Abstract
Social exclusion is known to cause alterations in neural activity and perceptions of social distress. However, previous research is largely limited to examining social interactions as a unitary phenomenon without investigating adjustments in neural and attentional processes that occur during social interactions. To address this limitation, we examined neural activity on a trial-by-trial basis during different social interactions. Our results show conflict monitoring neural alarm activation, indexed by the N2, in response to specific exclusionary events; even during interactions that are inclusionary overall and in the absence of self-reported feelings of social pain. Furthermore, we show enhanced attentional activation to exclusionary events, indexed by the P3b, during exclusionary, compared with inclusionary, interactions, and this P3b activation was associated with self-reported social distress following prolonged social exclusion. Finally, both the N2 and P3b showed larger amplitudes in the earlier stages of exclusion compared with later stages, suggesting heightened early sensitivity for both components. Together, these findings provide novel insights into the dynamic neural and perceptual processes of exclusion that exist during social interactions and the relationship between discrete events within interactions and the more general contexts of the social interactions.
Keywords: social exclusion, event-related brain potentials (ERPs), N2, P3b, conflict monitoring
INTRODUCTION
Social exclusion gives rise to a diffuse pattern of behavioral and neural changes that can lead to severe emotional, cognitive, social and developmental impairments in targets of exclusion (Williams, 2001; Baumeister et al., 2002; Eisenberger et al., 2003; Masten et al., 2009). These effects include increases in aggressive social behavior, anxiety and depression (MacDonald and Leary, 2005; Williams et al., 2005) and decreases in self-esteem and the fulfillment of needs (Williams et al., 2001). Additionally, different patterns of neural activation are present during exclusion compared with inclusion, with enhanced activation of the anterior cingulate cortex (ACC) and right ventral prefrontal cortex (RVPFC) during exclusion (Eisenberger et al., 2003, 2007). In these studies, measures of neural activation were aggregated within blocks of social interactions, which show the overall patterns of activation for each type of interaction (i.e. inclusionary and exclusionary) but not the alterations in neural activation over the course of the interactions. This allows for general characterizations of the relationship between neural activation and self-reported feelings following exclusion but does not allow for the examination of adjustments in neural processes during social interactions. To address this issue, we conducted an event-related brain potential (ERP) study of social exclusion. ERP measurement allows for the examination of specific events within a larger social interaction due to the excellent temporal resolution of ERPs compared with other neuroimaging techniques and methodologies (e.g. functional magnetic resonance imaging; fMRI), which are temporally limited to examinations of social interactions at the level of the entire interaction. Therefore, we were able to examine specific patterns of neural activity in response to discrete events during ongoing social interactions, including neural alarm activation and related task-relevant attentional activations, within the larger contexts of different types of social interactions.
Neural alarm and conflict monitoring
The neural alarm is derived from conflict monitoring theory (Botvinick et al., 2001; Yeung et al., 2004), which describes the neural alarm as a conflict-based system implemented by the ACC that detects (or monitors) levels of conflict between actual outcomes and intended or desired outcomes during information processing. The activation of this ACC-based alarm then triggers adjustments in compensatory cognitive control to more successfully regulate thoughts and behaviors to obtain desired outcomes. Accordingly, conflict monitoring theory has suggested that there are at least two functionally linked but dissociable systems of cognitive control (see Botvinick et al., 2001, for review). The first is the ACC-based evaluative system, mentioned earlier and characterized as the neural alarm, which acts as a conflict monitor during information processing events (Botvinick et al., 2001; Yeung et al., 2004). Neuroimaging research has shown that the ACC is involved in the evaluative system by indicating when adjustments in control are warranted (MacDonald et al., 2000; Kerns et al., 2004). The second system is the regulative system, which exerts flexible adjustments in top–down control and attentional allocation during subsequent information processing. Available evidence indicates that this support is likely provided by the prefrontal cortex (MacDonald et al., 2000; Kerns et al., 2004), with different control processes associated with different regions within the prefrontal cortex. These control processes lead to compensatory activations in other attentional networks to improve subsequent behavioral outcomes during cognitive task execution or following a task when undesired or unwanted outcomes are perceived.
Research has shown that the conflict-driven ACC activation is present during difficult tasks or task conditions (e.g. Stroop task) resulting in either correct or incorrect behavioral outcomes (Botvinick et al., 2001; Kerns et al., 2004; Yeung et al., 2004). This suggests that the neural alarm is not error or pain specific but is responsive to conflict regardless of response outcomes. Further, studies have indicated that one’s neural alarm circuitry is responsive to the errors or negative outcomes of others (von Schie et al., 2004; Shane et al., 2008) independent of the individual’s own behavioral or emotional responses. Conflict monitoring, then, is not solely reactive to personal negative outcomes. Rather, it is a constant and ongoing preconscious process that is present throughout environmental interactions that can be positive or negative in nature or can be personally experienced or observed. Alternatively, the regulation of conflict is a conscious process meant to modify behavior to achieve desired outcomes through the implementation of cognitive control, which adjusts the activation of attentional control networks to deal with the sources of the conflict or to cope with the consequences of the behavior.
Therefore, conflict monitoring theory suggests that the ACC-based neural alarm system, similar to the one activated during social exclusion (Eisenberger et al., 2003, 2007), is active in response to conflict regardless of the behavioral or emotional outcomes and can be present even before the outcome of an event or interaction is determined (Botvinick et al., 2001; Yeung et al., 2004). Thus, any specific exclusionary event, even a brief moment of exclusion within the context of a largely inclusionary interaction, should be sufficient to elicit neural alarm activation, without leading to perceptions of exclusion and corresponding self-reported feelings of social distress. Alternatively, the conscious control and allocation of attention toward perceptions of exclusion and exclusionary events would be more specifically associated with negative feelings and reports of social pain.
The neuropsychology of social exclusion
As indicated earlier, neuroimaging studies have examined neural responses to social exclusion (Eisenberger et al., 2003, 2007), with the ACC and RVPFC showing greater activation during exclusionary interactions compared with inclusionary interactions. Participants’ reports of social distress following exclusion were positively correlated with ACC activation during exclusion, suggesting a strong relationship between ACC activation and the social pain of exclusion (Eisenberger et al., 2003). Given previous research showing ACC involvement with the distressing affective experience of physical pain (Foltz and White, 1962; Rainville et al., 1997; Sawamoto et al., 2000) and research detailing the ACC as a conflict monitor that detects discrepancies between desired and actual task outcomes (Botvinick et al., 2001; Kerns et al., 2004; Yeung et al., 2004), the authors suggested that the ACC sounds a neural alarm (described earlier) in response to the social pain felt from being excluded (Eisenberger et al., 2003).
Conversely, RVPFC activation was negatively correlated with both social distress and ACC activation during exclusion (Eisenberger et al., 2003). The RVPFC has been associated with the regulation of distress from both physical pain and more general negative emotional experiences (Hariri et al., 2000; Petrovic and Ingvar, 2002; Lieberman et al., 2007). Further, the RVPFC has been shown to have efferent connections with the ACC (Vogt and Pandya, 1987; Preibisch et al., 2003). These combined findings suggest that the RVPFC is activated to suppress neural alarm activation and disrupt the pain-related distress in response to exclusion (Eisenberger et al., 2003; Eisenberger and Lieberman, 2004).
Although very informative, these neuroimaging investigations only report on neural activation at the level of the social interactions, and inferences regarding dynamic responses to specific social events within the interaction cannot be drawn from this type of analysis. Specifically, it remains unclear whether ACC-based neural alarm activity seen during exclusionary interactions is particularly associated with prolonged exposure to exclusion and feelings of social distress characterized by entire interactions that are largely exclusionary or whether the neural alarm is also sensitive to each instance of exclusion within an interaction, regardless of whether that interaction as a whole is largely exclusionary or inclusionary in nature. Therefore, we suggest that a different level of analysis, one with a greater temporal resolution that can examine the specific events within ongoing social interactions (i.e. ERPs), may yield additional important information regarding neural activation during social exclusion.
We suggest that the neural alarm is active in response to social pain experienced following exclusion, consistent with the findings of Eisenberger et al. (2003, 2007). However, we suggest that the neural alarm is not exclusion specific but is a more sensitive and generic conflict monitor that is also proactively sensitive to exclusionary events similar to pain-inducing phenomena that may, or may not, lead to complete exclusion. Further, we suggest that the enhanced activation of the remaining self-regulatory neural circuitry following exclusion, including the prefrontal cortex and related parietal attentional network regions, is more closely associated with self-reported perceptions of exclusion and feelings of social distress.
Current study
To examine the dynamic relationships between neural and behavioral indices of social exclusion, we conducted an ERP study that assessed the responsiveness of neural alarm activity and other self-regulatory attentional processes to exclusionary events within the larger contexts of different social interactions. ACC-based neural alarm activation was indexed by the N2 component of the stimulus-locked ERP, whereas conscious cognitive control and attentional processes were indexed by the P3b component. The N2 component is a multifaceted component that has been linked to multiple cognitive processes (Folstein and Van Petten, 2008). Recently, a differentiation in N2 functionality has separated anterior N2s from posterior N2s, with anterior N2s related with wither the detection of novelty and mismatch or with error/conflict detection and cognitive control processes (Folstein and Van Petten, 2008). In the current investigation, we will be examining the influence of conflict derived from social interactions on the anterior cognitive control N2. This ‘conflict N2’ is negative-going component that is maximal over fronto-central recording sites, peaks between 150 and 350 ms after stimulus presentation and is believed to be a psychophysiological index of conflict monitoring that originates from the ACC (van Veen and Carter, 2002; Yeung et al., 2004; Folstein and Van Petten, 2008). Scalp recordings of this component reflect the detection of conflict that occurs without action errors or error feedback, including conflict associated with the inhibition of action (Braver et al., 2001) and conflict existing outside one’s awareness (Leuthold and Kopp, 1998) during correct task execution. Thus, the conflict N2 reflects the activity of a pre-conscious conflict monitoring system that can be activated before the execution of unintended behavioral responses (Yeung et al., 2004). The P3b is a consciousness-dependent ERP component that is sensitive to task difficulty and the subjective probability of task stimuli or conditions (Kok, 2001; Polich, 2007). The P3b is believed to reflect neuronal activity involved with basic cognitive functions such as memory updating and event categorization (Polich and Kok, 1995) and has been theorized to index the allocation of attention to task-relevant external stimuli (Donchin, 1981; Kok, 2001; Polich, 2007). The P3b is a positive-going component that is maximal over parietal recording sites and peaks between 300 and 800 ms after stimulus presentation. The P3b has multiple neural generators, including frontal and parietal activations (Polich, 2007). Therefore, in our investigation of the conflict N2 and P3b, we examined conflict monitoring neural alarm activation during the course of social interactions and activation associated with ongoing alterations in attentional allocation. On the basis of previous event-related research examining conflict monitoring and cognitive control and studies detailing the neural correlates of social exclusion, we hypothesized that: (i) neural alarm activation, indexed by the conflict N2, would be present in response to any event where the participant was excluded, regardless of the larger context of the interaction; (ii) enhanced activation of conscious attentional processes, indexed by the P3b, would be present in response to ongoing social exclusion from an interaction but not in response to ongoing social inclusion, reflecting the allocation of attention toward the undesired exclusionary experience and (iii) the modulation of the P3b component from the inclusionary to the exclusionary interactions described earlier would be associated with changes in self-reported social distress from the inclusionary to the exclusionary interaction.
METHODS
Participants
Twenty-five undergraduate students between the ages of 18 and 23 years were recruited to participate in this study. Participants in the study were awarded research credit toward a class requirement but no other compensation was provided. Three participants were excluded from the analyses due to excessive noise and artifacts obtained during ERP data collection, resulting in a sample size of 22 participants (15 women and 7 men). The study was approved by the Institutional Review Board of Illinois Wesleyan University.
Self-report assessments
After obtaining informed consent, each participant completed a series of questionnaires. These self-reports included a simple demographics questionnaire, the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) and a brief Need-Threat Scale (NTS) and feelings assessment that has been used in previous social exclusion research (Williams et al., 2000; Zadro et al., 2004). Both the PANAS and NTS were administered before the Cyberball task began and after each of the three subsequent blocks of the Cyberball task during the experiment. The NTS administered before the task instructed participants to represent the feelings they have ‘right now’ and used the present tense ‘feel’, whereas the NTS used after each Cyberball block asked participants to report how they ‘felt’ during the game and included the manipulation check questions used by Zadro et al. (2004).
Cyberball manipulation
Following the completion of the first set of questionnaires, participants were told that they would be playing an online game of ‘catch’ (Cyberball; Williams et al., 2000) with two other undergraduate participants, each located at different nearby universities. Unknown to the participants, the two other players in the Cyberball game were actually computer-generated players controlled by a computer program. During Cyberball, participants’ neuroelectric activity was recorded for data analysis. Every participant completed the same three blocks of the Cyberball paradigm (inclusion, exclusion and re-inclusion), completing the needs and feelings questionnaire and PANAS assessment before the first block and after each block. In each block, the Cyberball game was set for 80 throws, with the computerized players waiting between 2 and 3 s after receiving the ball to make a throw to enhance the sense that the player was actually playing the game and making a choice about which other player should receive the ball. In the first block (inclusion), the participant had a 50% chance of receiving the ball each throw. In the second block (exclusion), the participant had the same 50% chance of receiving the ball until receiving a total of 10 throws from the other participants. Following this initial inclusionary phase, the participant was no longer included in any of the remaining approximately 50 throws in the block. The third block (re-inclusion) returned the game to the original probability parameters described for the inclusion block.
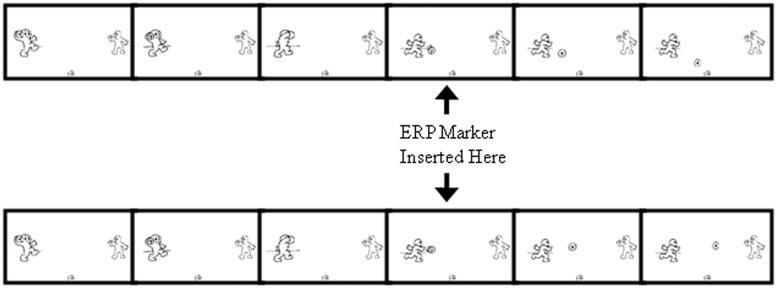
Event-related markers were created on a computer collecting ERP data from the participants while they were engaged in the Cyberball paradigm. The event markers were inserted at the first frame in each ball toss where information was provided on which player was going to be the recipient of the ball toss (i.e. throw to human participant and throw to computerized player). The inclusion of these event markers allowed for the quantification of moment-to-moment ERP activity in response to inclusionary (throw to human participant) or exclusionary (throw to computerized player) events that were present in the context of larger interactions that were either generally inclusive or exclusive for the participant overall (Figure 1).
Fig. 1.
Timing of ERP markers during throws in ongoing Cyberball game. Markers were inserted at the first informational frame providing information about the recipient of each throw. The remaining throw frames displaying the completion of the throw are not displayed.
Neuroelectric assessment
The electroencephalogram (EEG) was recorded from 64 sintered Ag–AgCl electrodes with an average-ear reference and forehead ground (AFz). Vertical and horizontal bipolar electrooculographic activity was recorded to monitor eye movements. A Neuroscan Synamps2 bioamplifier (Neuro Inc., El Paso, TX, USA) was used to continuously digitize (500 Hz sampling rate) and low-pass filter (30 Hz; 24 dB/octave) the raw EEG signal. Offline processing of the stimulus-locked ERP included eye blink correction using a spatial filter (Compumedics Neuroscan, 2003), creation of stimulus-locked epochs (−800 to 2500 ms relative to the event marker), baseline removal (800 ms pre-stimulus interval) and artifact rejection (epochs with signal that exceeded ±75 µV were rejected). The N2 component was quantified as the average amplitude in the discrete latency window running from 200 to 320 ms after the event marker at FCz, whereas the P3b component was quantified as the average amplitude in the discrete latency window running from 320 to 450 ms following the event marker at Pz. EEG activity was recorded using Neuroscan Scan software (v4.3.1). Stimulus presentation, timing and the recording of participants’ responses for the Cyberball paradigm were controlled by Neuroscan Stim (v2.0) software.
Statistical analyses
Omnibus 3 (block: inclusion, exclusion and re-inclusion) × 2 [throw type: inclusionary throws (ITs) to the participant and exclusionary throws (ETs) ignoring the participant] repeated-measures analyses of variance (ANOVAs) were conducted separately to compare the average amplitude of the N2 and P3b components across the different trial blocks and types of throw within the Cyberball paradigm. Self-report measures were examined in a four-level (time: baseline, inclusion block, exclusion block and re-inclusion block) repeated-measures ANOVAs, and manipulation check measures were examined in a three-level (block: inclusion, exclusion and re-inclusion) repeated-measures ANOVA to verify the expected pattern of behavioral findings associated with social inclusion and exclusion. Follow-up analyses used repeated-measures ANOVAs and two-tailed paired-samples t tests with Bonferroni correction as appropriate. An experiment-wise alpha level of P ≤ 0.05 was set for all analyses before Bonferroni correction.
RESULTS
Behavioral measures
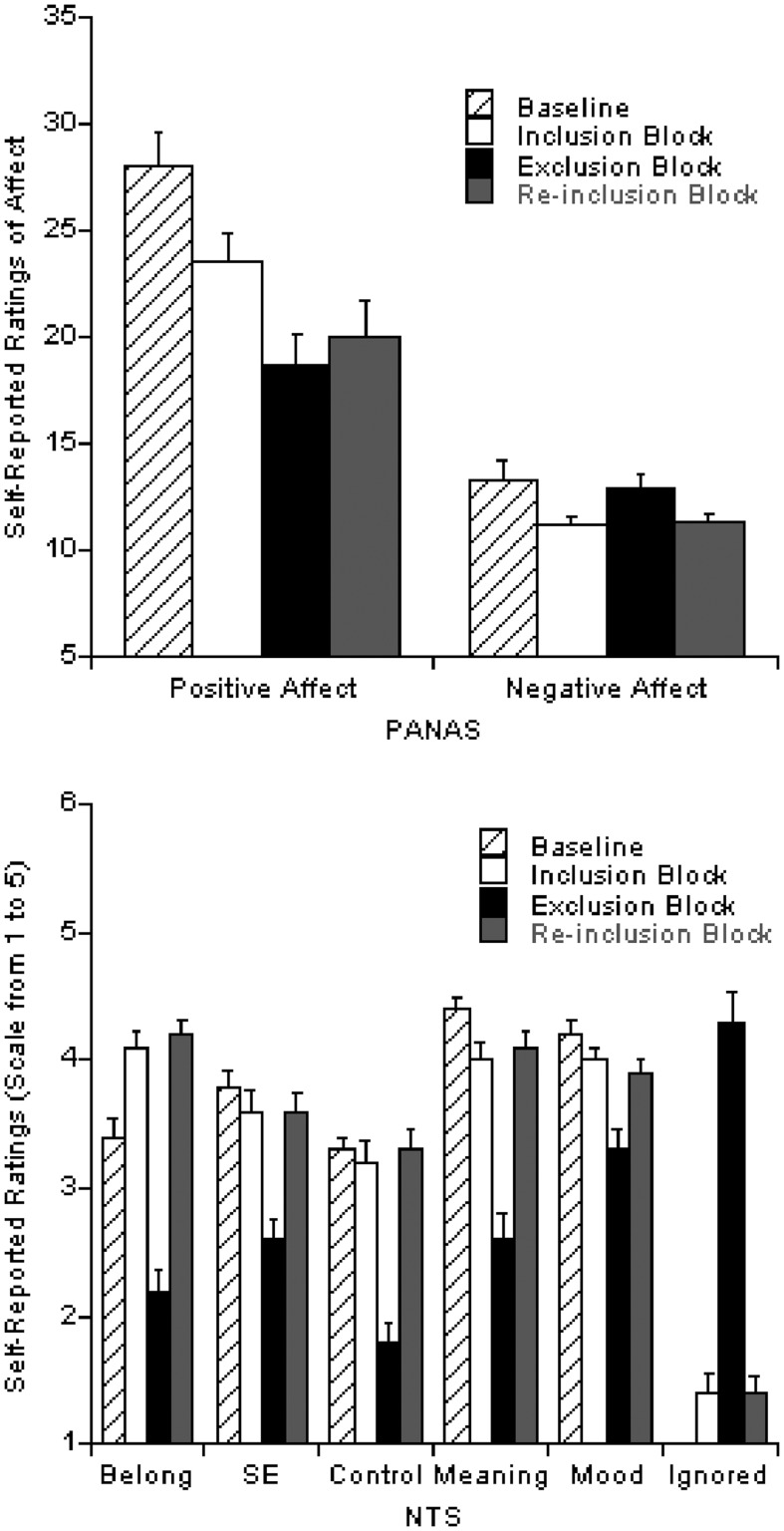
Omnibus analyses revealed the expected block effects for all scales on both the PANAS and NTS, F’s(3,19) ≥ 7.5, P’s ≤ 0.002 and the manipulation check measures in the NTS, F’s(2,20) ≥ 70.1, P’s ≤ 0.001 (Figure 2). Examining pairwise comparisons between different Cyberball blocks and baseline measures for the PANAS and NTS revealed that measures taken following the exclusion block were significantly different from all other measurements on all scales, t’s(21) ≥ 3.6, P’s ≤ 0.002, with the exception of the positive affect (PA) scale of the PANAS, t(21) = 0.9, P = 0.38. These findings suggest that social exclusion resulted in a significant decrease in all needs fulfillment, positive mood and both positive and negative affect compared with baseline reports and measures taken following social inclusion. Further, these changes due to social exclusion were reversed when participants were re-included in the social interaction, with the exception of the influence on PA. The drop in PA due to exclusion persisted through the social re-inclusion, suggesting a lasting decrease in PA due to social exclusion. For the manipulation check measures (e.g. extent ignored/excluded) in which there was no baseline measurement, data obtained following the exclusion block showed significantly greater reporting of being ignored/excluded in comparison with both the inclusion and re-inclusion blocks, t’s(21) ≥ 10.8, P’s ≤ 0.001 (Figure 2).
Fig. 2.
Participants’ self-reported feelings relating to each scale of the Positive and Negative Affect Schedule (PANAS; A) and the Need-Threat Scale (NTS; B) during baseline and following each block of the Cyberball paradigm (inclusion, exclusion and re-inclusion). Error bars represent standard errors in both graphs. Note: Belong = Need for Belonging; SE = Need for self-esteem; Control = Need for Control; Meaning = Need for Meaningful Existence; Mood = Extent Feeling a Positive Mood and Ignored = Extent Feeling Ignored and Excluded.
Neural measures
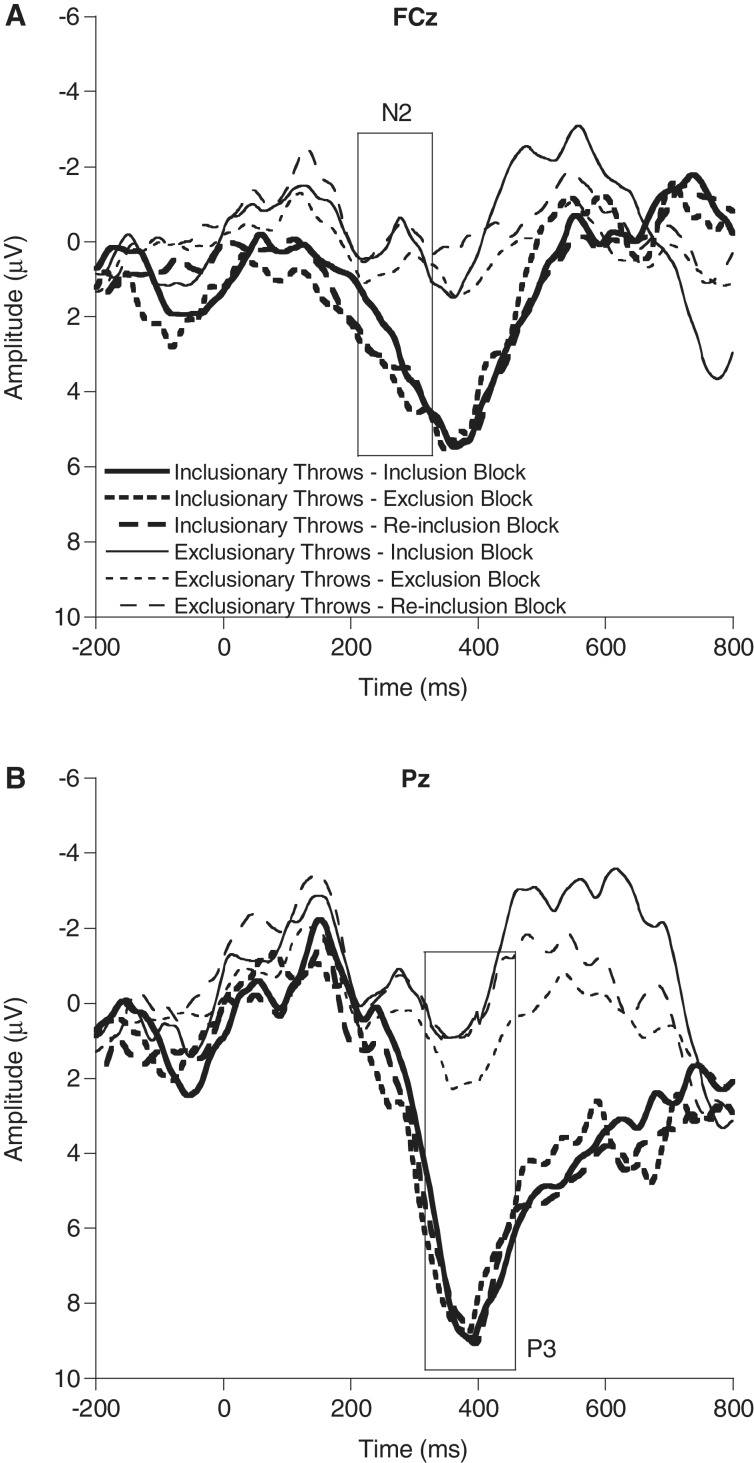
Omnibus ANOVAs revealed that neither the N2 nor the P3b showed a main effect for block [F’s(2,20) ≤ 1.8, P’s ≥ 0.20], indicating the larger context of being included or excluded had no significant impact on the average activation of either the ACC-based conflict monitoring activation theorized to be indexed by the conflict N2 (Yeung et al., 2004) or any adjustments in attentional allocation during the task as indexed by the P3b (Polich, 2007). Importantly, because more exclusionary events would be aggregated together in the exclusion block, the aggregated total of N2 activation would be greater in the exclusion compared with the inclusion block, corroborating previous studies showing block effects for ACC activation (Eisenberger et al., 2003, 2007). In contrast to the block findings, both the N2 [F(1,21) = 57.6, P < 0.001, partial η2 = 0.73] and P3b [F(1,21) = 111.9, P < 0.001, partial η2 = 0.84] revealed significant effects of throw type, with larger N2 amplitude and smaller P3b amplitude for ETs [N2 M(SD) = 0.3(1.7) μV; P3b M(SD) = 0.8(1.9) μV] compared with ITs [N2 M(SD) = 3.1(2.0) μV; P3b M(SD) = 7.6(2.9) μV]. These findings indicate that the neural responses to conflict and the related adaptations in attentional allocation were active during each social interaction (inclusion, exclusion and re-inclusion) but were sensitive to the specific momentary exclusionary events during each of the social interactions rather than the larger overall contexts of the social exchanges. Figure 3 provides ERP waveforms by Cyberball block and throw type, highlighting the observed differences in N2 and P3b amplitudes.
Fig. 3.
Grand-averaged stimulus-locked ERP waveforms during Cyberball for the inclusion, exclusion and re-inclusion blocks (solid, dotted and dashed lines, respectively) for inclusionary throws (thick lines) and exclusionary throws (thin lines) at FCz (A) and Pz (B) electrode sites. Relative to inclusionary throws, exclusionary throws are characterized by larger N2 amplitudes at FCz and smaller P3b amplitudes at Pz. Additionally, P3b amplitude to exclusionary throws was larger during the exclusion block compared with both the inclusion and re-inclusion blocks, with no differences present in P3b amplitude across blocks for inclusionary throws.
Additionally, analyses showed a significant interaction of block by throw type for the P3b [F(2,20) = 3.6, P < 0.05, partial η2 = 0.27]. Separate analyses were conducted across blocks for each throw type (ETs and ITs) and revealed a significant difference in P3b amplitude for ETs across the different blocks [F(2,20) = 15.7, P < 0.001, partial η2 = 0.61], whereas no difference was present in P3b amplitude across blocks for ITs [F(2,20) < 0.1, P = 0.99, partial η2 < 0.01]. Post hoc contrasts among ETs across blocks (t’s(21) ≥ 3.5, P’s ≤ 0.002) revealed that P3b amplitude to ETs during the exclusion block [M(SD) = 2.1(2.0) μV] was larger compared with P3b amplitude in both the inclusion [M(SD) = 0.2(2.4) μV)] and re-inclusion blocks [M(SD) = 0.1(3.0) μV], respectively. No significant interaction was present for the N2. These findings illustrate that the attentional processes reflected by P3b amplitude are heightened to ETs during the exclusion block compared with ETs during either the inclusion or re-inclusion blocks (bottom of Figure 3). This pattern of neural findings justified an examination of the ETs during the exclusion block to determine the nature of the relations between exclusion-specific neural and behavioral processes over time.
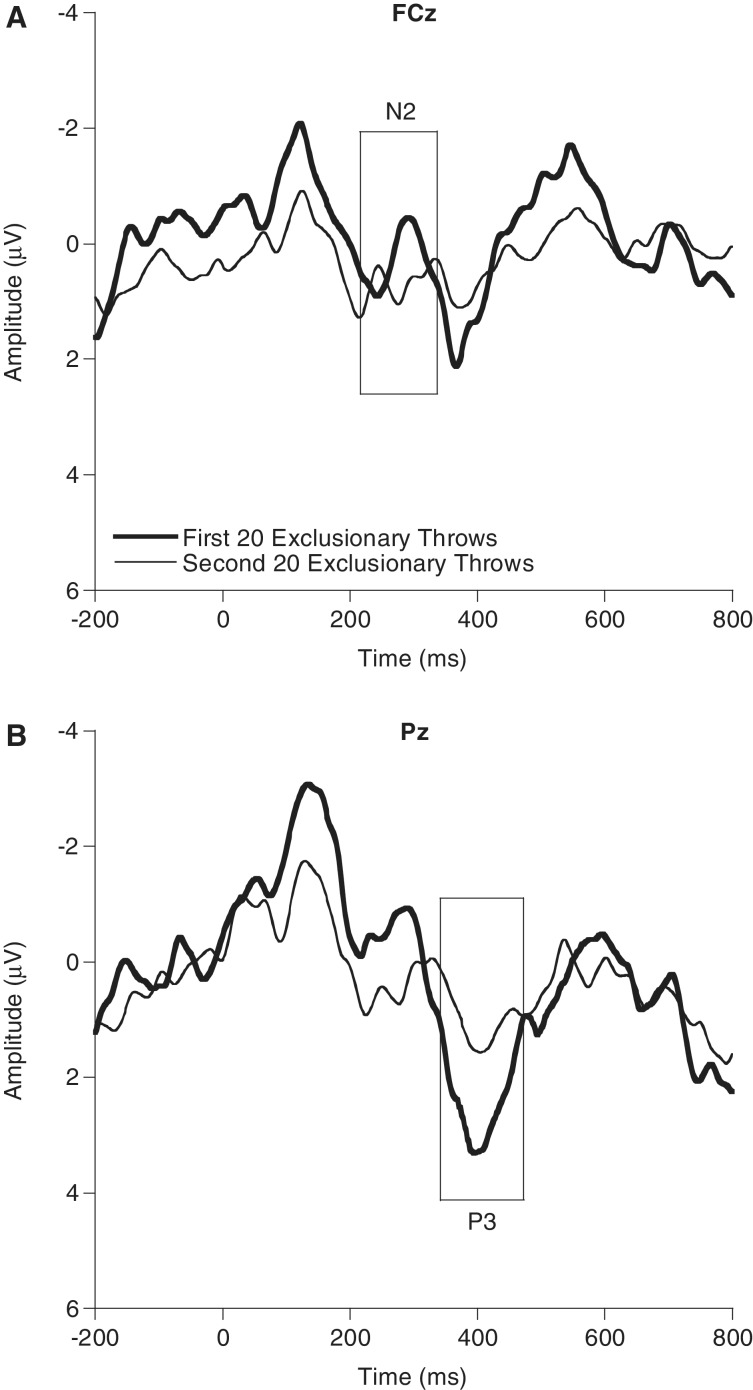
Accordingly, separate two-level repeated-measures ANOVAs were conducted for the N2 and P3b components to examine the potential alterations in neural activation to ETs over the course of the exclusion process. Following the onset of the complete exclusion phase of the exclusion block, the remaining ET trials were examined in 20-trial blocks across time (time: first 20 ETs and second 20 ETs) allowing for the two-level analysis for each components. These analyses identified differences in both the N2 [F(1,21) = 4.7, P = 0.04, partial η2 = 0.18] and P3b [F(1,21) = 5.0, P = 0.04, partial η2 = 0.19] during the course of the exclusionary exchange (Figure 4). Specifically, following the initial social exchange among all players, both N2 and P3b amplitudes were larger (N2 more negative and P3b more positive) during the first 20 complete-exclusion ETs [N2 M(SD) = −0.1(2.2) μV; P3b M(SD) = 3.0(3.6) μV] compared with the second 20 complete-exclusion ETs [N2 M(SD) = 1.0(1.7) μV; P3b M(SD) = 1.0(2.4) μV].
Fig. 4.
Larger N2 amplitude at FCz (A) and P3b amplitude at Pz (B) during the first 20 (thick lines) versus second 20 (thin lines) complete-exclusion exclusionary throws during the exclusion block.
Additionally, the increase in P3b amplitude for ETs from inclusion to the heightened levels in the initial complete-exclusion phase was correlated with participants’ self-reported decreases in PA (r = −0.43, P < 0.05) and feelings of control (r = −0.44, P = 0.04) from inclusion to exclusion. There were no significant correlations for the increase in N2 amplitude. Taken together, these results indicate that exclusion is a dynamic process (Williams et al., 2005) with alterations in the neural response to exclusionary events over the course of ongoing social exclusion. Further, they illustrate that the explicit awareness of exclusion and the related allocation of attention to exclusionary experiences, not the mere activation of the ACC-based conflict monitoring neural alarm, may be more closely related to self-reported feelings in response to social exclusion.
DISCUSSION
Our findings provide evidence for differences in neural activation to specific events within social interactions, regardless of the larger contexts of the interactions. Specifically, we found conflict monitoring neural alarm activation, indexed by the conflict N2, to exclusionary throws (ETs) within largely inclusionary social interactions. This event-related N2 activation did not differ in amplitude to the activation evidenced during ETs within largely exclusionary social interactions and was present in the absence of self-reported social distress. Further, differences in the allocation of attention, indexed by the P3b, were identified within the larger context of the social interactions, with greater attention paid to ETs when they occurred during exclusion compared with inclusion. Additionally, the modulation of the P3b from inclusion to exclusion was associated with the modulation of self-reported social distress from inclusion to exclusion. Finally, patterns of neural activation changed over the course of the ongoing exclusion experienced during the exclusion block, with heightened neural activity earlier, compared with later, in the exclusion.
The conflict N2 was activated by the specific act of being excluded from a social interaction, even if the individual was largely included throughout the social exchange. Similar levels of N2 activation to ETs occurred during both social inclusion and exclusion, suggesting that conflict-based neural alarm activation by itself is triggered by something other than self-reported feelings of distress as there were no reports of social distress following the inclusionary interactions. Our results provide evidence that conflict monitoring is a more sensitive and general process that is not specifically reactive following overtly negative outcomes (i.e. exclusion) but is more broadly activated by any undesired event during the course of an interaction. Consistent with theories of conflict monitoring and cognitive control (Cohen et al., 2000; Botvinick et al., 2001; Yeung et al., 2004; Braver et al., 2007), our data suggest that this system can be activated before the outcome of a task or social interaction to address any conflicting information among ongoing processes (actual/undesired versus intended/desired) and facilitate the optimal completion of a task or adjust attention during task engagement (Botvinick et al., 2001). In this study, we were able to measure the conflict-based neural alarm activation that occurred before the outcome of the larger social interaction, during generally positive and inclusive interactions and independent from self-reported feelings resulting from the larger interactions.
Further, our results indicate that the findings associating ACC-based conflict monitoring activation with exclusion and social distress (Eisenberger et al., 2003) are not due to the singular activation of the neural alarm circuitry during exclusion or an increased magnitude or strength of that activation resulting from negative feelings of social distress. Instead, it seems that the association is due to the increased frequency of conflict-based neural activations over repeated ETs during exclusion compared with inclusion, which were aggregated over the duration of the entire social interaction.
Moreover, our findings suggest the explicit awareness or perception of being excluded and the related allocation of attention to the exclusionary experience, indexed by the P3b, may be more closely associated with self-reported social distress outcomes of exclusion. Thus, in contrast to previous hypotheses (Eisenberger et al., 2003), our findings indicate that reports of social pain would not be reported following an ‘implicit social exclusion’ condition (Eisenberger et al., 2003) even though conflict-based ACC activation was present. Rather, in this condition, we suggest that self-reported levels of social distress would be similar to levels shown during an inclusion condition as the participant would not develop explicit perceptions of being excluded and would not display enhanced neural activations in parietal attentional networks reflecting the conscious allocation of attention toward being excluded.
These findings are consistent with the hypothesis that any event, or string of events, that is powerful enough to warrant the perception of exclusion and direct one’s attention toward being excluded is powerful enough to elicit the damaging cognitive and emotional consequences of exclusion. This is similar to how exclusion can work in the real world (Williams, 2001), where one could be fully included for an interaction or series of interactions, but one important exclusionary moment or event can lead to devastating outcomes (Williams, 2001; Baumeister et al., 2002; MacDonald and Leary, 2005; Williams et al., 2005). Accordingly, perceptions of social exclusion and feelings of social distress could result from almost any type of social interaction, even those that are largely inclusionary, as long as conscious self-regulatory control processes, and the related allocation of attention toward the exclusionary events, are engaged.
With respect to alterations in neural activity over the course of extended exclusionary events, these changes could reflect a decrease in exclusion-related conflict and attentional allocation over time, implying that the participants effectively became habituated or desensitized to the exclusionary experience over time (Rule et al., 2002; Nieuwenhuis et al., 2003). Alternatively, the prolonged repetition of exclusionary events could have depleted the neural alarm and self-regulatory attentional systems, resulting in social cognitive deficits similar to those hypothesized in cognitive deconstruction (Baumeister et al., 2002), leaving targets of exclusion unable to properly respond to being excluded from an interaction. On the basis of the present data, we cannot determine which explanation is most likely as both could potentially elicit the observed reductions in N2 and P3b amplitudes over the course of exclusion.
Limitations and future directions
Although our analyses were able to determine the extent to which patterns of neural activation were independently associated with specific events during social interactions, it is important to note the limitations of this study. The relatively small sample size, the severe constraints used to create the exclusionary interaction and the poor spatial resolution of ERPs limit the strength of the findings. Future examinations should implement a broader array of exclusion manipulations and even combined multiple measures of neural activation (fMRI and ERP) to more completely assess the relationships between neural alarm activation, attentional allocation and self-reported social distress during a variety of exclusionary social interactions. Further, future studies examining more participants and their individual differences are warranted to investigate key variables that may moderate or mediate exclusion-related effects on neural activity and behavioral responses to social interactions.
Summary
This study offers new empirical insights into the dynamic nature of social interactions by exploring the event-related differences in neural activation present during social inclusion and exclusion. We have shown that conflict-based neural activation is present during largely inclusionary interactions, and social distress is associated with shifts in attention toward exclusionary events in the midst of a social exchange. Further, we showed that alterations in attentional processing toward exclusion occurred immediately in response to an increase in exclusionary events. This implies that perceptions and consequences of social exclusion can develop rapidly from a variety of interactions not only from social interactions that are largely exclusionary but also from any interaction in which explicit perceptions of exclusion may develop. Thus, these data call for the re-examination of how perceptions of exclusion occur in terms of the momentary dynamic processes within social interactions in addition to the larger contexts of social exchanges.
Conflict of Interest
None declared.
Acknowledgments
This research was supported by a grant from the National Science Foundation (MRI 0722526) to Illinois Wesleyan University (PI: Joseph Williams) and a grant from Illinois Wesleyan University to J.R.T.
REFERENCES
- Baumeister RF, Twenge JM, Nuss C. Effects of social exclusion on cognitive processes: anticipated aloneness reduces intelligent thought. Journal of Personality and Social Psychology. 2002;83:817–27. doi: 10.1037//0022-3514.83.4.817. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition, and errors. Cerebral Cortex. 2001;11:825–36. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who’s in control? Nature Neuroscience. 2000;3:421–3. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Compumedics Neuroscan. Offline Analysis of Acquired Data (SCAN 4.3 – Vol. II, EDIT 4.3) 2003. [Software Manual]. El Paso, TX: Compumedics Neuroscan. [Google Scholar]
- Donchin E. Surprise! … Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;7:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt: an fMRI study in social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: clues from the brain’s response to social exclusion. Biological Psychiatry. 2007;61:1100–1108. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–70. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz EL, White LE., Jr Pain “relief” by frontal cingulumotomy. Journal of Neurosurgery. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport: For Rapid Communication of Neuroscience Research. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–77. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Kopp B. Mechanisms of priming by masked stimuli: inferences from event-related brain potentials. Psychological Science. 1998;9:263–9. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdale activity to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131:202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Polich JP. Updating P300: an integrative theory of P3a and P3b. Clinical Neuropsychology. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biological Psychology. 1995;41:103–46. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Raab P, Neumann K, et al. Event-related fMRI for the suppression of speech-associated artifacts in stuttering. Neuroimage. 2003;19:1076–84. doi: 10.1016/s1053-8119(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:264–70. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, et al. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MS, Stevens M, Harenski CL, Kiehl KA. Neural correlates of the processing of another’s mistakes: a possible underpinning for social and observational learning. Neuroimage. 2008;42:450–9. doi: 10.1016/j.neuroimage.2007.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. Journal of Comparative Neurolology. 1987;262:271–89. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- von Schie HT, Mars RB, Coles MGH, Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nature Neuroscience. 2004;7:549–54. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism: The Power of Silence. New York: Guilford Press; 2001. [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–62. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Forgas JP, von Hippel W, Zadro L. The social outcast: an overview. In: Williams KD, Forgas JP, von Hippel W, editors. The Social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying. New York: Psychology Press; 2005. pp. 1–16. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer lowers belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40:560–7. [Google Scholar]