Abstract
The purpose of this study was to examine the results of implementing a rapid counselor-based HIV testing program in community pharmacies. A prospective cross-sectional study was conducted on a convenience sample of clients at five community pharmacies in New York City (NYC). In 294 days of pharmacy testing, 2805 clients were eligible to receive testing, and 2030 individuals agreed to test. The average age was 33±15 years, 41% were male, 59% were Hispanic, 77% had been previously tested for HIV, and 34% were uninsured. HIV incidence was 0.3%, median CD4 cell count was 622.0, and the average age of the newly diagnosed positives was 36.0±13.9 years. Participants were satisfied with a counselor-based rapid HIV testing program in community-based pharmacies.
Introduction
According to the Centers for Disease Control and Prevention (CDC), in 2009 there were an estimated 784,701 persons living with HIV in the United States and 48,100 new infections.1 In 2011, nearly 1 in 5 HIV-positive (about 240,000) individuals in the US were unaware of their infection.2 Most recent national surveillance reports show that from 2008 to 2010, the estimated number of persons living with AIDS has increased, with 487,692 persons living with an AIDS diagnosis at the end of 2010.3
In 2006, the CDC revised their HIV testing guidelines, expanding routine opt-out screening testing recommendations to all individuals between the ages of 13 and 64 visiting healthcare facilities. In response, cities and boroughs such as Washington D.C.4 and the Bronx, respectively, have implemented campaigns to scale-up HIV testing. Some states, such as New York, mandate an offer of HIV testing to all patients receiving treatment for non-life-threatening conditions in a hospital emergency department (ED), or primary care setting, such as a doctor's office or outpatient clinic.5 Despite these efforts to expand testing, CDC data from August 2011 shows that the numbers of new infections and proportion of late diagnoses has remained relatively stable.6
The reason why scale-up testing has not resulted in a major decrease in the number of individuals unaware of their serostatus or diagnosed late is multi-factorial, but access to testing likely plays a large role. Access to healthcare remains low among low-income and minority populations in which the burden of HIV is also the highest.7 When access to care is limited, these high risk populations may have fewer opportunities to test. While the reasons behind limited access to care are many—including lack of insurance coverage, feeling unwelcome at medical practices, and unaffordable costs—it is undeniable that these limitations negatively impact HIV testing and diagnosis.8 Barriers to care are evident in most epicenters of the HIV epidemic. One study in NYC found that in the year before diagnosis, only 20% of participants reported being offered an HIV test, only 40% reported having a primary care provider, and only 50% reported having health insurance during all or part of the year before concurrent HIV/AIDS diagnoses.9 Studies have shown that an opt-out approach, routinizing HIV screening, is one way to decrease the stigma with HIV testing.10–11 Furthermore, studies show that community-based testing models have the potential to decrease stigma.12,13 Still, stigma associated with HIV continues to be a major barrier to HIV prevention and care in the United States and globally.14,15
Populations with the least access to care continue to suffer from HIV/AIDS in a disproportionate way. African Americans have a seven times greater incidence rate of HIV/AIDS compared to whites, and the highest rate of new HIV of any racial group, and according to the most recent CDC data, the highest rate of persons living with infection ever classified as AIDS were among blacks/African Americans (551.2 per 100,000) and Hispanics (196.1 per 100,000).3
The 2010 National HIV/AIDS Strategy for the United States (NHAS) called for intensified prevention efforts in highly effected communities where HIV is most heavily concentrated.16 Since the most at-risk communities are also those with the least access to care, one way to increase testing access for these populations is to move testing outside traditional healthcare settings. Community-based testing can complement current healthcare facility-based HIV testing, potentially identifying both early HIV infection and HIV-positive patients in high-risk populations that do not regularly access care.
Data show that NYC is particularly affected by HIV; more than 107,000 NYC residents live with HIV, thousands do not know they are infected, and NYC's AIDS case rates are almost three times the national average.17 Pharmacies can serve as a confidential workspace for testing, are familiar with the Health Insurance Portability and Accountability Act (HIPAA) and preservation of confidentiality, and many already have working relationships with medical centers and are perceived as a “safe space” for a confidential test. Pharmacies have a large client base to test for HIV that includes individuals filling prescriptions for medications, individuals browsing the pharmacy for supplies other than medicine, and individuals passing by in front of the pharmacy. While individuals filling prescriptions may be more likely to have access to a physician, the other groups mentioned reflect a more general population that may lack access to primary care.
We previously demonstrated that HIV testing can be greatly expanded by using a model where trained Public Health Advocates (PHAs) actively recruit patients for HIV testing and counseling in a hospital setting, referred to in that article as Project BRIEF.18 This article examines the results of implementing counselor-based HIV testing and linkage to care components in five urban, NYC pharmacies located in communities highly affected by HIV, in areas with some of the highest rates of poverty in the United States. Twenty-eight percent (28%) of the population lives below the poverty level, and many individuals in this community are uninsured with poor access to healthcare.19 The community is comprised of a predominantly minority population (53.8% Hispanic and 43.3% black).20 In this study, indicators included the number of clients tested for HIV, CD4 count at diagnosis, and participant satisfaction.
Methods
Study design and setting
This study took place in New York City (specifically in the Bronx and Manhattan). According to the NYC Department of Health and Mental Hygiene (DOHMH) 2010 surveillance data, the Manhattan pharmacies (n=2) where HIV testing was conducted were in zip codes with the top quintile of HIV prevalence in NYC.21 The Bronx pharmacies (n=3) were located in communities with high HIV prevalence (923–1628 per 100,000).22 None of the pharmacies participated in the New York Syringe Exchange Program; however, participants that used injection drugs were referred to nearby centers offering needle exchange services.
We conducted a prospective cross-sectional study in five urban inner city community pharmacies from 10/26/2009 to 06/15/2011. We measured the number of HIV tests performed, HIV risk behavior, participant satisfaction, and linkage to care for HIV-positive individuals. Data were collected using paper surveys administered by PHAs in both English and Spanish. The institutional review board (IRB) of the medical school and the hospital participating in the study determined that the study protocol qualified for exempt status because it was considered a public health implementation rather than human subjects research.
Selection and staffing of pharmacies
Participating pharmacies were chosen based on objective criteria. Objective criteria were determined by the researchers and discussed in-depth with the pharmacy staff. Pharmacies were determined working closely with and following recommendations from the NYC DOHMH. At least three researchers visited each pharmacy site and evaluated the pharmacies based on four elements, including HIV seroprevalence of service area; safety of neighborhood for PHAs; availability of a confidential area for testing and counseling to be performed; and ability to link diagnosed HIV-positive patients easily to care. Evaluations were then reviewed and the final testing site determination was made by consensus decision. Due to staffing and space constraints, PHAs alternated between pharmacies on a daily basis; thus, not every pharmacy was staffed every day. On average, two of the pharmacies were covered three times a week, one was covered twice a week, and two were covered once a week.
Selection of clients
English- or Spanish-speaking individuals 13 years of age or older either inside or passing by the pharmacies between the hours of 10 am and 3:30 pm Monday–Friday (range 4–6 h a day) were approached for testing by PHAs specially trained in HIV counseling, testing, and linkage to care. In New York State, minors 13 years old and older are allowed to consent for themselves for HIV testing. Testing hours overlapped with the hours of the HIV clinic at the hospital to ensure that any patients that tested preliminary positive would have the opportunity to be linked to care on the same day. Individuals recruited in the pharmacies and on the street are referred to as “clients.” Clients had the option to refuse testing. If clients chose not to test, the PHA asked them to voluntarily provide demographic information as well as their reason for refusal. Clients who refused to provide this information were asked no further questions. Clients were considered ineligible if they had tested within the last 6 months, already had an HIV positive diagnosis, or were unable to understand the consent process for HIV testing. The main reasons for refusal included perception of no HIV risk, no time to test, afraid of the test, perception that testing would slow down visit and with family or friends. Clients were not offered any incentives or compensation other than taking the free HIV test and provided with free condoms.
For those that agreed to test, the PHA escorted the client to a private space within each pharmacy to conduct the test. During the 20 min it takes to perform the HIV test, the PHA asked clients to complete risk factor and satisfaction questionnaire and conducted tailored risk-reduction counseling based on the answers to the risk factor questionnaire. After delivering results, PHAs would provide any additional risk-reduction post-test counseling. In order to ensure confidentiality, PHAs tested one client at a time and stayed with the client throughout the duration of the test.
HIV testing and linkage procedures
HIV testing was performed by oral fluid specimen collection using the Ora-Quick Advance Rapid HIV-1/2 Antibody Test. If a preliminary positive result was obtained, the Director of the HIV clinic was notified immediately. The PHA informed the patient of his/her preliminary positive result and escorted the patient by taxicab to the outpatient HIV clinic at the hospital. Patients were registered at the clinic, a second OraQuick test using whole blood from a finger stick or venipuncture was performed, a confirmatory Western Blot, CD4 cell count, and Viral Load were obtained, and the patient was then seen by an HIV specialist. Patients were given an appointment to return in 3 days to receive confirmatory test results. Patients had the option to refuse linkage to care.
Data collection and processing
As a standard part of the Project BRIEF testing model, all pharmacy clients answered questions about demographics, risk factors, and satisfaction. Clients with low literacy or who struggled to complete the survey alone were assisted by the PHA. Pharmacy data were entered into a secure database by a trained research assistant.
Data analysis
Population characteristics were analyzed using descriptive statistics. Means and standard deviations were calculated for continuous variables and proportions for categorical variables. All data analysis was performed using Stata version 10.0 (StataCorp, College Station, TX) and SPSS software (IBM, Armonk, NY).
Results
Demographics and risk factors of pharmacy clients
During 294 testing days, 4227 clients were approached for testing. Denominators vary due to the fact that not all participants answered all demographic and risk factor questions. Average age was 33±15 years. Less than half (40.5%; 818/2012) of clients tested were male. Eight clients were transgender male to female. Many clients were Hispanic (59.1%; 1170/1981), spoke English (72.7%; 1449/1993), had tested for HIV before (76.6%; 1524/1990), and/or had a regular doctor (78.0%; 1520/1949) (Table 1). Approximately 1/3 of clients (33.9%; 674/1986) lacked health insurance, and 3.5% (69/1976) of clients had been homeless in the past six months.
Table 1.
Demographics and Risk Factors of Clients Tested for HIV in Community-Based Pharmacies in New York City from 10/26/2009 to 06/15/2011 Broken Down by Self-Identified Gender and HIV Status
| Male(n=822) | Female (n=1183) | Transgender (n=8) | ||
|---|---|---|---|---|
| Age | HIV−(n=2019) | 32.9±15.0 | 32.4±14.4 | 36.1±15.0 |
| HIV+(n=6) | 29.8±5.0 | 48.5±20.5 | N/A | |
| Gender | HIV−(n=2019) | 40.5% (818/2019) | 58.5% (1181/2019) | 0.4% (8/2019) |
| HIV+(n=6) | 66.7% (4/6) | 33.3% (2/6) | N/A | |
| Hispanic | HIV−(n=2019) | 56.4% (456/808) | 60.9% (707/1160) | 50.0% (4/8) |
| HIV+(n=6) | 75.0% (3/4) | 50% (1/2) | N/A | |
| Race | HIV – (n=2019) | (n=446) | (n=639) | (n=7) |
| White | 20.2% (90) | 32.2% (119) | 14.3% (1) | |
| Black | 74.4% (332) | 78.6% (502) | 71.4% (5) | |
| Asian | 2.7% (12) | 1.6% (10) | 0% (0) | |
| Other | 2.7% (12) | 1.3% (8) | 14.3% (1) | |
| HIV+(n=6) | (n=2) | (n=2) | N/A | |
| White | 0.0% (0) | 0.0% (0) | ||
| Black | 100.0% (2) | 50.0% (1) | ||
| Asian | 0.0% (0) | 0.0% (0) | ||
| Other | 0.0% (0) | 50.0% (1) | ||
| Language | HIV – (n=2019) | (n=807) | (n=1174) | (n=8) |
| English | 74.2% (599) | 71.6% (840) | 87.5% (7) | |
| Spanish | 22.9% (185) | 26.1% (307) | 12.5% (1) | |
| Other | 2.9% (23) | 2.2% (26) | 0% (0) | |
| HIV+(n=6) | (n=3) | (n=2) | N/A | |
| English | 66.7% (2) | 50.0% (1) | ||
| Spanish | 33.3% (1) | 50.0% (1) | ||
| Other | 0.0% (0) | 0.0% (0) | ||
| Ever tested before for HIV | HIV−(n=2019) | 70.0% (565/808) | 72.5% (847/1169) | 87.5% (7/8) |
| HIV+(n=6) | 100.0% (0/4) | 100.0% (2/2) | N/A | |
| Uninsured | HIV−(n=2019) | 42.5% (341/802) | 28.1% (329/1169) | 37.5% (3/8) |
| HIV+(n=6) | 0.0% (0/4) | 50% (1/2) | N/A | |
| Been homeless in the past 6 months | HIV−(n=2019) | 3.8% (30/795) | 3.2% (37/1168) | 25.0% (2/8) |
| HIV+(n=6) | 0.0% (0/3) | 0.0% (0/2) | N/A | |
| Have a regular doctor | HIV−(n=2019) | 65.1% (512/786) | 87.8% (998/1150) | 87.5% (7/8) |
| HIV+(n=6) | 33.3% (1/3) | 100.0% (2/2) | N/A | |
| Previously had an STD (ever) | HIV−(n=2019) | 10.1% (80/794) | 11.9% (139/1167) | 12.5% (1/8) |
| HIV+(n=6) | 33.3% (1/3) | 0.0% (0/2) | N/A | |
| Sex with partner with HIV (ever) | HIV−(n=2019) | 2.1% (16/780) | 2.9% (33/1148) | 0% (0/8) |
| HIV+(n=6) | 33.3% (1/3) | 0.0% (0/2) | N/A | |
| Sex with PWID (ever) | HIV−(n=2019) | 2.3% (18/780) | 3.0% (35/1151) | 0% (0/8) |
| HIV+(n=6) | 0.0% (0/3) | 0.0% (0/2) | N/A | |
| Sex with known MSM (ever) | HIV−(n=2019) | 4.0% (31/780) | 1.0% (12/1150) | 12.5% (1/8) |
| HIV+(n=6) | 66.7% (2/3) | 0.0% (0/2) | N/A | |
| No. of male partners (past 12 months) | HIV−(n=2019) | (n=408) | (n=1049) | (n=8) |
| 0 | 72.3% (295) | 11.2% (118) | 50.5% (4) | |
| 1 | 13.7% (56) | 51.2% (537) | 37.5% (3) | |
| 2–5 | 11.0% (45) | 32.8% (344) | 12.5% (1) | |
| More than 6 | 2.9% (12) | 4.8% (50) | 0% (0) | |
| HIV+(n=6) | (n=3) | (n=1) | N/A | |
| 0 | 0.0% (0) | 0.0% (0) | ||
| 1 | 33.3% (1) | 0.0% (0) | ||
| 2–5 | 33.3% (1) | 100.0% (1) | ||
| More than 6 | 33.3% (1) | 0.0% (0) | ||
| No. of female partners (past 12 months) | HIV−(n=2019) | (n=656) | (n=581) | (n=5) |
| 0 | 10.1% (66) | 82.8% (481) | 80.0% (4) | |
| 1 | 40.1% (263) | 8.8% (51) | 20.0% (1) | |
| 2–5 | 37.3% (245) | 7.7% (45) | 0% (0) | |
| More than 6 | 12.5% (82) | 0.7% (4) | 0% (0) | |
| HIV+(n=6) | N/A | (n=1) | N/A | |
| 0 | 100.0% (1) | |||
| 1 | 0.0% (0) | |||
| 2–5 | 0.0% (0) | |||
| More than 6 | 0.0% (0) | |||
| Inconsistent condom use | HIV−(n=2019) | 72.4% (491/678) | 78.4% (749/955) | 60.0% (3/5) |
| HIV+(n=6) | 100.0% (3/3) | 100.0% (0/1) | N/A | |
| Exchanged sex for money or drugs (ever) | HIV−(n=2019) | 1.8% (14/794) | 1.2% (14/1167) | 12.5% (1/8) |
| HIV+(n=6) | 66.7% (2/3) | 0.0% (0/2) | N/A | |
| Used drugs before sex (ever) | HIV−(n=2019) | 12.2% (97/796) | 4.7% (55/1168) | 12.5% (1/8) |
| HIV+(n=6) | 66.7% (2/3) | 0.0% (0/2) | N/A | |
| Used drugs past (ever) | HIV−(n=2019) | 32.3% (257/796) | 21.0% (245/1165) | 25.0% (2/8) |
| HIV+(n=6) | 66.7% (2/3) | 0.0% (0/2) | N/A | |
| Used injection drugs (past 12 months) | HIV−(n=2019) | 1.1% (9/796) | 1.1% (13/1167) | 0% (0/8) |
| HIV+(n=6) | 25.0% (1/4) | 0.0% (0/2) | N/A | |
| Male who had sex with other men (past 12 months) | HIV−(n=2019) | 15.8% (115/727) | N/A | N/A |
| HIV+(n=6) | 100.0% (3/3) | N/A | N/A | |
| Been in jail (ever) | HIV−(n=2019) | 22.0% (175/795) | 6.8% (79/1166) | 37.5% (3/8) |
| HIV+(n=6) | 0.0% (0/3) | 0.0% (0/2) | N/A | |
| Been in prison (ever) | HIV−(n=2019) | 10.9% (87/795) | 2.8% (33/1164) | 12.5% (1/8) |
| HIV+(n=6) | 0.0% (0/3) | 0.0% (0/2) | N/A |
*Denominators do not equal total n's due to missing data.
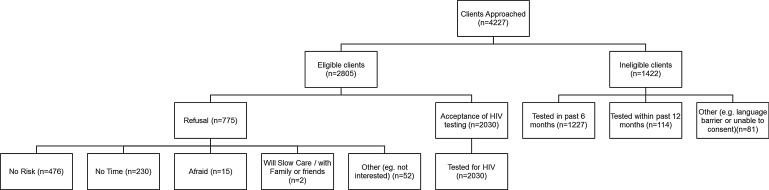
Two-thirds (66.4%; 2805/4227) of those approached were deemed eligible for testing. Most eligible clients agreed to test (72.4%; 2030/2,05) (Fig. 1). Of the 27.6% (775/2805) of clients who refused the HIV test, 61.4% (476/775) stated that they were not at risk and 29.7% (230/775) stated that they did not have time. Of the 33.8% (1422/4227) of clients who were ineligible, most [86.3% (1227/1422)] had tested in the past 6 months.
FIG. 1.
Pharmacy testing data.
Pharmacy clients reported various HIV risk factors. A majority (75.9%; 1247/1642) reported inconsistent condom use. About one-third had two or more male partners (31.0%; 455/1469) and about one-third had two or more female partners (30.2%; 376/1243); 11.2% (221/1974) previously had an STD; 2.6% (50/1941) had sex with a partner with HIV; 1.6% (31/1974) had sex in exchange for money or drugs; 2.7% (53/1944) had sex with a person who inject drugs (PWID), and 2.4% (46/1943) had sex with a known man who has sex with other men (MSM) (Table 1).
Demographic and risk factor data were broken down by self-identified gender and HIV status (Table 1). A greater proportion of males ever used drugs before sex (12.4% vs. 4.7%) and a greater proportion of males had ever been in jail (21.9% vs. 6.8%) or prison (10.9% vs. 2.8%). There was a greater proportion of transgender patients who had been homeless in the past 6 months, previously had an STD, had sex with a known MSM, had ever exchanged sex for money or drugs, and had ever been to jail or prison (Table 1).
HIV seropositivity in the pharmacies was 0.3% (6/2030). The average age of the newly diagnosed positives was 36.0±13.9 years old. Two-thirds (66.7%; 4/6) were Hispanic. The majority were male (66.7%; 4/6). Out of the three males that answered the risk factor questions, all three (100%) had sex with another man in the past 12 months. The median CD4 cell count at diagnosis was 622.0 white blood cells/mL (IQR 383.5 to 949.0) and median Viral Load was 14,725.0 (IQR 3,555.5 to 94,008.5). Median days to linkage to care in the pharmacies for those who were successfully linked (83%) was 0 days (IQR 0.0 to 1.0). The duration of time from the preliminary positive result to seeing an HIV specialist was, on average, less than an hour. Demographic and risk factor data for the newly diagnosed HIV positive participants were broken down by self-identified gender (Table 1).
Almost all pharmacy clients noted that the PHA made HIV testing easy (98.6%; 1884/1911). More than half (56.0%; 1061/1894) of clients felt they learned a small to moderate amount of new information with regards to HIV, and a third (30.7%; 582/1894) felt they learned a large amount of new information with regards to HIV.
Discussion
PHAs tested 2030 pharmacy clients for HIV. HIV testing in the pharmacies was feasible and successful based on our a priori measures. A large percentage of individuals approached agreed to get tested for HIV (72.9%) and an overwhelming majority (98.6%) felt that HIV testing with PHAs in pharmacies was easy. The data on acceptance to testing and satisfaction of testing strongly suggest that members of the highly affected community under study were open to and supportive of HIV testing in pharmacy venues. Last, we had a high linkage to care rate (83%), indicating that testing and counseling in pharmacies with immediate linkage can bring a larger proportion of newly diagnosed HIV patients into HIV specialist care.
The type of venue-based testing performed in this study has been used previously to reach out into the community to test for HIV. One study where researchers offered testing in places where young people congregate was successful in identifying HIV-positive adolescent men that have sex with men, 60% of whom did not know that they were HIV positive.23 There have also been successful innovative community-based testing programs in New York City (NYC), including search engine optimization and advertisements24 that uncovered 47 primary HIV infections, as well as recruiting for HIV testing in sex-on-premises venues, gay bars,25 public parks, and homeless shelters.26 Venue-based testing in bathhouses has shown success where, of the 493 men tested, 4% were found to be HIV positive.27 The bathhouse testing study tested a large proportion of White non-Hispanics (45.4%) and White Hispanics (27.6%);23 in comparison, our current study was able to test a much larger proportion of minorities, specifically Blacks (58.0%) and Hispanics (59.1%). These studies and our data demonstrate the importance of venue-based testing to target unique high risk populations successfully.
This study had some limitations. Not all pharmacies were staffed daily, limiting the number of people we were able to offer testing. Nevertheless, we believe that testing, on average 14 people a day, over 4–6 h a day, in a community-based setting, is a relatively high output. In the successful Bronx Knows testing initiative, 18 community-based organizations (CBO) tested a total of 54,648 people in the Bronx over a 3-year period.28 This amounts to about 1000 people tested per year per CBO. The pharmacy program was able to test 4227 people in a span of less than 2 years, amounting to more than 2000 participants per year. In the pharmacies, it was also difficult to count refusals accurately because there is a large volume of patients passing by the front of the pharmacy on any given day. For the purposes of this study, we classified refusals only those individuals the PHAs were able to assess the reason for refusal.
A second limitation of the study concerns sustainability of such a program without the presence of ancillary personnel to perform testing. Our ability to implement HIV testing in community pharmacies successfully has led to partnerships with community-based organizations and pharmacists. In the future, we hope to ensure sustainability by moving to a model where pharmacists and pharmacy staff conduct HIV testing and counseling and community-based organizations or case managers from partnered hospitals will provide linkage to care and retention in care services. In New York, pharmacists already played a role in other preventative services. Many New York State pharmacies participate in the Expanded Syringe Access Program, allowing provision of nonprescription syringes in order to decrease the spread of HIV through needle sharing by injection drug users.29 Since 2008, pharmacists in New York State have also been authorized to administer immunizations. One study showed that vaccination efforts with high foreign-born, immigrant populations can reduce disparities in vaccination rates.25 Pharmacies already play an important role in HIV care, helping patients achieve greater adherence and persistence with antiretroviral therapy.30 Qualitative research shows that support for in-pharmacy HIV testing is high among pharmacy staff,31 and patient-centered pharmacy services can help patients take greater responsibility for self-managing their HIV infections.32 Therefore, logistics of pharmacy-based testing is an area that merits more research. Furthermore, with HIV testing diagnostics advancing rapidly, including over-the-counter (OTC) tests available in pharmacies and rapid 1-minute tests that shorten the duration of time needed before receiving results, the pharmacy may be an important and viable venue for HIV testing and linkage to care. The OTC test, in particular, may provide a major entry point for self-testing and may naturally give pharmacists an expanded role in providing HIV information, promoting testing, and helping to facilitate testing and linkage to care. Our counselor-based model exposed pharmacists to community-based testing that could potentially help them facilitate education and linkage to care in the future.
A third limitation of this study is that we do not have data on which of the participants were pharmacy patrons and which participants were recruited off the street in front of the pharmacy, as well as data on reasons for pharmacy attendance for those that were pharmacy clients. However, our risk factor data show that many of the participants tested for HIV reported high-risk behaviors, including inconsistent condom use, being in jail or prison, and men having had sex with other men in the past 12 months.
The community pharmacies we partnered with were chosen based on a rating system that has not been validated; however, we believe the pharmacy and community characteristics used as selection criteria have face validity when applied to HIV testing. For example, we chose locations that offered both a safe and private location for our staff and clients. We also choose sites that were in high HIV prevalence areas and proximate to large numbers of young, potentially at risk individuals.
Our pharmacy-based program tests individuals that may have been missed through traditional clinical-based settings. Due in large part to the Bronx Knows HIV testing campaign, conducted from 2007 to 2009, the Bronx has the highest rate of HIV testing in New York City. Since our community-testing program was Bronx-based, a large proportion of our sample reported having had a previous HIV test. However, one community-based testing initiative cannot reach all individuals in need of HIV testing. The pharmacy model would be an important addition to current community-based testing programs to identify individuals that are not being tested through other programs, while providing a professional health venue to help participants feel safe when receiving the test, counseling, and linkage to care services. Furthermore, pharmacy participants reported having had a previous HIV test (70.0% for males and 72.5% for females) at rates lower than overall testing rates reported in the Bronx, 79.1%.
In conclusion, implementing a rapid HIV testing program in community pharmacies is feasible and identifies individuals with unique risk factor characteristics. Expansion of HIV screening initiatives into community pharmacies is one way to increase access to HIV testing for individuals who might not otherwise interact with the healthcare system.
Acknowledgments
The authors would like to thank all of the clients and patients who were a part of this retrospective study. They would also like to thank the Public Health Advocates who collected data and helped to conduct HIV testing and counseling in the pharmacies. The grant for this project was given by of the New York City Department of Health and Mental Hygiene Contract # 11-PPT-583 for HIV Priority Population Testing and Gilead Sciences, Inc.
Author Disclosure Statement
There are no conflicts of interest for any of the listed authors.
References
- 1.U.S. Centers for Disease Control and Prevention. Late HIV testing-34 states, 1996–2005. MMWR Morb Mortal Wkly Rep. 2009;58:661–665. [PubMed] [Google Scholar]
- 2.U.S. Centers for Disease Control and Prevention. Vital signs: new hope for stopping HIV. 2011. http://www.cdc.gov/vitalsigns/HIVTesting/ [Jan 5;2013 ]. http://www.cdc.gov/vitalsigns/HIVTesting/
- 3.U.S. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. 2013. http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf#Page=21. [May 14;2013 ]. http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf#Page=21
- 4.Castel AD. Magnus M. Peterson J, et al. Implementing a novel citywide rapid HIV testing campaign in Washington, DC: findings and lessons learned. Public Health Rep. 2012;127:422–431. doi: 10.1177/003335491212700410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.New York State Department of Health. How New York State's New HIV Testing Law Affects Consumers. 2011. http://www.health.ny.gov/diseases/aids/testing/law/q_and_a_for_consumers.htm. [Jan 5;2013 ]. http://www.health.ny.gov/diseases/aids/testing/law/q_and_a_for_consumers.htm
- 6.U.S. Centers for Disease Control and Prevention. HIV in the United States: an overview. 2011. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/pdf/HIV-US-overview.pdf. [Jan 5;2013 ]. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/pdf/HIV-US-overview.pdf
- 7.New York City Department of Health and Mental Hygiene. HIV Testing is now a routine part of health care in New York. 2010. http://www.nyc.gov/html/doh/html/pr2010/pr043-10.shtml. [Jan 5;2013 ]. http://www.nyc.gov/html/doh/html/pr2010/pr043-10.shtml
- 8.DeVoe JE. Baez A. Angier H, et al. Insurance+access≠health care: typology of barriers to health care access for low-income families. Ann Fam Med. 2007;5:511–518. doi: 10.1370/afm.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Centers for Disease Control. HIV Surveillance Report, 2009. http://www.cdc.gov/hiv/surveillance/resources/reports/2009report/pdf/2009SurveillanceReport.pdf. [Jan 5;2013 ];2011 21 [Google Scholar]
- 10.Bokhour BG. Solomon JL. Knapp H, et al. Barriers and facilitators to routine HIV testing in VA primary care. J Gen Intern Med. 2009;24:1109–1114. doi: 10.1007/s11606-009-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musheke M. Ntalasha H. Gari S, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Saharan Africa. BMC Public Health. 2013;13:220–235. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurgensen M. Sandoy IF. Michelo C, et al. Effects of home-based voluntary counseling and testing on HIV-related stigma: findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81:18–25. doi: 10.1016/j.socscimed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Mall S. Middelkoop K. Mark D, et al. Changing patterns in HIV/AIDS stigma and uptake of voluntary counseling and testing services: the results of two consecutive community surveys conducted in the Western Cape, South Africa. AIDS Care. 2013;25:194–201. doi: 10.1080/09540121.2012.689810. [DOI] [PubMed] [Google Scholar]
- 14.Bluthenthal RN. Palar K. Mendel P, et al. Attitudes and beliefs related to HIV/AIDS in urban religious congregation: barriers and opportunities for HIV-related interventions. Soc Sci Med. 2013;74:1520–1527. doi: 10.1016/j.socscimed.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turan JM. Nyblade L. HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: A review of the evidence. AIDS Behav. 2013 Mar 9; doi: 10.1007/s10461-013-0446-8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.National HIV/AIDS Strategy for the United States. The White House. 2010. http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf. [May 14;2013 ]. http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf
- 17.New York City Department of Health and Mental Hygiene. 2013. http://www.nyc.gov/html/doh/html/living/std-hiv.shtml. [Jan 5;2013 ]. http://www.nyc.gov/html/doh/html/living/std-hiv.shtml
- 18.Calderon Y. Leider J. Hailpern S, et al. High-volume rapid HIV testing in an urban emergency department. AIDS Patient Care STDs. 2009;23:749–755. doi: 10.1089/apc.2008.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.County Health Rankings. Bronx, New York: 2012. 2012. [Jan 5;2013 ]. Uninsured. [Google Scholar]
- 20.United States Census Bureau. Bronx County, New York: 2012. [Jan 5;2013 ]. State & County Quick facts. Bronx County, New York. [Google Scholar]
- 21.New York City Department of Health and Mental Hygiene. Epi Data Tables. 2012. http://www.nyc.gov/html/doh/downloads/pdf/epi/datatable3.pdf. [Jan 5;2013 ]. http://www.nyc.gov/html/doh/downloads/pdf/epi/datatable3.pdf
- 22.Sweeney M. Overview of HIV Epidemiology in the Bronx. Presentation at HPTN 065 (TLC-Plus) Investigators Meeting.2010. [Google Scholar]
- 23.Barnes W. D'Angelo L. Yamazaki M, et al. Identification of HIV-infected 12- to 24- year-old men and women in 15 cities through venue-based testing. Arch Pediatr Adolesc Med. 2012;164:273–276. doi: 10.1001/archpediatrics.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvera R. Stein D. Hutt R, et al. The development and implementation of an outreach program to identify acute and recent HIV infections in New York City. Open AIDS J. 2012;4:76–83. doi: 10.2174/1874613601004010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birrell F. Staunton S. Debattista J, et al. Pilot of non-invasive (oral fluid) testing for HIV within a community setting. Sex Health. 2010;7:11–16. doi: 10.1071/SH09029. [DOI] [PubMed] [Google Scholar]
- 26.Bowles KE. Clark HA. Tai E, et al. Implementing rapid HIV testing in outreach and community settings: results from an advancing HIV prevention demonstration project conducted in seven U.S. cities. Public Health Rep. 2008;123:78–85. doi: 10.1177/00333549081230S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daskalakis D. Silvera R. Bernstein K, et al. Implementation of HIV testing at 2 New York City bathhouses: from pilot to clinical service. Clin Infect Dis. 2009;48:1609–1616. doi: 10.1086/598979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New York City Department of Health and Mental Hygiene. The Bronx Knows HIV Testing Initiative Final Report. 2011. http://www.nyc.gov/html/doh/downloads/pdf/ah/bronx-knows-summary-report.pdf. [May 23;2013 ]. http://www.nyc.gov/html/doh/downloads/pdf/ah/bronx-knows-summary-report.pdf
- 29.Crawford ND. Blaney S. Amesty S, et al. Individual- and neighborhood-level characteristics associated with support of in-pharmacy vaccination among ESAP-registered pharmacies: pharmacists' role in reducing racial/ethnic disparities in influenza vaccinations in New York City. J Urban Health. 2011;88:176–185. doi: 10.1007/s11524-010-9541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy P. Cocohoba J. Tang A, et al. Impact of HIV-specialized pharamcies on adherence and persistence with antiretroviral therapy. AIDS Patient Care STDs. 2012;26:526–531. doi: 10.1089/apc.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amesty S. Blaney S. Crawford ND, et al. Pharmacy staff characteristics associated with support for pharmacy-based HIV testing. J Am Pharm Assoc. 2003;52:472–479. doi: 10.1331/JAPhA.2012.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kibicho J. Owczarzak J. A patient-centered pharmacy services model of HIV patient care in community pharmacy settings: a theoretical and empirical framework. AIDS Patient Care STDs. 2012;26:20–28. doi: 10.1089/apc.2011.0212. [DOI] [PubMed] [Google Scholar]