Dear Editor,
Worldwide human immunodeficiency virus type 1 (HIV-1) affects approximately 34 million people and accounts for around 3 million newly infected persons per year.1 In Brazil, approximately 600,000 individuals are infected with HIV-1 and the annual incidence in 2010 was 17.9/100,000 inhabitants.2 Heterosexual HIV-1 transmission has increased significantly among young women, and in Brazil the rate of male:female infection has changed from 25:1 to 1.5:1 during the last two decades.2 This shift raises concerns since infected women can disseminate HIV-1 by sexual route and by mother-to-child transmission (MTCT). HIV-1 MTCT is a multifactorial event; however, maternal viral load alone is considered the main risk factor.3 In this context, prophylactic use of antiretroviral drugs (ARV) to reduce maternal viral load has proved effective to avoid MTCT. Nevertheless, emergence of HIV drug-resistant strains has increased with the scaling up population-based treatment regimens based on nucleoside/non-nucleoside reverse transcriptase inhibitors (NRTI/NNRTI) and protease inhibitors (PI) and due to the widespread use of standard first-line regimens based on low genetic barrier NNRTI.4 During pregnancy, low adherence to ARV therapy may also contribute to drug resistance development.5 Transmitted HIV-drug resistance mutations (TDR) in ARV naïve pregnant women can compromise both MTCT preventive measures and future treatment options. In this regard, genotypic data among pregnant women is crucial for effective MTCT prophylaxis and future ARV regimens.
In central western Brazil, a comprehensive public health antenatal program including over 240 municipalities from Goias State (“Program for the Protection of Pregnant Women/PPPW”) screens ∼90,000 pregnant women/year for seven infectious diseases, including HIV-1. This program has contributed to HIV-1 MTCT rates of 1%, the lowest in Brazil.2 In this setting, between 2004–2005, the prevalence of HIV-1 infection among 28,000 pregnant women was around 0.1%.6 More recently (2010–2011) among 54,139 pregnant women from this setting the prevalence of HIV-1 infection was 1.58 case/1000 women and the estimated incidence was 0.47/1000 person-years based on HIV-specific antibody affinity (Calypte Aware BED EIA HIV-1 Incidence Test), representing 20% of the recently diagnosed cases (Costa et al., 2013, submitted). A subsample of 22 HIV-1 infected women was proportionally selected in the recruitment site to include participants from both the capital metropolitan area and from other settings. In the current study, we report HIV-1 molecular data including drug resistance and genetic subtypes and socio-demographic profiles in this subsample of HIV-1 infected pregnant women.7
Participants were interviewed using a standardized questionnaire to collect socio-demographic data. Clinical and laboratory data (CD4+ cell counts, viral loads) were retrieved from medical files at the reference public health hospital (Hospital Anuar Auad/HDT/SUS, Goiania/GO). The pol gene was sequenced as previously described.7 In brief, plasma RNA was extracted (QIAamp® Viral RNA Mini Kit, Qiagen, Germany), reverse transcribed into complementary DNA (Invitrogen), and used for nested polymerase chain reaction employing HIV-1 protease (PR) and reverse transcriptase (RT) K1/K2 external primers and DP10/F2 internal primers. The entire PR and ∼750 bp fragment of RT were amplified, purified (QIAquick® PCR Purification Kit/Qiagen, Germany) and sequenced (BigDye Terminator kit, ABI Prism 3130 Genetic Analyzer, Applied Biosystems, USA). Transmitted and secondary drug resistance mutations were analyzed according to International AIDS Society-USA and Stanford HIV Drug Resistance Databases (hivdb.stanford.edu). TDR was identified by the Calibrated Population Resistance Tool. ARV mutation susceptibility profile was analyzed by Stanford HIV Drug Resistance Database. GenBank accession numbers of these sequences are KC249749-KC249766. HIV-1 subtypes were defined by the REGA tool and phylogenetic inference (Neighbor-Joining under Kimura's 2-parameter). Isolates with discordant subtypes in PR/RT regions were analyzed by SIMPLOT software (200 bp window, 20 bp step, 1000 replicates). Local institutional review board (Universidade Federal de Goiás: protocol #073/05) approved this study and signed informed consents were obtained. Frequencies and medians of main variables were calculated using Epi Info7 (CDC, Atlanta, GA, USA).
The median age of study group was 25 years (15–38 range), 5 were adolescents (≤19 years). There was no difference in age between this sample and the entire group of 86 HIV-1 infected women. Twelve participants had a stable sexual partner, one of the participants was a prostitute. All pregnant women reported heterosexual route, 5/22 declared a sexual partner with known HIV-1 infection, however none was aware of ARV use by the partner. Two participants have had a partner who had been in prison, and three had a bisexual partner. Sexually transmitted disease was reported by two patients (HPV) and two patients have previously received blood transfusion. Half of the participants had low educational level (<8 years), none had a university degree. Most patients (n=11) have had from 1 to 4 previous pregnancies. Most patients (14/22, 63.3%) were from the great metropolitan area (capital Goiânia city, 1.6 million inhabitants). For 18 out of 22 patients recruited HIV-1 pol gene could be amplified and sequenced, including 6 cases of recent seroconversion (<155 days) and 12 cases of long-term seroconversion identified by BED EIA. Main characteristics of patients are summarized in Table 1.
Table 1.
Main Characteristics of HIV-1 Infected Pregnant Women with Recent and Long-Term Seroconversion
| Variables | Recent seroconversion (n=6) | Long-term seroconversion (n=12) |
|---|---|---|
| Age | ||
| Median | 24 | 24 |
| (range) | (19–32) | (15–36) |
| CD4 count (cells/mm3) | ||
| Median | 532 | 427 |
| (range) | (310–666) | (146–1302) |
| Viral load (copies/mL) | ||
| Median | 28,700 | 36,370 |
| (range) | (14,828–55,299) | (342–219,750) |
| ARV status | ||
| Previously exposed | – | 02 (16.7%) |
| Naïve | 06 (100%) | 10 (83.3%) |
| Clinical status | ||
| Asymptomatic | 6 (100%) | 10 (83.3%) |
| Symptomatic | – | 02 (16.7%) |
| HIV-1 subtype (PR/TR) | ||
| B/B | 05 (83.3%) | 05 (41.6%) |
| C/C | 01 (16.7%) | 02 (16.7%) |
| F1/F1 | – | 01 (8.3%) |
| B/F1 or F1/B | – | 04 (33.3%) |
| Drug resistance | 02 (2/6, 33.3%) | 02 (2/12, 16.6%) |
| PI | 02 (L90M) | 01 (M46I) |
| NRTI | 02 (M41L/T215C/D) | 01 (T215S) |
| NNRTI | – | – |
ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PR, protease; RT, reverse transcriptase.
Recent seroconversion: ≤155 days, long-term seroconversion: ≥155 days as defined by BED EIA HIV-1 Incidence Test.
Only two long-term seroconverted women had been exposed once to intravenous AZT during a previous delivery and none had secondary drug resistance mutation. Among 16 ARV naïve pregnant women, there were four cases of TDR (25%, 4/16) (GenBank accession numbers: KC249752, KC249759, KC249760, KC249761). Among TDR cases, single class mutation (NRTI) was detected in two patients (PI: M46I and NRTI: T215S) and dual class (PI+NRTI) mutations were identified in two patients: L90M+M41L and T215C/D mutations. Two out of six recently infected patients had TDR mutations: PI (L90M) and NRTI mutations (M41L and T215C). M46I is a major PI mutation that confers low susceptibility to nelfinavir (NFV) but in non-subtype B, M46I is usually the consensus amino acid. T215S/C are revertant mutations related to the emergence of T215Y, that increases the risk of virologic failure to AZT and stavudine (d4T).8 M41L when associated with T215Y confers high level of resistance to these two drugs and decreases susceptibility to all nucleoside reverse transcriptase inhibitors.9 L90M is associated with high level of resistance to NFV.8
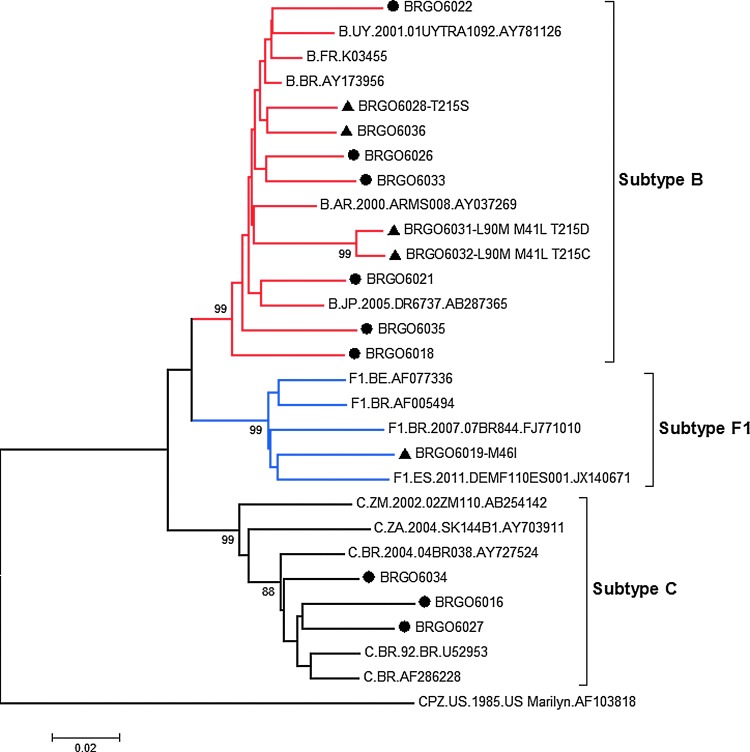
Regarding HIV-1 diversity, despite the predominance of subtype B (10/18), subtype C represented the second most frequent subtype (3/18), followed by BF1 recombinants and subtype F1 (Table 1, Fig. 1). These findings corroborate the recently described heterosexual spread of HIV-1 subtype C in central western Brazil, which contrasts with its low prevalence in previous studies from this geographic area.10,11
FIG. 1.
Phylogenetic classification of 14 HIV-1 isolates from pregnant women in PR and TR genes: (●) HIV-1 sequences without drug resistance mutations; (▲) HIV-1 sequences with TDR mutations. Recombinant HIV-1 sequences were excluded from the phylogenetic analysis. Brackets indicate different monophyletic clusters. Bootstrap values (1000 replicates) above 70% are shown. GenBank accession numbers of reference sequences are: Subtype B (AY173956, K03455, AY781126; AB287365; AY037269), Subtype F1 (AF077336; AF005494; FJ771010; JX140671), and Subtype C (U52953; AF286228; AY727524; AY703911; AB254142). The tree was rooted using the simian immunodeficiency virus sequence from chimpanzee (SIVcpz, GenBank accession number: AF103818) as an outgroup. (Color image can be found at www.liebertonline.com/apc).
The profile of the Brazilian pregnant women infected with HIV-1 depicted in this report raises concerns since it includes adolescents, mostly with low educational levels, high risk behavior represented by unprotected sex with HIV-1 infected patients and prisoners. Moreover, despite the small sample size, a high rate of transmitted drug resistance mutations (25%) was observed, including dual class resistance mutations. This rate may be underestimated since bulk sequencing detects only strains that represent >20%. Timing of genotypic testing among long-term seroconverters may also interfere since in the absence of ARV drug pressure, resistance mutations may decline and become undetectable. The high rate of TDR mutations identified in this group represents an alert since great efforts by public health programs in central western Brazil have promoted the lowest HIV-1 MTCT in the country, which is an important achievement towards an AIDS-free generation.
Acknowledgments
This research was sponsored by Fundação de Apoio a Pesquisa do Estado de Goiás (FAPEG 008/2009) and the authors' institutions. We thank our colleagues of Secretariat of Health and APAE for their support. CMT Martelli and MMA Stefani are recipients of fellowships from the CNPq/Bolsa de Produtividade em Pesquisa (Grants #306489/2010-4 and #310582/2011-3). CMT Martelli is a research member from the National Institute of Science and Technology for Health Technology Assessment.
Ethical approval: The project was approved by the Ethical Committee of the Federal University of Goias, Brazil (CEPMHA/HC/UFG; number 174/08). All of the participants (or the legal guardians of underage participants) signed an opt-in informed consent form before receiving the screening, which was part of the routine protocol. The Study was also approved by regional APAE Ethical Committee.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. World AIDS Day Report. UNAIDS. 2011 [Google Scholar]
- 2.Ministério da Saúde., editor. Brasil, Boletim Epidemiológico 2010. Ano VII n°1 in AIDS.DST. 2010.
- 3.Tubiana R. Le Chenadec J. Rouzioux C, et al. Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load <500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1) Clin Infect Dis. 2010;50:585–596. doi: 10.1086/650005. [DOI] [PubMed] [Google Scholar]
- 4.Sungkanuparph S. Sukasem C. Kiertiburanakul S, et al. Emergence of HIV-1 drug resistance mutations among antiretroviral-naïve HIV-1-infected patients after rapid scaling up of antiretroviral therapy in Thailand. J Int AIDS Soc. 2012;15:12. doi: 10.1186/1758-2652-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreitchmann R. Harris DR. Kakehasi F, et al. Antiretroviral adherence during pregnancy and postpartum in Latin America. AIDS Patient Care STDS. 2012;26:486–495. doi: 10.1089/apc.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa ZB. Machado GC. Avelino MM, et al. Prevalence and risk factors for hepatitis C and HIV-1 infections among pregnant women in Central Brazil. BMC Infect Dis. 2009;9:116. doi: 10.1186/1471-2334-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso LP. Queiroz BB. Stefani MM. HIV-1 pol phylogenetic diversity and antiretroviral resistance mutations in treated naive patients from Central West Brazil. J Clin Virol. 2009;46:134–139. doi: 10.1016/j.jcv.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Johnson VA. Calvez V. Gunthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19:156–164. [PMC free article] [PubMed] [Google Scholar]
- 9.Whitcomb JM. Parkin NT. Chappey C, et al. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188:992–1000. doi: 10.1086/378281. [DOI] [PubMed] [Google Scholar]
- 10.Alcântara KC. Lins JB. Albuquerque M, et al. HIV-1 mother-to-child transmission and drug resistance among Brazilian pregnant women with high access to diagnosis and prophylactic measures. J Clin Virol. 2012;54:15–20. doi: 10.1016/j.jcv.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Alcântara KC. Reis MN. Cardoso LP, et al. Increasing heterosexual transmission of HIV-1 subtype C in Inland Central western Brazil. J Med Virol. 2013;85:396–404. doi: 10.1002/jmv.23474. [DOI] [PubMed] [Google Scholar]