Abstract
Background
We hypothesized that the severity of resting perfusion abnormalities assessed by the summed rest score (SRS) would be associated with a higher rate of adverse outcomesin patients with heart failure (HF) and reduced left ventricular (LV) ejection fraction (EF).
Methods
A subset of 240 subjects from HF-ACTION underwent resting Tc99m tetrofosmin gated single photon emission computed tomography (SPECT) myocardial perfusion imaging(MPI). Images were evaluated using a 17-segment model to derive the SRS and additional nuclear variables.
Results
After adjusting for pre-specified covariates, SRS was significantly associated with the primary endpoint (hazard ratio [HR] 0.98; 95% confidence interval [CI] 0.97–1.00, P=0.04), with a higher SRS corresponding to lower risk of an event. This association was not present in the unadjusted analysis. The relationship between SRS and the primary outcome was likely due to a higher event ratein patients with ischemic HF and a low SRS. The LV phase standard deviation (SD) was not predictive of the primary outcome (HR 1.00; 95% CI 0.99–1.01, P=0.49). In a post hoc analysis, nuclear variables provided incremental prognostic information when added to clinical information (P=0.006).
Conclusions
Gated SPECT MPI provides important information in patients with HF and reduced LVEF. In the adjusted analysis, SRS has an unexpected relationship with the primary endpoint. Phase SD was not associated with the primary endpoint. Rest gated SPECT MPI provides incrementally greater prognostic information than clinical information alone.
Keywords: heart failure, SPECT, outcomes, coronary artery disease, cardiomyopathy
Despite significant advances in medical and device therapy, the morbidity, mortality, and economic burden of heart failure (HF) remain high.1–8 Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) is the largest randomized study of exercise therapy in HF patients to date. The overall HF-ACTION study design and primary results have been published.9,10 The HF-ACTION nuclear substudy was designed to test the hypotheses that the extent and severity of resting perfusion abnormalities will predict outcomes.
The prognostic significance of perfusion defects as assessed by single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) has been well established.11–13 Recent developments in gated SPECT imaging have ledto a method for objectively quantifying dyssynchrony.14–21
The primary hypothesis of the study was that the severity of resting perfusion abnormalities assessed by the summed rest score (SRS) would be associated with a higher endpoint rate for the substudy. A secondary hypothesis was that SPECT dyssynchrony analysis would correlate with adverse events.22
Methods
The primary endpoint was combined all-cause mortality and cardiovascular hospitalization. Ischemic etiology was defined in HF-ACTION as 1 of the following: ≥75% lesion in at least 1 major epicardial vessel, history of myocardial infarction (MI), history of revascularization, or perfusion defects in the setting of ischemic symptoms.
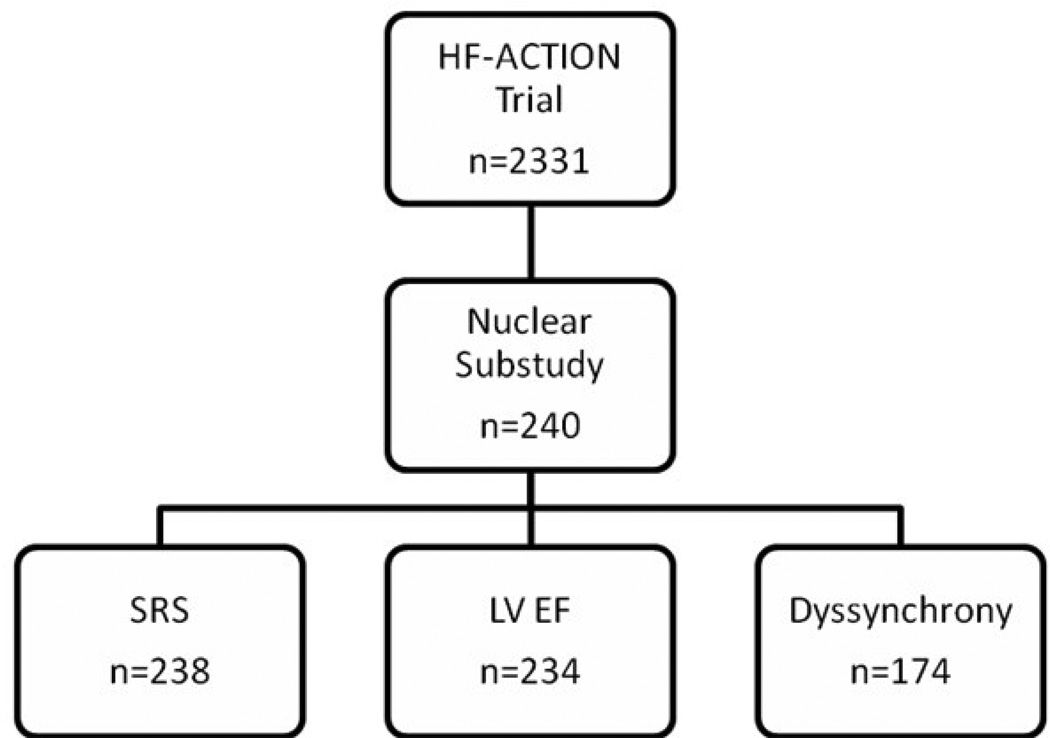
A total of 240 patients were enrolled in the substudy (Figure 1). All subjects underwent resting gated SPECT MPI with Tc-99m tetrofosmin. Image acquisition and processing has been previously described.11,14 No stress imaging was obtained to prevent interference with the main trial. Studies were independently interpreted by 2 blinded nuclear cardiologists using a 17-segment model and a 5-grade severity score. Differences between the 2 interpreters were resolved by consensus. To evaluate left ventricle (LV) mechanical dyssynchrony, a phase standard deviation (SD) >40° was used as the cutoff point.18
Figure 1.
Patient enrollment: 240 patients from the HF-ACTION trial were enrolled in the nuclear substudy; 2 studies were not interpretable and were not included in the SRS analysis; LV ejection fraction and volumes were not available in 6 studies; 29 studies were not gated and an additional 37 patients with biventricular pacemakers were excluded from the dyssynchrony analysis.
This study was supported by extramural funds from GE Health.
Statistical analyses
Statistical analyses were performed using SAS software version 8.2 (SAS Institute Inc., Cary, NC). Baseline characteristics were summarized using medians and interquartile ranges for continuous variables and frequencies and percentages for categorical variables. All statistical tests were 2-tailed with a significance level α=0.05.
The principal analysis of the pre-specified primary endpoint was performed using Cox proportional hazards regression with baseline SRS as the independent variable of interest, with the covariates age, sex, New York Heart Association class, baseline LV ejection fraction, HF etiology, and body mass index. An additional pre-specified analysis investigated the univariate relationship between baseline SRS and the primary endpoint (no covariates).
The secondary hypothesis involving dyssynchrony was assessed with a Cox proportional hazards regression model including phase SD as the independent variable of interest, with the same covariates as above. Subjects with a biventricular pacemaker at baseline were excluded from dyssynchrony analyses. A Kaplan-Meier curve of the primary endpoint by high versus low phase SD (based on a cut point of 40°) was examined as well.
The primary endpoint results were further assessed with additional post hoc analyses. Kaplan-Meier curves of the primary endpoint by HF etiology were examined, as well as curves of the primary endpoint by etiology and high/low SRS, with an SRS cut-point of 15, suggested by a spline fit of the log hazard ratio versus SRS. All P values for Kaplan-Meier curves were generated using the log-rank test, except for the plot by etiology and SRS, for which the interaction P value was also examined, keeping SRS as a dichotomous variable. Also, the main analysis of baseline SRS was repeated with the composite endpoint of cardiovascular mortality and HF hospitalization, with and without covariates. Another post hoc analysis involved examining the incremental prognostic value of 3 gated SPECT variables (SRS, LV ejection fraction, and end systolic volume) beyond baseline clinical variables (age, sex, New York Heart Association class, etiology of HF, and body mass index) in predicting the primary endpoint. Finally, the incremental prognostic value of phase SD beyond the combined set of clinical variables and the 3 gated SPECT variables was investigated. These 2 analyses were performed by assessing Cox proportional hazards models of the primary endpoint, ensuring the same sample size in each model, and comparing the likelihood ratio chi-square statistics.
Results
Patient demographics can be found in Table I. Most patients were optimally treated with standard medical therapy (Table II). Table III highlights baseline clinical and gated SPECT information.
Table I.
Demographics
| HF-ACTION Nuclear Substudy (n=240) |
|
|---|---|
| Age, median (25th, 75th), yrs | 59 (51, 68) |
| Female | 74 (31) |
| Race | |
| Black | 78 (33) |
| White | 150 (63) |
| Body mass index, median (25th, 75th), kg/m2 | 30 (26, 35) |
| Ischemic etiology | 129 (54) |
| Atrial fibrillation/flutter | 38 (16) |
| Hypertension | 154 (65) |
| Hyperlipidemia | 160 (67) |
| Stroke | 27 (11) |
| Diabetes | 78 (33) |
| Chronic obstructive pulmonary disease | 39 (17) |
| Peripheral vascular disease | 15 (6) |
| Coronary artery bypass grafting | 61 (25) |
| Percutaneous coronary intervention | 63 (26) |
| Pacemaker | 41 (17) |
| Automated internal cardioverter defibrillator | 128 (53) |
| Biventricular pacemaker | 44 (18) |
| Automated internal cardioverter defibrillator | 136 (57) |
| or biventricular pacemaker |
All data presented as n (%), unless otherwise noted.
Table II.
Medications
| HF-ACTION Nuclear Substudy | |
|---|---|
| (n=240) | |
| Angiotensin-converting enzyme inhibitor | 168 (70) |
| Angiotensin receptor blocker | 63 (26) |
| Angiotensin-converting enzyme inhibitor | 225 (94) |
| or angiotensin receptor blocker | |
| Beta-blocker | 230 (96) |
| Digoxin | 96 (40) |
| Spironolactone | 108 (45) |
| Aspirin | 175 (73) |
| Loop diuretic | 172 (72) |
| Non-loop diuretic | 17 (7) |
| Lipid-lowering agent | 157 (65) |
| Statin | 125 (52) |
| Nitrate | 53 (22) |
| Calcium channel blocker | 7 (3) |
| Glitazone | 7 (3) |
| Selective serotonin reuptake inhibitor | 40 (17) |
All data presented as n (%).
Table III.
Baseline variables
| HF-ACTION Nuclear Substudy | |
|---|---|
| (n=240) | |
| Clinical and exercise | |
| New York Heart Association class, n (%) | |
| II | 154 (64) |
| III | 86 (36) |
| Resting heart rate, beats/min | 69 (64, 76) |
| Resting systolic blood pressure, mm Hg | 112 (102, 126) |
| Resting diastolic blood pressure, mm Hg | 70 (64, 80) |
| Peak VO2, mL/kg/min | 15.3 (12.3, 18.5) |
| Exercise duration, min | 10.0 (7.4, 12.5) |
| 6-minute walk distance, meters | 381 (305, 454) |
| Gated SPECT | |
| SRS | 20 (5, 31) |
| LV ejection fraction, % | 26 (21, 34) |
| End systolic volume, mL | 165 (117, 238) |
| End diastolic volume, mL | 226 (168, 297) |
| Phase SD, ° | 41 (23, 63) |
| Bandwidth, ° | 111 (71, 207) |
Data presented as median (25th, 75th), unless otherwise noted. LV, left ventricular; SD, standard deviation; SPECT, single photon emission computed tomography; SRS, summed rest score.
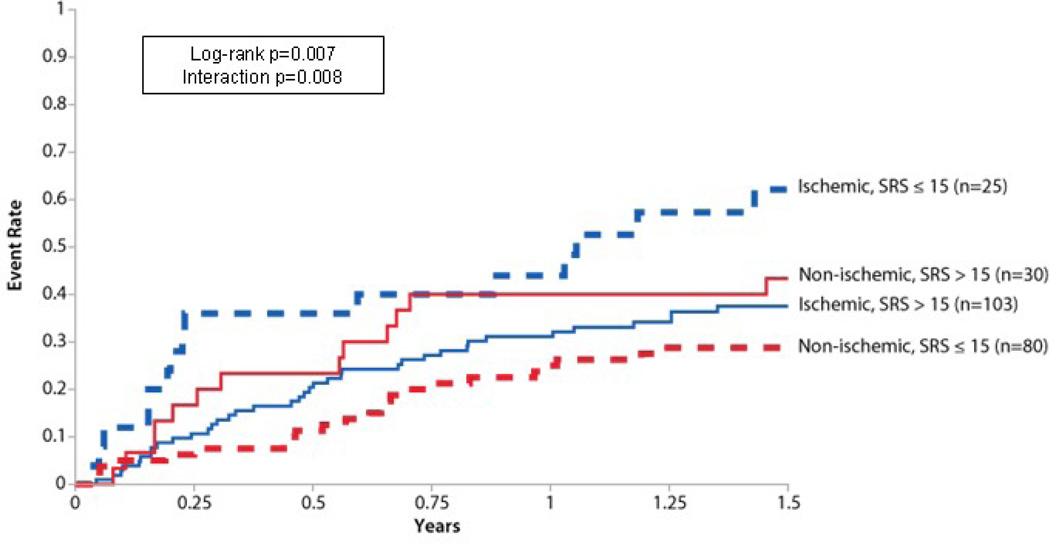
Baseline SRS had a statistically significant association with the primary endpoint with a higher SRS corresponding to a lower risk of event (Table IV). This relationship was not observed in the unadjusted analysis (hazard ration [HR] 1.00; 95% confidence interval [CI] 0.99–1.01, P=0.94). In a post hoc analysis of the primary endpoint, differences were identified within the cohort based on the etiology of HF (ischemic versus nonischemic) and SRS (high versus low) (Figure 2). Patients with an ischemic etiology of HF and a low SRS had the highest event rate, primarily driven by cardiovascular hospitalization. Patients with a high SRS had a similar event rate regardless of the HF etiology, and patients with a nonischemic etiology and a low SRS had the lowest event rate.
Table IV.
Multivariable analysis of SRS and the primary endpoint
| Parameter | Chi-square | P Value | HR | (95% CI) |
|---|---|---|---|---|
| SRS | 4.24 | 0.040 | 0.98 | (0.97, 1.00) |
| Age | 3.66 | 0.056 | 1.02 | (1.00, 1.04) |
| Body mass index | 0.75 | 0.385 | 1.01 | (0.98, 1.04) |
| Sex | 0.94 | 0.333 | 1.24 | (0.80, 1.90) |
| New York Heart Association class | 2.63 | 0.105 | 1.38 | (0.94, 2.03) |
| HF etiology | 5.23 | 0.022 | 0.56 | (0.35, 0.92) |
| LV ejection fraction | 8.32 | 0.004 | 0.97 | (0.95, 0.99) |
CI, confidence interval; HF, heart failure; HR, hazard ratio; LV, left ventricular; SRS, summed rest score.
Figure 2.
Primary endpoint by etiology and SRS Kaplan-Meier curves for the primary endpoint stratified by HF etiology and SRS. Patients with a nonischemic etiology and a low SRS had the lowest event rate. Those with a high SRS, regardless of etiology, had similar event rates, with the subjects with nonischemic HF having slightly higher rates. Patients with an ischemic etiology and a low SRS had the highest event rate, likely from a high underlying ischemic burden.
Overall, 115 (48%) of the 240 patients enrolled experienced the primary endpoint of all-cause mortality or cardiovascular hospitalization. All-cause mortality occurred in 14% (n=33) of the entire cohort whereas 45% (n=107) were hospitalized for cardiovascular causes. The primary endpoint occurred in 68 (53%) of those with an ischemic etiology (n=129). Among those with an ischemic etiology, all-cause mortality occurred in 18% (n=23) and hospitalization for cardiovascular causes occurred in 49% (n=63). For those with a nonischemic etiology (n=111), the primary endpoint occurred in 47 (42%) patients. Among those with a nonischemic etiology, all-cause mortality occurred in 9% (n=10) and hospitalization for cardiovascular causes occurred in 40% (n=44).
Other outcome measures pertinent to this study included the composite endpoint of cardiovascular mortality and HF hospitalization (n=67 [28%]). Baseline SRS was not predictive of this post hoc endpoint (unadjusted HR 1.01; 95% CI 0.99–1.02, P=0.25). This relationship persisted when adjusted for age, sex, New York Heart Association class, HF etiology, and body mass index (adjusted HR 0.99; 95% CI 0.97–1.01, P=0.27). There were 56 subjects with at least 1 HF hospitalization (23%), which included 13 subjects with a subsequent cardiovascular death. Cardiovascular mortality occurred in 22 subjects (9%), and there were 3 deaths of unknown cause classified as cardiovascular death for purposes of this analysis.
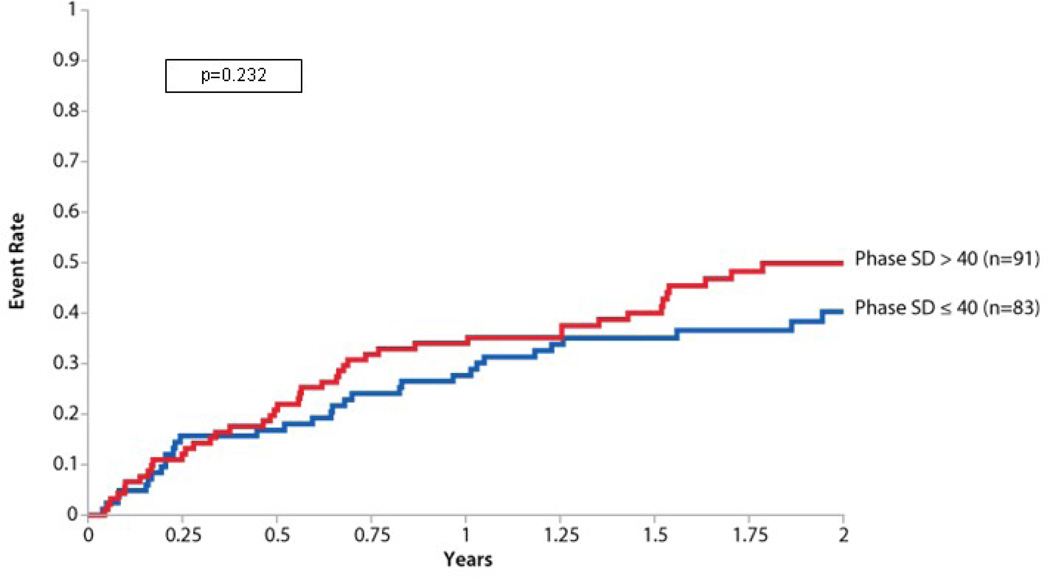
In the analysis of dyssynchrony, phase SD was not significantly associated with the primary endpoint(Table V). When examined as a dichotomous variable split at 40° in a post hoc analysis, there was a nonsignificant trend toward increased adverse events in those with a higher phase SD (Figure 3).
Table V.
Multivariable analysis of phase SD and the primary endpoint
| Parameter | Chi-square | P Value | HR | (95% CI) |
|---|---|---|---|---|
| Dyssynchrony phase SD | 0.48 | 0.49 | 1.00 | (0.99, 1.01) |
| Age | 0.45 | 0.50 | 1.01 | (0.99, 1.03) |
| Body mass index | 0.72 | 0.39 | 1.01 | (0.98, 1.05) |
| Sex | 1.17 | 0.28 | 1.34 | (0.79, 2.26) |
| New York Heart Association class | 4.06 | 0.044 | 1.60 | (1.01, 2.51) |
| HF etiology | 5.69 | 0.017 | 0.50 | (0.28, 0.88) |
| LV ejection fraction | 2.15 | 0.14 | 0.98 | (0.95, 1.01) |
CI, confidence interval; HF, heart failure; HR, hazard ratio; LV, left ventricular; SD, standard deviation.
Figure 3.
Primary endpoint by phase SD Kaplan-Meier curve for the primary endpoint in those with significant dyssynchrony (phase SD >40°) and those without significant dyssynchrony (phase SD ≤ 40°). In this post hoc analysis, there was no significant difference in the primary endpoint of death and cardiovascular hospitalization between these groups (log-rank P=0.232). The P value was computed treating dyssynchrony as a dichotomous variable with a phase SD cutoff of 40°. Subjects with a biventricular pacemaker at baseline were excluded (n=174).
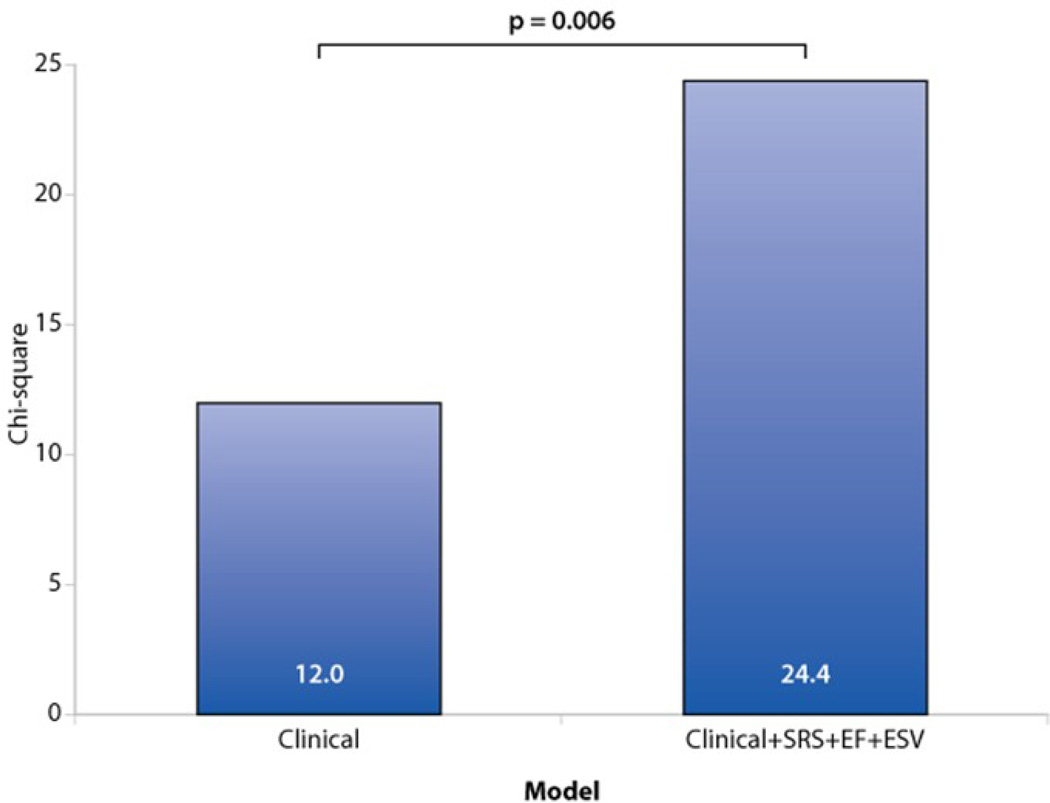
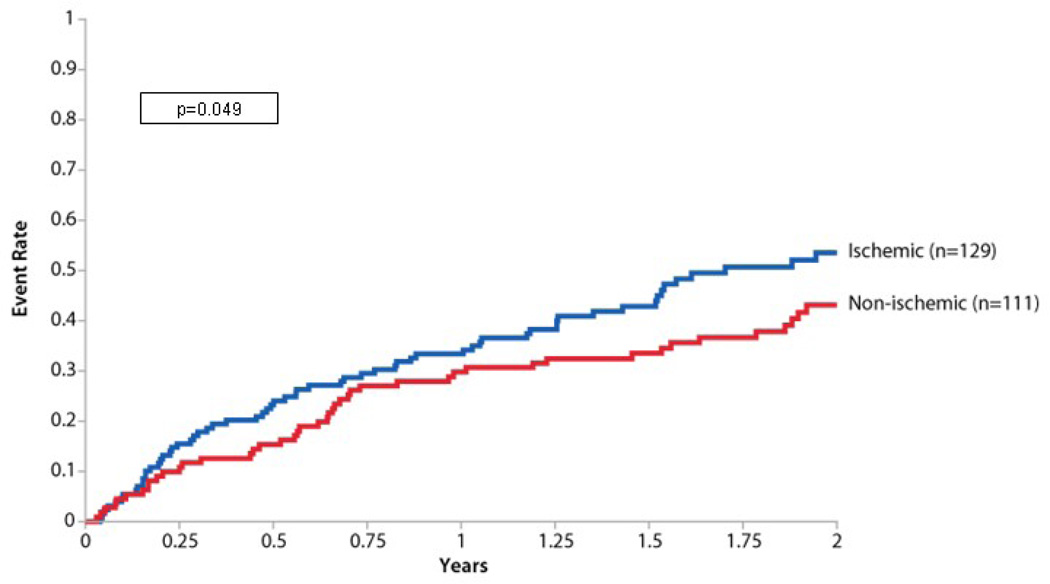
In other post hoc analyses, key variables obtained from gated SPECT imaging (SRS, LV ejection fraction (EF), and end systolic volume (ESV) added prognostic value to clinical information (P=0.006) (Figure 4). Phase SD did not contribute significantly to the incremental information obtained from gated SPECT imaging (P=0.72). Figure 5 presents a Kaplan-Meier curve for the primary endpoint by HF etiology where patients with an ischemic etiology had a higher event rate than those with a nonischemic etiology (P=0.049).
Figure 4.
Incremental value of nuclear variables gated SPECT variables (SRS, LV ejection fraction, and end systolic volume) provides significant prognostic value beyond baseline clinical information for the prediction of the primary endpoint (death and cardiovascular hospitalization). In both models, the sample size was limited to subjects with non-missing values of SRS, ejection fraction, and end systolic volume (n=234). Clinical variables used were age, sex, New York Heart Association class, body mass index, and etiology of HF. Phase SD did not contribute significantly to the incremental information obtained from gated SPECT imaging (P=0.72), analysis not shown.
Figure 5.
Primary endpoint by ischemic and nonischemic etiology Kaplan-Meier curve for the primary endpoint in patients with an ischemic etiology of HF compared with those with a nonischemic etiology. Patients with ischemic etiology had a significantly higher rate of the primary endpoint (P=0.049).
Discussion
The main conclusions of this study are as follows. First, in the adjusted analysis, SRS was predictive of the primary outcome, but the point estimate was contrary to our original hypothesis. Second, post hoc analysis of the unexpected results between SRS and the primary outcome showed that this relationship was driven primarily by cardiovascular hospitalization in patients with an ischemic etiology and low SRS. Third, mechanical dyssynchrony was not significantly associated with death and cardiovascular hospitalization. Fourth, key variables (SRS, EF, and end systolic volume) derived from gated SPECT MPI added prognostic information to baseline clinical characteristics.
Ischemic etiology
SPECT MPI provides independent prognostic information in patients with cardiovascular disease.12,13,23–25 Furthermore, the ability to use gated SPECT MPI as a noninvasive method to distinguish ischemic from nonischemic etiologies of HF has also been described.26–33 Soman et al. demonstrated an excellent negative predictive value (96%) for the presence of significant coronary artery disease (CAD)in patients with newly diagnosed HF.34 Additionally, both the summed stress score (SSS) and summed difference score (SDS) have been shown to be independent predictive variables of adverse outcomes.35–37
This study demonstrated an inverse association between SRS and the primary endpoint, a relationship that appears to have been driven by cardiovascular hospitalizations in patients with known CAD and a low SRS. This likely represents patients with a higher degree of underlying ischemia where the SRS alone was not sufficient to fully ascertain the risk of adverse events. This hypothesis is supported by Bouzas-Mosquera et al., which demonstrated an increased risk of death or hospitalization in patients with resting wall motion abnormality and evidence of ischemia by new stress-induced wall motion abnormality versus the ones with no further evidence of ischemia.38
Nonischemic etiology
The prognostic significance of scar and/or fibrosis in HF patients with a nonischemic etiology is under investigation. In a study by Wu et al., patients with nonischemic cardiomyopathy with a high degree of myocardial scar (>10% of LV) had an 8-fold increased risk of HF hospitalization, appropriate implantable cardioverter defibrillator therapy, or death compared with those with lower scar burdens.39 There are currently limited data with respect to scar assessment and outcomes in nonischemic cardiomyopathy by nuclear imaging. To our knowledge, this is the first study to correlate outcomes in nonischemic cardiomyopathy with resting perfusion defects by gated SPECT imaging. Patients with a nonischemic etiology of HF and a high SRS had a trend toward increased mortality and cardiovascular hospitalization compared with those who had a low SRS (Figure 2).
Dyssynchrony
Dyssynchrony has been shown to be a predictor of adverse outcomes in HF patients.40 However, the measurement of dyssynchrony by echocardiography has suffered from a lack of standardization and high variability. There is a growing body of literature with respect to the clinical applicability of phase analysis of gated SPECT MPI to assess LV dyssynchrony.14,18,20,21,41 One early study, demonstrated that a phase SD≥43° (normal 9°±3°) had a sensitivity and specificity of 74% for predicting response to cardiac resynchronization therapy.18,42 Previous analysis from HF-ACTION have also shown an association between the severity of dyssynchrony and worsening New York Heart Association class.43,44
This study demonstrated a nonsignificant relationship between mortality and cardiovascular hospitalization in those with more severe dyssynchrony despite a trend over the 2-year follow-up period.
Incremental prognostic value
The ability of gated SPECT imaging to provide prognostic information above and beyond clinical information has been well established.11,37,45–49 In this study, gated SPECT variables more than doubled the prognostic power of baseline clinical information. These findings are worth noting when considering that the clinical information used in this analysis was robust and included both New York Heart Association functional class and HF etiology, both of which are independent predictors of adverse outcomes in HF patients with reduced LV EF.
Clinical implications
Results from this study suggest the following clinical implications. In patients with heart failure, a resting gated SPECT MPI Tc-99m Myo view study has the ability to identify the ischemic versus non-ischemic etiology of cardiomyopathy. However, to detect any obstructive CAD in HF patients, a stress gated SPECT MPI study would be recommended. The ability to simultaneously evaluate perfusion, function, volumes, and dyssynchrony makes SPECT MPI a potential gatekeeper in the evaluation of HF patients.
Limitations
Although relatively large for an HF nuclear imaging study, the sample size is approximately 10% of the overall study population. There may also have been selection bias as only patients willing to provide informed consent were enrolled in the nuclear substudy. The limited sample size obtained for the substudy means that the subgroups were small and potentially subject to variability. Another limitation is that only resting images were obtained and there was no imaging performed for assessment of stress-induced ischemia. This was mandated by the overall HF-ACTION trial design as it was felt that it could lead to alterations in medical care, particularly revascularization, which could introduce bias in endpoints related to the overall study intervention. There may be unmeasured variables that are related to both the nuclear variables and the primary endpoint that affected the results. Finally, dyssynchrony as assessed by SPECT MPI has not yet been integrated into routine clinical care. This is primarily due to a relative lack of data regarding the prognostic significance of this modality and its ability to predict other clinically relevant outcomes such as response to cardiac resynchronization therapy or appropriate defibrillator therapy.
Conclusion
Rest Tc-99m tetrofosmin gated SPECT MPI provides important information in patients with HF and reduced LV ejection fraction. The degree of resting perfusion defects demonstrated an unexpected relationship with the primary endpoint—a higher SRS corresponding to a lower risk of event, although this relationship was not observed in the unadjusted analysis. This relationship appears to have been driven by an underlying ischemic burden not assessed in this study. No significant relationship was documented between dyssynchrony and the primary endpoint, although an apparent trend toward worsened outcomes with more severe dyssynchrony was noted. Finally, gated SPECT MPI provides prognostic information in HF patients with reduced LV function beyond that provided by clinical variables.
Acknowledgments
We want to express our gratitude to Morgan deBlecourt for her editorial work in the preparation of this manuscript.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written. Dr. Atchley is a fellow in training and is funded by a National Institutes of Health T32 research grant. Dr. Borges-Neto is part of the speakers’ bureau and advisory board and receives grants from GE Health. Dr. Ellis receives funding support from GE Health.
Funding sources: This study was funded by the National Institutes of Health, National Heart Lung and Blood Institute, and GE Healthcare. This research was supported by National Institutes of Health grants: 5U01HL063747, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, 5U01HL064264, 5U01HL066461, R37AG18915, P60AG10484.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure information
Drs. Kraus, Iskandrian, Whellan, O’Connor, Ewald, Gardin, Kao, Bensimhon, Walsh, Abdul-Nour, and Kitzman have no conflicts of interest to disclose.
References
- 1.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 9.Whellan DJ, O'Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borges-Neto S, Shaw LK, Tuttle RH, et al. Incremental prognostic power of single-photon emission computed tomographic myocardial perfusion imaging in patients with known or suspected coronary artery disease. Am J Cardiol. 2005;95:182–188. doi: 10.1016/j.amjcard.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 13.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Garcia EV, Folks RD, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 15.Cooke CD, Ziffer J, Folks RD, et al. A count-based method for quantifying myocardial thickening from SPECT 99m Tc sestamibi studies: description of the method (abstract) J Nucl Med. 1991;32:1068. [Google Scholar]
- 16.Cooke CD, Garcia EV, Cullom SJ, et al. Determining the accuracy of calculating systolic wall thickening using a fast Fourier transform approximation: a simulation study based on canine and patient data. J Nucl Med. 1994;35:1185–1192. [PubMed] [Google Scholar]
- 17.Cooke CD, Garcia EV, Cullom SJ, et al. Technetium-99m sestamibi myocardial gated SPECT simulation for determining the accuracy of count-based systolic wall thickening measurements (abstract) J Nucl Med. 1993;34:96. [Google Scholar]
- 18.Henneman MM, Chen J, Ypenburg C, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–1714. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Garcia EV, Lerakis S, et al. Left ventricular mechanical dyssynchrony as assessed by phase analysis of ECG-gated SPECT myocardial perfusion imaging. Echocardiography. 2008;25:1186–1194. doi: 10.1111/j.1540-8175.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 20.Trimble MA, Velazquez EJ, Adams GL, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun. 2008;29:374–381. doi: 10.1097/MNM.0b013e3282f81380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trimble MA, Borges-Neto S, Smallheiser S, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated SPECT myocardial perfusion imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14:298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Bensimhon DR, Adams GL, Whellan DJ, et al. Effect of exercise training on ventricular function, dyssynchrony, resting myocardial perfusion, and clinical outcomes in patients with heart failure: a nuclear ancillary study of Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION); design and rationale. Am Heart J. 2007;154:46–53. doi: 10.1016/j.ahj.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Marwick TH, Shaw LJ, Lauer MS, et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999;106:172–178. doi: 10.1016/s0002-9343(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 24.Shaw LJ, Hendel RC, Heller GV, et al. Prognostic estimation of coronary artery disease risk with resting perfusion abnormalities and stress ischemia on myocardial perfusion SPECT. J Nucl Cardiol. 2008;15:762–773. doi: 10.1007/BF03007357. [DOI] [PubMed] [Google Scholar]
- 25.Sharir T, Berman DS, Lewin HC, et al. Incremental prognostic value of rest-redistribution (201)Tl single-photon emission computed tomography. Circulation. 1999;100:1964–1970. doi: 10.1161/01.cir.100.19.1964. [DOI] [PubMed] [Google Scholar]
- 26.Saltissi S, Hockings B, Croft DN, et al. Thallium-201 myocardial imaging in patients with dilated and ischaemic cardiomyopathy. Br Heart J. 1981;46:290–295. doi: 10.1136/hrt.46.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danias PG, Ahlberg AW, Clark BA, 3rd, et al. Combined assessment of myocardial perfusion and left ventricular function with exercise technetium-99m sestamibi gated single-photon emission computed tomography can differentiate between ischemic and nonischemic dilated cardiomyopathy. Am J Cardiol. 1998;82:1253–1258. doi: 10.1016/s0002-9149(98)00609-2. [DOI] [PubMed] [Google Scholar]
- 28.Danias PG, Papaioannou GI, Ahlberg AW, et al. Usefulness of electrocardiographic-gated stress technetium-99m sestamibi single-photon emission computed tomography to differentiate ischemic from nonischemic cardiomyopathy. Am J Cardiol. 2004;94:14–19. doi: 10.1016/j.amjcard.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Bulkley BH, Hutchins GM, Bailey I, et al. Thallium 201 imaging and gated cardiac blood pool scans in patients with ischemic and idiopathic congestive cardiomyopathy. A clinical and pathologic study. Circulation. 1977;55:753–760. doi: 10.1161/01.cir.55.5.753. [DOI] [PubMed] [Google Scholar]
- 30.Dunn RF, Uren RF, Sadick N, et al. Comparison of thallium-201 scanning in idiopathic dilated cardiomyopathy and severe coronary artery disease. Circulation. 1982;66:804–810. doi: 10.1161/01.cir.66.4.804. [DOI] [PubMed] [Google Scholar]
- 31.Tauberg SG, Orie JE, Bartlett BE, et al. Usefulness of thallium-201 for distinction of ischemic from idiopathic dilated cardiomyopathy. Am J Cardiol. 1993;71:674–680. doi: 10.1016/0002-9149(93)91009-7. [DOI] [PubMed] [Google Scholar]
- 32.Iskandrian AS, Hakki AH, Kane S. Resting thallium-201 myocardial perfusion patterns in patients with severe left ventricular dysfunction: differences between patients with primary cardiomyopathy, chronic coronary artery disease, or acute myocardial infarction. Am Heart J. 1986;111:760–767. doi: 10.1016/0002-8703(86)90113-4. [DOI] [PubMed] [Google Scholar]
- 33.Chikamori T, Doi YL, Yonezawa Y, et al. Value of dipyridamole thallium-201 imaging in noninvasive differentiation of idiopathic dilated cardiomyopathy from coronary artery disease with left ventricular dysfunction. Am J Cardiol. 1992;69:650–653. doi: 10.1016/0002-9149(92)90158-u. [DOI] [PubMed] [Google Scholar]
- 34.Soman P, Lahiri A, Mieres JH, et al. Etiology and pathophysiology of new-onset heart failure: evaluation by myocardial perfusion imaging. J Nucl Cardiol. 2009;16:82–91. doi: 10.1007/s12350-008-9010-8. [DOI] [PubMed] [Google Scholar]
- 35.Zellweger MJ, Lewin HC, Lai S, et al. When to stress patients after coronary artery bypass surgery? Risk stratification in patients early and late post-CABG using stress myocardial perfusion SPECT: implications of appropriate clinical strategies. J Am Coll Cardiol. 2001;37:144–152. doi: 10.1016/s0735-1097(00)01104-9. [DOI] [PubMed] [Google Scholar]
- 36.Elhendy A, Schinkel AF, van Domburg RT, et al. Prognostic significance of fixed perfusion abnormalities on stress technetium-99m sestamibi single-photon emission computed tomography in patients without known coronary artery disease. Am J Cardiol. 2003;92:1165–1170. doi: 10.1016/j.amjcard.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Gimelli A, Rossi G, Landi P, et al. Stress/Rest Myocardial Perfusion Abnormalities by Gated SPECT: Still the Best Predictor of Cardiac Events in Stable Ischemic Heart Disease. J Nucl Med. 2009;50:546–553. doi: 10.2967/jnumed.108.055954. [DOI] [PubMed] [Google Scholar]
- 38.Bouzas-Mosquera A, Peteiro J, Alvarez-Garcia N, et al. Prediction of mortality and major cardiac events by exercise echocardiography in patients with normal exercise electrocardiographic testing. J Am Coll Cardiol. 2009;53:1981–1990. doi: 10.1016/j.jacc.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 39.Mahrholdt H, Wagner A, Judd RM, et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 40.Cho GY, Song JK, Park WJ, et al. Mechanical dyssynchrony assessed by tissue Doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol. 2005;46:2237–2243. doi: 10.1016/j.jacc.2004.11.074. [DOI] [PubMed] [Google Scholar]
- 41.Trimble MA, Borges-Neto S, Honeycutt EF, et al. Evaluation of mechanical dyssynchrony and myocardial perfusion using phase analysis of gated SPECT imaging in patients with left ventricular dysfunction. J Nucl Cardiol. 2008;15:663–670. doi: 10.1016/j.nuclcard.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atchley AE, Trimble MA, Samad Z, et al. Use of phase analysis of gated SPECT perfusion imaging to quantify dyssynchrony in patients with mild-to-moderate left ventricular dysfunction. J Nucl Cardiol. 2009;16:888–894. doi: 10.1007/s12350-009-9136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atchley AE, Kitzman DW, Whellan DJ, et al. Myocardial perfusion, function, and dyssynchrony in patients with heart failure: baseline results from the single-photon emission computed tomography imaging ancillary study of the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Trial. Am Heart J. 2009;158:S53–S63. doi: 10.1016/j.ahj.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ypenburg C, van Bommel RJ, Borleffs CJ, et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53:483–490. doi: 10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Schinkel AF, Elhendy A, van Domburg RT, et al. Incremental value of exercise technetium-99m tetrofosmin myocardial perfusion single-photon emission computed tomography for the prediction of cardiac events. Am J Cardiol. 2003;91:408–411. doi: 10.1016/s0002-9149(02)03234-4. [DOI] [PubMed] [Google Scholar]
- 46.Ho KT, Miller TD, Christian TF, et al. Prediction of severe coronary artery disease and long-term outcome in patients undergoing vasodilator SPECT. J Nucl Cardiol. 2001;8:438–444. doi: 10.1067/mnc.2001.114520. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto N, Sato Y, Suzuki Y, et al. Incremental prognostic value of cardiac function assessed by ECG-gated myocardial perfusion SPECT for the prediction of future acute coronary syndrome. Circ J. 2008;72:2035–2039. doi: 10.1253/circj.cj-08-0488. [DOI] [PubMed] [Google Scholar]
- 48.Petix NR, Sestini S, Coppola A, et al. Prognostic value of combined perfusion and function by stress technetium-99m sestamibi gated SPECT myocardial perfusion imaging in patients with suspected or known coronary artery disease. Am J Cardiol. 2005;95:1351–1357. doi: 10.1016/j.amjcard.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 49.Leslie WD, Tully SA, Yogendran MS, et al. Prognostic value of automated quantification of 99mTc-sestamibi myocardial perfusion imaging. J Nucl Med. 2005;46:204–211. [PubMed] [Google Scholar]