Seeley et al., 2007), or medial temporal lobe (MTL) network incorporating the amygdala (Damoiseaux et al., 2008), have not yet been studied. Furthermore, since the brain regions identified in Di Martino’s meta-analysis (2009) are present in spatially distinct resting state networks, it is important to determine to what extent the connectivity at rest between these networks might also be affected. Between-network connectivity has been shown to reveal functional clustering of resting state networks, such that networks that are engaged in similar types of brain processes are more highly correlated with each other (Allen et al., 2011). In individuals with ASC, between-network connectivity may provide insight into the ease with which these networks interact and share functionally relevant information.
It is also interesting that most previous studies that have investigated resting functional connectivity in ASC have used a seed-based region-of-interest (ROI) approach (Cherkassky et al., 2006; Kennedy and Courchesne, 2008b; Monk et al., 2009; Ebisch et al., 2011; Di Martino et al., 2011). Unlike the purely data-driven ICA approach, seed-based analyses identify correlations in resting brain activity of a specific seed region (e.g. mPFC) with other brain regions. Thus, while ICA provides information about whole-brain functional networks and how tightly intertwined these are, seed-based analyses provide a measure of connectivity for specific ROIs. These methods should therefore provide complementary information where ICA identifies networks affected in ASC and seed-based analyses identify the specific brain regions within or between these networks that are affected.
The aims of this study were 2-fold: first, to use ICA to determine whether established resting state networks, which include brain regions showing task-related hypoactivity in individuals with ASC, show differences in intrinsic functional connectivity both within and between networks relative to typical controls; second, to perform a seed-based correlation analysis in order to clarify whether resting functional connectivity analyses using a directed seed-based approach and a larger network-based approach reveal complementary information. We hypothesised that there would be reduced functional connectivity both within and between resting state networks incorporating ‘social’ brain regions, and that both ICA and seed-based analyses would reveal similar results.
MATERIALS AND METHODS
Participants
A total of 25 typical male participants and 18 males with ASC (two with High-functioning Autism and 16 with Asperger Syndrome) participated in this study for payment. All ASC subjects had a confirmed diagnosis of ASC: five by Autism Diagnostic Interview-Revised (Lord et al., 1994) and two by Autism Diagnostic Observation Schedule (Lord et al., 2000) as part of previous studies at the Autism Research Centre, University of Cambridge, and the remainder provided written confirmation of independent clinical diagnosis. Neither of the control participants had a diagnosis of ASC nor were they on any kind of medication. One participant with ASC was on anti-depressant medication. All participants completed the Wechsler Abbreviated Scale of Intelligence (WASI; The Psychological Corporation, 1999) and all scored 85 or higher. Participants also completed the Autism Spectrum Quotient (AQ), a validated measure of autism spectrum characteristics found within both the typical population and individuals with a diagnosis of autism (Baron-Cohen et al., 2001; Woodbury-Smith et al., 2005). One control participant and one participant with ASC were left-handed; the remainder of participants were right-handed. This study was approved by the Cambridgeshire Local Research Ethics Committee and all participants provided written, informed consent according to the Declaration of Helsinki.
fMRI data acquisition
Data were acquired using a 3T Tim Trio (Siemens, Erlangen, Germany) scanner. For the resting state scan, whole brain T2*-weighted echo planar images (EPIs) were acquired with a repetition time (TR) = 2190 ms, echo time (TE) = 30 ms, flip angle = 78°, 36 oblique slices and 3 mm isotropic resolution. A total of 273 volumes were acquired for a total imaging time of 9 min 58 s. Participants were instructed to relax but keep still. A high-resolution structural magnetization-prepared rapid-acquisition gradient echo scan for normalization purposes was also acquired (voxel size 1 × 1 × 1 mm, repetition time = 2250 ms, echo time = 2.99 ms, inversion time = 900 ms, flip angle = 9°, total scan time 4 min 16 s).
Pre-processing
fMRI data pre-processing was carried out in SPM5 (www.fil.ion.ucl.ac.uk/spm). The first three volumes were discarded to allow for equilibration effects. The EPI images were sinc interpolated in time to correct for slice time differences and realigned to the first scan by rigid body transformations to correct for head movements. EPI and structural scans were co-registered and normalised to the T1 standard template in Montreal Neurological Institute (MNI) space (MNI: International Consortium for Brain Mapping) using linear and nonlinear transformations and smoothed with a Gaussian kernel of 8 mm full width at half maximum. Data were bandpass filtered to include only frequencies between 0.0078 and 0.08 Hz (Cordes et al., 2001; Fox and Raichle, 2007).
Three subjects with ASC and one control had to be removed from the analysis due to excessive head movement (>3 mm). As a result, our previously age-matched groups were no longer exactly age-matched (Table 1) and age was therefore included as a covariate in our analyses. For the remaining participants, the root mean square movement (translation and rotation) was determined and entered into between-groups ANOVA. There were no group differences in movement (F < 1, P = 0.8).
Table 1.
Participant details
| Age | WASI |
AQ | |||
|---|---|---|---|---|---|
| Full | Verbal | Performance | |||
| Controls (n = 24) | 25 ± 6 range (19–36) | 118 ± 13 | 115 ± 13 | 117 ± 13 | 15 ± 6 range (5–25) |
| ASC (n = 15) | 30 ± 8 range (19–40) | 116 ± 12 | 112 ± 13 | 117 ± 10 | 36 ± 8 range (16–45) |
| P (t-test) | 0.04 | 0.70 | 0.48 | 0.98 | <0.001 |
Independent component analysis
Group spatial independent component analysis (ICA) was carried out using the Group ICA for fMRI Toolbox (GIFT v1.3g, icatb.sourceforge.net) using the Infomax algorithm (Bell and Sejnowski, 1995). The mean number of components for all subjects was estimated using the minimum description length criteria for source estimation (Li et al., 2007) and was found to be 30 components. Group ICA was carried out in three main stages: data reduction, ICA and back reconstruction. First, individual subjects’ data were reduced by principal components analysis (PCA). The subjects’ data were then concatenated, followed by a further PCA data reduction step. ICA was then applied to this reduced data set. The resulting components reflect group components across all subjects. Using the assumption that individual subjects’ data form statistically independent observations and that the unmixing matrix (group ICA output) is therefore separable across subjects, GIFT performs a back reconstruction of individual subjects’ time courses and spatial maps based on the output group components and information from the PCA data reduction steps (Calhoun et al., 2001, 2009). Group ICA was run treating all subjects as one group to ensure that the same components were identified in each subject. However, to ensure that the same components would be identified in each group, we also ran a group ICA in controls and ASC separately and identified the same components of interest in each.
As several components appeared to overlap spatially with DMN regions, we performed a spatial template matching procedure using the DMN template provided within the GIFT toolbox. Three components were identified as having a high spatial overlap with the template [correlation values: 0.36, 0.35, 0.35, which are similar to previously reported values (Assaf et al., 2010)], and each comprised one or more brain regions associated with the DMN. We identified two further networks of interest based on visual inspection for inclusion of the amygdala or insula, as well as ensuring similarity with previously identified resting state networks. One of these was the ‘salience’ network (Seeley et al., 2007), which includes the insula, anterior cingulate cortex and lateral prefrontal regions. The other network comprised the MTLs (Damoiseaux et al., 2008), including the amygdala, a structure important for the processing of emotions, as well as the cerebellum.
Statistical analysis of independent components
Prior to statistical analysis, the resting state network components were transformed to z-scores using the GIFT software and then exported to SPM5 (Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) where statistical analyses were performed. Independent components of interest for each subject were entered into a two-sample t-test, where controls were contrasted with individuals with ASC. Age was included as a covariate due to the significant group difference in age. All results were masked with the whole group’s (all subjects) average independent component thresholded at P < 0.005 uncorrected, to ensure that only effects restricted to the spatial extent of the component were included in the analyses; however, it is important to note that no significant group differences were found outside the masked regions, neither were there any differences in the results when data were unmasked. Group results were corrected for multiple comparisons (P < 0.05 false discovery rate, FDR) based on a priori regions-of-interest in the mPFC, PCC, angular gyrus/TPJ, anterior insula and amygdala. For regions outside the ROIs, we applied a whole-brain corrected threshold of P < 0.05 (FDR) with a 10 voxel extent threshold. Spherical ROIs were created centred on DMN coordinates identified in Shulman et al.’s (1997) meta-analysis as follows: mPFC (−1, 49, −2), PCC (−5, −53, 41) and left (−45, −71, 35) and right (45, −71, 35) angular gyri. ROIs for anterior insula were centred on Taylor et al.’s (2009) study of insula resting connectivity (left, x = −34, y = 14, z = 3; right, x = 36, y = 16, z = 3), and we used an anatomically based ROI for the amygdala from the Automated Anatomic Labelling Atlas (Tzourio-Mazoyer et al., 2002). All spherical ROIs had a radius of 10 mm, except for the bilateral angular gyrus (radius 20 mm) as we wanted to include neighbouring TPJ/posterior superior temporal sulcus (pSTS) which forms part of the wider posterior lateral cortices implicated in the DMN (Gusnard et al., 2001).
Functional network connectivity
Functional network connectivity (FNC) analyses were performed using the FNC Toolbox (version 2.2), an add-on to the GIFT software. The toolbox has previously been used to examine between-network connectivity in schizophrenia (Jafri et al., 2008). The toolbox computes a constrained maximal lag correlation between each pair of networks of interest by calculating Pearson’s correlation and constraining the lag between the time courses (Jafri et al., 2008). With five networks of interest, the number of possible pair-wise combinations to examine between-network connectivity is 10. Maximal lag for the correlation calculation was 4.4 s (2 TR), and results were corrected for multiple comparisons between networks using P < 0.05 (FDR). A two-sample t-test was used to determine differences in between-network connectivity between groups (FDR corrected at P < 0.05). Age was included as a covariate due to the significant group difference in age.
Seed-based correlation analysis
Seeds for the seed-based correlation analysis were based on regions reported to show a consistent reduction in task-related activation in individuals with ASC: mPFC, PCC, angular gyrus, anterior insula and amygdala (Di Martino et al., 2009). The coordinates for the seed-based analyses were identical to those used for the ICA multiple comparisons corrections described above. Each seed consisted of a 6 mm radius sphere, in line with previous seed-based correlation studies (Fox et al., 2005; Kennedy and Courchesne, 2008b; Ebisch et al., 2011). The mean time course from each region-of-interest was extracted and entered into a general linear model. Global signal and six movement parameters were included as additional regressors in the model. The resulting correlation maps for each subject were z-transformed (Fisher’s Z) prior to the group comparisons, which were performed using two-sample t-tests in SPM5. Age was included as a covariate in the model due to the significant group difference in age. Results were corrected for multiple comparisons using a small-volume correction based on the a priori ROI mentioned above.
Correlation with AQ
In order to investigate the relationship between functional connectivity and autism spectrum traits across both the control and ASC groups, we performed additional analyses where AQ was included in the second-level model as a covariate. In this way, we were able to determine the relationship of autism spectrum traits to functional connectivity over and above group membership.
RESULTS
Subject details for the participants included in the analyses (24 controls, 15 ASC) are listed in Table 1.
Independent component analysis
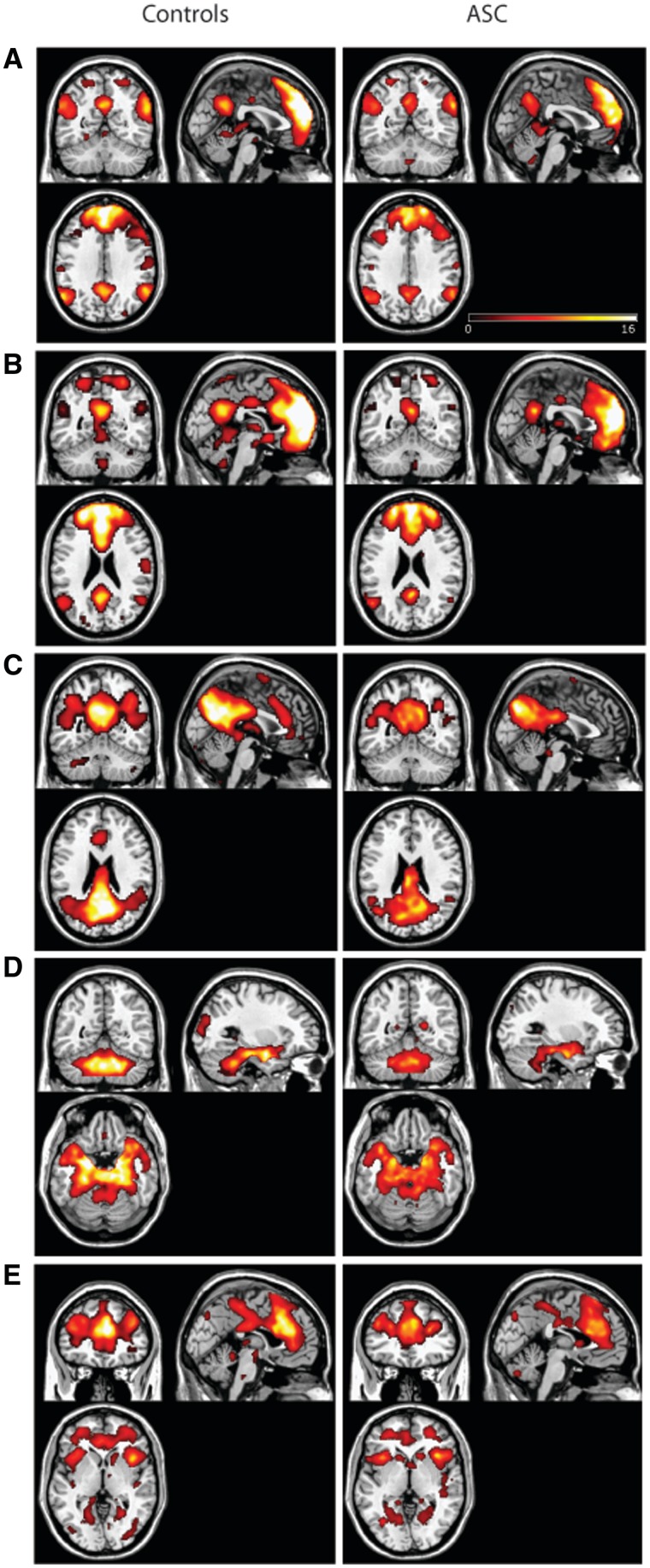
Three default mode network components, DMNa, DMNb and DMNc, a ‘salience’ network including the insula, and a medial temporal lobe network including the amygdala were identified as networks of interest (Figure 1). While it is more common for the DMN to be contained within one component, there have been several reports of the DMN being broken into several sub-components (Damoiseaux et al., 2008; Assaf et al., 2010; Allen et al., 2011), particularly as the data set becomes larger and is characterised by a larger number of independent components (Allen et al., 2011). The salience network (Seeley et al., 2007) and medial temporal lobe network (MTL; Damoiseaux et al., 2008) are also resting state networks that have previously been identified.
Fig. 1.
Independent components representing aspects of the default mode network DMNa (A), DMNb (B), DMNc (C), the MTL and cerebellar network (D) and the salience network (E). Average components for the control group are shown in the left column; average components for the ASC group are shown in the right column. Maps are thresholded at P < 0.05 (whole-brain FDR corrected).
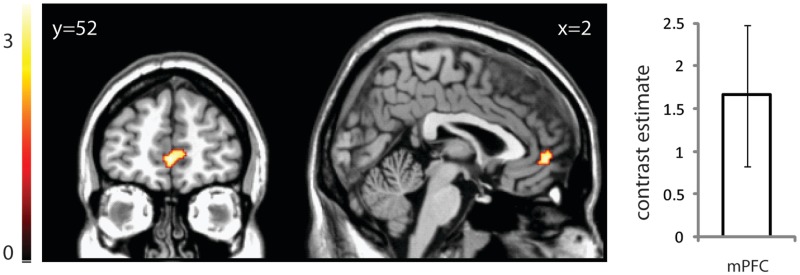
When DMNa was compared between groups, there was significantly reduced functional connectivity in mPFC in ASC compared with controls (t = 3.39, x = 0, y = 52, z = −2, 84 voxels, P < 0.05 svc; Figure 2). In other words, the mPFC’s time course of activity showed reduced correlation between the component’s overall mean time courses. There were neither significant group differences in DMNb, DMNc, salience and MTL networks within our ROIs, nor any group differences outside the ROIs.
Fig. 2.
The mPFC within DMNa displays reduced connectivity in individuals with ASC compared with controls. Image is thresholded at P < 0.005 uncorrected for display purposes. Graph displays the contrast estimate and 95% confidence intervals for the peak voxel following small volume correction.
Functional network connectivity
The control group showed significant correlations between each of the DMN components, as well as between the DMN components and the MTL network, and between the salience and MTL networks (P < 0.05, FDR). There was no significant correlation between the salience network and the three DMN components (Table 2 for list of correlation coefficients). For the ASC group, a similar pattern was apparent (Table 2), but the salience network was not significantly correlated with the MTL network. Moreover, a group comparison in FNC revealed significantly reduced correlation between the salience network and the MTL network in the ASC group relative to controls (P < 0.05, FDR). In addition, there was a borderline but non-significant increase in the correlation between the DMNc and MTL networks in the ASC group.
Table 2.
Correlation coefficients and p values from FNC analysis
| Component combination | Controls | ASC | P (FDR) between group |
|---|---|---|---|
| DMNa–DMNb | 0.58* | 0.49* | 0.31 |
| DMNa–DMNc | 0.45* | 0.38* | 0.31 |
| DMNb–DMNc | 0.39* | 0.35* | 0.53 |
| MTL–DMNa | 0.07* | 0.12* | 0.46 |
| MTL–DMNb | 0.19* | 0.29* | 0.31 |
| MTL–DMNc | 0.12* | 0.23* | 0.07 |
| MTL–Salience | 0.10* | −0.03 | 0.02† |
| Salience–DMNa | −0.02 | −0.02 | 0.92 |
| Salience–DMNb | 0.03 | 0.01 | 0.87 |
| Salience–DMNc | 0.07 | 0.05 | 0.87 |
MTL, medial temporal lobe; DMN, default mode network.
*Denotes significant (P < 0.05 FDR corrected) correlation between components within each group, †denotes significant group difference in the magnitude of the correlation between components.
Seed-based analysis
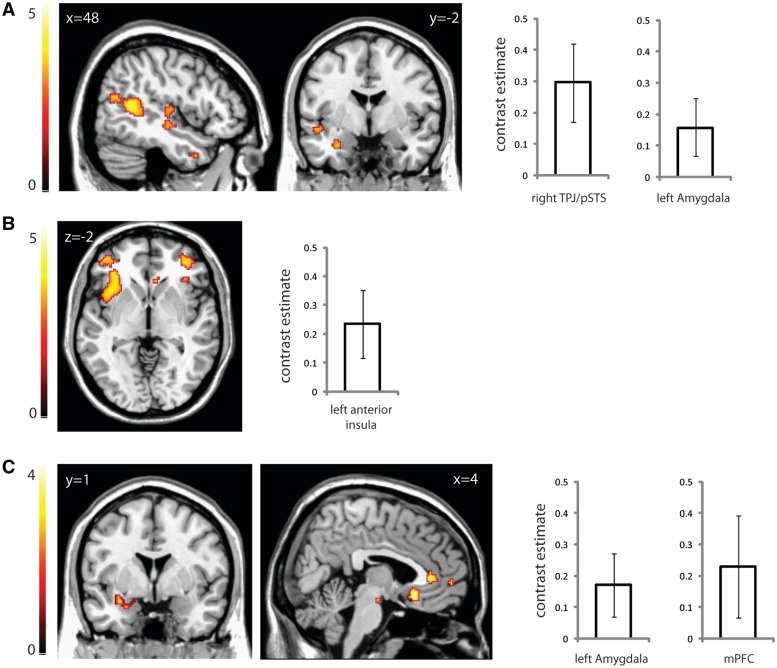
Using mPFC as a seed, there was significantly reduced connectivity between mPFC and TPJ/pSTS in the ASC group compared with the control group, similar to the reduced within-network connectivity between the mPFC and other areas of this network observed by ICA. Additionally, there was reduced connectivity between mPFC and left amygdala, and between mPFC and left anterior insula (Figure 3a and Table 3).
Fig. 3.
Seed-based connectivity analyses showing areas correlated with a seed region based in mPFC (A), left amygdala (B) and left anterior insula (C). Images display regions of greater connectivity with the seed region in the control group compared with the ASC group. Graphs display the contrast estimates and 95% confidence intervals for the peak voxel following small volume correction. Significantly greater connectivity was found (A) between mPFC and right TPJ/pSTS and left amygdala, (B) between left amygdala and left anterior insula and (C) between left anterior insula and left amygdala and mPFC. Images are thresholded at P < 0.005 uncorrected for display purposes.
Table 3.
List of brain regions showing significantly reduced functional connectivity in ASC with a given seed region (P < 0.05 FDR svc)
| Brain region | T | Cluster size (voxels) | MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| mPFC seed | |||||
| TPJ / Posterior STS (R) | 4.84 | 67 | 54 | −68 | 18 |
| Anterior insula (L) | 3.49 | 54 | −34 | 8 | −4 |
| Amygdala (L) | 3.50 | 32 | −30 | −2 | −24 |
| Amygdala (R) seed | |||||
| Anterior insula (L) | 4.04 | 116 | −32 | 22 | 0 |
| Amygdala (L) seed | |||||
| Anterior insula (L) | 4.01 | 172 | −32 | 22 | 0 |
| Anterior insula (R) seed | |||||
| mPFC | 4.32 | 155 | 2 | 58 | −2 |
| Angular gyrus (R) | 4.39 | 101 | 36 | −66 | 52 |
| Anterior insula (L) seed | |||||
| mPFC | 2.86 | 9 | 6 | 54 | −2 |
| Amygdala (L) | 3.39 | 50 | −20 | −10 | −16 |
Using a seed based in the left amygdala, there was significantly reduced connectivity with left anterior insula in individuals with ASC compared with controls (Figure 3b and Table 3), and a similarly reduced connectivity between right amygdala and left anterior insula (Table 3). This agrees with the ICA-based observation of reduced connectivity between the MTL and salience networks.
With a seed based in left anterior insula, the ASC group showed reduced connectivity with the left amygdala and mPFC (Figure 3c and Table 3). There was also a borderline reduction in connectivity in the ASC group between left insula and right amygdala (Table 3). A right anterior insula seed revealed reduced connectivity in the ASC group with mPFC and right angular gyrus (Table 3).
Neither the PCC nor angular gyrus seeds revealed any significant differences in functional connectivity between the groups. There were neither regions of significantly increased connectivity in the ASC group relative to the control group for any of the seed regions, nor any differences between the groups outside of the ROIs.
In order to ensure that the difference in group sizes were not unduly biasing our ICA and seed-based results, we repeated all analyses using the 15 subjects with ASC and the first 15 control subjects that were recruited. The ICA results showed the same pattern, with significantly reduced mPFC connectivity in ASC. For the seed-based analyses, all results also remained significant or borderline significant, with the exception of the reduced connectivity between mPFC and insula (see Supplementary Data).
Furthermore, although age was included as a covariate in all of our primary analyses, the results were unchanged when we repeated the analyses without age as a covariate, suggesting that age is not playing a significant role in the results. Finally, the removal of the ASC participant who was medicated also did not affect the results.
Relationship with AQ
We examined the relationship between autism spectrum traits and functional connectivity measures by including AQ scores as a covariate in our analyses. Since there was a significant group difference in AQ (Table 1), we factored out group membership to ensure that any correlation between AQ and functional connectivity across all subjects was not being driven by the main effect of group. We found a significant negative relationship between AQ score and seed-based mPFC connectivity with left and right angular gyrus, extending into right TPJ/pSTS (left: x = −32, y = −64, z = 38; t = 4.83, 336 voxels, P < 0.05 svc FDR; right: x = 54, y = −56, z = 38; t = 4.50, 85 voxels, P < 0.05 svc FDR; see Supplementary Figure S1). Interestingly, there was no significant relationship between AQ and functional connectivity identified by ICA.
DISCUSSION
We have shown that individuals with ASC display reduced functional connectivity in resting state networks that contain brain regions relevant to social processing. Using an ICA approach to investigate group differences in within-network connectivity, we replicated previous research showing that the mPFC region of the DMN shows atypical connectivity or activation in ASC (Kennedy et al., 2006; Kennedy and Courchesne, 2008a; Assaf et al., 2010). In addition, we showed for the first time that people with ASC show significant reductions in between-network connectivity. Specifically, the MTL network, which includes the amygdala, showed reduced connectivity, and by implication reduced communication, with the salience network, which includes the insula. Consistent with the reduced connectivity between these networks, the seed-based approach showed reduced connectivity between the amygdala and insula. Taken together, the results show that ASC is associated with specific differences in connectivity at rest. These differences are reflected within specific networks containing regions relevant to social processing, and globally in terms of the manner in which these networks interact. We suggest that difficulties in communication or information sharing both within and between networks relevant to social processing may underlie some of the social difficulties associated with ASC.
Previous resting state studies in ASC have looked primarily at the DMN (Cherkassky et al., 2006; Kennedy et al., 2006; Kennedy and Courchesne, 2008a, b; Monk et al., 2009; Assaf et al., 2010), as this network is thought to reflect activity resulting from internally focussed thought processes which are impaired in ASC (Buckner et al., 2008). Only one of these earlier studies used ICA, but several of them converge on identifying mPFC as a locus of reduced functional connectivity or resting activity in ASC (Kennedy et al., 2006; Kennedy and Courchesne, 2008a; Assaf et al., 2010).
Our results reinforce these earlier findings, but go further by showing that seed-based analyses reveal that the reduction in connectivity of mPFC within the DMN appears to reflect reduced connectivity with the TPJ/pSTS. The same analysis also revealed that individuals with ASC showed decreased connectivity between mPFC and areas outside the DMN (insula and amygdala), and decreased connectivity between the amygdala and insula; see also Ebisch et al. (2011). Although our investigation of other networks in addition to the DMN showed no evidence of impaired within-network connectivity, we provide new evidence suggesting that the reduced connectivity between the amygdala and insula, identified by seed-based analyses, reflects reduced between-network connectivity between the salience and MTL networks. A reduction in connectivity between, rather than within, networks suggests difficulties in integrating information across networks and hence across different cognitive processes. The salience network, and in particular, the insula, is thought to be involved in integrating information about emotion and bodily states of arousal (Craig, 2002), and plays a role in redirecting attention to unexpected but salient stimuli (Corbetta et al., 2008). Reduced connectivity between the salience network and limbic regions, such as the amygdala, may therefore reflect reduced integration of information between these networks. This may underlie some of the abnormalities that are observed in ASC, such as impaired emotional awareness of the self and others and impaired reorienting to salient social or emotional stimuli.
Autism spectrum traits
We found evidence that traits associated with the autism spectrum, as measured by the AQ, predict reduced connectivity across both the control and ASC population between mPFC and bilateral angular gyrus and extending into right TPJ/pSTS. These results complement previous studies (Kennedy et al., 2006; Assaf et al., 2010) that found reduced mPFC resting connectivity associated with autistic traits, but our results show that this relationship also exists within the typical, non-ASC, population.
ASC: a condition of underconnectivity?
Our results also reinforce the notion that ASC is a condition of underconnectivity since the changes we found reflected reduced connectivity in the ASC group. The idea that abnormalities associated with ASC can be reduced to underconnectivity has been suggested previously (Just et al., 2004; Muller et al., 2011), and while evidence in support of this theory is largely based on functional connectivity analyses, there are also a number of studies that have looked at anatomical connectivity in ASC using diffusion-tensor-imaging (Barnea-Goraly et al., 2004; Lee et al., 2007; Cheung et al., 2009). Overall, these studies show reduced fractional anisotropy in ASC across different brain regions, most notably in mPFC (Barnea-Goraly et al., 2004; Lee et al., 2007; Cheung et al., 2009); reduced fractional anisotropy is thought to reflect changes to white matter tracts, such as a reduced fibre density, reduced myelination or increased branching. One study (van den Heuvel et al., 2009) also found that well-described white matter tracts link brain areas within several of the common resting state networks, suggesting that the regions in these networks are also structurally connected. However, it is unclear to what extent changes in functional connectivity within these networks might mirror changes in underlying anatomical connectivity, and whether reduced functional connectivity between networks may reflect weaker or indirect structural connections.
ICA or seed-based analysis
Our results shed light on the type of information that can be obtained using two different techniques for investigating functional connectivity at rest. Although both ICA and seed-based analyses are prevalent methods for studying resting connectivity, there had been no examination of the relationship between the two methods until recently (Joel et al., 2011). Joel et al. (2011) showed that seed-based measures of functional connectivity do not distinguish within- and between-network connectivity while ICA-based measures do. Our results support these observations by revealing that our seed-based analyses reflect a combination of within- and between-network connectivity, as identified by ICA. However, our seed-based analyses also revealed underconnectivity in the ASC group between regions (e.g. mPFC and amygdala) that was not reflected by ICA, evidenced by the absence of a significant difference in connectivity between the DMN and MTL networks. Similarly, we observed a correlation between AQ score and functional connectivity of mPFC with angular gyrus and TPJ/pSTS, but did not observe a similar relationship with AQ in mPFC as identified by ICA. These discrepancies may reflect the fact that in ICA it is the mean time course of a whole network (e.g. DMNa) which is compared with the time course of individual voxels within that network, whereas seed-based analyses identify correlations between the time courses of activity of one small group of voxels with other voxels. Whether these observations suggest that seed-based analyses provide a more sensitive measure of resting functional connectivity remains to be determined. However, it seems that an approach that combines both ICA- and seed-based methods provides not only insight into connectivity measures at the level of networks and how these networks interact, but also identifies which specific brain areas within or between those networks are primarily involved.
Limitations
As three ASC participants had to be removed from our analyses due to an excess movement during the scan, our ASC group size may be a limitation of this study. However, as our resting state scan duration was roughly double the usual length, the power lost by our reduced subject numbers may be offset by the longer scan duration for each subject (Mumford and Nichols, 2008). In addition, repeating our analyses with matched group sizes (i.e. reducing the number of control subjects) showed a similar pattern of results.
Although resting state connectivity analyses provide a means for studying brain ‘function’ in the absence of task-related performance confounds, it is still unclear what resting brain activity represents. Based on our results, which show extensive underconnectivity at rest of brain regions that are reported to show task-related hypoactivity in ASC, there may be a strong link between task-related activation of one or more regions and the strength of underlying ‘resting’ functional connectivity between those regions. A key next step will be to examine directly how underconnectivity at rest in ASC relates to observed differences in task-related brain activity.
Finally, it is important to remember that our sample was restricted to individuals with High-Functioning Autism or Asperger Syndrome with an IQ of 85 or higher. As a result, it is unclear whether these results would also generalise to the broader spectrum of autism, i.e. individuals with a diagnosis of ASC but with IQ below 85. Given the heterogeneous nature of ASC, further research is needed to address this issue.
CONCLUSIONS
While the vast majority of previous research has concentrated on the DMN, we have shown that there is a marked reduction in resting functional connectivity both within and between other ‘social brain’ networks in individuals with ASC. The reduced communication or information sharing within and between these networks relevant to social processing may underlie some of the difficulties observed in processing social cues in ASC.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online
CONFLICT OF INTEREST
None declared.
Supplementary Material
Acknowledgments
We would like to thank the participants for volunteering their time, Sally Wheelwright and Michael Lombardo for help with participant recruitment, and the radiographers at the MRC Cognition & Brain Sciences Unit. Supported by the UK Medical Research Council (MC_US_A060_0017 to AJC and a program grant to SBC).
REFERENCES
- Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7:1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–72. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. Journal of Child Psychology and Psychiatry. 2009;50:1102–12. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. Biological Psychiatry. 2011;69:847–56. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biological Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Human Brain Mapping. 2011;32:1013–28. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–79. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel SE, Caffo BS, van Zijl PC, Pekar JJ. On the relationship between seed-based and ICA-based measures of functional connectivity. Magnetic Resonance in Medicine. 2011;66:644–57. doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognitive and Affective Neuroscience. 2008a;3:177–90. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008b;39:1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424:127–32. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping. 2007;28:1251–66. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–72. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Nichols TE. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage. 2008;39:261–8. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–48. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks .2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Social Neuroscience. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping. 2009;30:3127–41. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury-Smith MR, Robinson J, Wheelwright S, Baron-Cohen S. Screening adults for Asperger Syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders. 2005;35:331–5. doi: 10.1007/s10803-005-3300-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.