Abstract
RGS14 is a 60 kDa protein that contains a regulator of G protein signalling (RGS) domain near its N-terminus, a central region containing a pair of tandem Ras binding domains (RBD), and a GPSM (G protein signalling modulator) domain (a.k.a. Gi/o-Loco binding (GoLoco) motif) near its C-terminus. The RGS domain of RGS14 exhibits GTPase accelerating protein (GAP) activity toward Gαi/o proteins, while its GPSM domain acts as a guanine nucleotide dissociation inhibitor (GDI) on Gαi1 and Gαi3. In the current study, we investigate the contribution of different domains of RGS14 to its biochemical functions. Here we show that the full-length protein has a greater GTPase activating activity but a weaker inhibition of nucleotide dissociation relative to its isolated RGS and GPSM regions, respectively. Our data suggest that these differences may be attributable to an inter-domain interaction within RGS14 that promotes the activity of the RGS domain, but simultaneously inhibits the activity of the GPSM domain. The RBD region seems to play an essential role in this regulatory activity. Moreover, this region of RGS14 is also able to bind to members of the B/R4 subfamily of RGS proteins and enhance their effects on GPCR-activated Gi/o proteins. Overall, our results suggest a mechanism wherein the RBD region associates with the RGS domain region, producing an intramolecular interaction within RGS14 that enhances the GTPase activating function of its RGS domain while disfavoring the negative effect of its GPSM domain on nucleotide dissociation.
Keywords: RGS proteins, GAP activity, GDI activity, GAP-enhancing activity, Ras binding domain, intramolecular interaction
Heterotrimeric G proteins are involved in many important cellular processes. The binding of an activating ligand to a G protein-coupled receptor leads to the exchange of the nucleotide on the Gα subunit of G protein, which further regulates many downstream effectors, such as adenylyl cyclase and ion channels (Neves et al., 2002). Nucleotide exchange and GTP hydrolysis are the two major events that control the duration of G protein signalling. The fact that signal termination in vivo tends to be more rapid than observed rates of GTP hydrolysis in vitro suggested that other mechanisms may exist to regulate the duration of G protein signalling (Ross and Wilkie, 2000). Many factors have been identified that are able to modulate the G protein cycle such as GTPase accelerating proteins (GAPs), guanine nucleotide exchange factors (GEFs) and guanine nucleotide dissociation inhibitors (GDIs) (Siderovski and Willard, 2005). Some modulators are relatively simple in structure and only contain one functional domain, however, several proteins such as GPSM3 (also called G18 or AGS4) (Zhao et al., 2010) and RGS14 (Hollinger et al., 2001; Mittal and Linder, 2006) have been found to contain more than one heterotrimeric G protein regulatory domain. The net effects of these complex proteins on signalling events still remain poorly understood.
RGS14 is a relatively large RGS protein (∼60 kDa) that belongs to the D/R12 subfamily. Two members of this subfamily, RGS12 and RGS14, are multidomain proteins. Besides an RGS domain, each contains a second Gα binding region (GPSM domain) near the C-terminus, as well as a central Ras-binding domain (RBD) region that contains a pair of tandem ∼70 amino acid residue Raf homology regions, at least one of which can selectively bind to activated Ras-like small G proteins (Mittal and Linder, 2006). RGS12 and RGS14 are among the largest RGS proteins, while the remaining D/R12 member, RGS10, is similar in size to the B/R4 subfamily of RGS proteins (Ross and Wilkie, 2000). Most studies on the physiological function of RGS14 have focused on its roles in the brain and in cell division (Lee et al., 2010; Martin-McCaffrey et al., 2004a; Martin-McCaffrey et al., 2004b; Martin-McCaffrey et al., 2005; Rodriguez-Munoz et al., 2007). For example, RGS14 is a mitotic spindle protein that associates with microtubules (Martin-McCaffrey et al., 2004a; Martin-McCaffrey et al., 2004b; Martin-McCaffrey et al., 2005). In addition, RGS14 may play an important role in hippocampal-based learning and memory by acting as a natural suppressor of synaptic plasticity in CA2 neurons (Lee et al., 2010).
The individual biochemical activities of the two heterotrimeric G protein binding domains of RGS14 have been well studied (Hollinger et al., 2003; Kimple et al., 2001; Mittal and Linder, 2004; Shu et al., 2007). The RGS domain exhibits GAP activity in single-turnover GTPase assays in solution, whereas the C-terminal GPSM domain interferes with the nucleotide exchange from isolated Gαi1 and Gαi3 in vitro. Interestingly, the GAP activity of full-length RGS14 for Gαi/o was apparently greater than that of the isolated RGS domain (Hollinger et al., 2001). This could conceivably reflect differences in their intrinsic activities and/or affinities for G proteins, however the exact mechanism remains to be elucidated. Regarding full-length RGS14, either its RGS or its GPSM domain effect on heterotrimeric G proteins may predominate under a given set of circumstances (Hollinger et al., 2001; Traver et al., 2004; Vellano et al., 2011), although how this happens is not known.
As noted above, both RGS12 and RGS14 contain two tandem binding domains for activated Ras-like monomeric G proteins (Ponting, 1999). Recent studies have shown that both H-Ras and, surprisingly, Raf-1 can bind in a positively cooperative manner to the RBD region of RGS14 and modulate signalling through Ras/Raf/MAP kinase cascades (Shu et al., 2010;Willard et al., 2009). Since RGS14 contains two distinct Gα binding sites as well as two Ras binding sites, it also has been proposed that RGS14 may act as a scaffolding protein that integrates heterotrimeric G protein and small G protein pathways (Shu et al., 2010; Willard et al., 2009). Indeed, the binding of Gαi1 to RGS14 appears to modulate its ability to govern H-Ras signalling (Shu et al., 2010). Given the complexity of its structure, other interdomain effects could potentially occur between the various domains of RGS14 (Mittal and Linder, 2006).
Besides the full-length protein, various potential alternative splice variants of RGS14 have been tentatively identified (see Discussion), although no specific activities have yet been attributed to them (Martin-McCaffrey et al., 2004a). In several RGS14 variants, the RGS domain is missing or incomplete, suggesting that these RGS14 isoforms may have undiscovered functions that either do not require the RGS domain or alternatively may co-opt another RGS protein in substitution.
Although the biochemical activities of different domains of RGS14 have been studied individually, their aggregate contribution to the function of the full-length RGS14 has not been well established. In the current study, we compared the effects of RGS14 on G protein activities using the full-length protein and its isolated domains, and our data point to a novel intramolecular regulation mechanism which strengthens the RGS domain GAP activity but interferes with the GPSM domain function of the protein. The region between the RGS domain and the GPSM domain seems to be responsible for this function (amino acid residues 300-444). In addition to regulating RGS14 function, our data also suggest that this region of RGS14 might have a general GAP enhancing effect on other RGS proteins as well.
Materials and Methods
Protein expression and purification
Hexahistidine (H6)-tagged thioredoxin (Tx), Tx- and hexahistidine (H6)-tagged full-length RGS14 (TxH6-RGS14), truncated versions of the protein which contain the N-terminus and RGS domain (aa1-205, H6-R14-RGS), the RBD region (aa205-490, TxH6-R14-RBD), the RBD region with active or inactive GPSM domain (aa299-544, TxH6-R14-RBD/GPSM, TxH6-R14-RBD/GPSM(LLAA)), or the active or inactivated GPSM domain (aa444-544, TxH6-R14-GPSM or TxH6-R14-GPSM(LLAA)), were constructed and expressed. Proteins were purified from BL21/DE3 bacterial cells as described (Hepler et al., 2005). The cells were grown to mid-log phase, and protein production was induced with 1 mM IPTG for 2 h. Cells were lysed using the French Press method, and the supernatant was recovered, loaded to a Ni2+ HiTrap affinity column (Amersham Pharmacia, NJ), and purified by FPLC. Proteins were eluted with an imidazole gradient from 20 to 200 mM imidazole in 50 mM HEPES at pH 7.4 and 150 mM NaCl. For TxH6-R14, the cell supernatant was loaded onto Ni-NTA agarose beads, washed and eluted using 200 mM imidazole, and further purified by FPLC using a superdex-200 column (Pharmacia-Biotech). Histidine-tagged RGS4, RGS5, RGS16, and Gi1α, as well as Glutathione S-transferase (GST) control protein and GST-tagged RGS4 and RGS5 were grown in E. coli and purified as described previously (Abramow-Newerly et al., 2006).
Receptor- and agonist-stimulated GTPase assay
Sf9 membranes overexpressing M2 muscarinic receptor or α2a-adrenergic receptor and heterotrimeric G proteins were prepared as indicated previously (Cladman and Chidiac, 2002). Baculovirus encoding the α2a-adrenergic receptor was generously provided by Dr. Johnny Näsman (Åbo Akademi University, Turku, Finland), and other baculoviruses were as described previously (Cladman and Chidiac, 2002; Mao et al., 2004). Sf9 cell membranes (8 μg/tube) were assayed for agonist-stimulated GTP hydrolysis at 30 °C for 5 minutes in the absence or presence of the indicated purified proteins in reaction buffer (20 mM Hepes, pH 7.5, 1 mM EDTA, 1mM DTT, 0.1 mM PMSF, 10 mM NaCl, 2 mM MgCl2 (1 mM free Mg2+) 1 μM GTP, 1 mM ATP, [γ-32P] GTP (1×106 cpm per assay) and protease inhibitors) in a total reaction volume of 50-70 μL. The assay was stopped by adding ice-cold 5% (w/v) Norit in 0.05 M NaH2PO4. The reaction mixture was centrifuged and the level of 32Pi in the resulting supernatant was determined by liquid-scintillation counting. The nonspecific GTPase activity was defined as that in the presence of either the M2 muscarinic receptor inverse agonist tropicamide (10 μM) or the α2-adrenergic receptor inverse agonist rauwolscine (10 μM) as appropriate, and these values were subtracted from the total counts per minute to yield the agonist- and receptor-dependent GTP hydrolysis rates.
GTPγS binding assay
Purified H6-Gαi1 (100 nM) was incubated for 1 hour at 4°C in binding buffer (20 mM Hepes (pH 8.0), 1 mM EDTA (pH 8.0), 100 mM NaCl, 1 mM DTT, 2 mM MgCl2, 0.1 mg/ml BSA, 0.1% Lubrol, PMSF and 1 μg/ml leupeptin, 10 μg/ml aprotinin) in the presence or absence of 1 μM RGS14 or one of its mutants. Binding assays were initiated by adding 0.5 μM [35S]-GTPγS (1.25×105 cpm/pmol). The incubation continued for various time up to 30 minutes at 30°C. The assay was terminated by adding ice-cold stop buffer (20 mM Tris (pH 8.0), 10 mM MgCl2, 100 mM NaCl, 0.1% Lubrol, 1 mM GTP and 0.1 mM DTT). Samples were filtered through nitrocellulose membranes (Millipore) followed by washing four times with 2 mL ice-cold wash buffer (20 mM Tris (pH 8.0), 100 mM NaCl, 10 mM MgCl2). Radioactivity was measured using liquid-scintillation counting. The nonspecific binding was determined in the presence of 100 μM unlabeled GTPγS, and these values were subtracted to yield specific binding.
Protein-protein interaction assay
Individual truncated mutants of RGS14 (500 nM) were incubated with an equimolar amount (500 nM) of GST-tagged RGS4 or RGS5. The protein mixture was incubated on a rotating platform at 4°C for 2 hours in binding buffer (50 mM Tris (pH 7.5), 0.6 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.1% Triton-X 100, PMSF 2 μg/ml leupeptin, and 20 μg/ml aprotinin). Glutathione Sepharose 4B beads (20 μL bed volume) were then added into the protein mixture and incubated overnight. The protein mixture was washed three times with binding buffer and the pelleted beads were resuspended in 2× Laemmli buffer. Eluted proteins were separated on a 12% SDS gel and transferred to a Polyvinylidene Fluoride Transfer (PVDF) membrane (Pall Corporation) for immunoblotting.
Immunoblotting
Membranes were incubated with blocking buffer (Tris-Buffered Saline Tween-20 (TBST) with 5% skim milk) for 1 hour and then probed with anti-His or anti-GST antibody (1:1000) (Santa Cruz biotechnology) diluted in blocking buffer overnight on a rotating platform at 4°C. Blots were subsequently washed 3 times with TBST and then incubated with HRP-conjugated secondary antibody (1:2000) (Promega) diluted in TBST for 1 hour at room temperature. After another 3 washes with TBST, the blot was visualized by LumiGLO Reserve Chemiluminescence substrate (KPL, Inc) using a FluorChem 8000 imaging system.
Statistics
Data are presented as Mean±SEM. Data were analysed using T-test, One way ANOVA, or Non-Linear regression. Differences were considered significant at P < 0.05.
Results
Full-length RGS14 stimulates M2 muscarinic receptor-activated G protein GTPase activity
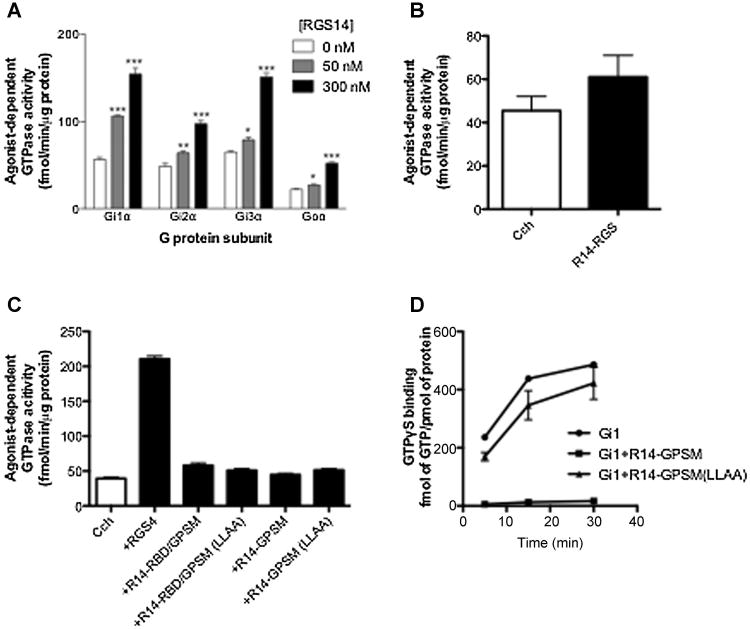
To characterize the effect of RGS14 on the cyclical binding and hydrolysis of GTP by G proteins, and the contributions of its various domains to these activities, agonist and receptor-stimulated steady-state GTPase assays were carried out (Figure 2). Full length RGS14 degrades rapidly (Cho et al., 2005; Hollinger et al., 2001), but purification was made possible by the addition of a thioredoxin (Tx) moiety (see Figure 1, Construct No. 1), which itself does not appear to bind to Gα proteins (Hepler et al., 2005). As shown in Figure 2A, full-length RGS14 enhanced the GTPase activity of all four Gi/o proteins in a dose dependent manner.
Fig. 2. Effect of RGS14 on M2 muscarinic receptor stimulated GTPase activity.
Membranes were derived from Sf9 cells co-expressing the M2 muscarinic acetylcholine receptor plus heterotrimeric Gi1, Gi2, Gi3 or Go. Receptor-/agonist stimulated GTPase assays were carried out at 30°C for 5 minutes with the agonist carbachol (100 μM) either alone or in the presence of full-length RGS14 at indicated concentration (A), R14-RGS (300 nM) (B), or different truncated mutants of RGS14 (1 μM) (C). Nonspecific signal with each membrane was defined as that observed in the absence of RGS protein and in the presence of the inverse agonist tropicamide (10 μM) and this was subtracted to yield the values indicated. (D) Purified H6-Gαi1 (100 nM) was pre-incubated for 1 hour at 4°C in binding buffer in the presence or absence of RGS14 or one of its mutants (1 μM). Binding assays were initiated by adding [35S]-GTPγS. The incubation continued for the indicated times at 30°C. The assay was terminated by adding ice-cold stop buffer. Samples were vacuum filtered through nitrocellulose membranes (Millipore) and washed. Radioactivity was measured using liquid scintillation counting. The nonspecific signal was determined in the presence of 100 μM unlabeled GTPγS, and these values were subtracted to yield specific binding. Data represent mean values ± SEM of at least three independent experiments, #, p < 0.05, compared with agonist in the presence of R14-RGS, **, p<0.01; ***, p<0.001, compared with agonist alone (one-way ANOVA with Tukey's multiple comparison test).
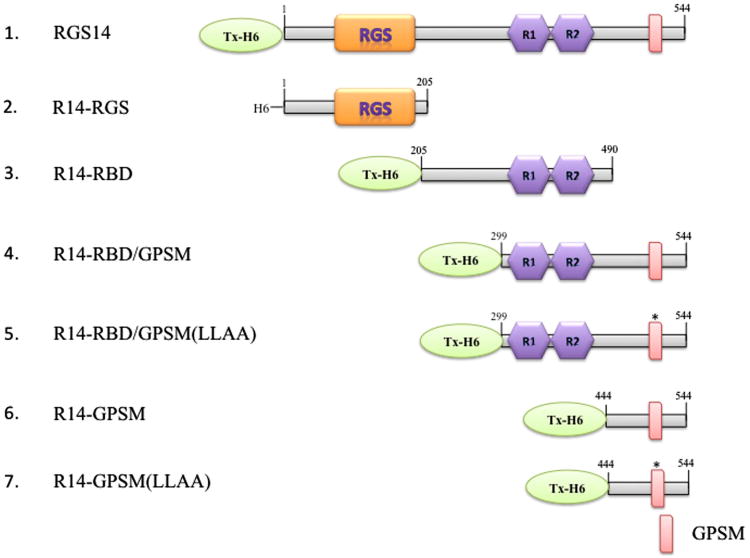
Fig. 1. Diagram of constructs used in this study.
Tx- and H6-tagged full-length RGS14 (1), and truncated versions of the protein which contain the RGS domain (2) (aa1-205, R14-RGS), the RBD region (3) (aa205-490, R14-RBD), the RBD region and an active (4) or inactive GPSM domain (5) (aa299-544, R14-RBD/GPSM, R14-RBD/GPSM(LLAA)), or the wild type (6) or inactivated GPSM domain (7) (aa444-544, R14-GPSM, R14-GPSM(LLAA)). Abbreviations: RGS: regulator of G protein signalling; R1,R2: Ras binding domains 1 and 2. GPSM, G protein signalling modulator.
To permit comparisons with our previous work with RGS4 (Hepler et al., 2005), the RGS domain of RGS14 was purified as a polyhistidine-tagged protein (R14-RGS, Figure 1, Construct 2). In contrast to the effects of full-length RGS14 shown in Figure 2A, the GAP activity of R14-RGS on receptor-stimulated Gαi3 (Figure 2B), Gαi1, Gαi2 and Gαo (not shown) was difficult to reliably detect. This could not be attributed to a lack of GAP activity per se, as we and others have shown that this and a similar RGS14 construct have robust activity in solution-based, pre-steady-state GTPase assays with isolated Gαi/o proteins (Cho et al., 2005; Hollinger et al., 2001).
The poor activity of R14-RGS in receptor- and membrane-based assays suggested two possibilities: 1) The observed GAP activity of full-length RGS14 comes from a region or regions other than the RGS domain. 2) The isolated RGS domain of RGS14 on its own is not sufficient to act as a GAP under such conditions, thus amino acid residues outside the RGS domain may act allosterically to enhance its GAP activity. To examine the first of these possibilities, we tested the effects of various truncated mutants (Figure 1, Constructs 4-7) containing a wild type or mutationally inactivated GPSM domain, either with or without the Ras binding domain (RBD) region that makes up the middle portion of the protein. As shown in Figure 2C, none of these truncated forms of RGS14 produced any effect on muscarinic receptor-stimulated GTPase activity, implying that the observed GTPase accelerating activity of full-length RGS14 (Figure 2A) does not arise independently from a region outside of the RGS domain. The failure of constructs containing an active GPSM domain to increase steady-state GTPase activity is not surprising given the inhibitory effect of this domain on guanine nucleotide exchange under pre-steady-state conditions; mutation of the GPSM domain abrogated the latter activity (Figure 2D) and had no apparent effect on GTP turnover (Figure 2C).
Interdomain regulation of RGS14 GAP and GDI activities
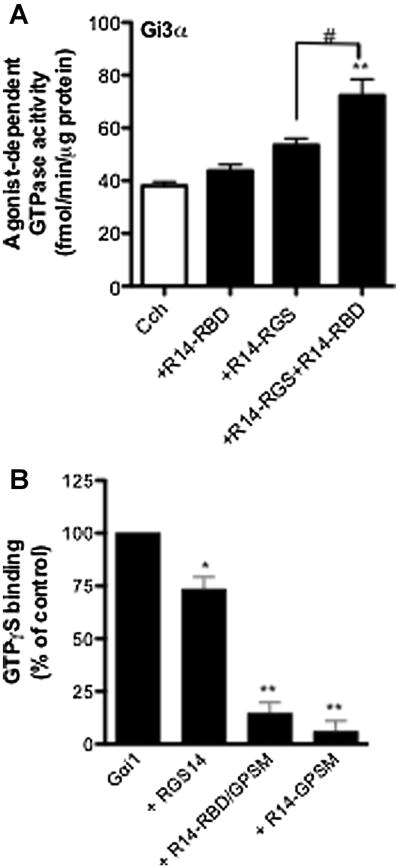
To test the second possibility noted above, i.e., that elements outside of the RGS domain of RGS14 might be able to promote its GTPase accelerating effects, we examined the activity of the isolated RGS14 RGS domain on its own and in the presence of a construct spanning the regions found between the RGS domain and the GPSM domain (Figure 1, R14-RBD, construct No. 3). This portion of the protein, which contains two sequential ∼70 amino acid residue stretches that show homology to the small G protein-activated kinase Raf, would not on its own be expected to produce any effects on heterotrimeric G protein activity. Figure 3A shows that purified R14-RBD alone has no appreciable effect on the GTPase activity of M2 muscarinic receptor-stimulated Gαi3. R14-RGS by itself once again showed only a marginal ability to promote GTPase activity, however, combining the two purified proteins produced a complementary increase in GTP turnover. This finding suggests that a part or parts of RGS14 outside of its conserved RGS domain can promote its ability to serve as a GTPase accelerating protein for receptor-coupled heterotrimeric G proteins.
Fig 3. Interdomain regulation of RGS14 GAP and GDI activity.
(A) Membranes were derived from Sf9 cells co-expressing the M2 muscarinic acetylcholine receptor plus heterotrimeric Gi3. Receptor-/agonist stimulated GTPase assays were carried out at 30°C for 5 minutes with the agonist carbachol (100 μM) either alone or in the presence of R14-RGS (300nM) and/or R14-RBD (1μM). Nonspecific signal with each membrane was defined as that observed in the absence of RGS protein and in the presence of the inverse agonist tropicamide (10 μM) and this was subtracted to yield the values indicated. (B) Purified H6-Gαi1 (100 nM) was pre-incubated for 1 hour at 4°C in binding buffer in the presence or absence of RGS14 or one of its mutants (1 μM). Binding assays were initiated by adding [35S]-GTPγS. The incubation continued for 30 minutes at 30°C. The assay was terminated by adding ice-cold stop buffer. Samples were vacuum filtered through nitrocellulose membranes (Millipore) and washed. Radioactivity was measured using liquid scintillation counting. The nonspecific signal was determined in the presence of 100 μM unlabeled GTPγS, and these values were subtracted to yield specific binding. Measurements acquired using G protein alone were taken as 100% in each experiment, and all the other values were normalized to those controls. Data represent mean values ± SEM of three independent experiments *, p < 0.05, **, p<0.01, compared with G protein alone, (one-way ANOVA with Tukey's multiple comparison test)
The fact that R14-RBD is able to enhance the GTPase accelerating activity of R14-RGS suggests that intramolecular effects within RGS14 may influence its interactions with target G proteins. Therefore, we examined whether the function of the GPSM domain of RGS14 might be regulated by either the RGS domain or the RBD region. To do this we compared full-length RGS14 and truncated forms lacking the RGS domain or both the RGS domain and the RBD region (Figure 1, Constructs No.1, 4, and 6) with respect to their abilities to inhibit nucleotide exchange on Gαi1. We found that full-length RGS14 clearly impeded guanine nucleotide exchange on Gαi1. However, the inhibitory effect of the full-length protein was significantly smaller than that of an equimolar amount of R14-RBD/GPSM, while the additional removal of the RBD region resulted in little further change (Figure 3B). This result suggests that the RGS domain and/or its surrounding amino acid residues may interfere with the physical and/or functional interaction between the GPSM domain and its G protein binding partner.
Taken together, the observations shown in Figures 2 and 3 suggest that the respective activities of the RGS and GPSM domains of RGS14 can vary depending on which other parts of the protein are present. This may reflect an interdomain effect wherein contact between the RBD region and the RGS domain enhances the GAP activity of the latter, while this interaction at the same time limits the ability of the C-terminal GPSM domain to inhibit GDP dissociation. Further experiments were carried out to investigate this apparent intramolecular regulatory mechanism.
The RBD region of RGS14 enhances RGS4 GAP activity
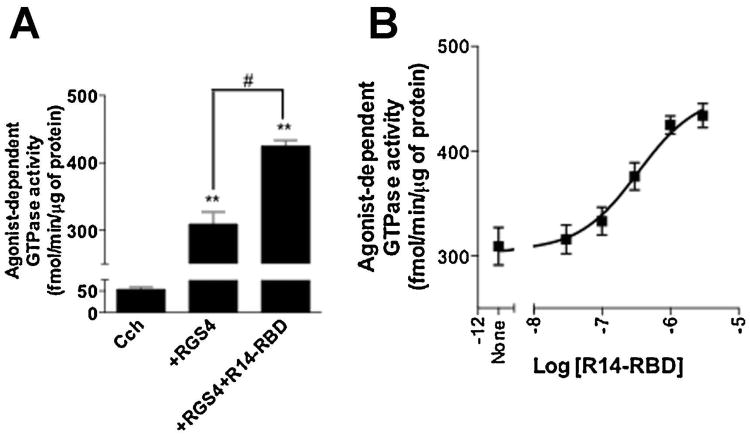
To better understand how the GTPase accelerating activity of the RGS domain of RGS14 is enhanced by other parts of the protein (Figure 3A), we examined and compared the effects of various RGS14 truncation mutants lacking the RGS domain (Figure 1, constructs 3-7) using the steady-state GAP activity of RGS4 as a readout. This small RGS protein has been shown previously to be positively modulated by R14-RBD/GPSM (Hepler et al., 2005), and its reliably robust effect on agonist- and receptor-stimulated GTP hydrolysis (Cladman and Chidiac, 2002) allows for a relatively broad window through which to examine the GAP-enhancing properties of RGS14. As shown in Figure 4A, R14-RBD significantly increased the effect of RGS4 on Gαi3, and this was found to be dose dependent, with an EC50 of ∼350 nM (Figure 4B). This finding indicates that the observed GAP-enhancing function of R14-RBD is not limited to R14-RGS.
Fig. 4. The RGS14 Ras-binding region enhances the GAP activity of RGS4.
Membranes derived from Sf9 cells co-expressing the M2 muscarinic acetylcholine receptor plus heterotrimeric Gi3 were assayed at 30°C for 5 minutes with the agonist carbachol (100 μM) either alone or in the presence of RGS4 (300 nM) with and without R14-RBD at a single concentration of 1 μM (A) or at multiple concentrations as indicated (B) . Nonspecific signal in A and B was defined as that observed in the absence of RGS protein and in the presence of tropicamide (10 μM) and this was subtracted to yield the values indicated. Data represent mean values ± SEM of three independent experiments #, p < 0.05, compared with agonist in the presence of RGS4, **, p<0.01, compared with agonist alone, (one-way ANOVA with Tukey's multiple comparison test)
The GPSM domain of RGS14 does not inhibit receptor-stimulated GTPase activity
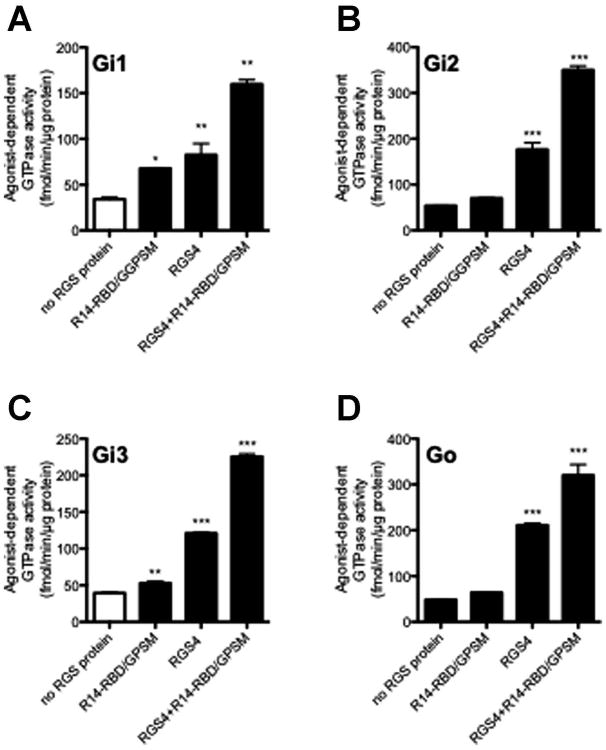
In spite of the ability of its GPSM domain to inhibit nucleotide exchange (Figure 3B), full-length RGS14 clearly accelerated receptor-stimulated, steady-state G protein GTPase activity in membrane-based assays (Figure 2A). However, given that the activity of the RGS14 GPSM domain was increased by the removal of the RGS domain (Figure 3B), it follows that an inhibitory effect on steady-state GTPase activity might be more readily detected with R14-RBD/GPSM than with the full length protein. To study this possibility we compared the effect of the R14-RBD/GPSM on GDI sensitive G proteins (Gαi1 and Gαi3) with GDI insensitive G proteins (Gαi2, and Gαo). We hypothesized that since they are unresponsive to the inhibitory effect of the GPSM domain on nucleotide exchange (Mittal and Linder, 2004), Gαi2 and Gαo might exhibit increased receptor- and RGS4-stimulated GTP turnover relative to Gαi1 and Gαi3. We found that R14-RBD/GPSM enhances the GAP activity of a submaximally activating concentration of RGS4 on all four Gi/o protein subtypes (Figure 5), leading to 70-160% increases under the conditions tested. In contrast to the behaviour predicted, effects on Gi2 and Go GTPase activity were no greater than those on Gi1 and Gi3.
Fig. 5. Effect of R14-RBD/GPSM on RGS protein GAP activity.
Membranes derived from Sf9 cells co-expressing the M2 muscarinic acetylcholine receptor plus heterotrimeric Gi1, Gi2, Gi3 or Go were assayed at 30°C for 5 minutes with the agonist carbachol (100 μM) either alone or in the presence of either R14-RBD/GPSM (1 μM), RGS4 (300 nM) or both . Nonspecific signal with each membrane was defined as that observed in the absence of RGS protein and in the presence of tropicamide (10 μM) and this was subtracted to yield the values indicated. Data represent mean values ± SEM of at least three independent experiments. *, p<0.05, **, p<0.01, ***, p<0.001, compared with agonist alone, (one-way ANOVA with Tukey's multiple comparison test).
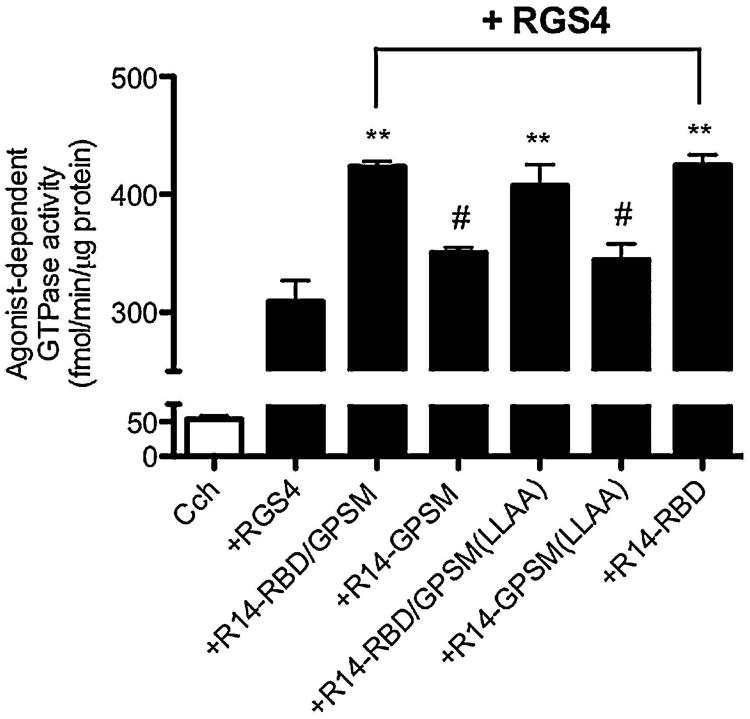
Both active and inactive forms of R14-RBD/GPSM as well as R14-GPSM (Figure 1, constructs 4-7) were also used to further probe the potential effects of the GPSM domain on RGS-stimulated GTPase activity. Neither the active (R14-GPSM) nor the mutationally inactived GPSM domain of RGS14 (R14-GPSM(LLAA)) had any appreciable effect on the steady-state GTPase activity of receptor-activated Gi3 in the presence of RGS4 (Figure 6). Removal of the GPSM domain also did not augment the GAP enhancing activity of RBD region of RGS14, as results obtained with R14-RBD/GPSM and R14-RBD were equivalent. Finally, it is conceivable that the extent of the GAP-enhancing effect of R14-RBD/GPSM on Gi3 (Figure 5) could have been limited by an inhibitory GPSM domain effect on GTP binding, in which case mutationally inactivating the GPSM domain would be expected to further enhance GTP turnover. The latter possibility can be ruled out, however, as R14-RBD/GPSM(LLAA) produced an effect indistinguishable from that of R14-RBD/GPSM (Figure 6). Overall, we observed no evidence of an inhibitory effect on guanine nucleotide exchange of the RGS14 GPSM domain in any of our receptor-based experiments.
Fig. 6. The GPSM domain of RGS14 does not inhibit receptor-stimulated GTPase activity.
Membranes derived from Sf9 cells co-expressing the M2 muscarinic acetylcholine receptor plus heterotrimeric Gi3 were assayed at 30°C for 5 minutes with the agonist carbachol (100 μM) either alone or in the presence of RGS4 (300nM) and different mutants of RGS14 (1 μM). Nonspecific signal with each membrane was defined as that observed in the absence of RGS protein and in the presence of the antagonist tropicamide (10 μM) and this was subtracted to yield the values indicated. Data represent mean values ± SEM of at least three independent experiments. **, p <0.01, compared with agonist in the presence of RGS4, #, p<0.01compared with agonist in the presence of RGS4 and R14-RBD/GPSM, (one-way ANOVA with Tukey's multiple comparison test).
Direct interaction between RGS proteins and R14-RBD region
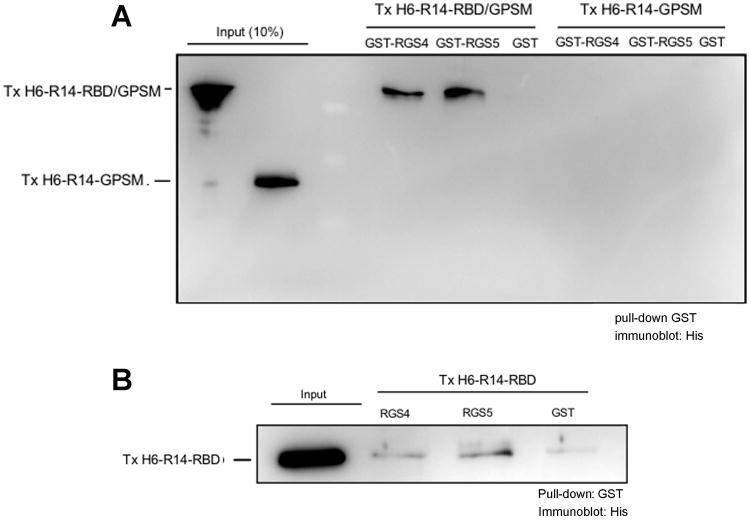
Based on our findings suggesting that the GAP-enhancing effect of R14-RBD may reflect a physical interaction with the RGS domain region of RGS14, we hypothesized that a direct protein-protein interaction might occur between R14-RBD and RGS4. This notion is supported by the experimental findings shown in Figure 7A, wherein R14-RBD/GPSM or R14-GPSM, which carry a polyhistidine tag, was co-incubated with GST control protein or GST-fusion proteins of RGS4 or RGS5, followed by precipitation using glutathione-sepharose beads. R14-RBD/GPSM directly interacted with RGS4 and RGS5, but the GPSM domain alone showed no appreciable binding to any of the RGS proteins (Figure 7A). We further confirmed this RBD-RGS complex using isolated R14-RBD region and RGS proteins. As shown in Figure 7B, indeed, we observed a direct interaction between R14-RBD and both RGS4 and RGS5, although the signal is not as robust as with the R14-RBD/GPSM construct. Overall, our findings suggest that residues 300-444, where both of the Ras binding domains are located, is primarily responsible for the observed enhancement of RGS GAP activity, and that this effect in full-length RGS14 may be due to a direct intramolecular interaction between the RBD region and amino acid residues in and around the RGS domain.
Fig. 7. Direct interaction between RGS proteins and R14-RBD region.
Purified TxH6-R14-RBD/GPSM (500 nM), TxH6-R14-GPSM (500 nM) (A) or TxH6-R14-RBD (500 nM) (B) was incubated with an equimolar amount of GST-tagged RGS4 or RGS5 for 2 hours at 4°C. Glutathione Sepharose 4B beads were then added into the protein mixture and incubation continued overnight. The protein mixture was washed three times with binding buffer and the beads were separated and transferred to a PVDF membrane for immunoblotting. A representative blot of three independent experiments is shown.
A generalized RGS14 GAP-enhancing effect
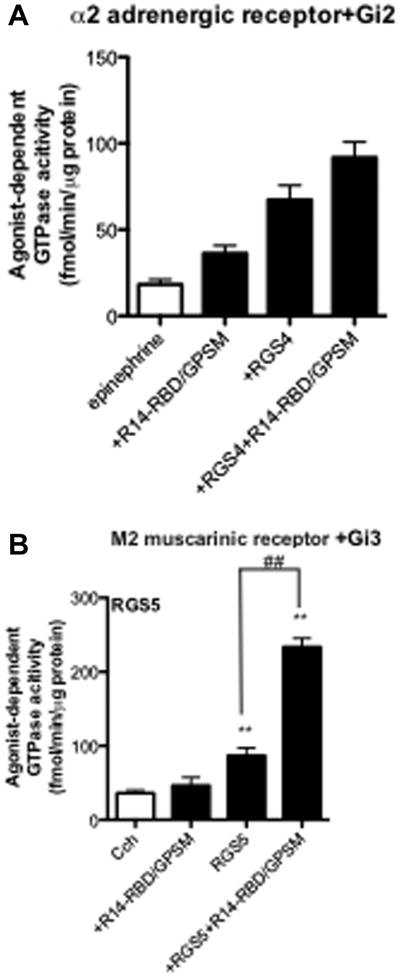
The results shown in Figure 5 indicate that the GAP-enhancing effect of RGS14 occurs with all four members of the Gi/o family that were tested. In a final series of experiments, we sought to determine whether the GAP-enhancing effect of RGS14 could be further generalized to a broader range of signalling partners. R14-RBD/GPSM was found to promote the effect of RGS4 on α2-adrenergic receptor-activated Gi3, and thus the potentiating effect does not appear to be dependent on the M2 muscarinic receptor per se (Figure 8A). We also examined whether R14-RBD/GPSM could enhance the GAP activities of other RGS proteins. Indeed, R14-RBD/GPSM facilitated the GAP activity of each B/R4 subfamily RGS protein that we tested, including RGS4, RGS5 (Figure 8B), and RGS16 (data not shown). The latter results are consistent with the observed physical interactions between RGS5 and truncated forms of RGS14 containing the RBD region (Figure 7A, B). Overall, our results indicate that the GAP-enhancing effect of RGS14 can occur with multiple different combinations of receptor, G protein and RGS protein.
Fig. 8. General effect of R14-RBD/GPSM on RGS-promoted steady-state GTPase activity.
Membranes derived from Sf9 cells co-expressing the α2-adrenergic receptor plus heterotrimeric Gi2 (A) or M2 muscarinic acetylcholine receptor plus heterotrimeric Gi3 (B) were assayed with the agonists epinephrine (α2 adrenergic receptor (10 μM)), or carbachol (M2 muscarinic acetylcholine receptor (100 μM)) either alone or in the presence of RGS4 (300 nM) or RGS5 (100 nM). Nonspecific signal was defined as that observed in the absence of RGS protein and in the presence of the antagonists rauwolscine (α2 adrenergic receptor (10 μM)), or tropicamide (M2 muscarinic acetylcholine receptor (10 μM)) and this was subtracted to yield the values indicated. Data represent mean values ± SEM of at least three independent experiments. ##, p < 0.01, compared with agonist in the presence of RGS protein, **, p<0.01, compared with agonist alone, (one-way ANOVA with Tukey's multiple comparison test).
Discussion
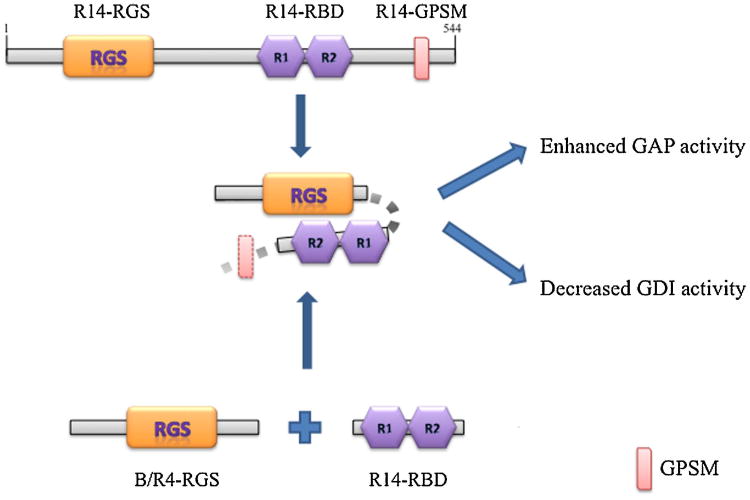
Our study characterizes a novel biochemical property of the RBD region of RGS14 (amino acids 300-444), and suggests that it may have functions other than binding to small G proteins. This region of RGS14 is able to promote the GTPase activating activities of various RGS proteins, including the RGS domain of RGS14 itself and also members of the B/R4 RGS protein subfamily. Moreover, we observed a direct interaction between N-terminally truncated forms of RGS14 and different RGS proteins, which seems to be abolished upon removal of the RBD region. Thus the increase in GAP activity may reflect an increase in the affinity between the RGS domain and its target G protein mediated by the RBD and its surrounding regions (Hepler et al., 2005). This enhancing effect of the RBD region of RGS14 on RGS-mediated GTPase accelerating effects is evident with multiple types of Gi/o proteins, RGS proteins, and GPCRs. In contrast, this interdomain interaction seems to interfere with the GDI activity of the RGS14 GPSM domain, as truncated forms of RGS14 lacking the RGS domain were found to have increased effects on Gαi/Gαo nucleotide exchange compared to full-length RGS14. Figure 9 illustrates a simple model that seems to agree with all of the data described in the present study. Briefly, our data suggest that the RBD region of RGS14 is able to directly interact with amino acid residues in and around its target RGS domains and enhance their GTPase accelerating activities. At the same time, this interaction may inhibit the activity of the GPSM domain.
Fig. 9. Illustration of RBD region regulation of RGS14 and other RGS proteins activities.
The present findings suggest that an intramolecular interaction between elements in and around the RGS domain and the RBD region of RGS14 enhances the GAP activity of the RGS domain while diminishing the GDI activity of the GPSM domain. Analogously, the RBD region of RGS14 can interact with RGS proteins of the B/R4 subfamily and enhance their GAP activities as well. Abbreviations: RGS: regulator of G protein signalling; R1,R2: Ras binding domains 1 and 2; GPSM, G protein signalling modulator.
The apparent ability of the RBD region of RGS14 to enhance RGS-GAP activity, as well as the complex effects of Gαi1 on the ability of RGS14 to modulate Ras/Raf signalling reported by Shu and co-workers (Shu et al., 2010) imply that interdomain interactions within RGS14 or between the RBD region of RGS14 and other RGS proteins regulate the function of each domain. Such a mechanism would be consistent with observations that 1) unlike full-length RGS14 (Figure 2A), the isolated RGS domain has minimal GTPase accelerating activity in membrane-based assays of receptor-driven G protein activity (Figure 2B), while the presence of the RBD region greatly enhances RGS14 GAP activity (Figure 3A), and 2) full-length RGS14 is approximately 10-fold more potent as a GAP compared to the isolated RGS14 RGS domain in single turnover GTP hydrolysis assays with Gαi1 and Gαo (Hollinger et al., 2001).
The ability of the RGS14 RBD region to enhance GAP activity is not limited to the RGS domain of RGS14, as similar effects were observed with RGS4 and RGS5. Thus the RBD region of RGS14 might serve a general role in regulating RGS protein function. This might not be expected to occur with full-length RGS14, but could happen if the RGS domain were absent, for example due to partial proteolysis or alternative splicing of RGS14 mRNA. Available sequence data suggest that RGS14 in primates can exist as four or more splice variants. Studies to date have examined the full-length form (isoform 1; UniProtKB/Swiss-Prot O43566-7; GenBank EAW85012.1) and a short variant (isoform 2; UniProtKB/Swiss-Prot O43566-4; GenBank AAM12650.1) containing part of the RGS domain and part of the first small G protein binding domain whose function remains unclear (Cho et al., 2005; Martin-McCaffrey et al., 2004a; Martin-McCaffrey et al., 2005). In addition, there are two putatively identified intermediate-length variants that contain the full RBD region and the GPSM domain and intervening sequence but from which the RGS domain is either mostly missing (human isoform 3; UniProtKB/Swiss-Prot O43566-5; GenBank: AAY26402.1, Chimpanzee isoform 3; NCBI Reference Sequence: XP_001141818.1) or completely absent (human isoform 4; UniProtKB/Swiss-Prot O43566-6; GenBank: BAC85600.1, Chimpanzee isoform isoform 2; NCBI Reference Sequence: XP_001141745.1). The possible existence of natural variants comparable to our truncated mutants suggests that isoforms of RGS14 lacking the RGS domain may function in a way distinct from the full-length protein, and thus the present results may shed light on the functions of these putative uncharacterized protein species. Although the existence of some variants still remains to be confirmed experimentally, we hypothesize that isoforms of RGS14 lacking the RGS domain may a) promote the GAP activity of subfamily B/R4 RGS proteins at GPCR-activated Gi/o proteins, and b) have increased GPSM activity relative to full-length RGS14.
The exact mechanism underlying the observed enhancement in GAP activity remains to be determined. Our data would appear to imply that the R14-RBD regulates RGS activity via an allosteric mechanism. The crystal structure of RGS4 reveals one potential allosteric modulation site (Site B) located opposite to the G protein-binding surface of the RGS domain (Sjogren and Neubig, 2010). This region within the RGS domain includes two pairs of positively charged residues which appear to be responsible for RGS binding to calmodulin (Ishii et al., 2005; Tesmer, 2009). Interestingly, a sequence BLAST between calmodulin and the RBD region of RGS14 reveals an 11 amino acid region of 64% similarity, and this region in both cases is intensively negatively charged. Thus, it is possible that the RBD region of RGS14 binds to site B on the RGS domain.
The interdomain interactions that affect RGS14 function may vary according to the experimental context or cellular environment. Receptor-activated G proteins appear to be insensitive to the GDI effect of the GPSM domain of RGS14 (Figures 2C and 6). In contrast, the steady-state GTPase activity of free Gαi1 was found to be inhibited by the addition of full-length RGS14 irrespective of the presence of the guanine nucleotide exchange factor Ric-8A, implying that the activity of the GPSM domain is favoured over that of the RGS domain under such conditions (Vellano et al., 2011). Moreover, essentially no difference in basal or Ric-8A-stimulated Gαi1 GTPase activity was evident between full-length RGS14 versus R14-RBD/GPSM, which again implies that the GAP activity of the RGS domain of RGS14 was secondary to the GDI activity of its GPSM domain. It follows that assay conditions can strongly influence RGS14 function. Apart from using different nucleotide exchange factors than Vellano et al. (Vellano et al., 2011) (i.e., agonist-activated GPCRs vs Ric-8A), GTPase assays carried out in the present study also utilized Sf9 cell membranes that included Gβγ, phospholipids, and possibly other relevant cellular constituents, and one or more of these factors might influence RGS14 activity. Overall the data seem to suggest that the interdomain interaction that facilitates RGS GAP activity while limiting GPSM GDI activity is less pronounced in detergent solution and may be increased in the solid-phase context of a plasma membrane that includes GPCR, Gβγ and other membrane components.
Overall, the results of our lab and others indicate that each of the multiple functions of RGS14 may manifest itself or remain quiescent under a given set of conditions depending upon intramolecular interactions, post-translational modifications, interactions with other proteins, and cellular context. The present findings advance our understanding of RGS14 function, and also raise a number of interesting questions about RGS14 and its possible splice variants. Future studies will aim to further elucidate how intramolecular interactions within RGS14 (and possibly, by analogy, within RGS12) are altered by the binding of various partners such as heterotrimeric and small G proteins, and how this impacts the ability of RGS14 to function as a signal integrator between these two types of guanine nucleotide-dependent signalling pathways.
Acknowledgments
This work was supported by funds from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research (to PC) and from the US National Institutes of Health R01-NS37112 and R01-NS049195 (to JRH). PC holds a Career Investigator Award from the Heart and Stroke Foundation of Canada.
Abbreviations used
- GAP
GTPase accelerating proteins
- GEF
Guanine nucleotide exchange factors
- GDI
Guanine nucleotide dissociation inhibitors
- RGS protein
Regulators of G protein signaling proteins
- GPSM
G protein signaling modulator
- RBD
Ras-binding domain(s)
- ERK
Extracellular signal-regulated kinases
Footnotes
The authors declare that they have no conflict of interest.
Reference List
- Abramow-Newerly M, Ming H, Chidiac P. Modulation of subfamily B/R4 RGS protein function by 14-3-3 proteins. Cellular signalling. 2006;18:2209–2222. doi: 10.1016/j.cellsig.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Cho H, Kim DU, Kehrl JH. RGS14 is a centrosomal and nuclear cytoplasmic shuttling protein that traffics to promyelocytic leukemia nuclear bodies following heat shock. The Journal of biological chemistry. 2005;280:805–814. doi: 10.1074/jbc.M408163200. [DOI] [PubMed] [Google Scholar]
- Cladman W, Chidiac P. Characterization and comparison of RGS2 and RGS4 as GTPase-activating proteins for m2 muscarinic receptor-stimulated G(i) Molecular pharmacology. 2002;62:654–659. doi: 10.1124/mol.62.3.654. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Cladman W, Ramineni S, Hollinger S, Chidiac P. Novel activity of RGS14 on Goalpha and Gialpha nucleotide binding and hydrolysis distinct from its RGS domain and GDI activity. Biochemistry. 2005;44:5495–5502. doi: 10.1021/bi048359d. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Ramineni S, Hepler JR. Phosphorylation of RGS14 by protein kinase A potentiates its activity toward G alpha i. Biochemistry. 2003;42:811–819. doi: 10.1021/bi026664y. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Taylor JB, Goldman EH, Hepler JR. RGS14 is a bifunctional regulator of Galphai/o activity that exists in multiple populations in brain. Journal of neurochemistry. 2001;79:941–949. doi: 10.1046/j.1471-4159.2001.00629.x. [DOI] [PubMed] [Google Scholar]
- Ishii M, Fujita S, Yamada M, Hosaka Y, Kurachi Y. Phosphatidylinositol 3,4,5-trisphosphate and Ca2+/calmodulin competitively bind to the regulators of G-protein-signalling (RGS) domain of RGS4 and reciprocally regulate its action. The Biochemical journal. 2005;385:65–73. doi: 10.1042/BJ20040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple RJ, De Vries L, Tronchere H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity. The Journal of biological chemistry. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, Cowan DP, Ramineni S, Yates CK, Feng Y, Smith Y, Sweatt JD, Weinshenker D, Ressler KJ, Dudek SM, Hepler JR. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Zhao Q, Daigle M, Ghahremani MH, Chidiac P, Albert PR. RGS17/RGSZ2, a novel regulator of Gi/o, Gz, and Gq signaling. The Journal of biological chemistry. 2004;279:26314–26322. doi: 10.1074/jbc.M401800200. [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Willard FS, Oliveira-dos-Santos AJ, Natale DR, Snow BE, Kimple RJ, Pajak A, Watson AJ, Dagnino L, Penninger JM, Siderovski DP, D'Souza SJ. RGS14 is a mitotic spindle protein essential from the first division of the mammalian zygote. Developmental cell. 2004a;7:763–769. doi: 10.1016/j.devcel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Willard FS, Pajak A, Dagnino L, Siderovski DP, D'Souza SJ. Analysis of interactions between regulator of G-protein signaling-14 and microtubules. Methods in enzymology. 2004b;390:240–258. doi: 10.1016/S0076-6879(04)90016-X. [DOI] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Willard FS, Pajak A, Dagnino L, Siderovski DP, D'Souza SJ. RGS14 is a microtubule-associated protein. Cell Cycle. 2005;4:953–960. doi: 10.4161/cc.4.7.1787. [DOI] [PubMed] [Google Scholar]
- Mittal V, Linder ME. The RGS14 GoLoco domain discriminates among Galphai isoforms. The Journal of biological chemistry. 2004;279:46772–46778. doi: 10.1074/jbc.M407409200. [DOI] [PubMed] [Google Scholar]
- Mittal V, Linder ME. Biochemical characterization of RGS14: RGS14 activity towards G-protein alpha subunits is independent of its binding to Rap2A. The Biochemical journal. 2006;394:309–315. doi: 10.1042/BJ20051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Ponting CP. Raf-like Ras/Rap-binding domains in RGS12- and still-life-like signalling proteins. J Mol Med (Berl) 1999;77:695–698. doi: 10.1007/s001099900054. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, de la Torre-Madrid E, Gaitan G, Sanchez-Blazquez P, Garzon J. RGS14 prevents morphine from internalizing Mu-opioid receptors in periaqueductal gray neurons. Cellular signalling. 2007;19:2558–2571. doi: 10.1016/j.cellsig.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annual review of biochemistry. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- Shu FJ, Ramineni S, Amyot W, Hepler JR. Selective interactions between Gi alpha1 and Gi alpha3 and the GoLoco/GPR domain of RGS14 influence its dynamic subcellular localization. Cellular signalling. 2007;19:163–176. doi: 10.1016/j.cellsig.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Shu FJ, Ramineni S, Hepler JR. RGS14 is a multifunctional scaffold that integrates G protein and Ras/Raf MAPkinase signalling pathways. Cellular signalling. 2010;22:366–376. doi: 10.1016/j.cellsig.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. International journal of biological sciences. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren B, Neubig RR. Thinking outside of the “RGS box”: new approaches to therapeutic targeting of regulators of G protein signaling. Molecular pharmacology. 2010;78:550–557. doi: 10.1124/mol.110.065219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJ. Structure and function of regulator of G protein signaling homology domains. Progress in molecular biology and translational science. 2009;86:75–113. doi: 10.1016/S1877-1173(09)86004-3. [DOI] [PubMed] [Google Scholar]
- Traver S, Splingard A, Gaudriault G, De Gunzburg J. The RGS (regulator of G-protein signalling) and GoLoco domains of RGS14 co-operate to regulate Gi-mediated signalling. The Biochemical journal. 2004;379:627–632. doi: 10.1042/BJ20031889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellano CP, Shu FJ, Ramineni S, Yates CK, Tall GG, Hepler JR. Activation of the regulator of G protein signaling 14-Galphai1-GDP signaling complex is regulated by resistance to inhibitors of cholinesterase-8A. Biochemistry. 2011;50:752–762. doi: 10.1021/bi101910n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Willard MD, Kimple AJ, Soundararajan M, Oestreich EA, Li X, Sowa NA, Kimple RJ, Doyle DA, Der CJ, Zylka MJ, Snider WD, Siderovski DP. Regulator of G-protein signaling 14 (RGS14) is a selective H-Ras effector. PloS one. 2009;4:e4884. doi: 10.1371/journal.pone.0004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Nguyen CH, Chidiac P. The proline-rich N-terminal domain of G18 exhibits a novel G protein regulatory function. The Journal of biological chemistry. 2010;285:9008–9017. doi: 10.1074/jbc.M109.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]