Abstract
The human transferrin receptor (hTfR) is a target for cancer immunotherapy due to its overexpression on the surface of cancer cells. We previously developed an antibody-avidin fusion protein that targets hTfR (anti-hTfR IgG3-Av) and exhibits intrinsic cytotoxicity against certain malignant cells. Gambogic acid (GA), a drug that also binds hTfR, induces cytotoxicity in several malignant cell lines. We now report that anti-hTfR IgG3-Av and GA induce cytotoxicity in a new broader panel of hematopoietic malignant cell lines. Our results show that the effect of anti-hTfR IgG3-Av is iron-dependent whereas that of GA is iron-independent in all cells tested. In addition, we observed that GA exerts a TfR-independent cytotoxicity. We also found that GA increases the generation of reactive oxygen species that may play a role in the cytotoxicity induced by this drug. Additive cytotoxicity was observed by simultaneous combination treatment with these drugs and synergy by using anti-hTfR IgG3-Av as a chemosensitizing agent. In addition, we found a concentration of GA that is toxic to malignant hematopoietic cells but not to human hematopoietic progenitor cells. Our results suggest that these two compounds may be effective, alone or in combination, for the treatment of human hematopoietic malignancies.
Keywords: TfR, iron, antibody fusion protein, gambogic acid, hematopoietic malignances, ROS
Introduction
Iron is an important cofactor of enzymes that participate in cellular metabolism including those of the respiratory chain and DNA synthesis.1 Iron associated with transferrin is internalized by cells through transferrin receptor (TfR)-mediated endocytosis.2 The TfR family consists of TfR1 (CD71) and TfR2, which are type II transmembrane glycoproteins. TfR1 is expressed at different levels on the surface of most normal cells in which the majority show low TfR1 expression level.2 However, dividing cells such as basal epidermis and intestinal epithelium cells express high levels of TfR1.2 In contrast, although TfR2 mRNA is present in different tissues,3 the TfR2 protein is limited to hepatocytes and enterocytes of the small intestine.2,4 As cancer cells require large amounts of iron to maintain their high rate of cell proliferation, TfR1 is overexpressed on their surface.2 The TfR1 overexpression in malignant cells, its ability to internalize and its central role in the cellular pathology of human cancer make this receptor an attractive target for the therapy of cancer.2,5
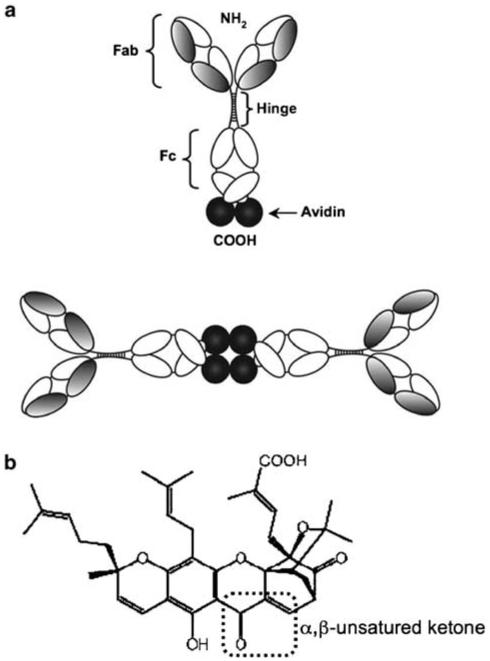
TfR has been targeted by monoclonal antibodies that either induce cytotoxicity2 or are used for drug delivery.5 Our group developed earlier an antibody fusion protein that specifically recognizes hTfR1 and is composed of chicken avidin, genetically fused to the CH3 domain of a mouse/human chimeric IgG3 (anti-hTfR IgG3-Av, Figure 1a), to be used as a universal delivery system of biotinylated drugs into cancer cells.6-8 Unexpectedly, we discovered that this fusion protein exhibits a significant intrinsic cytotoxicity against certain hematopoietic malignant cell lines compared with the antibody alone (anti-hTfR IgG3).6,7,9
Figure 1.
Schematic representation of anti-hTfR IgG3-Av and chemical structure of GA. (a) In the anti-hTfR IgG3-Av, chicken avidin was fused to the carboxy terminus of the heavy chain of mouse/human chimeric anti-hTfR1 IgG3 containing human kappa (k) light chain constant region. The antibody fusion protein forms homodimers in solution due to non-covalent tetrameric interaction of four avidin moieties.6 (b) Chemical structure of GA (C38H44O8, MW.: 628.75). The α,β-unsaturated ketone is marked by the punctuated box.12
Anti-hTfR IgG3-Av disrupts TfR recycling and redirects it into the lysosomes where it is presumably degraded, promoting iron starvation and the induction of apoptosis that can be blocked by iron supplementation.7,9 As avidin is a non-covalent tetrameric protein, the presence of two avidin moieties in anti-hTfR IgG3-Av favors the dimerization of this fusion protein.6 Although the structural requirements responsible for the cytotoxicity of anti-hTfR IgG3-Av are still unclear, the activity of this fusion protein is considered to be, at least in part, due to its dimeric structure (Figure 1a).7 We have recently shown that anti-hTfR IgG3-Av conjugated to biotinylated saporin (b-SO6), a plant toxin of Saponaria officinialis, increases its cytotoxicity in malignant cells and more importantly, overcomes the resistance of cells to the fusion protein alone.9 In addition, as anti-hTfR IgG3-Av specifically binds hTfR1 (not hTfR2), this antibody fusion protein delivers b-SO6 into cells that express hTfR1.8 Therefore, the fusion of avidin to anti-hTfR IgG3 results in a relevant tool that can be used in cancer immunotherapy as it elicits cytotoxic effects by itself and can also deliver biotinylated cytotoxic compounds such as b-SO6 into cancer cells that overexpress hTfR1.
We are also interested in the effect of the combination of anti-hTfR IgG3-Av with non-biotinylated cytotoxic drugs. We have focused on gambogic acid (GA) as it can also bind hTfR.10 GA is a xanthone isolated from a resin obtained from the Garcinia hanburyi tree that is used in oriental traditional medicine (Figure 1b).11 GA induces cytotoxicity in malignant epithelial cell lines and also in malignant hematopoietic cell lines such as JURKAT and HL-60 cells.12-21 GA can also inhibit the growth of human lung carcinoma and hepatoma xenografts in nude mice.21 This drug blocks hTfR internalization and induces cytotoxicity in an iron-independent manner in JURKAT cells.10 When hTfR is downregulated by RNA interference, the effect of GA is decreased suggesting that this drug induces cytotoxicity through hTfR.10,22
As anti-hTfR IgG3-Av and GA have shown cytotoxicity to certain malignant cells, the first objective of this study was to evaluate the cytotoxicity of anti-hTfR IgG3-Av and GA in a broader panel of human hematopoietic malignant cell lines including leukemia, lymphoma and multiple myeloma cells. The second objective was to analyze the role of iron and hTfR in the anti-hTfR IgG3-Av- and GA-mediated cytotoxicity. The third and last objective was to evaluate the effect of the combination of both agents to determine if their potential therapeutic use could be increased. Here, we report the cytotoxicity of anti-hTfR IgG3-Av and GA alone or in combination in a variety of malignant hematopoietic cell lines. The toxic effects of either agent alone or in combination on normal hematopoietic progenitor cells were also evaluated.
Material and methods
Antibodies, antibody fusion protein and gambogic acid
The mouse/human chimeric anti-hTfR IgG3-Av was described earlier6-9 and consists of chicken avidin fused to the CH3 domains of human IgG3 (Figure 1a). This fusion protein was expressed in murine myeloma cells, purified and characterized as described earlier.6,7,23 Phycoerythrin-conjugated anti-human CD71 (PE anti-hCD71) and its isotype control (phycoerythrinconjugated mouse IgG2a k) were purchased from BD Pharmigen (BD Bioscience, Franklin Lakes, NJ, USA). GA was purchased from BIOMOL International L.P (MW: 628.75; Plymouth meeting, PA, USA).
Cell lines
The human cell lines, JURKAT (acute T-cell leukemia), HL-60 (acute promyelocytic leukemia), IM-9 (EBV-transformed B-lymphoblastoid), U266 (multiple myeloma), RAMOS (American Burkitt's lymphoma), RAJI and HS-SULTAN (Burkitt's lymphoma) were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). The cell lines were maintained in RPMI 1640 medium (Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA) and cultured at 37 °C in 5% CO2. IM-9 and U266 cell lines were used as controls of highly sensitive and resistant cells to anti-hTfR IgG3-Av-induced cytotoxicity, respectively.7,9 JURKAT and HL-60 cell lines were used as positive controls10 to compare the cytotoxicty of GA in cells not tested earlier. CHO-TRVb-hTfR1 cells (a cell line that only expresses hTfR1) and CHO-TRVb-neo cells (a cell line transfected with the empty neomycin vector) are derived from CHO-TRVb cells, a TfR-deficient mutant Chinese hamster ovary (CHO) cell line,24 were kindly donated by Dr H Phillip Koeffler of Cedars Sinai Medical Center, Los Angeles, CA, USA. The CHO cell lines were cultured in F-12 (Hams' medium; Invitrogen) medium supplemented with 10% fetal bovine serum plus 1 mg/ml G418 antibiotic as a selectable marker (Invitrogen). Cell images were captured using a Zeiss Axivovert 40 CFL PlasDIC Inverted Microscope using a × 20 objective (Mikron Instruments Inc, San Marcos, CA, USA) and a Canon PowerShot A620 digital camera (Mikron Instruments Inc.).
Detection of TfR1 by flow cytometry
Chinese hamster ovary (CHO) cells and hematopoietic cells (4 × 105) were incubated with phycoerythrin-conjugated anti-human CD71 (anti-hTfR1) or with the isotype control antibody phycoerythrin-conjugated mouse IgG2a k for 15 min on ice in 0.5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid phosphate-buffered saline. Cells were washed and fixed with 4% paraformaldehyde in phosphate-buffered saline. Cells were analyzed on a FACScan Analytic Flow Cytometer (BD-Bioscience). Data were analyzed using the WinMDI 2.8 software (The Scripps Research Institute, La Jolla, CA, USA).
Evaluation of cytotoxic effects Proliferation assay.
The antiproliferative effects induced by either anti-hTfR IgG3-Av or GA were measured by the [3H]-Thymidine incorporation assay as described earlier.9 Cells were incubated with anti-hTfR IgG3-Av or GA for the indicated times, then harvested and the radioactivity was counted as described earlier.9 Negative controls for each agent were tested in each experiment and included their diluents, buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.8) for anti-hTfR IgG3-Av or dimethylsulfoxide (DMSO) for GA. The amount of buffer or DMSO added in control treatments was consistent with the highest volume of each drug used in their respective assays. For iron supplementation, cells were incubated with or without 25 μM ferric ammonium citrate (Sigma-Aldrich Co., St Louis, MI, USA) simultaneously with each treatment.
Apoptosis assay.
Cells (105 per well for hematopoietic cells and 2000 cells for CHO cells) were incubated in 24-well plates (total volume per well = 1 ml) with the different treatments for 48 h. Cells were washed in cold phosphate-buffered saline and stained with Alexa Flour 488-labeled Annexin V and propidium iodide (PI) contained in the Vybrant Apoptosis Assay Kit no.2 (Invitrogen) following the procedure suggested by the manufacturer. In total, 104 events were recorded for each flow cytometry measurement using FACScan Analytic Flow Cytometer (BD-Bioscience). The cells Annexin V+/PI− and Annexin V+/PI+ were considered as apoptotic cells. The level of apoptosis of cells treated with anti-hTfR IgG3-Av or GA is calculated by the subtraction of the total percentage of apoptosis of cells treated with buffer or DMSO alone from the percentage of apoptosis of cells treated with the fusion protein and GA, respectively. The WinMDI 2.8 software (The Scripps Research Institute) was used for data analysis.
Evaluation of the cytotoxic effect induced by the combination of anti-hTfR IgG3-Av and GA.
The effect of the simultaneous combination or the chemosensitization treatments of anti-hTfR IgG3-Av and GA was determined by isobolographic analysis as described.25 For simultaneous treatments 0.31, 0.625, 1.25, 2.5, 5.0 and 10 nM anti-hTfR IgG3-Av plus 0.0375, 0.075, 0.15, 0.3 and 0.6 μM GA were incubated simultaneously at 37 °C for 48 h. In chemosensitization treatments, cells were pre-incubated with the same concentrations mentioned above of anti-hTfR IgG3-Av at 37 °C for 24 h, followed by the treatment with the same concentrations mentioned above of GA for additional 24 h. The additive effect is shown by the concentration of both agents represented by the points that lie on the straight line that connect the concentration of both agents required to produce a defined single-agent effect (concentration at which 90% of cells show inhibition of proliferation [IC90]). The points that lie below or above the straight line represent the concentrations of both agents that produce synergistic or antagonistic effects, respectively.
Detection of intracellular reactive oxygen species generation
Cells treated with 0.3 or 1 μM GA for 6 h were co-incubated with or without 3 mM dithiotheritol (Invitrogen) or with or without 2000 U/ml catalase from bovine liver (Sigma-Aldrich, St. Louis, MO, USA) to block reactive oxygen species (ROS) induction. As a positive control, a sample of untreated cells was incubated with 100 μM tert-butyl hydroperoxide (Sigma) for 30 min at 37 °C. All of the cells from the different treatments were incubated with 5 μM of the dye 5-(and-6)-carboxy-2’, 7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA: Invitrogen) in serum-free medium at 37 °C for 20 min. Cells were washed and analyzed by flow cytometry using FACScan Analytic Flow Cytometer (BD-Bioscience). Data were analyzed using the WinMDI 2.8 software (The Scripps Research Institute). The oxidation of CM-H2DCFDA is detected by the increase of fluorescence that is proportional to the amount of total intracellular ROS generated. The value of ROS generation is represented as the mean of relative fluorescence units.
Human colony forming assay
The human colony forming assay was performed to determine the effect of GA, anti-hTfR IgG3-Av or their combination on normal human hematopoietic progenitor cells as described earlier.9 Human bone marrow mononuclear cells were purchased from StemCell Technologies (StemCell Technologies Inc., Vancouver, BC, Canada) and were plated in quadruplicate with diluent alone (buffer or DMSO: negative controls), GA (0.1, 0.3, 0.5 or 1 μM), anti-hTfR IgG3-Av (1 or 10 nM) or their combination. In total, 2 × 104 cells were seeded per 35 mm dish in MethoCult GF H4434 (‘Complete’ Methylcellulose Medium with Recombinant Cytokines and Erythropoietin; StemCell Technologies). Cells were incubated for 14 days at 37°C in 5% CO2 following the instructions of the manufacturer. The number of CFU-E (colony forming unit-erythroid, mature erythroid progenitors), BFU-E (burst forming unit-erythroid, more primitive progenitor than CFU-E), CFU-GM (colony forming unit-granulocyte/macrophage, more mature than CFU-GEMM) and CFU-GEMM (colony forming unit granulocyte/erythroid/macrophage/megakaryocyte) was determined using an Olympus CK2 inverted microscope (Olympus America Inc., Center Valley, PA, USA) and the criteria defined by StemCell Technologies for each colony type.
Statistical analysis
The student’s t-test was used to determine the significant differences in the data, using Microsoft Excel 2004 (Microsoft Co., Redmond, WA, USA). P<0.05 were considered to be significant.
Results
Cytotoxic effect of anti-hTfR IgG3-Av in hematopoietic malignant cell lines
We have reported earlier the cytotoxic effect of anti-hTfR IgG3-Av against myeloma and B-lymphoblastoid cell lines.7,9 To explore the cytotoxic effect of anti-hTfR IgG3-Av in a new broader panel of human hematopoietic malignant cell lines including leukemia and lymphoma cells, we first evaluated the expression level of hTfR1 on the surface of the cells. The anti-hTfR IgG-Av binds specifically to TfR1,8 which is expressed on the surface of all hematopoietic cell lines used in this study (Supplementary Figure 1). This fusion protein elicited a significant (P < 0.001) antiproliferative effect in all lymphoma and leukemia cell lines tested (Figure 2a). It is important to note that although the IM-9 cell line has a lower TfR1-expression level compared with RAJI and HL-60 (Supplementary Figure 1), IM-9 cells are highly sensitive to the fusion protein. Changes in cell morphology suggest that the treatment with anti-hTfR IgG3-Av induces cell death (Supplementary Figure 2). Induction of cell death by anti-hTfR IgG3-Av was confirmed by an increase of apoptosis (Annexin V+/PI−, early apoptotic cells; and Annexin V+/PI+, late apoptotic cells) in the panel of the cell lines tested with the exception of U266 cells that also express hTfR1 (resistant to the fusion protein as reported earlier7,9) (Figure 2b). Different sensitivity levels of apoptosis ranging from 10 to 40% compared with buffer alone was observed, however, it is important to note that although the level of apoptosis induced by anti-hTfR IgG3-Av is low in some cancer cell lines, cells in the bottom left quadrant (Annexin V−/PI−) show a shift to the right compared with buffer alone treated cells suggesting that the apoptosis has been initiated in most cells.
Figure 2.
Anti-hTfR IgG3-Av induces cytotoxic effects in malignant hematopoietic cells. (a) Proliferation assay of hematopoietic malignant cells incubated with 10 nM anti-hTfR IgG3-Av or buffer for 48 h. The punctuated line represents the percentage of [3H]-Thymidine incorporation in cells treated with buffer alone. Data shown are the average of quadruplicates and are representative of three independent experiments. Error bars indicate the standard deviation. Statistically significant (P<0.001) growth inhibition compared with buffer alone treated cells is marked(*). (b) Induction of apoptosis in the panel of cells tested above incubated with 10 nM anti-hTfR IgG3-Av for 48 h. The percentage of cells in each quadrant is localized in the corner. The cells in the right quadrants (Annexin V+/PI− and Annexin V+/PI+) are considered as apoptotic cells. Data are representative of three independent experiments.
Cytotoxic effect of GA in hematopoietic malignant cell lines
The cytotoxic effect of GA, a drug that also binds hTfR1,10 has been tested in several cancer cell lines. We analyzed the toxic effect of GA over a period of 72 h in the panel of malignant hematopoietic cell lines at concentrations below 1 μM used in previous studies. Figure 3a shows the dose–response of GA over time in IM-9 cells. Cells incubated with 0.3 μM GA, concentration chosen for subsequent experiments, for 48 h blocked 80–95% of the proliferation in all the cell lines tested including U266 cells (Figure 3b), which are resistant to anti-hTfR IgG3-Av (Figure 2). At this concentration, GA also induces high levels of apoptosis ranging from 30 to 80% compared with the innocuous effect of DMSO alone in all cell lines (Figure 3c), which was consistent with the morphological changes observed by the treatment with this drug (Supplementary Figure 2).
Figure 3.
GA induces cytotoxic effects in hematopoietic malignant cells. (a) Anti-proliferative effect of GA at the concentrations and times indicated in IM-9 cells. Cell proliferation was determined by [3H]-Thymidine incorporation assay. (b) Proliferation assay of hematopoietic malignant cells incubated with 0.3 μM GA for 48 h. The punctuated line represents the percentage of [3H]-Thymidine incorporation in cells treated with DMSO alone. Data represent the average of quadruplicates and are representative of three independent experiments. Statistically significant (P<0.001) growth inhibition compared with DMSO alone treated cells is marked (*). Error bars indicate the standard deviation. (c) Detection of apoptosis in the panel of cells tested above incubated with 0.3 μM GA or DMSO alone for 48 h. The percentage of cells in each quadrant is localized in the corner. The cells in the right quadrants (Annexin V+/PI− and Annexin V+/PI+) are considered as apoptotic cells. Data represents the results of three independent experiments. DMSO, dimethylsulfoxide; GA, gambogic acid.
Role of iron in the cytotoxicity induced by anti-hTfR IgG3-Av and GA
Previous studies from our group have shown that the cytotoxic effect of anti-hTfR IgG3-Av against the B-lymphoblastoid cell lines ARH-77 and IM-9 is mediated by iron starvation.7,9 To determine whether this is also the case in lymphoma and leukemia cell lines, we added exogenous iron using 25 μM ferric ammonium citrate plus the fusion protein in proliferation assays. The results showed that the cytotoxic effect of anti-hTfR IgG3-Av was blocked (P<0.001) by iron supplementation in all the cell lines that are sensitive to the fusion protein (Figure 4a), confirming that the cytotoxic effect of anti-hTfR IgG3-Av in malignant hematopoietic cells is mediated by iron starvation. Using the same rationale, we tested the effect of iron on the cytotoxicty induced by GA. Iron supplementation did not block the GA-induced antiproliferative effect (Figure 4b), demonstrating that the effect of GA is iron-independent.
Figure 4.
Role of iron in the cytotoxicity induced by anti-hTfR IgG3-Av and GA in hematopoietic malignant cells. (a) Cells were incubated with 10 nM anti-hTfR IgG3-Av or (b) 0.3 μM GA for 48 h in the presence or absence of 25 μM FAC. The punctuated line represents the percentage of [3H]-Thymidine incorporation in cells treated with buffer or DMSO alone. Each value is the mean of quadruplicate samples and is representative of three independent experiments. Statistically significant (P<0.001) growth inhibition compared with buffer or DMSO alone treated cells is marked (*). Error bars indicate the standard deviation. GA, gambogic acid; DMSO, dimethylsulfoxide; FAC, ferric ammonium citrate.
GA cytotoxicity in the absence of the TfR
Previous studies have shown that GA targets hTfR1.10,22 Therefore, we explored the cytotoxicity of GA in CHO cells that do not express endogenous TfR (hamster) (CHO-TRVb neo) and in CHO cells that express exogenous hTfR1 (CHO-TRVb-TfR1) (Figure 5a). Despite previous studies suggesting that the cytotoxic effect of GA is TfR-mediated, 0.3 μM GA blocks the proliferation (Figure 5b) and induces apoptosis (Figure 5c) of both TfR-negative and TfR1-positive CHO cell lines. The CHO cell lines treated with 0.3 μM GA showed less cell number and morphological changes such as the loss of adherence that are consistent with cell death (Figure 5d).
Figure 5.
GA induces cytotoxic effects in the absence of TfR. (a) CHO-TRVb-neo (no TfR expression) or CHO-TRVb-hTfR1 (exogenous hTfR1 (hCD71) expression) cells were incubated with PE-conjugated anti-hCD71 (thick lines) or PE-conjugated isotype control antibody (thin lines). CD71 cell surface expression correlates with the increase of relative fluorescence that was detected by flow cytometry. (b) Proliferation assay in CHO-TRVb-neo and CHO-TRVb-hTfR1 cells incubated with 0.3 μM GA or DMSO alone for 48 h. Statistically significant (P<0.001) growth inhibition compared to DMSO alone treated cells is marked (*). Error bars indicate the standard deviation. (c) Detection of apoptosis in the indicated CHO cells incubated with 0.3 μM GA or DMSO alone for 48 h. The percentage of cells in each quadrant is localized in the corner. The cells in the right quadrants (Annexin V+/PI− and Annexin V+/PI+) are considered as apoptotic cells. (d) Morphological changes of the cells treated with 0.3 μM GA for 48 h analyzed by differential interferential contrast microscopy using a × 20 objective. Data are representative of two independent experiments. GA, gambogic acid; DMSO, dimethylsulfoxide; TfR, transferrin receptor; CHO, Chinese hamster ovary; PE, phycoerythrin.
GA induces ROS generation in hematopoietic cell lines
Structure–activity relationship analysis showed that the tricyclic ring and α,β-unsaturated ketone present in the structure of GA are relevant for its cytotoxicity.10,16 Therefore, the presence and putative activity of the α,β-unsaturated ketone suggests that GA can induce the generation of ROS, which can be signaling messengers that promote apoptosis.26,27 Using the cell permeant CM-H2DCFDA dye, we demonstrate that GA triggers the generation of ROS in a dose-dependent manner in U266 cells as shown by the significant increase of the relative fluorescence compared with cells treated with the control (Figures 6a and b). To confirm the observed generation of ROS induced by GA, cells were co-incubated with catalase or dithiotheritol simultaneously. The co-incubation of GA with catalase, an enzyme that protects the cells from oxidative stress by breaking down hydrogen peroxide (H2O2) into water and oxygen, significantly decreased the amount of ROS generated by GA measured directly by flow cytometry (Figure 6a and b). In addition, the co-incubation of GA with dithiotheritol, a reducing agent that inhibits oxidative reactions, decreased the amount of ROS to basal levels (Figures 6a and b). GA-induced apoptosis was partially blocked in U266 cells (Figure 6c) as well as in IM-9, HL-60 and RAMOS cells (Supplementary Figure 3) by co-incubation with catalase. These findings suggest that the generation of ROS plays a significant role in the apoptosis induced by GA in malignant hematopoietic cells.
Figure 6.
GA induces ROS generation. The oxidation of the dye by ROS generated by GA was measured by flow cytometry. (a) U266 cells were incubated with 0.3 μM for 6 h in the presence or absence of 2000 U/ml catalase or 3 mM DTT. Cells were incubated with 5 μM of the dye CM-H2DCFDA for 20 min at 37°C. (b) Mean of relative fluorescence units (RFU) generated by the effect of 0.3 μM (data presented in (a)) or 1 μM GA in U266 cells. As positive control for the induction of ROS, 100 μM TBHP was used. Statistically significant (P<0.05) data is marked (*). Error bars indicate the standard error of two independent experiments. (c) Detection of apoptosis in U266 cells treated with 0.3 μM GA in the presence or absence of 2000 U/ml of catalase. The percentage of cells in each quadrant is localized in the corner. The cells in the right quadrants (Annexin V+/PI− and Annexin V+/PI+) are considered as apoptotic cells. The data of cells treated with GA is the same as Figure 2 because these experiments were performed simultaneously. Data are representative of two independent experiments. DTT, dithiotheritol; TBHP, tert-butyl hydroperoxide; ROS, reactive oxygen species; GA, gambogic acid.
Anti-hTfR IgG3-Av enhances the cytotoxic effect of GA on malignant hematopoietic cell lines
As we demonstrated that both anti-hTfR IgG3-Av and GA induce cytotoxic effects in human malignant hematopoietic cells, we analyzed the effect of the combination of these drugs. The combination of both components showed an enhanced anti-proliferative effect compared with either compound alone (Figure 7 for IM-9 cells and Supplementary Table 1 for the panel of cell lines). All cells pre-treated with 10 nM anti-hTfR IgG3-Av, but not with 2.5 nM of the fusion protein, were more sensitive to the effect of GA (chemosensitization treatments, Figure 7a for IM-9 cells and Supplementary Table 1a for the panel of cell lines). These results were confirmed by the increased apoptosis observed in IM-9 cells in the chemosensitization treatments of anti-hTfR IgG3-Av and GA (Supplementary Figure 4). The simultaneous combination of 2.5 or 10 nM anti-hTfR IgG3-Av and 0.1 μM GA enhances the antiproliferative effect observed in most of the malignant cells tested (Figure 7b for IM-9 cells and Supplementary Table 1b for the panel of cell lines), however, this enhanced effect was less compared with that observed in chemosensitization treatments. Isobologram analysis showed synergistic enhancement of cytotoxicity by the combination of GA and anti-hTfR IgG3-Av in chemosensitization treatments (Figure 7c) represented by the points that lie below the straight line. Although simultaneous combination treatments showed enhanced cytotoxicity, its isobologram (Figure 7d) suggests an overall additive effect (the points that lie on the straight line) of the combination of anti-hTfR IgG3-Av and GA, which is consistent with the results mentioned above (Figure 7b). Iron supplementation in cell proliferation assays in both chemosensitization (Figure 8a) and simultaneous (Figure 8b) combination treatments partially blocked the cytotoxicity, suggesting that anti-hTfR IgG3-Av contributes to the enhanced cytotoxicity of the combination treatment, at least in part though iron starvation.
Figure 7.
The combination of anti-hTfR IgG3-Av and GA enhances the cytotoxic effects in IM-9 cells. IM-9 cells were treated as follows: (a) Chemosensitization treatments. For cytotoxocity induced by GA alone, cells were incubated for 24 h in medium alone followed by the incubation with 0.1 or 0.3 μM GA for additional 24 h; for the cytotoxicity mediated by anti-hTfR IgG3-Av, cells were incubated with 2.5 or 10nM of the fusion protein alone for 48 h; for chemosensitization treatment combinations, cells were pre-incubated with 2.5 or 10 nM of anti-hTfR IgG3-Av alone for 24 h followed by the incubation with 0.1 or 0.3 μM GA for additional 24 h in the presence of anti-hTfR IgG3-Av. (b) Simultaneous combination treatments. Cells were treated with 0.1 μM GA and 2.5 or 10 nM anti-hTfR IgG3-Av for 48 h as controls. For the combination treatments, cells were treated with 0.1 μM GA and 2.5 or 10 nM anti-hTfR IgG3-Av simultaneously for 48 h. The punctuated line represents the percentage of [3H]-Thymidine incorporation in cells treated with buffer alone. Each value is the mean of quadruplicate samples and is representative of three independent experiments. Error bars indicate the standard deviation. Statistically significant growth inhibition compared with buffer plus DMSO treated cells is marked (**P<0.001 or *P<0.05). (c) Isobologram curve obtained from the chemosensitization treatments with 0.0375, 0.075, 0.15, 0.3 or 0.6 μM GA with 1.25, 2.5, 5 or 10 nM anti-hTfR IgG3-Av, shows the synergistic effect of these drugs. (d) Isobologram curve obtained from the simultaneous combination of anti-hTfR IgG3-Av and GA with the same concentrations used in (c), shows a mostly an additive effect of these drugs. The concentrations of each drug in the combination that produce the specified effect is expressed as a fractional inhibitory concentration (F.C.I.) that produce the same effect by the treatment with each drug alone. Points below and above to the straight line mean synergy or antagonism respectively. The additive effect is represented by the points that lie on the straight line. GA, gambogic acid; DMSO, dimethylsulfoxide.
Figure 8.
Role of iron in the combination of anti-hTfR IgG3-Av and GA in IM-9 cells. IM-9 cells were treated in the presence or absence of 25 μM FAC in the following treatments: (a) Chemosensitization treatments. For cytotoxocity induced by GA alone, cells were incubated for 24 h in medium alone followed by the incubation with 0.1 or 0.3 μM GA for additional 24 h; for the cytotoxicity mediated by anti-hTfR IgG3-Av, cells were incubated with 2.5, or 10 nM of the fusion protein alone for 48 h; for chemosensitization treatment combinations, cells were pre-incubated with 2.5 or 10 nM of anti-hTfR IgG3-Av alone for 24 h followed by the incubation with 0.1 or 0.3 μM GA for additional 24 h in the presence of anti-hTfR IgG3-Av. (b) Simultaneous combination treatments. Cells were simultaneously treated with 0.1 μM GA and 2.5 or 10 nM anti-hTfR IgG3-Av for 48 h as controls. For the combination treatments, cells were treated with 0.1 μM GA and 2.5 or 10 nM anti-hTfR IgG3-Av simultaneously for 48 h. The punctuated line represents the percentage of [3H]-Thymidine incorporation in cells treated with buffer alone. Error bars indicate the standard deviation. Statistically significant growth inhibition compared with buffer plus DMSO treated cells is marked (**P<0.001 or *P<0.05). Each value is the mean of quadruplicate samples and is representative of three independent experiments. GA, gambogic acid; DMSO, dimethylsulfoxide; FAC, ferric ammonium citrate.
Toxicity of GA and anti-hTfR IgG3-Av alone or in combination in human hematopoietic progenitor cells
As GA is highly toxic toward malignant hematopoietic cell lines, we explored the effect of this drug on normal hematopoietic progenitor cells to help determine the clinical relevance of GA as a potential treatment for human cancers. The effect of GA on progenitor cells was determined using the colony forming assay, which tests the ability of human progenitor cells to proliferate and differentiate into colonies in a semisolid medium. Table 1a shows that GA is not cytotoxic to human progenitor cells at concentrations ⩽0.3 μM. However, although 0.5 μM GA is not toxic to most progenitor cells tested, this concentration induced a significant inhibition of the formation of CFU-GM and CFU-GEMM colonies. As 0.3 μM GA, a concentration toxic to cancer cells, is not toxic to human normal hematopoietic progenitor cells (Table 1a), GA might not have an effect on hematopoiesis at certain therapeutic doses in vivo. The concentration used in various previous studies from other groups, 1 μM GA,13,15,18-20 completely inhibits colony formation of all types (Table 1) indicating that GA at 1 μM or above is highly toxic to normal human hematopoietic progenitor cells. We also tested the cytotoxic effect of anti-hTfR IgG3-Av on progenitor cells. Anti-hTfR IgG3-Av showed dose-dependent toxicity on the progenitor cells in which 1 nM anti-hTfR IgG3-Av was toxic to erythroid progenitor cells (CFU-E and BFU-E) as well as CFU-GM and CFU-GEMM (P<0.001). 10nM anti-hTfR IgG3-Av was toxic to all colony types (P<0.001). The combination of 10nM anti-hTfR IgG3-Av with 0.3 μM GA, the maximum concentrations used in all combination treatments, also demonstrates toxicity that appears to be due to the effects of anti-hTfR IgG3-Av as the results of the combination treatments are not significantly different from those of anti-hTfR IgG3-Av alone, with the exception of CFU-GM colonies, which are more sensitive to the combination of both agents. However, we still observed colonies of cells treated with anti-hTfR IgG3-Av and GA.
Table 1.
Cytotoxicity of gambogic acid, anti-hTfR IgG3-Av and their combination on human hematopoietic progenitor cells
| CFU-E | BFU-E | CFU-GM | CFU-GEMM | |
|---|---|---|---|---|
| (a) Cytotoxicity of GA | ||||
| Untreated | 44±10.6 | 41±4.4 | 90±13.2 | 15±1.6 |
| DMSO alone | 44±4.0 | 47±7.0 | 89±7.7 | 15±4.2 |
| 0.1 mM GA | 40±3.7 | 35±2.1 | 94±10.0 | 19±3.1 |
| 0.3 μM GA | 40±3.0 | 39±5.0 | 89±6.7 | 12±3.5 |
| 0.5 μM GA | 30±4.5 | 37±6.5 | 70±2.1* | 9±3.0* |
| 1.0 μM GA | 0* | 0** | 0** | 0** |
| (b) Cytotoxicity of anti-hTfR IgG3-Av alone or in combination with GA | ||||
| Untreated | 37±1.9 | 26±2.5 | 91±7.9 | 9±1.0 |
| Buffer alone | 42±6.2 | 28±4.2 | 92±7.1 | 8±1.5 |
| 1nM anti-hTfR IgG3-Av | 32±9.9 | 19±4.7* | 56±9.6** | 1±1.0** |
| 10 nM anti-hTfR IgG3-Av | 15±5.3* | 5±2.9** | 39±6.8** | 0** |
| 10 nM anti-hTfR IgG3-Av + 0.3μM GA | 11±4.5** | 2±1.2** | 24±2.9*,♣ | 0** |
anti-hTfR IgG3-Av, antibody-avidin fusion protein that targets hTfR; BFU-E: burst forming units-erythroid; CFU-E: colony forming units-erythroid; CFU-GM: colony forming units-granulocyte/macrophage; CFU-GEMM: colony-forming units-granulocyte/erythroid/macrophage/megakaryocyte; GA, gambogic acid.
Each value represents the mean of the number of colonies±the s.d. of quadruplicate samples of two independent experiments.
P<0.05
P<0.001 compared with buffer alone
♣P<0.05 compared with anti-hTfR IgG3-Av.
Discussion
Initially, anti-hTfR IgG3-Av was developed by our group as a universal drug delivery system.6,8,9 Our previous studies in multiple myeloma (MM. 1S, S6B45, OCI-My5, 8226/S, 8226/ DOX40), B-lymphoblastoid (IM-9 and ARH-77), and K562 human erythroleukemia cell lines, demonstrated that anti-hTfR IgG3-Av elicits significant cytotoxic effects by itself.6,7,9 In the present work, we demonstrate that anti-hTfR IgG3-Av is also cytotoxic to a broader variety of human malignant hematopoietic cells including leukemia (HL-60 and JURKAT) and B-cell lymphoma (RAMOS, HS-SULTAN and RAJI) cell lines. We found that this cytotoxicity is iron-dependent consistent with our previous reports using IM-9 and ARH-77 cells,7,9 suggesting that iron starvation may be a general mechanism of cytotoxicity induced by anti-hTfR IgG3-Av in human malignant hematopoietic cells. Even though all cell lines tested express significant levels of hTfR1, the variable sensitivity to anti-hTfR IgG3-Av did not correlate with the level of TfR1 cell surface expression. Studies from our group are now in progress to analyze this variable sensitivity described in this report and earlier.7 Toxicity of anti-hTfR IgG3-Av on normal hematopoiesis (bone marrow mono-nuclear cells for a 14-day incubation) showed a cytotoxic effect as evidenced by a significant decrease in colony formation of all colony types tested. Our previous results showed that 1 nM anti-hTfR IgG3-Av does not have cytotoxic effects on human progenitor cells if they are treated for 1 h in liquid culture followed by washing to remove unbound fusion protein prior to the 14-day incubation in semi-solid medium required for colony formation.9 The colony forming assay tests the toxicity of a compound on late progenitor cells, many of which express hTfR1 (CD71) at various stages of differentiation.2,28 Therefore, it is expected that anti-hTfR IgG3-Av would have an effect on these proliferating cells during the extended 14-day incubation period. The hematopoietic pluripotent stem cells and the early progenitor cells may be less or not sensitive to the fusion protein because these cells express low to no TfR.28-31 In fact, early progenitor cells are distinguished from late progenitor cells by surface antigen expression, including the lack of hTfR1 expression.28,29,31 The low number of pluripotent cells in the bone marrow makes it difficult to study their sensitivity to anti-hTfR IgG3-Av. In this case, although anti-hTfR IgG3-Av had cytotoxic effects on committed progenitor cells, it is expected that they could be repopulated by their early counterparts. However, a transient myelosuppresion may be expected for the duration of the treatment in patients. Other anticancer drugs such as homoharringtonine and the aurora kinase inhibitor (VX-680), used for the treatment of hematopoietic malignances including leukemia and multiple myeloma, have shown a dose–response cytotoxicity on human progenitor cells in vitro.32-34
Immunogenicity of potential biological therapeutic agents such as antibodies and antibody fusion proteins is always a concern. In our case, to avoid the immune response that is often observed with the use of murine therapeutic monoclonal antibodies, anti-hTfR IgG3-Av was developed using human IgG3 and human kappa (κ) light chain constant regions. In addition, the human Fc can elicit effector functions such as complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicty in patients.35 The chicken avidin present in anti-hTfR IgG-Av is potentially immunogenic. However, avidin was used instead of the highly immunogenic bacterial protein streptavidin.6,35 The frequent exposure of humans to avidin by eating eggs, may decrease its immunogenecity compared with streptavidin, as it is known that oral antigens induce tolerance.36 Consistent with this possibility, there is a report describing the use of avidin/111 Indium-biotin as an imaging method for diagnosis of prosthetic vascular graft infection. In this study, immunogenicity analysis showed that none of the 25 patients studied developed a human anti-avidin humoral response.37 However, in case that anti-hTfR IgG3-Av induces an antibody response in patients, this response can be prevented by the use of rituximab (Rituxan, Genentech Inc., San Francisco, CA, USA and Biogen IDEC, San Diego, CA, USA), a therapeutic antibody used to treat B-cell non-Hodgkin's lymphoma,38 which also depletes normal B cells and, therefore, the humoral immunity.39,40 Additionally, patients with hematopoietic malignances such as multiple myeloma and lymphoma show an impaired immune response;41,42 therefore, the immunogenicity of anti-hTfR IgG3-Av may not be an issue in these patients.
Previous reports have shown that GA binds hTfR110,22 and blocks its internalization.10 In addition, it has been reported that GA induces apoptosis in several types of cancer cells, including JURKAT and HL-60 cell lines.12-22 In this study, we demonstrate the cytotoxicity of GA on malignant hematopoietic cell lines including multiple myeloma, lymphoma and leukemia. Iron supplementation showed that the cytotoxic effect of GA was iron-independent, as previously described in JURKAT cells.10 It is important to note that we observed a strong cytotoxic effect of GA using a lower concentration (0.3 μM GA) compared with those of previous studies conducted by other groups that used concentrations of at least 1 μM GA. Moreover, using human colony-forming assays, we found that 1 μM GA inhibits the growth of human hematopoietic progenitor cells. Thus, it is possible that the use of GA at concentrations at or above 0.5 μM would induce severe hematopoietic toxicity in patients. However, in this study we demonstrated that there is an effective concentration range, where GA can specifically induce apoptosis in malignant hematopoietic cells without toxicity to normal hematopoiesis. Toxicologic studies have demonstrated the innocuous effect of GA in rats, mice and dogs,43-45 suggesting that GA can be a viable treatment option for multiple myeloma, lymphoma and leukemia in humans.
It has been suggested that GA induces cytotoxic effects through its ability to target hTfR1.10,22 In the present study, we show that GA induces cytotoxicity independent of cell surface TfR expression as demonstrated using CHO cells expressing hTfR1or not. Our results are consistent with several mechanisms of cytotoxicity mediated by GA such as the induction of cell cycle arrest in G2/M phase19,20 by the deregulation of CDK7 phosphorylation, a cell cycle kinase of the mitosis-promoting factor.19 In addition, GA increases the level of Bax (a pro-apoptotic protein) and decreases the level of Bcl-2 (anti-apoptotic protein).17,20 GA also blocks the activity of the transcription factor NF-κB through inhibition of IκBα kinase22 resulting in the induction of apoptosis. Moreover, GA reduces the expression of the human telomerase reverse transcriptase resulting in decreased telomerase activity, which is required to maintain malignant cell proliferation.12,18,21 Therefore, GA can be considered to be a pleiotropic drug that induces cytotoxicity through multiple mechanisms.
The structure of GA shows the presence of an α,β-unsatured ketone (Figure 1b) that has been shown to play an important role in the GA cytotoxicity.10 As α,β-unsatured ketone groups are present in some drugs that induce apoptosis through the generation of ROS,46,47 we explored the possibility that the cytotoxic effects of GA could be also mediated through the generation of ROS. We found that GA increases the ROS generation in malignant hematopoietic cell lines. In addition, the effect of GA can be partially blocked by its co-incubation with catalase, an enzyme that decreases the presence of H2O2, a potent ROS. The partial protective effect of catalase is related to the specific break down of H2O2, suggesting the presence of other ROS, such as hydroxyl (.OH), alkoxyl (RO.), peroxyl (ROO.), superoxide () or nitroxyl radical (NO.),27 which may be also generated by GA. As signaling messengers, ROS are involved in pathways that result in apoptosis.26,27,48 It has been shown that ROS induce the oxidation of sulfydryl groups present in NF-κB, inhibiting its DNA-binding activity in vitro.49-51 As we mentioned earlier, GA also inhibits the NF-κB activity by blocking its translocation to the nucleus.22 As we demonstrated that GA induces ROS generation, we suggest that in addition to its ability to inhibit NF-κB translocation, GA can also block NF-κB activity through abolishing its DNA-binding activity resulting in cooperative mechanisms of GA-mediated apoptosis. Additionally, it has been reported that GA-mediated apoptosis involves the mitochondrial pathway as evidenced by the release of cytochrome c,10 which can be related with the ability of ROS to induce mitochondrial membrane permeability that results in the release of pro-apoptotic proteins such as apoptosis inducing factor, caspase activators (Smac/DIABLO) as well as cytochrome c.48 Taken together, our results suggest that the generation of ROS by GA is an additional mechanism involved in the strong cytotoxic effect of this drug in cancer cells.
Previous studies demonstrated that anti-hTfR IgG3-Av disrupts the cycling of hTfR1 leading to its degradation,7 in contrast with GA, which has been reported to block the internalization of hTfR1.10 Despite the potential antagonist effect of these agents, the simultaneous combination of anti-hTfR IgG3-Av and GA enhances their cytotoxicity mainly by an additive effect in the panel of cells tested, including U266 that is resistant to anti-hTfR IgG3-Av alone, which can be explained by the multiple mechanisms of GA to induce cytotoxicity including ROS generation. The incomplete blocking effect of iron supplementation showed the partial role of this fusion protein in the additive cytotoxic effect. The simultaneous combination treatment shows an enhancement of the cytotoxicity on CFU-GM progenitor cells compared with the fusion protein alone. Even though the simultaneous combination treatment resulted in fewer CFU-E and BFU-E colonies than those of anti-hTfR IgG3-Av alone this variation was not significantly different, suggesting that the cytotoxicity observed by the combination of anti-hTfR IgG3-Av and GA is mostly due to the effect of the antibody-avidin fusion protein alone. However, colonies are still formed with the treatment of the combination of anti-hTfR IgG3-Av and GA.
Some reports have shown that therapeutic antibodies, such as trastuzumab (Herceptin Genentech Inc.) and rituximab (Rituxan), induce chemosensitization of drug-resistant cancer cells and thus can be used in combination with other antitumor agents to overcome drug resistance.52-54 Although GA alone has a cytotoxic effect, the pre-treatment with anti-hTfR IgG3-Av can sensitize cells to the effect of GA through a synergistic cytotoxicity. In addition, iron supplementation confirms the role of anti-hTfR IgG3-Av in chemosensitization treatments, because of this fusion protein induces cytotoxicity in an iron-dependent manner, suggesting for the first time that the iron stress induced by anti-hTfR IgG3-Av can sensitize cancer cells to GA. These results suggest that in addition to the intrinsic cytotoxicity and delivery capabilities of anti-hTfR IgG3-Av, it may also be used as a chemosensitizing agent. However, this chemosensitizing effect of anti-hTfR IgG3-Av needs to be further explored using other chemotherapeutic agents to determine if this is a general phenomenon. In addition, owing to the universal delivery capability of anti-hTfR IgG3-Av, it can also be used to deliver drugs that are able to promote chemosensitization. In fact, a broad spectrum of therapeutics including antisense oligonuleotides and toxins have been successfully delivered into tumor cells by targeting TfR.5
In summary, we have demonstrated the cytotoxicity of anti-hTfR IgG3-Av in new human malignant hematopoietic cell lines including leukemia and lymphoma cells through an iron-dependent mechanism. We also demonstrated the cytotoxic effect of GA in leukemia, lymphoma and multiple myeloma cells through an iron-independent mechanism. We show that GA can also induce cytotoxicity by a hTfR-independent mechanism. Moreover, we demonstrate that GA induces the generation of ROS, suggesting that oxidative stress is part of the mechanism of action of this pleiotropic drug. We also found a concentration of GA that is cytotoxic to malignant hematopoietic cells, but not to human hematopoietic progenitor cells. Importantly, additive cytotoxicity was observed with the simultaneous combination of anti-hTfR IgG3-Av and GA, and synergistic cytotoxicty in chemosentitization treatments, suggesting these combination strategies may be effective treatments for a wide variety hematopoietic malignances.
Supplementary Material
Acknowledgments
We would like to thank Dr Sara Huerta-Yapez (Hospital Infantil de México ‘Federico Gomez’, Mexico City), Dr Mario I Vega (Hospital de Infectología ‘Dr Daniel Mendez Hernández’ Centro Médico ‘La Raza’ del Instituto Mexicano del Seguro Social, Mexico City) and José A. Rodríguez (University of California, Los Angeles) for assistance with isobolographic analysis. We also would like to thank Dr H Phillip Koeffler (Cedars Sinai Medical Center, Los Angeles, CA) for providing us with the CHO-TRVb cells. Our study was supported in part by the NIH/NCI Grants CA86915, CA107023 and SPORE in Lymphoma P50CA096888; and also by the NIH/NCI research supplements CA107023-02S1, CA057152-13S1 and Fogarty AITRP AIDS Malignancies Program D43-TW000013-S1.
References
- 1.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 2.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 4.Deaglio S, Capobianco A, Cali A, Bellora F, Alberti F, Righi L, et al. Structural, functional, and tissue distribution analysis of human transferrin receptor-2 by murine monoclonal antibodies and a polyclonal antiserum. Blood. 2002;100:3782–3789. doi: 10.1182/blood-2002-01-0076. [DOI] [PubMed] [Google Scholar]
- 5.Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121:159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ng PP, Dela Cruz JS, Sorour DN, Stinebaugh JM, Shin SU, Shin DS, et al. An anti-transferrin receptor-avidin fusion protein exhibits both strong proapoptotic activity and the ability to deliver various molecules into cancer cells. Proc Natl Acad Sci USA. 2002;99:10706–10711. doi: 10.1073/pnas.162362999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng PP, Helguera G, Daniels TR, Lomas SZ, Rodriguez JA, Schiller G, et al. Molecular events contributing to cell death in malignant human hematopoietic cells elicited by an IgG3-avidin fusion protein targeting the transferrin receptor. Blood. 2006;108:2745–2754. doi: 10.1182/blood-2006-04-020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez JA, Helguera G, Daniels TR, Neacato II, Lopez-Valdes HE, Charles AC, et al. Binding specificity and internalization properties of an antibody-avidin fusion protein targeting the human transferrin receptor. J Control Release. 2007;124:35–42. doi: 10.1016/j.jconrel.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Daniels TR, Ng PP, Delgado T, Lynch MR, Schiller G, Helguera G, et al. Conjugation of an anti transferrin receptor IgG3-avidin fusion protein with biotinylated saporin results in significant enhancement of its cytotoxicity against malignant hematopoietic cells. Mol Cancer Ther. 2007;6:2995–3008. doi: 10.1158/1535-7163.MCT-07-0330. [DOI] [PubMed] [Google Scholar]
- 10.Kasibhatla S, Jessen KA, Maliartchouk S, Wang JY, English NM, Drewe J, et al. A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci USA. 2005;102:12095–12100. doi: 10.1073/pnas.0406731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asano J, Chiba K, Tada M, Yoshii T. Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry. 1996;41:815–820. doi: 10.1016/0031-9422(95)00682-6. [DOI] [PubMed] [Google Scholar]
- 12.Guo QL, Lin SS, You QD, Gu HY, Yu J, Zhao L, et al. Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma SMMC-7721 cells. Life Sci. 2006;78:1238–1245. doi: 10.1016/j.lfs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 13.Lu N, Yang Y, You QD, Ling Y, Gao Y, Gu HY, et al. Gambogic acid inhibits angiogenesis through suppressing vascular endothelial growth factor-induced tyrosine phosphorylation of KDR/Flk-1. Cancer Lett. 2007;258:80–89. doi: 10.1016/j.canlet.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Qin Y, Meng L, Hu C, Duan W, Zuo Z, Lin L, et al. Gambogic acid inhibits the catalytic activity of human topoisomerase IIalpha by binding to its ATPase domain. Mol Cancer Ther. 2007;6:2429–2440. doi: 10.1158/1535-7163.MCT-07-0147. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Yang L, You QD, Nie FF, Gu HY, Zhao L, et al. Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007;256:259–266. doi: 10.1016/j.canlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HZ, Kasibhatla S, Wang Y, Herich J, Guastella J, Tseng B, et al. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem. 2004;12:309–317. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol. 2005;11:3655–3659. doi: 10.3748/wjg.v11.i24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Guo QL, You QD, Lin SS, Li Z, Gu HY, et al. Repression of telomerase reverse transcriptase mRNA and hTERT promoter by gambogic acid in human gastric carcinoma cells. Cancer Chemother Pharmacol. 2006;58:434–443. doi: 10.1007/s00280-005-0177-2. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang Y, et al. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis. 2007;28:632–638. doi: 10.1093/carcin/bgl168. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull. 2004;27:998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 21.Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol Pharm Bull. 2004;27:1769–1774. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- 22.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-\{kappa\}B signaling pathway. Blood. 2007;110:3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helguera G, Penichet ML. Antibody-Cytokine Fusion Proteins for the Therapy of Cancer. Adoptive Immunotherapy. Methods Mol Med. 2005;109:347–3774. doi: 10.1385/1-59259-862-5:347. [DOI] [PubMed] [Google Scholar]
- 24.McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berenbaum MC. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 27.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 28.Lansdorp PM, Dragowska W. Long-term erythropoiesis from constant numbers of CD34+ cells in serum-free cultures initiated with highly purified progenitor cells from human bone marrow. J Exp Med. 1992;175:1501–1509. doi: 10.1084/jem.175.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews RG, Singer JW, Bernstein ID. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989;169:1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender JG, Unverzagt K, Walker DE, Lee W, Smith S, Williams S, et al. Phenotypic analysis and characterization of CD34+ cells from normal human bone marrow, cord blood, peripheral blood, and mobilized peripheral blood from patients undergoing autologous stem cell transplantation. Clin Immunol Immunopathol. 1994;70:10–18. doi: 10.1006/clin.1994.1003. [DOI] [PubMed] [Google Scholar]
- 31.Gross S, Helm K, Gruntmeir JJ, Stillman WS, Pyatt DW, Irons RD. Characterization and phenotypic analysis of differentiating CD34+ human bone marrow cells in liquid culture. Eur J Haematol. 1997;59:318–326. doi: 10.1111/j.1600-0609.1997.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 32.Mi Y, Xue Y, Yu W, Liu S, Zhao Y, Meng Q, et al. Therapeutic experience of adult acute myeloid leukemia in a single institution of China and its relationship with chromosome karyotype. Leuk Lymphoma. 2008;49:524–530. doi: 10.1080/10428190701836852. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Reiman T, Li W, Maxwell CA, Sen S, Pilarski L, et al. Targeting aurora kinases as therapy in multiple myeloma. Blood. 2007;109:3915–3921. doi: 10.1182/blood-2006-07-037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umbach GE, Hug V, Spitzer G, Thames H, Drewinko B. Responses of human bone marrow progenitor cells to fluoro-ara-AMP, homoharringtonine, and elliptinium. Invest New Drugs. 1984;2:263–265. doi: 10.1007/BF00175374. [DOI] [PubMed] [Google Scholar]
- 35.Penichet ML, Morrison SL. Design and Engineering Human Forms of Monoclonal Antibodies. Drug Development Research. 2004;61:121–136. [Google Scholar]
- 36.Weiner HL. Oral tolerance. Proc Natl Acad Sci USA. 1994;91:10762–10765. doi: 10.1073/pnas.91.23.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuel A, Paganelli G, Chiesa R, Sudati F, Calvitto M, Melissano G, et al. Detection of prosthetic vascular graft infection using avidin/indium-111-biotin scintigraphy. J Nucl Med. 1996;37:55–61. [PubMed] [Google Scholar]
- 38.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 39.Frankel AE. Reducing the immune response to immunotoxin. Clin Cancer Res. 2004;10:13–15. doi: 10.1158/1078-0432.ccr-1216-3. 1 Part 1. [DOI] [PubMed] [Google Scholar]
- 40.van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100:2257–2259. [PubMed] [Google Scholar]
- 41.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:405–408. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- 42.Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138:563–579. doi: 10.1111/j.1365-2141.2007.06705.x. [DOI] [PubMed] [Google Scholar]
- 43.Guo Q, Qi Q, You Q, Gu H, Zhao L, Wu Z. Toxicological studies of gambogic acid and its potential targets in experimental animals. Basic Clin Pharmacol Toxicol. 2006;99:178–184. doi: 10.1111/j.1742-7843.2006.pto_485.x. [DOI] [PubMed] [Google Scholar]
- 44.Hao K, Liu XQ, Wang GJ, Zhao XP. Pharmacokinetics, tissue distribution and excretion of gambogic acid in rats. Eur J Drug Metab Pharmacokinet. 2007;32:63–68. doi: 10.1007/BF03190993. [DOI] [PubMed] [Google Scholar]
- 45.Qi Q, You Q, Gu H, Zhao L, Liu W, Lu N, et al. Studies on the toxicity of gambogic acid in rats. J Ethnopharmacol. 2008;117:433–438. doi: 10.1016/j.jep.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 46.Chen YC, Shen SC, Tsai SH. Prostaglandin D(2) and J(2) induce apoptosis in human leukemia cells via activation of the caspase 3 cascade and production of reactive oxygen species. Biochim Biophys Acta. 2005;1743:291–304. doi: 10.1016/j.bbamcr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, et al. 15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci USA. 2002;99:7367–7372. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 49.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. Embo J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–737. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- 51.Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci USA. 1991;88:4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Yang W, Lan KH, Sellappan S, Klos K, Hortobagyi G, et al. Enhanced sensitization to taxol-induced apoptosis by herceptin pretreatment in ErbB2-overexpressing breast cancer cells. Cancer Res. 2002;62:5703–5710. [PubMed] [Google Scholar]
- 53.Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-\{kappa\}B signaling pathway. Cancer Res. 2005;65:264–276. [PubMed] [Google Scholar]
- 54.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin’s lymphoma B cells by Rituximab. Cancer Res. 2004;64:7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.