Abstract
Significance: Platyhelminth parasites cause chronic infections that are a major cause of disability, mortality, and economic losses in developing countries. Maintaining redox homeostasis is a major adaptive problem faced by parasites and its disruption can shift the biochemical balance toward the host. Platyhelminth parasites possess a streamlined thiol-based redox system in which a single enzyme, thioredoxin glutathione reductase (TGR), a fusion of a glutaredoxin (Grx) domain to canonical thioredoxin reductase (TR) domains, supplies electrons to oxidized glutathione (GSSG) and thioredoxin (Trx). TGR has been validated as a drug target for schistosomiasis. Recent Advances: In addition to glutathione (GSH) and Trx reduction, TGR supports GSH-independent deglutathionylation conferring an additional advantage to the TGR redox array. Biochemical and structural studies have shown that the TR activity does not require the Grx domain, while the glutathione reductase and deglutathionylase activities depend on the Grx domain, which receives electrons from the TR domains. The search for TGR inhibitors has identified promising drug leads, notably oxadiazole N-oxides. Critical Issues: A conspicuous feature of platyhelminth TGRs is that their Grx-dependent activities are temporarily inhibited at high GSSG concentrations. The mechanism underlying the phenomenon and its biological relevance are not completely understood. Future Directions: The functional diversity of Trxs and Grxs encoded in platyhelminth genomes remains to be further assessed to thoroughly understand the TGR-dependent redox network. Optimization of TGR inhibitors and identification of compounds targeting other parasite redox enzymes are good options to clinically develop relevant drugs for these neglected, but important diseases. Antioxid. Redox Signal. 19, 735–745.
Introduction
Platyhelminthes is a phylum of metazoan organisms that includes two major classes of human and livestock parasites: trematodes (flukes) and cestodes (tapeworms). Platyhelminth parasites have complex life cycles with both sexual and asexual reproductive phases and undergo metamorphoses and migrations within their multiple hosts. Platyhelminth infections can result in chronic (lasting for years) infections. The disability and poverty generated by platyhelminth infections are an enormous health and economic burden for low-income countries. Schistosomiasis alone, the most prevalent and devastating infection caused by trematodes, affects more than 200 million people and causes more than 200,000 deaths in tropical and subtropical areas of Africa, South America, and Asia. It is estimated that the global prevalence of food-borne trematodiases is >40 million people (25). These infectious agents include Opisthorchis spp. and Clonorchis spp. (liver flukes causing cholangitis and bile duct cancer), Paragonimus spp. (lung flukes), and Fasciolopsis buski and Fasciola hepatica (intestinal and liver flukes, respectively). Cestode infections are less prevalent, yet some of them, such as cysticercosis and hydatid disease, constitute significant problems to human health; for example, neurocysticercosis caused by Taenia solium is the major cause of acquired epilepsy worldwide (19). Platyhelminthes also result in widespread infections in economically important domestic animals.
Parasites causing chronic infections, such as platyhelminthes, are excellent model organisms to study pathways involved in redox homeostasis. These organisms are exposed not only to the oxidant species endogenously generated as a result of their own metabolism, but also to those derived from the immune response mounted by their hosts. The study of antioxidant defenses in platyhelminth parasites has revealed a unique biochemical scenario and novel drug targets for platyhelminth infections. This is highly relevant for the neglected diseases caused by these infections: few drugs are currently available for the treatment of platyhelminth infections. Schistosomiasis treatment relies on the use of only one drug, praziquantel, which is also effective against other platyhelminthes. However, this drug is massively used to treat millions of people every year (there is little acquired immunity to reinfection) and there is justified concern for the emergence of resistance (12).
Antioxidant Defenses in Platyhelminth Parasites Are More Precarious Than Anticipated
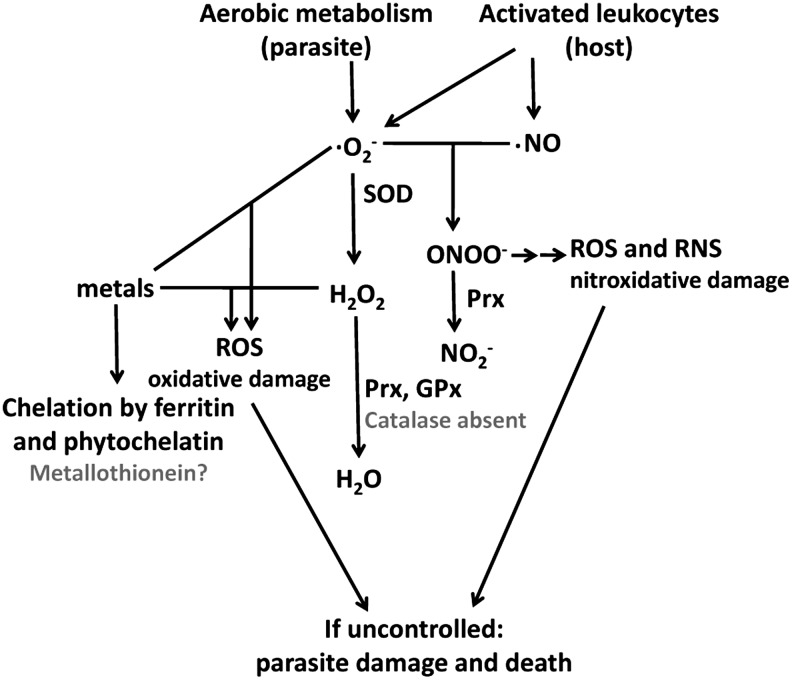
Platyhelminth parasites must cope with the reactive oxygen species (ROS) derived from their aerobic metabolism, and should withstand ROS and reactive nitrogen species (RNS) derived from the host's activated immune cells. Once activated by parasites, recruited leukocytes and resident macrophages can release large amounts of nitric oxide radical (•NO) and superoxide radical anion (O2•−) (Fig. 1). These species are not intrinsically harmful, but can react with each other giving rise to peroxynitrite, a highly reactive oxidant and nitrating species that generates nitrosative stress. Superoxide anion radical dismutation (spontaneous and catalyzed by superoxide dismutase [SOD]) leads to hydrogen peroxide (H2O2) production. H2O2 can react with metal ions (Fenton reaction) to generate hydroxyl radical (•OH), a highly oxidizing species (Fig. 1). Recruited and activated neutrophils and eosinophils also release the heme-containing enzymes myeloperoxidase (EC 1.11.2.2) and eosinophil peroxidase (EC 1.11.1.7), respectively. These enzymes catalyze the conversion of H2O2 and halides into hypohalous acids that are also powerful oxidants (18, 57). Collectively, ROS and RNS impair protein function, nitrate lipids and initiate lipid peroxidation, which leads to radical chain reactions further damaging membranes, nucleic acids, and proteins. These processes may ultimately lead to parasite death. To withstand oxidative stress parasites rely on antioxidant enzymes. Parasitic platyhelminth antioxidant enzymes have been characterized in recent years revealing both similarities and striking differences with those of parasitic platyhelminth's hosts and free-living platyhelminthes.
FIG. 1.
Simplified map of oxidants and platyhelminth antioxidant defenses. Superoxide radical anion (O2•−) is a by-product of aerobic metabolism. Large amounts of O2•− and nitric oxide (•NO) are generated by activated host leukocytes through NADPH oxidase and inducible NO synthase. Superoxide anion and NO radicals can give rise to ROS and RNS. SOD prevents peroxynitrite formation, but generates H2O2 that in the presence of metals (Fenton reaction) can generate hydroxyl radical, a highly damaging ROS. Superoxide is able to reduce metal ions, therefore, contributing to hydroxyl radical generation by H2O2. H2O2 is reduced by Prx and GPx; protection against metals in platyhelminthes is provided by ferritins and phytochelatins. Some Prxs can also reduce peroxynitrite, although Kumagai et al. (31) have suggested that Schistosoma japonicum Prxs may not be protective against •NO-derived oxidants. Metallothionein and catalase (common eukaryotic defenses against metals and H2O2, respectively) are shown in gray; catalase is absent in platyhelminth parasites and metallothionein is likely absent (see text). GPx, glutathione peroxidase; H2O2, hydrogen peroxide; •NO, nitric oxide; Prx, peroxiredoxin; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase.
Similar to most eukaryotic organisms, parasitic platyhelminthes possess Cu/Zn and Mn SOD that decompose O2•− in the cytosolic and mitochondrial compartments, respectively. Surprisingly, early biochemical observations suggested that parasitic platyhelminthes lack catalase (36), a highly efficient enzyme that converts H2O2 to oxygen and water. The sequencing of platyhelminth genomes from class Trematoda (Schistosoma spp.) and class Cestoda (Echinococcus spp.) confirmed the absence of genes encoding catalase (Fig. 2). It is interesting to note that the catalase gene is present in free-living platyhelminthes (e.g., Schmidtea mediterranea), and other animal lineages, suggesting that this gene has been lost in the parasitic neodermata lineage. Detoxification of peroxides in parasitic platyhelminthes relies on thiol- and selenol-dependent peroxidases: 2-Cys peroxiredoxins (Prxs) and selenocysteine (Sec)-containing glutathione peroxidases (GPxs), which are able to reduce H2O2 to water and lipid hydroperoxides to alcohols. There are three Prx genes in parasitic platyhelminth genomes, two of them encode cytosolic proteins and a third one encodes a mitochondrial protein (51). These Prxs have been characterized in Schistosoma mansoni and Schistosoma japonicum (30–31, 49, 51), all of them reduce H2O2 and receive electrons from thioredoxin (Trx), and two of them can also receive electrons from glutathione (GSH) (51). One Prx in each schistosome species is found at the worm surface or in egg secretions, areas of increased oxidative stress. Interestingly, these specific Prx proteins show a biochemical transition toward increased activity under higher oxidizing conditions (51). GPxs reduce H2O2 and lipid hydroperoxides to water and the corresponding alcohols, respectively. There are two Sec-containing GPxs present in parasitic platyhelminth genomes corresponding to cytosolic and secreted isoforms, both of them most closely related to mammalian GPx4, which is active mostly against lipid peroxides (49). Indeed, the biochemically characterized GPx protein from S. mansoni is more active with lipid peroxides than with H2O2 (35).
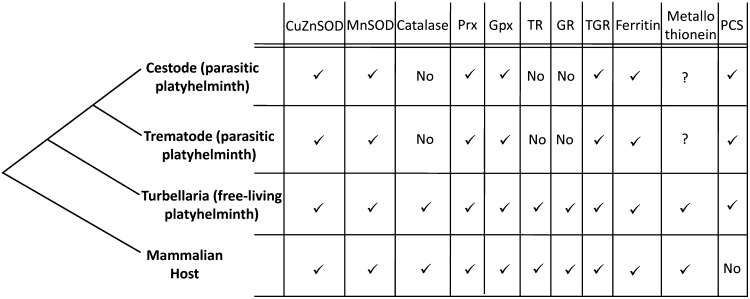
FIG. 2.
Antioxidant defenses of parasitic and free-living platyhelminthes and their mammalian hosts. Genes encoding antioxidant defenses are shown in a phylogenetic context. Class Cestoda and Class Trematoda constitute the neodermata monophyletic lineage of the platyhelminth phylum, which comprises exclusively parasitic organisms. The class Turbellaria of platyhelminthes includes free-living organisms such as the planaria Schmidtea mediterranea, whose genome has been sequenced, and nonhuman parasitic organisms, none of which has been sequenced. Remarkably, platyhelminth parasites have lost genes involved in antioxidant defenses such as catalase, GR, and TR and possess streamlined redox pathways. GR, glutathione reductase; PCS, phytochelatin synthase; TGR, thioredoxin glutathione reductase; TR, thioredoxin reductase.
As already mentioned, metals ions (in particular Fe2+ and Cu+1) are potentially dangerous because they can drive redox reactions and generate damaging species (Fig. 1). Therefore, sequestration of metal ions under conditions of stress is an important antioxidant defense strategy. In mammals, among other proteins, ferritins (an “iron cage”) and metallothioneins (Cys-rich proteins that chelate copper and heavy metals) are the key players in maintaining intracellular iron and copper ions tightly bound and redox inactive, helping to suppress their pro-oxidant effects. Platyhelminth parasites have genes encoding ferritins (16, 28), but metallothioneins have not been described (and their identification by similarity is not trivial). Interestingly, it has recently been proposed that copper and heavy metal sequestration in platyhelminth parasites probably relies on phytochelatins, Cys-rich oligopeptides with the basic structure of (γ-GluCys)nGly, with n=2–11 (43). Phytochelatins are synthesized from GSH by phytochelatin synthase; this enzyme recently described in S. mansoni is absent in humans, and therefore it is a potential new target enzyme for drug development (43). This finding also highlights the key role of thiol-dependent antioxidant defenses in these organisms. Figure 2 compares the antioxidant enzymes and proteins present in free-living- and parasitic platyhelminthes and in mammalian genomes.
Thioredoxin Glutathione Reductase Is the Sole Enzyme That Supports Both Trx and GSH Pathways in Platyhelminth Parasites
In most organisms, including the mammalian hosts of platyhelminthes, the Trx and GSH pathways play a key role in a variety of cellular processes, such as DNA synthesis, defense against oxidative stress, detoxification, protein folding, and repair (Fig. 3A). Both systems operate through redox cascades that involve transfer of reducing equivalents from NADPH to targets through reversible dithiol-disulfide reactions.
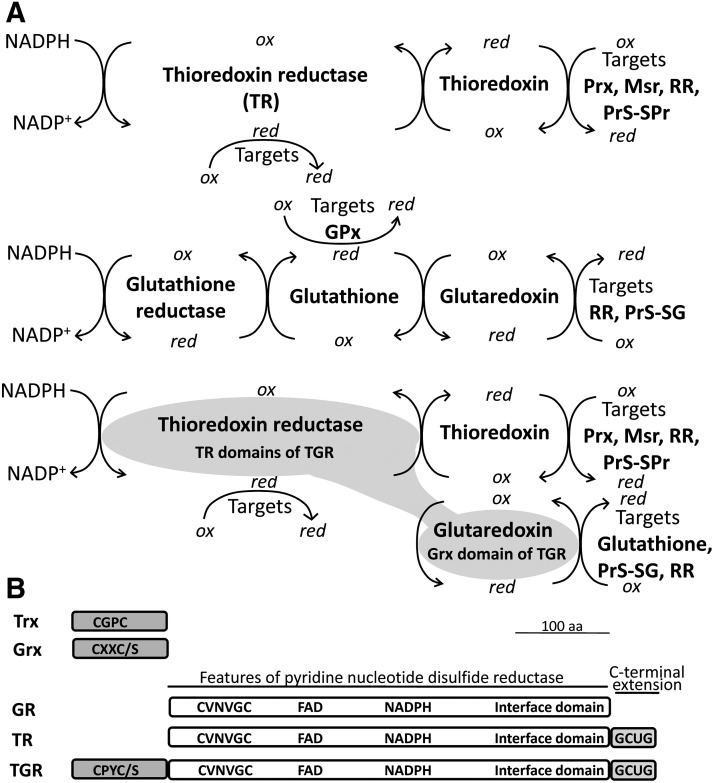
FIG. 3.
Simplified Trx, GSH, and thioredoxin-glutathione linked systems. (A) Electron flow in the Trx, GSH, and linked Trx-GSH systems. The Trx system comprises TR and Trx; the GSH system is composed by GR, GSH, and Grx; and in the linked Trx-GSH system TGR, a fusion of an N-terminal Grx domain to TR domains shown in grey functionally replaces TR, GR, and Grx, and provides reducing equivalents to targets of both pathways. Note that while electrons to the oxidized Grx in the GSH system are provided by reduced GSH, this step of electron flow is reversed in the Trx-GSH linked system: the Grx domain of TGR reduces GSSG to its reduced form. (B) Scheme of Trx, Grx, GR, TR, and TGR domain organization and redox centers (see text for detailed description). Adapted from ref. (48). GSH, glutathione; GSSG, oxidized glutathione; Msr, methionine sulfoxide reductase; PrS-SG, glutathione-protein mixed disulfide; Prx, peroxiredoxin; RR, ribonucleotide reductase; Trx, thioredoxin.
The Trx system comprises the flavoenzyme thioredoxin reductase (TR) and Trx, a powerful disulfide reductase with a catalytic dithiol/disulfide. Trx provides reducing equivalents to a variety of enzymes containing redox active cysteine pairs as part of their catalytic cycle, including ribonucleotide reductase, an essential catalyst for the synthesis of deoxynucleotides; antioxidant enzymes such as Prxs; and repair enzymes such as methionine sulfoxide reductases (Msrs, see below). Trx is also directly involved in blocking oxidative stress as a generic protein disulfide reductase. In addition, Trx exerts redox control of regulatory proteins involved in signal transduction and gene transcription (24). The reduction of the disulfide of Trx redox-active site by NADPH is catalyzed by TR. Animal TRs belong to class I pyridine-nucleotide disulfide oxidoreductases. They contain an N-terminal CX4C motif at the active site in the FAD-binding domain and a carboxy-terminal redox active center that mediates electron transfer from the CX4C redox active site to TR substrates. The carboxy-terminal center usually contains redox active Cys and Sec residues, within the conserved motif GCUG (U denotes Sec). In addition to Trx, TR can reduce other substrates including low-molecular weight antioxidants such as the oxidized forms of lipoamide, lipoic acid, and ascorbic acid (dehydroascorbate). It has been suggested that most of the NADPH-dependent lipoamide and lipoic acid dehydrogenase activities in mammalian cells should be attributable to TR (7), although the physiological relevance of lipoic acid as antioxidant has been questioned due to the low concentration of its free lipoic acid.
The GSH system consists of glutathione reductase (GR), GSH, and glutaredoxin (Grx). As with TR, GR is a class I pyridine-nucleotide disulfide oxidoreductase that contains a CX4C motif at the N-terminal FAD-binding domain, but lacks the C-terminal redox center present in TR and thioredoxin glutathione reductase (TGR). GR transfers the reducing equivalents of NADPH exclusively to the dimeric oxidized form of GSH (oxidized glutathione [GSSG]). GSH constitutes the most abundant thiol-containing compound in cells (ranging from 0.5 to 10 mM), being the major non-protein thiol-based redox buffer. GSH acts as a general antioxidant molecule within the cell, provides electrons to GPx, and recycles Grx to its reduced state. In addition, GSH serves a detoxifying role for hydrophobic electrophiles (including products of lipid peroxidation and xenobiotics) and methylglyoxal (a side product of glycolysis and other metabolic pathways) in reactions catalyzed by GSH S-transferases and the glyoxalase system, respectively. Grxs are small thiol-disulfide oxidoreductases that belong to the Trx superfamily, have a similar redox active site, and transfer electrons to their substrates and substrate reductases such as protein-GSH mixed disulfides and ribonucleotide reductase. A particular type of Grxs, the monothiol group, plays an important role in Fe/S cluster assembly and mobilization in the mitochondria.
Typically, Trx and GSH systems are present in both the cytosol and the mitochondria. In mammals three TR isoenzymes are present. TR1 (also known as TrxR1 and Txnrd1) and TR3 (also known as TrxR2 and Txnrd2), encoded by different genes, function in the cytosol and the mitochondria, respectively. A third gene encodes a TGR (also known as TR2, TrxR3, and Txnrd3) a versatile enzyme whose expression is largely restricted to testis (54). TGR also belongs to class I pyridine-nucleotide disulfide oxidoreductases and consists of a natural fusion of conventional TR domains to an N-terminal Grx domain (56). This enzyme contains a CX4C motif proximal to the FAD-binding domain, a carboxy-terminal GCUG redox active center and an additional redox center at the N-terminus extension in a Grx-like domain containing a redox-active CX2C or CX2S motif (56). GR exists as a distinct gene and is alternatively spliced to produce both cytoplasmic and mitochondrial variants (29). GSH and isozymes of Trx and Grx are present in both cytosolic and mitochondrial compartments. Figure 3B depicts a scheme of primary sequence and redox centers of TR, GR, TGR, Trx, and Grx.
In contrast to their hosts, parasitic platyhelminthes lack conventional TR and GR (1, 3, 21, 44). Instead, they rely exclusively on a linked Trx-GSH system in which TGR provides reducing equivalents to both pathways (Fig. 3A). The early biochemical studies were later supported by the genomic information of platyhelminth parasites. In these organisms, cytosolic and mitochondrial TGR variants are derived from a single TGR gene and have identical amino acid sequences (1). Nevertheless, the cytosolic isoform of T. crassiceps TGR showed a higher sensitivity to calcium than the mitochondrial variant, suggesting that these isoforms may be conditioned by their environments (e.g., by post-translational modifications) (20). It is interesting to note that the genome of S. mediterranea encodes TGR and conventional GR and TR, suggesting that GR and TR genes were lost in the neodermata lineage (39) (Fig. 2).
Structural and Biochemical Studies Have Revealed Details of TGR, a Complex Selenoenzyme
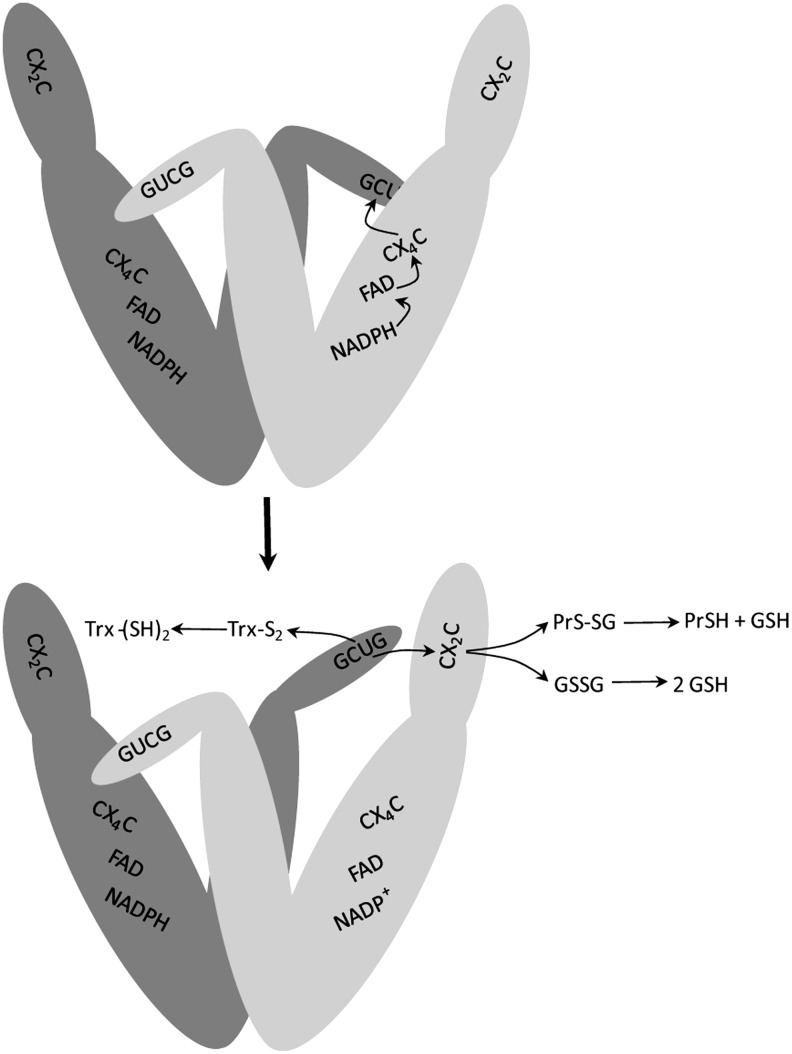
TGR, like GR and TR, is a homodimer with monomers oriented in an inverted manner forming a twisted “W” shape (5). The current model for the reaction mechanism of TGR, based on biochemical and structural studies, indicates that electrons flow from NADPH to FAD, to the CX4C redox center, and then to the C-terminal GCUG redox center of the second subunit, as it is the case in mammalian TRs. The reduced GCUG redox center can shuttle electrons to the CX2C redox center of oxidized Trx (TR activity) or to the CX2C redox center of the Grx domain of the first subunit, which then can reduce GSSG (GR activity; Fig. 4) (4, 5, 8, 26). The proposed electron pathway implies that the Sec-containing redox center is within a flexible “whip” at the carboxy terminus of TGR; consistent with this view, the C-terminal tail of TGR has not been visualized in the available structures of TGR (4, 6).
FIG. 4.
Model of the proposed reaction mechanism of TGR. The active form of TGR is a dimer of identical subunits. In the upper part, the flow of reducing equivalents in the TR module of TGR is shown. In the lower part, reduction of the GCUG redox center produces a structural change in the flexible carboxy-terminal arm allowing it to be repositioned to either react with the glutaredoxin domain active site (CX2C) or directly with oxidized Trx (Trx-S2). The reduced glutaredoxin domain can then interact with its substrates, glutathione disulfide (GSSG) or glutathionylated proteins (PrS-SG). Adapted from ref. (41).
It has been recently shown that, in addition to having TR and GR activities, TGR can also reduce glutathionylated peptides (8). The reversible protein S-thiolation by GSH is emerging as a new mechanism involved in redox control of protein function and in the protection of key Cys residues from over-oxidation (15). It is thought that glutathionylation occurs spontaneously, while deglutathionylation is achieved by thiol-disulfide oxidoreductases, mainly by Grxs. Deglutathionylation by Grxs involves a glutathionylated intermediate generated by the nucleophilic attack of the N-terminal Cys of the CX2C active site of the Grx domain, that is then reduced by GSH (17). Recent studies have shown that in platyhelminth TGRs, the Grx domain can deglutathionylate substrates receiving electrons not only from GSH, but also from the TR domains (1, 8, 26). This dual electron donor specificity has only been observed for Grxs in a few cases (e.g., human Grx2) (27). In TGR, the efficiency of this process would be optimized since the electron transfer from TR to Grx occurs intramolecularly, avoiding the need for diffusion. Through this particular array deglutathionylation may occur under a broader range of conditions (e.g., when reduction of the Grx domain by GSH might be thermodynamically unfavorable because of a low [GSH]/[GSSG] ratio).
Recently, the role of the CX2C active site of the Grx domain and of the Sec residue of TGR in GR and deglutathionylase activities has been examined in recombinant E. granulosus and S. mansoni TGRs (EgTGR and SmTGR, respectively) (8, 26). The N-terminal nucleophilic Cys residue of the Grx domain is essential for both functions; Cys to Ser/Ala mutants have marginal activity. Therefore, for both activities, the mechanism of catalysis involves a glutathionylated intermediate with the N-terminal Cys of the Grx domain. Cys to Ser mutants in the carboxy-terminal Cys residue of EgTGR and SmTGR are fully active in GSSG reduction, while a Cys to Ala mutant in SmTGR has significantly lower activity. These results suggest that the C-terminal Cys residue of the Grx domain may stabilize the thiolate of the N-terminal Cys and that resolution can be achieved without the formation of the disulfide intermediate within the CX2C motif. These observations, together with the absence of GR activity of the Sec (the penultimate residue) to stop mutants of EgTGR and SmTGR and the marginal activity of the Sec to Cys mutants, indicate that the Sec-containing redox center of the TR module can resolve the glutathionylated intermediate (Fig. 5, route A) (8, 26). Studies on the deglutathionylase activity have revealed that alternative routes for the resolution of the glutathionylated intermediate may be operative (26). Deglutathionylation has been studied by different methods for EgTGR and SmTGR. The deglutathionylase activity of EgTGR was measured using a glutathionylated peptide and NADPH as substrates (8), while the activity of SmTGR has been evaluated using the classical hydroxyethyl disulfide (HED) assay (23), which includes GSH in the reaction mix (Fig. 6 shows a scheme of deglutathionylase activity assays). Similar to the GR activity, the Cys to Ser mutant in the C-terminal Cys residue of the Grx domain redox center of EgTGR had full deglutathionylase activity on a glutathionylated peptide while the Sec to Cys mutant is virtually inactive. Using the HED assay, both the Sec to Cys mutant and the Cys to Ser mutant in the C-terminal Cys of the Grx domain of SmTGR had markedly decreased activities, indicating that this Cys would participate in the resolution of the mechanism (Fig. 5, route B) (26). Taken together, these results indicate that Sec is vital to the GCUG shuttling electrons to the Grx active site; as for the GR activity, the glutathionylated intermediate can be resolved by the Sec-containing redox center, either directly or indirectly (Fig. 5, routes A and B, respectively). The fact that in SmTGR 40% of the activity was preserved in the Sec to Cys mutant, indicates that the Grx domain can function uncoupled from the TR module when GSH is present in the reaction mix (Fig. 5, route C) (26). Thus, resolution of the glutathionylated intermediate could take place by three different routes, and some of the differences observed between EgTGR and SmTGR may be inherent to the enzymes or, more likely, due to the experimental conditions of the different assays (e.g., presence or absence of GSH).
FIG. 5.
TGR deglutathionylation routes. The figure depicts three possible routes for deglutathionylation. In all cases the N-terminal Cys residue (CN) of the Grx domain (depicted in white) is glutathionylated. Then, the glutathionylated enzyme intermediate can be resolved by the carboxy-terminal redox center in the TR module (of the other subunit of the homodimer, depicted in dark gray) regenerating the reduced form of the Grx domain (Route A). Alternatively, the resolution of the intermediate might be achieved by the carboxy-terminal Cys residue (CC) of the Grx redox center forming a disulfide, which would then be resolved by the Sec-containing redox center of the TR module (Route B). At high GSH concentrations the glutathionylated intermediate could be resolved by GSH, as in conventional Grxs (Route C). U; selenocysteine.
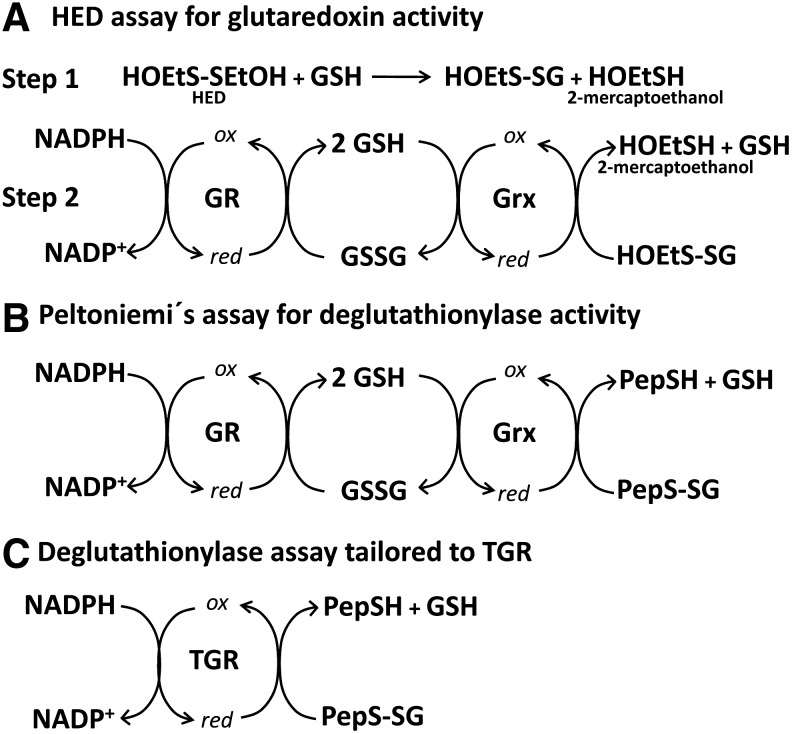
FIG. 6.
Enzymatic assays for measuring the ability of conventional Grxs and TGR to reduce GSH-mixed disulfides. (A) In the HED assay (23) (the method traditionally used to evaluate Grx activity of conventional Grxs), the ability of these enzymes to reduce an artificial low-molecular weight mixed-disulfide (hydroxyethylmercaptan-GSH) prepared by mixing GSH and HED (Step 1), using GSH as an electron donor, is evaluated. The reaction is followed by coupling it to the NADPH-dependent reduction of GSSG by a GR (Step 2). The rate of NADPH oxidation (decrease in the absorbance at 340 nm) is measured. (B) The deglutathionylation assay described by Peltoniemi et al. (40) uses a glutathionylated heptapeptide (PepS-SG) as a physiological GSH-mixed disulfide. This peptide is glutathionylated at a cysteine residue that is adjacent to a tryptophan. The fluorescence of this tryptophan at 356 nm after being excited at 280 nm is partially quenched by the presence of the GSH moiety in the adjacent Cys. The rate of reduction of this substrate by Grxs using GSH as an electron donor is followed by measuring the increase in the intensity of fluorescence at 356 nm. Since this reaction is reversible it must be coupled to the NADPH-dependent removal of GSSG by a GR. (C) A deglutathionylase assay “tailored” to TGRs based on Peltoniemi's assay (40). The same glutathionylated heptapeptide (PepS-SG) substrate is used but in this case the rate of the NADPH-dependent (and GSH-independent) reduction of this substrate by TGR is evaluated by following NADPH oxidation (decrease in absorbance at 340 nm). This last method avoids the need for GSH and therefore allows the GSH-independent deglutathionylase activity of TGR to be evaluated. HED, hydroxyethyl disulfide.
Hysteresis Is a Conspicuous Feature of Platyhelminth TGRs
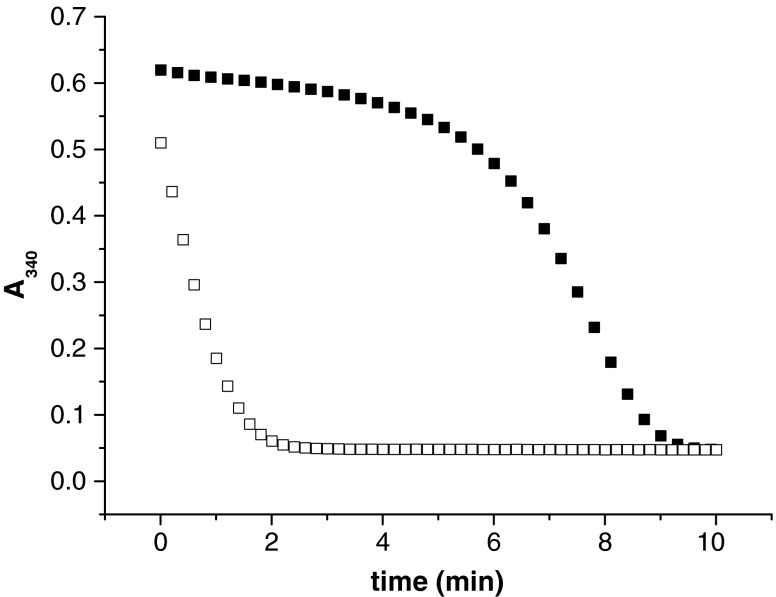
Another piece of the puzzle, to understand how the Grx domain of platyhelminth TGRs functions and the role of the C-terminal Cys, arises from the hysteretic behavior of the GR activity, first described for T. crassiceps TGR (44) and then observed in all platyhelminth TGRs characterized so far (9, 21, 26). The GR activity of these TGRs, but not their TR activity, is temporarily inhibited at high [GSSG], exhibiting a lag before achieving full activity (Fig. 7). The higher the [GSH]/[GSSG] ratio the lower is the lag time. The lag is abolished not only by GSH, but also by Trx and dithiothreitol (DTT) (9, 44); and there is evidence that GSH and Trx decrease hysteresis without the need of preincubation (9). Further, the hysteretic behavior persists after GSSG removal by size exclusion chromatography of a TGR sample treated with GSSG at conditions under which there is hysteresis (9). Taken together, these observations indicate that in all likelihood the phenomenon involves thiol-disulfide exchange reactions (9, 44) (Fig. 7). Mutants in the C-terminal Cys of the Grx domain of EgTGR and SmTGR abolish most of GR hysteresis, implicating this Cys residue in the observed behavior (8, 26). This suggests that at high GSSG concentrations a disulfide intermediate involving C-terminal Cys residue would be rapidly formed, but slowly resolved, resulting in decreased enzyme activity. The identity of this alternative disulfide intermediate (i.e., which is the pairing Cys residue of the C-terminal Cys and how its formation would be favored at high GSSG concentrations remains to be elucidated. If the hysteretic behavior observed for this enzyme in vitro occurs under physiological conditions, the Grx domain-dependent functions of TGR would be inhibited under certain circumstances, while Trx reduction is preserved. Further, while the functions of the Grx domain are inhibited TGR can support Trx-based GSSG reduction and deglutathionylation through alternative pathways that involve its TR module (8). Therefore, if hysteresis occurs in vivo, this inhibition would be transient: Trxs can reduce GSSG, protein disulfides, and protein–GSH mixed disulfides, and consequently relieve hysteresis. If hysteresis would have a physiological function, it might serve a role in enzyme regulation or signaling, or it might have a protective role for the active Cys residue from irreversible oxidation.
FIG. 7.
Hysteretic behavior of the GR activity of platyhelminth TGRs. Full time courses for NADPH oxidation (decrease in absorbance at 340 nm) by Echinococcus granulosus TGR are shown at different conditions. At 1 mM GSSG concentration, the GR activity of platyhelminth TGRs exhibits hysteretic behavior (filled squares); that is, a lag time before becoming fully active. At 100 μM GSSG concentration no hysteretic behavior is evident (open squares). A 10 nM TGR concentration was used in the assays. Hysteretic behavior is favored at high GSSG concentrations and is relieved by GSH, Trx, and dithiothreitol (DTT). The hysteresis observed at 1 mM GSSG is completely relieved by the addition of DTT, 10 μM Trx and 100 μM GSH.
Expanding the Thiol-Dependent Redox Network: More Players Than Anticipated
As already mentioned, a first line of antioxidant defenses in platyhelminth parasites relies on the thiol- and selenol-dependent peroxidases: Prxs and GPxs. Msrs are a second line of defense against oxidative stress. Msrs are selenol- and thiol-dependent repair enzymes that reduce methionine sulfoxide (Met-SO) back to methionine and receive electrons from Trxs. Oxidation produces the S and R sulfoxide diastereomers of methionine, which are reduced by two distinct stereospecific Msrs: MsrA and MsrB that reduce Met-(S)-SO and Met-(R)-SO, respectively (33). In S. mansoni, two forms of MsrB were recently cloned and characterized (38). They are able to reduce Met-SO to Met, and were found to be expressed in all stages of the parasite's life cycle. The search for MsrA gene in the S. mansoni genome and transcriptomic data did not reveal any homolog. In contrast, Echinococcus spp. genomes contain one copy of each MsrA and MsrB genes (our unpublished observations). The absence of MsrA gene in Schistosoma is intriguing, and it suggests an incomplete genome sequence or a novel mechanism to repair Met-(S)-SO. In contrast to mammalian MsrB proteins, none of the platyhelminth MsrB proteins contain Sec.
The sequencing of parasitic platyhelminth genomes has provided a view of the complexity of the redox network dependent on TGR. So far, cytosolic and mitochondrial Trxs have been characterized in platyhelminth parasites (2, 10, 13, 34). Data mining of the Echinococcus spp. genome revealed the existence of five additional cytosolic monodomain Trxs, which are actively expressed (our unpublished observations). One of them possesses an atypical CPHS active site, found in human Trx3, which participates in disulfide isomerization in mammalian testis and can accept electrons from GSH or from TR. Interestingly, the Grx domain of mammalian TGR, which is also expressed in testis and participates in disulfide isomerization contains a CPHS active site too. The data mining of E. granulosus genome also revealed the existence of Grx diversity. We identified two Grxs with CGFS active site and two monodomain cytosolic Grxs with CPYC redox active site, and all four show evidence of expression (our unpublished information). Mitochondrial Grxs with CGFS active sites have been shown to participate in Fe/S cluster biogenesis and mobilization; they are dimeric and ligate a [2Fe-2S] cluster by the active site Cys of two Grx monomers and two GSH molecules (46). Grxs with CPYC active site have been shown to catalyze the GSH-dependent reduction of protein disulfides and GSH-protein mixed disulfides (17), and some of these Grxs have also been recently shown to bind Fe/S clusters (11). Despite these general considerations, the specific functions and targets of these newly identified Trxs and Grxs remain to be explored.
Finally, platyhelminth parasite genomes encode a Sec-containing selenoprotein W (SelW) (41, 47). The members of this class of selenoproteins have a redoxin domain related to Trx domain and have been shown to be GSH-dependent antioxidant proteins in vivo, although their precise function is not known. Transcriptomic surveys indicate that this gene is highly expressed.
TGR Inhibition Has Led to the Identification of Promising Drug Leads for Platyhelminth Infections
Given that both TGR (32) and Prx (49) had been shown to be essential S. mansoni proteins, the redox pathway, NADPH→TGR→GSH→Prx2→H2O2, was reconstituted and miniaturized and used in a quantitative high throughput screen of 70,000 compounds (52). The screen identified several potent TGR inhibitors (but no Prx inhibitors), including oxadiazole 2-oxides (also known as furoxans), phosphinic amides, and isoxazolones (Fig. 8). It was subsequently shown that the furoxan 4-phenyl-1,2,5-oxadiazole-3-carbonitrile-2-oxide (Fx) was (i) active against adult ex vivo S. mansoni, S. japonicum, and S. haematobium; (ii) active against all developmental stages of ex vivo S. mansoni (skin, lung, liver, and adult); (iii) able to cure mice infected with S. mansoni at the skin, liver, and adult stages; and (iv) significantly reduce pathology (50). Fx proved to have a unique mechanism of action: •NO donation through TGR activity was followed by TGR inhibition and worm death (50). Although •NO donation was not essential for Fx activity, it significantly increased its potency. The reaction of TGR with Fx was more efficient at generating •NO and Fx killed worms more rapidly and at lower concentrations than other oxadiazole 2-oxides tested (50). Further, pretreating worms with an •NO scavenger reduced the worm-killing activity of Fx to that seen with less reactive oxadiazole 2-oxides (50). Recent evidence indicates that TGR can also be targeted in S. japonicum (22, 53). These results underscore the importance of TGR in trematode redox biology and its usefulness as a drug target. Structure-activity relationship studies on a wide range of Fx-related molecules determined the importance of the 2-oxide, a strong electron-withdrawing group at position 3 and less importance on the nature of the substitution at position 4 (42). Fx and its analogs show differential activity between SmTGR and mammalian TR and GR enzymes (42). As indicated, the mechanism of action of Fx involves release of •NO; this is followed by the S-nitrosylation and inactivation of TGR (42). Importantly, it has been recently shown that Fx inhibits other trematode and cestode TGRs and leads to parasite death (45), indicating that Fx can be used to target both flukes and tapeworms. These findings reinforce the concept that interference with parasite TGR can shift the biochemical balance in favor of the host. Although the evaluation of inhibitors on mammalian TGR is highly relevant, this has not been performed due to the high insolubility and poor expression of mammalian TGRs. It should be mentioned that platyhelminth TGRs have ∼59% identity and ∼73% similarity to both mammalian cytosolic TR and TGR. Other compounds found to inhibit SmTGR and to be active against worms include antimony potassium tartrate (32), long used to treat schistosomiasis; oltipraz, (32) a clinically relevant schistosomicide; and auranofin (3, 32), a gold-containing compound used for rheumatoid arthritis therapy (Fig. 8). Both antimony potassium tartrate and oltipraz are noncompetitive TGR inhibitors. Auranofin has been shown to be a prodrug; the actual inhibitor is gold, released from auranofin and bound at three different sites in TGR. It has been proposed that the Sec mediates the transfer of gold from its ligands in auranofin to the redox-active cysteines of the protein (6). Oltipraz activity has been correlated with GSH depletion and two-fold lower GR activity in S. mansoni (37). Because there is no classical GR in this parasite, the anti schistosomal effects of oltipraz are, at least in part, through inhibition of schistosome TGR (32). Antimony potassium tartrate was first administered to patients infected with S. haematobium in 1917 (14) and antimonials were the mainstay of schistosomiasis treatment until the introduction of praziquantel in the 1980s. Initially, the antischistosomal activity of antimony potassium tartrate was proposed to be due to the inhibition of phosphofructokinase (55). However, the inhibition of TGR (iC50=50 nM) by antimony potassium tartrate is three orders of magnitude lower than that for phosphofructokinase (iC50=16 μM), suggesting that its chemotherapeutic action is more likely to occur through inhibition of TGR. Cultured S. mansoni worms treated with auranofin have reduced TGR activity (GR, TR, and Grx) and lowered ratios of reduced GSSG (32) and the toxic effects can be reversed by the addition of N-acetyl cysteine (Williams, Unpublished), strongly suggesting that TGR is the main target of the action of auranofin.
FIG. 8.
Structures of representative compounds found to inhibit Schistosoma mansoni TGR. Oxadiazole 2-oxide (furoxan) (52); phosphinic amide (52); isoxazolone (52); antimony potassium tartrate (32); oltipraz (32); auranofin (3, 32).
Concluding Remarks and Perspectives
Platyhelminth parasites have a limited set of antioxidant defenses. They lack catalase and exclusively rely on thiol and selenol peroxidases for H2O2 detoxification. Remarkably, platyhelminth parasites have streamlined Trx and GSH systems. In contrast to their mammalian hosts and free-living platyhelminthes, they lack conventional GR and TR and possess only TGR. This selenoenzyme is the bottleneck enzyme for detoxification, redox homeostasis, and synthesis of deoxyribonucleotides in platyhelminth parasites (summarized in Fig. 9). The lack of redundancy in these pathways and the “drugability” of TGR have been exploited to shift the biochemical balance toward the host side. Oxadiazole N-oxides have been identified as platyhelminth TGR inhibitors with schistosomicidal and cestodicidal activity, and current efforts are directed to optimize this drug lead. In addition, phytochelatin synthase, an enzyme involved in detoxification, which also depends on GSH and is absent in platyhelminth parasite hosts, is a potential novel target enzyme. Finally, the genome information has revealed an unexpected functional diversity of Trxs and Grxs in parasitic platyhelminthes that deserves further investigation.
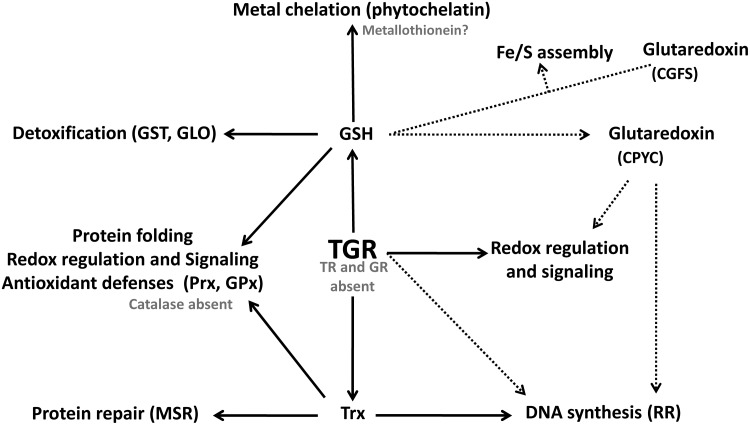
FIG. 9.
TGR: the selenium-containing central hub of the thiol-dependent redox networks of platyhelminth parasites. TGR is the sole enzyme responsible for Trx and GSH reduction in parasitic platyhelminthes: conventional TR and GR are absent (shown in gray). Therefore, DNA synthesis (through provision of electrons to RR), antioxidant defenses (through provision of electrons to GPx and Prx), protein repair (through Msr), thiol redox regulation (e.g., through deglutathionylation of mixed protein-GSH disulfides, PrS-SG), and redox signaling are fully dependent on this core enzyme. In addition, since GSH and Trx also participate in detoxification (via GST, and the GLO system), metal chelation (via phytochelatin) protein folding, and Fe/S clusters assembly and transfer (via Grx), TGR controls numerous metabolic processes in platyhelminth parasites. Discontinuous gray arrows indicate putative reactions that have not yet been proved experimentally. GLO, glyoxalase; GST, glutathione S-transferase.
Abbreviations Used
- DTT
dithiothreitol
- GLO
glyoxalase
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSSG
oxidized glutathione
- GST
glutathione S-transferase
- H2O2
hydrogen peroxide
- HED
hydroxyethyl disulfide
- iC50
half maximal inhibitory concentration
- Met-SO
methionine sulfoxide
- Msr
methionine sulfoxide reductase
- NO
nitric oxide
- PCS
phytochelatin synthase
- PrS-SG
glutathione-protein mixed disulfide
- Prx
peroxiredoxin
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RR
ribonucleotidereductase
- Sec
selenocysteine
- SOD
superoxide dismutase
- TGR
thioredoxin glutathione reductase
- TR
thioredoxin reductase
- Trx
thioredoxin
Acknowledgments
The authors are grateful to the Helminth Sequencing Group at the Sanger Institute for permitting us to work with the genomic assemblies. This work was supported by FIRCA-NIH grant TW008588, NIH grant GM065204, Universidad de la República, CSIC (grant CSIC 625), and U.S. National Institute of Allergy and Infectious Diseases grants AI065622 and AI081107.
References
- 1.Agorio A. Chalar C. Cardozo S. Salinas G. Alternative mRNAs arising from trans-splicing code for mitochondrial and cytosolic variants of Echinococcus granulosus thioredoxin-glutathione reductase. J Biol Chem. 2003;278:12920–12928. doi: 10.1074/jbc.M209266200. [DOI] [PubMed] [Google Scholar]
- 2.Alger HM. Sayed AA. Stadecker MJ. Williams DL. Molecular and enzymatic characterisation of Schistosoma mansoni thioredoxin. Int J Parasitol. 2002;32:1285–1292. doi: 10.1016/s0020-7519(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 3.Alger HM. Williams DL. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme: thioredoxin-glutathione reductase. Mol Biochem Parasitol. 2002;121:129–139. doi: 10.1016/s0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 4.Angelucci F. Dimastrogiovanni D. Boumis G. Brunori M. Miele AE. Saccoccia F. Bellelli A. Mapping the catalytic cycle of Schistosoma mansoni thioredoxin-glutathione reductase by X-ray crystallography. J Biol Chem. 2010;285:32557–32567. doi: 10.1074/jbc.M110.141960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelucci F. Miele AE. Boumis G. Dimastrogiovanni D. Brunori M. Bellelli A. Glutathione reductase and thioredoxin reductase at the crossroad: the structure of Schistosoma mansoni thioredoxin-glutathione reductase. Proteins. 2008;72:936–945. doi: 10.1002/prot.21986. [DOI] [PubMed] [Google Scholar]
- 6.Angelucci F. Sayed AA. Williams DL. Boumis G. Brunori M. Dimastrogiovanni D. Miele AE. Pauly F. Bellelli A. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J Biol Chem. 2009;284:28977–28985. doi: 10.1074/jbc.M109.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arner ES. Nordberg J. Holmgren A. Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem Biophys Res Commun. 1996;225:268–274. doi: 10.1006/bbrc.1996.1165. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla M. Denicola A. Marino SM. Gladyshev VN. Salinas G. Linked thioredoxin-glutathione systems in platyhelminth parasites: alternative pathways for glutathione reduction and deglutathionylation. J Biol Chem. 2011;286:4959–4967. doi: 10.1074/jbc.M110.170761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla M. Denicola A. Novoselov SV. Turanov AA. Protasio A. Izmendi D. Gladyshev VN. Salinas G. Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin-glutathione reductase and dependent on selenium and glutathione. J Biol Chem. 2008;283:17898–17907. doi: 10.1074/jbc.M710609200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boumis G. Angelucci F. Bellelli A. Brunori M. Dimastrogiovanni D. Miele AE. Structural and functional characterization of Schistosoma mansoni thioredoxin. Protein Sci. 2011;20:1069–1076. doi: 10.1002/pro.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceylan S. Seidel V. Ziebart N. Berndt C. Dirdjaja N. Krauth-Siegel RL. The dithiol glutaredoxins of african trypanosomes have distinct roles and are closely linked to the unique trypanothione metabolism. J Biol Chem. 2010;285:35224–35237. doi: 10.1074/jbc.M110.165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cioli D. Valle C. Angelucci F. Miele AE. Will new antischistosomal drugs finally emerge? Trends Parasitol. 2008;24:379–382. doi: 10.1016/j.pt.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Chalar C. Martinez C. Agorio A. Salinas G. Soto J. Ehrlich R. Molecular cloning and characterization of a thioredoxin gene from Echinococcus granulosus. Biochem Biophys Res Commun. 1999;262:302–307. doi: 10.1006/bbrc.1999.1168. [DOI] [PubMed] [Google Scholar]
- 14.Christopherson JB. The successful use of antimony in bilharziosis administered as intravenous injections of antimonium tartrate, tartar emetic. Lancet. 1918;ii:325–327. [Google Scholar]
- 15.Dalle-Donne I. Rossi R. Colombo G. Giustarini D. Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Dietzel J. Hirzmann J. Preis D. Symmons P. Kunz W. Ferritins of Schistosoma mansoni: sequence comparison and expression in female and male worms. Mol Biochem Parasitol. 1992;50:245–254. doi: 10.1016/0166-6851(92)90221-5. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes AP. Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 18.Furtmuller PG. Obinger C. Hsuanyu Y. Dunford HB. Mechanism of reaction of myeloperoxidase with hydrogen peroxide and chloride ion. Eur J Biochem. 2000;267:5858–5864. doi: 10.1046/j.1432-1327.2000.01491.x. [DOI] [PubMed] [Google Scholar]
- 19.García HH. Gonzalez AE. Evans CAW. Gilman RH. Taenia solium cysticercosis. Lancet. 2003;362:547–556. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guevara-Flores A. Del Arenal IP. Mendoza-Hernandez G. Pardo JP. Flores-Herrera O. Rendón JL. Mitochondrial thioredoxin-glutathione reductase from larval Taenia crassiceps (Cysticerci) J Parasitol Res. 2010 doi: 10.1155/2010/719856. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guevara-Flores A. Pardo JP. Rendón JL. Hysteresis in thioredoxin-glutathione reductase (TGR) from the adult stage of the liver fluke Fasciola hepatica. Parasitol Int. 2011;60:156–160. doi: 10.1016/j.parint.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Han Y. Zhang M. Hong Y. Zhu Z. Li D. Li X. Fu Z. Lin J. Characterization of thioredoxin glutathione reductase in Schiotosoma japonicum. Parasitol Int. 2012;61:475–480. doi: 10.1016/j.parint.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren A. Aslund F. Glutaredoxin. Methods Enzymol. 1995;252:283–292. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren A. Johansson C. Berndt C. Lonn ME. Hudemann C. Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 25.Hotez PJ. Brindley PJ. Bethony JM. King CH. Pearce EJ. Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H-H. Day L. Cass CL. Ballou DP. Williams CH. Williams DL. Investigations of the catalytic mechanism of thioredoxin glutathione reductase from Schistosoma mansoni. Biochemistry. 2011;50:5870–5882. doi: 10.1021/bi200107n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson C. Lillig CH. Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- 28.Jones MK. McManus DP. Sivadorai P. Glanfield A. Moertel L. Belli SI. Gobert GN. Tracking the fate of iron in early development of human blood flukes. Int J Biochem Cell Biol. 2007;39:1646–1658. doi: 10.1016/j.biocel.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelner MJ. Montoya MA. Structural organization of the human glutathione reductase gene: determination of correct cDNA sequence and identification of a mitochondrial leader sequence. Biochem Biophys Res Commun. 2000;269:366–368. doi: 10.1006/bbrc.2000.2267. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai T. Osada Y. Kanazawa T. 2-Cys peroxiredoxins from Schistosoma japonicum: the expression profile and localization in the life cycle. Mol Biochem Parasitol. 2006;149:135–143. doi: 10.1016/j.molbiopara.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai T. Osada Y. Ohta N. Kanazawa T. Peroxiredoxin-1 from Schistosoma japonicum functions as a scavenger against hydrogen peroxide but not nitric oxide. Mol Biochem Parasitol. 2009;164:26–31. doi: 10.1016/j.molbiopara.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Kuntz AN. Davioud-Charvet E. Sayed AA. Califf LL. Dessolin J. Arner ESJ. Williams DL. Thioredoxin-glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BC. Dikiy A. Kim HY. Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790:1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Line K. Isupov MN. Garcia-Rodriguez E. Maggioli G. Parra F. Littlechild JA. The Fasciola hepatica thioredoxin: high resolution structure reveals two oxidation states. Mol Biochem Parasitol. 2008;161:44–48. doi: 10.1016/j.molbiopara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Maiorino M. Roche C. Kiess M. Koenig K. Gawlik D. Matthes M. Naldini E. Pierce R. Flohe L. A selenium-containing phospholipid-hydroperoxide glutathione peroxidase in Schistosoma mansoni. Eur J Biochem. 1996;238:838–844. doi: 10.1111/j.1432-1033.1996.0838w.x. [DOI] [PubMed] [Google Scholar]
- 36.Mkoji GM. Smith JM. Prichard RK. Antioxidant systems in Schistosoma mansoni: evidence for their role in protection of the adult worms against oxidant killing. Int J Parasitol. 1988;18:667–673. doi: 10.1016/0020-7519(88)90102-6. [DOI] [PubMed] [Google Scholar]
- 37.Moreau N. Martens T. Fleury MB. Leroy JP. Metabolism of oltipraz and glutathione reductase inhibition. Biochem Pharmacol. 1990;40:1299–1305. doi: 10.1016/0006-2952(90)90396-3. [DOI] [PubMed] [Google Scholar]
- 38.Oke TT. Moskovitz J. Williams DL. Characterization of the methionine sulfoxide reductases of Schistosoma mansoni. J Parasitol. 2009;95:1421–1428. doi: 10.1645/GE-2062.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otero L. Bonilla M. Protasio A. Fernandez C. Gladyshev V. Salinas G. Thioredoxin and glutathione systems differ in parasitic and free-living platyhelminths. BMC Genomics. 2010;11:237. doi: 10.1186/1471-2164-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltoniemi MJ. Karala A-R. Jurvansuu JK. Kinnula VL. Ruddock LW. Insights into deglutathionylation reactions. J Biol Chem. 2006;281:33107–33114. doi: 10.1074/jbc.M605602200. [DOI] [PubMed] [Google Scholar]
- 41.Prast-Nielsen S. Huang H-H. Williams DL. Thioredoxin-glutathione reductase: its role in redox biology and potential as a target for drugs against neglected diseases. Biochim Biophys Acta. 2011;1810:1262–1271. doi: 10.1016/j.bbagen.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai G. Sayed AA. Lea WA. Luecke HF. Chakrapani H. Prast-Nielsen S. Jadhav A. Leister W. Shen M. Inglese J. Austin CP. Keefer L. Arner ES. Simeonov A. Maloney DJ. Williams DL. Thomas CJ. Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem. 2009;52:6474–6483. doi: 10.1021/jm901021k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray D. Williams DL. Characterization of the phytochelatin synthase of Schistosoma mansoni. PLoS Negl Trop Dis. 2011;5:e1168. doi: 10.1371/journal.pntd.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rendón JL. del Arenal IP. Guevara-Flores A. Uribe A. Plancarte A. Mendoza-Hernández G. Purification, characterization and kinetic properties of the multifunctional thioredoxin-glutathione reductase from Taenia crassiceps metacestode (cysticerci) Mol Biochem Parasitol. 2004;133:61–69. doi: 10.1016/j.molbiopara.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Ross F. Hernández P. Porcal W. López GV. Cerecetto H. González M. Basika T. Carmona C. Fló M. Maggioli G. Bonilla M. Gladyshev VN. Boiani M. Salinas G. Identification of thioredoxin glutathione reductase inhibitors that kill cestode and trematode parasites. PLoS One. 2012;7:e35033. doi: 10.1371/journal.pone.0035033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rouhier N. Couturier J. Johnson MK. Jacquot J-P. Glutaredoxins: roles in iron homeostasis. Trends Biochem Sci. 2010;35:43–52. doi: 10.1016/j.tibs.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salinas G. Bonilla M. Otero L. Lobanov AV. Gladyshev VN. selenoproteins in parasites. In: Hatfield DL, editor; Berry MJ, editor; Gladyshev VN, editor. Selenium: its Molecular Biology and Role in Human Health. New York: Springer-Verlag Inc.; 2011. [Google Scholar]
- 48.Salinas G. Selkirk ME. Chalar C. Maizels RM. Fernández C. Linked thioredoxin-glutathione systems in platyhelminths. Trends Parasitol. 2004;20:340–346. doi: 10.1016/j.pt.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Sayed AA. Cook SK. Williams DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and implicate peroxiredoxins as novel drug targets. J Biol Chem. 2006;281:17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- 50.Sayed AA. Simeonov A. Thomas CJ. Inglese J. Austin CP. Williams DL. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat Med. 2008;14:407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayed AA. Williams DL. Biochemical characterization of 2-Cys peroxiredoxins from Schistosoma mansoni. J Biol Chem. 2004;279:26159–26166. doi: 10.1074/jbc.M401748200. [DOI] [PubMed] [Google Scholar]
- 52.Simeonov A. Jadhav A. Sayed AA. Wang Y. Nelson ME. Thomas CJ. Inglese J. Williams DL. Austin CP. Quantitative high-throughput screen identifies inhibitors of the Schistosoma mansoni redox cascade. PLoS Negl Trop Dis. 2008;2:e127. doi: 10.1371/journal.pntd.0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song L. Li J. Xie S. Qian C. Wang J. Zhang W. Yin X. Hua Z. Yu C. Thioredoxin glutathione reductase as a novel drug target: evidence from Schistosoma japonicum. PLoS One. 2012;7:e31456. doi: 10.1371/journal.pone.0031456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su D. Novoselov SV. Sun Q-A. Moustafa ME. Zhou Y. Oko R. Hatfield DL. Gladyshev VN. Mammalian selenoprotein thioredoxin-glutathione reductase: roles in disulfide bond formation and sperm maturation. J Biol Chem. 2005;280:26491–26498. doi: 10.1074/jbc.M503638200. [DOI] [PubMed] [Google Scholar]
- 55.Su JG. Mansour JM. Mansour TE. Purification, kinetics and inhibition by antimonials of recombinant phosphofructokinase from Schistosoma mansoni. Mol Biochem Parasitol. 1996;81:171–178. doi: 10.1016/0166-6851(96)02702-8. [DOI] [PubMed] [Google Scholar]
- 56.Sun Q-A. Kirnarsky L. Sherman S. Gladyshev VN. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc Natl Acad Sci U S A. 2001;98:3673–3678. doi: 10.1073/pnas.051454398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dalen CJ. Kettle AJ. Substrates and products of eosinophil peroxidase. Biochem J. 2001;358:233–239. doi: 10.1042/0264-6021:3580233. [DOI] [PMC free article] [PubMed] [Google Scholar]