Abstract
Asymmetric cell division is a potential means by which cell fate choices during an immune response are orchestrated. Defining the molecular mechanisms that underlie asymmetric division of T cells is paramount for determining the role of this process in the generation of effector and memory T cell subsets. In other cell types, asymmetric cell division is regulated by conserved polarity protein complexes that control the localization of cell fate determinants and spindle orientation during division. We have developed a tractable, in vitro model of naïve CD8+ T cells undergoing initial division while attached to dendritic cells during antigen presentation to investigate whether similar mechanisms might regulate asymmetric division of T cells. Using this system, we show that direct interactions with antigen presenting cells provide the cue for polarization of T cells. Interestingly, the immunological synapse disseminates before division even though the T cells retain contact with the antigen presenting cell. The cue from the antigen presenting cell is translated into polarization of cell fate determinants via the polarity network of the Par3 and Scribble complexes and orientation of the mitotic spindle during division is orchestrated by the Pins/G protein complex. These findings suggest that T cells have selectively adapted a number of evolutionarily conserved mechanisms to generate diversity through asymmetric cell division.
INTRODUCTION
Upon activation, a naïve T cell proliferates to generate the different T cell subsets required for both an immediate response and an immune memory (1). How the activation of a single parent T cell can control multiple pathways of differentiation in the T cell progeny remains controversial. A single parental CD8+ T cell, for example, may have the potential to develop into both effector and memory cells, with the outcome determined by extrinsic factors such as environmental signals or stimulus strength (2). Alternatively, T cells may divide asymmetrically following antigen presentation, leading to molecularly distinct daughter cells with different effector and memory fate potential (3–5).
In vivo imaging has revealed much about the dynamics of T cell-DC interactions (6–8) and would be the ideal tool to analyse the molecular events following T cell conjugation with antigen presenting cells and subsequent activation and proliferation. Although current technology using 2-photon microscopy can accurately assess the duration of contacts and the functional consequences of these interactions (9–12), it does not have the resolution to assess the distribution of individual proteins in single cells. Fixed imaging analysis of dividing cells ex vivo in response to Listeria infection has revealed that asymmetric cell division (ACD) of T cells may dictate T cell memory and effector fates (4). However, in this instance, the history of the dividing cell is lost, making it difficult to extrapolate information about the mechanism of ACD, in particular, the cue for polarity.
To overcome these limitations, we have developed an experimental in vitro system that enables the molecular analysis of single progenitor T cells undergoing their first division during interaction with an antigen presenting cell. This model provides an excellent system with which to image individual T cells undergoing division in response to contact with antigen presenting cells, and evaluate the three requirements for ACD: (1) a cue to dictate the axis of polarity, (2) asymmetry of proteins along this axis and (3), alignment of the mitotic spindle with the axis of polarity (13–15). Using this system, we elucidate each of the three conditions required for ACD in T cells and show that T cells have adapted a number of evolutionarily conserved mechanisms to regulate polarity and mitotic spindle orientation during ACD.
MATERIALS AND METHODS
Antibodies and constructs
Primary antibodies used were rabbit anti-aPKC, rabbit anti-Scribble, rabbit anti-PKCθ (Santa Cruz Biotechnology); rabbit anti-ASIP/PAR-3 (Invitrogen); mouse anti-PSD-95 family (Upstate); goat anti-Numb, rat anti-tubulin (Abcam); mouse anti-Prox1 (Chemicon); mouse anti-tubulin (Sigma); rabbit anti-tubulin (Rockland); rat anti-CD8-Alexa-488, rat anti-CD45, rat anti-CD11a (LFA-1), hamster anti-CD69-FITC, rat anti-CD44-FITC, rat anti-Vα2 TCRPE, mouse anti-CD45.1-PE (BD Biosciences); rat anti-CD25-APC, rat anti-CD62L-APC, mouse anti-CD45.2-APC-Cy7, rat anti-CD45R-APC, hamster anti-TCRβ-PE-Cy5.5 (eBioscience). Secondary antibodies used were anti-rabbit, anti-rat, anti-mouse and anti-goat-Alexa Fluor 488, anti-rabbit, anti-mouse, anti-rat-Alexa Fluor 594/543 and anti-goatrhodamine (Molecular Probes). MSCV-β-ARK-C-terminal-GFP was subcloned from pRK5-Bark 1CT supplied by Robert Lefkowitz (16) and aurothiomalate (ATM) was supplied by Alan Fields. Biotin labelled hamster monoclonal antibodies to the Notch ligands, Delta 1, Delta 4, Jagged 1 and Jagged 2 (17) were supplied by Hideo Yagita (Juntendo University).
Mice and cells
C57BL/6 mice, B6-Ptpcra or OT-1 mice (C57BL/6 background) (18) of 8–12 weeks of age were used. Naïve OT-1 CD8+ T cells were purified from spleens of mice using MACS negative selection (Miltenyi Biotec). Bone marrow cells from hind limbs of C57Bl/6 mice were cultured in GM-CSF and IL-4 for 6 days to generate immature DCs (CD11c+, CD86low and MHC-IIlow) for use as antigen presenting cells (19). A proportion of these bone marrow derived APC expressed the Notch ligands Delta 1, Jagged and Jagged 2, but not Delta 4 which was moderately up-regulated following peptide pulsing (Fig. S1). All experiments on mice were performed in accordance with the Animal Experimentation Ethics Committee of the Peter MacCallum Cancer Centre. To generate effector and memory cells, OT-1 T cells were cultured with interleukin IL-2 or IL-15 as previously described (20) and analyzed by flow cytometry.
Transfections and Transductions
For generation of naïve CD8+ OT-1 T cells expressing GFP or the C-terminus of β-adrenergic kinase fused to GFP (16), hematopoietic stem cells were harvested from livers of OT-1 (Ly5.2) 13.5–14.5 embryos and cultured in IL-3, IL-6 and stem cell factor conditioned media with 20% Fetal Calf Serum (FCS) for 3 days. Phoenix-E cells were transfected by calcium phosphate and the supernatant containing recombinant retrovirus used to transduce the stem cells. Transduced cells were sorted by flow cytometry based on GFP expression and injected into the tail vein of lethally irradiated B6-Ptprca (Ly5.1) mice to reconstitute their hematopoietic system. Following reconstitution, CD8+ T cells were isolated from the spleen using MACS negative selection and the GFP+ cells sorted by flow cytometry for use in experiments. In some instances, 40 µM of ATM was added to the cultures 20 hours following addition of the T cells to the DCs.
Live imaging
For live cell imaging of dividing T cell-DC conjugates, 4 × 105 DCs were seeded into a glass bottom 35mm culture dish (MatTek Corporation) and left to adhere overnight. DCs were then incubated with 1 µM SIINFEKL (1 hour, 37°C), washed, and overlaid with 8 ×104 naïve OT-1 T cells. The co-cultures were left for 40 hrs prior to imaging. DIC and GFP images were captured on a TCS SP5 confocal microscope (Leica) fitted with a temperature controlled chamber maintained at 37°C and 5% CO2 using a 40× air objective (NA 0.85). Images were captured using Leica LAS AF Lite software every 2 minutes. All image analysis and manipulation was done using Leica LAS AF Lite software or MetaMorph® Imaging Series 7 software (Universal Imaging Corporation).
Immunofluorescent image analysis
For immunofluorescent staining of DC-T cell conjugates, DCs were adhered overnight onto 8-well chamber slides (Nalgene Nunc, IL, USA) and incubated with 1 µM SIINFEKL for 1 hour at 37°C. Naïve T cells were overlayed for 40 hours and non adherent cells washed off. Cells were then fixed with 3.7% (w/v) paraformaldehyde in 100 mM Pipes, 5 mM MgSO4, 10 mM EGTA and 2 mM DTT (10 min, RT), then washed twice, permeabilized in 0.1% Triton X-100 in 50 mM Tris-HCl (pH 7.6) (5 min, RT). Cells were then labelled with primary antibodies followed by detection with Alexa Fluor-conjugated secondary antibodies (Molecular Probes) and mounted in Prolong antifade (Molecular Probes). For examples of control staining for immunofluorescence, see supplementary data (Fig. S2). The slides were examined at room temperature using a Fluoview FV1000 confocal microscope (Olympus) mounted with a 60x oil immersion objective (NA 1.42). 3D images of the cells were acquired with an optical distance of 0.5 µm between slices. Maximum intensity projections of Z sections spanning the entire cell were used for all analyses and in each representative image throughout the text. Dividing T cells selected for analysis had a single contact site with the DC (DC shown at bottom of all images), enabling designation of proximal (P, adjacent to the DC) versus distal regions (D, away from the DC). Mitotic cells were identified by the pattern of α-tubulin fluorescence, which was also used to draw regions to define the two poles of the mitotic spindle in early mitotic cells and the proximal or distal daughter cells using MetaMorph® software. The regions were then overlaid onto the protein image. In order to remove background fluorescence from the images, a top hat filter was applied using a circular structure element with a radius equal to the average radius of the two marked cells. MetaMorph® software was using to calculate the integrated fluorescence in the proximal (P) or distal (D) daughter, the ratio (P-D/P+D) was applied and the results plotted using GraphPad Prism version 5.01 for Windows (GraphPad® Software, San Diego California USA).
Statistics
P values for polarization of individual proteins were generated using a one sample t test comparing (P-D/P+D) values to zero. To compare polarization between pairs of conditions, a 2 –tailed t test was used. P values smaller than 0.05 were considered significant.
RESULTS
T cells divide while attached to an antigen presenting cell and show asymmetry of the polarity network
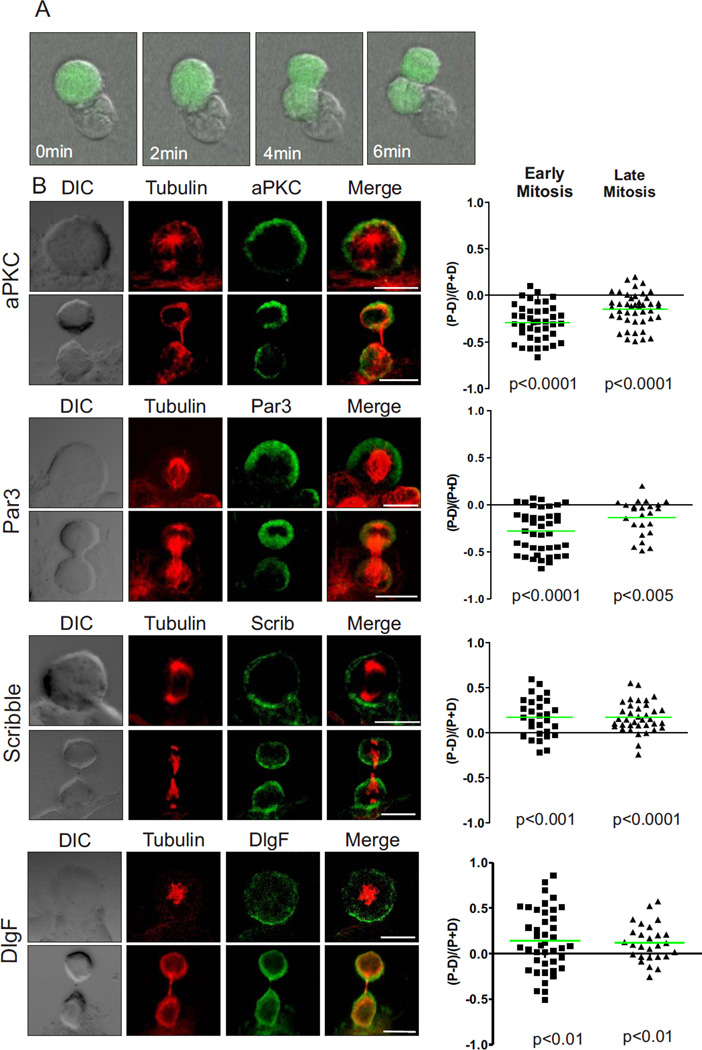
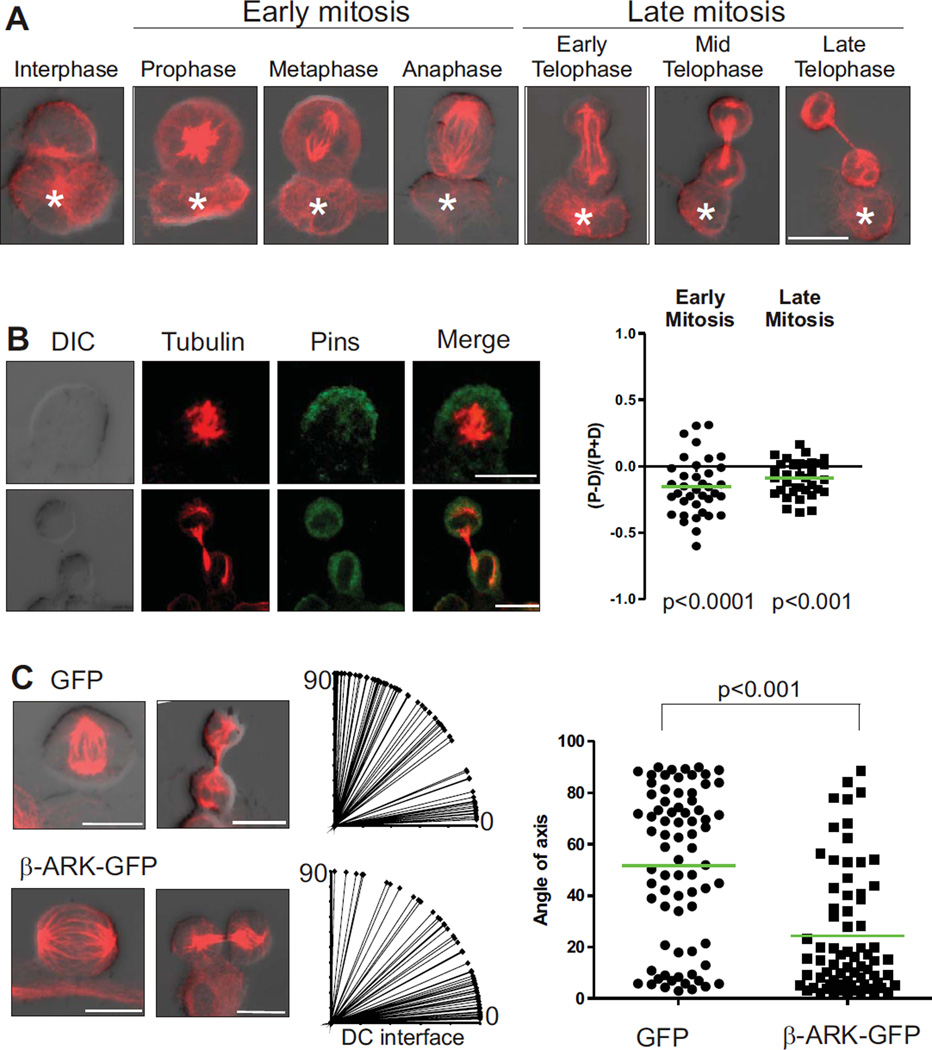
T cells maintain prolonged interactions with antigen presenting cells in vivo, and stable interactions are critical for T cell activation (9, 21). ACD following Listeria infection in vivo requires ICAM, suggesting that prolonged interactions between T cells and antigen presenting cells also orchestrate ACD (4), but the polarizing cue could not be identified in this experimental system. To determine whether antigen presenting cells can provide a cue for polarity during mitosis, we established an in vitro system to study long-term interactions between T cells and dendritic cells (DC). Naïve CD8+ T cells from OT-1 transgenic mice expressing a T cell receptor specific for an ovalbumin peptide were cultured with preadhered, peptide-pulsed DC. We seeded the cells at low densities to enable imaging of individual cells, and performed flow cytometric analysis of CFSE-labelled T cells to determine the time of first division under these conditions (40 hours, Fig. S3). Time-lapse microscopy of established T cell-DC conjugates over this time period showed that the nearly all the T cells remained attached to the DC during mitosis (172/182 cells from 5 experiments, for example see Fig. 1A). This data demonstrates that long-term interactions between T cells and DC in vitro can lead to division on the DC, suggesting that the DC can provide a cue for the establishment of the polarity required for a T cell to undergo ACD.
Figure 1.
Polarity proteins are asymmetric in mitotic T cells interacting with DC. (A) Naïve OT-1 CD8+ T cells expressing GFP were cultured with peptide-pulsed dendritic cells. The co-cultures were left for 40 hrs prior to imaging and then imaged by time-lapse microscopy every two minutes. Still images show an example of a T cell dividing while attached to a dendritic cell - time stamp shows time of progression through mitosis. Scale bar: 25 µm. (B) Naïve OT-1 CD8+ T cells dividing in response to antigen presentation by DC were fixed and stained to determine the ratio of proximal to distal polarization of aPKC, PAR-3, Scribble, and DlgF (3 experiments; 73, 61, 66 and 80 cells were analyzed respectively, representative images on the left). Positive and negative values indicate proximal and distal polarization respectively. Each point on the graph represents an individual cell and the green bar represents the mean. Scale bars: 10 µm.
Polarity in many cells is regulated by complexes of evolutionarily conserved polarity proteins known as the Scribble and Par complexes, which antagonize each other to define molecularly distinct regions of the cell (22). We, and others, have determined that both the Scribble complex (including Scribble, Lethal giant larvae (Lgl), and Discs large (Dlg)) and the Par complex (including Par3, Par6 and atypical Protein Kinase C (aPKC)) are expressed and polarized in T cells and are important in a number of T cell functions (19, 23–27). In support of a possible role in T cell ACD, we previously found that Scribble and aPKC were asymmetrically distributed in mitotic T cells responding to Listeria infection (4). To determine whether these, and other polarity proteins, were asymmetrically distributed in the dividing OT-1 T cells, and to definitively ascribe localization to proximal or distal cells relative to the DC, we fixed T cell-DC conjugates for immunofluorescent staining of α–tubulin and polarity proteins, and used confocal microscopy to capture fluorescent and differential interference contrast (DIC) images of dividing cells. For a description of the imaging protocol and quantification of fluorescence see supplementary data (Fig. S4). We used the DIC image and the pattern of tubulin staining to identify the phase of mitosis, to draw regions delineating the distal and proximal halves of the dividing T cells, and to quantitate fluorescence of the co-stained polarity proteins in each half of the dividing cells. The mitotic cells were classified into early (prophase, metaphase and anaphase) and late (early, mid, late telophase) mitotic phases to assess the polarization of each polarity protein over the course of division (Fig. 1B; images show representative early and late mitotic cells stained for the indicated proteins; scatter plots represent quantitation of dividing cells). Both aPKC and Par3 polarized significantly to the distal side of the cell in early mitotic T cells and maintained this asymmetry during late mitosis. The localization of Dlg showed greater spread between proximal and distal cells compared to Scribble, possibly due to the Dlg family (DlgF) antibody detecting all four Dlg isoforms (Dlg1–4) in T cells (19), which might localize differently. However, Scribble and DlgF were both significantly polarized to the proximal cell in early and late mitosis. These data show that T cells can divide asymmetrically while in contact with an antigen presenting cell.
ACD segregates Numb to the distal daughter, and affects effector and memory fate
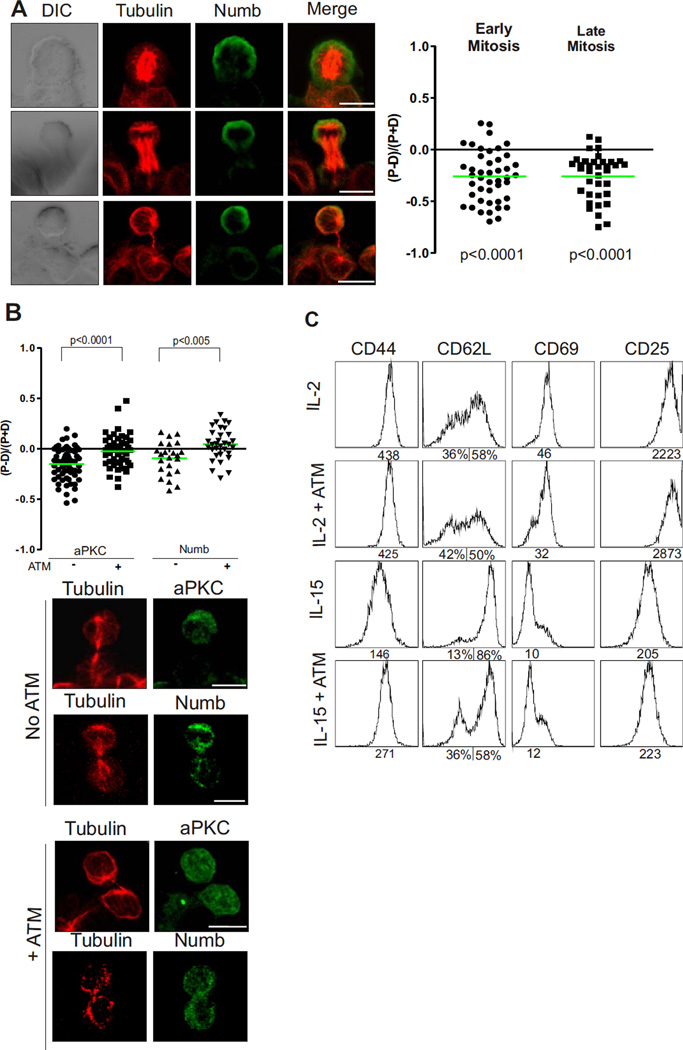
We next assessed whether ACD of OT-1 T cells correlated with any apparent differences in the daughters that could lead to distinct fates, such as differently sized daughter cells, or differences in distribution of cell fate determinants. We first measured the area of the proximal and distal daughters of 191 cells captured in late telophase and found the sizes to be very similar (Fig. S5, 13907 vs 13514 +/− 440 arbitrary units) indicating that ACD of T cells does not establish gross differences in size that might cause differences in cell fate. Unequal inheritance of Numb and Prospero can dictate cell fate in Drosophila sensory organ precursors and neuroblasts (28), and Numb is asymmetrically distributed in mitotic CD8+ T cells following Listeria infection (4). Staining for Prox-1 (the mammalian homolog of Prospero) (29) showed it was not polarized in dividing T cells (Fig. S6) whereas Numb was polarized to the distal pole of mitotic cells, and this asymmetry was maintained through to cytokinesis (Fig. 2A). These data demonstrate the differential segregation of the cell fate determinant, Numb, into the distal daughter cell of a dividing T cell attached to a DC.
Figure 2.
The polarity network orchestrates asymmetric distribution of the cell fate determinant, Numb. (A) The ratio of proximal to distal polarization was assessed as in Fig 2 for Numb in different phases of mitosis (3 experiments; 80 cells). Representative images on left, scale bars: 10 µm. (B) The ratio of proximal to distal polarization was assessed as in Fig 2 for aPKC (61 & 50 cells) and Numb (57 & 66 cells) with or without treatment with ATM (2 experiments). Representative images below, scale bars: 10 µm, NS = not significant. (C) Expression of the surface markers CD44, CD62L, CD69, CD25 on OT-1 CD8+ T cells cultured under effector (IL-2) and memory (IL-15) conditions with or without treatment with ATM. The geometric mean of the individual peaks, or % cells gated, is shown under the plots. Data is representative of 3 experiments.
The polarization of Numb with Par3 to the same daughter cell is similar to the co-localization of Numb with Par3 in migrating epithelial cells (30), but differs from the opposition of the Par3 complex and Numb in dividing neuroblasts (31). However, in both cases, the localization of Numb is dependent upon the Par3 complex, and specifically on phosphorylation by aPKC (32). To test whether Numb might be similarly regulated by aPKC in asymmetrically dividing T cells, we treated the T cell-DC conjugates with aurothiomalate (ATM). ATM (“Gold”) has been used to treat rheumatoid arthritis for decades, but its mechanism of action has been unclear (33, 34). However, ATM was recently shown to inhibit the interaction between aPKC and Par6, and is currently under investigation as a treatment for cancer (35). Treatment of the cells with ATM caused only negligible delay in T cell proliferation in response to antigen presentation (Fig. S7), but led to a significant reduction in the asymmetric polarization of aPKC to the distal pole of dividing T cells (Fig. 2B), indicating that ATM disrupted polarity during ACD. Compatible with a possible role for aPKC in regulating polarity of Numb during ACD, treatment with ATM also significantly reduced segregation of Numb to the distal cell (Fig 2B). Thus, although treatment with ATM enabled protracted interactions between T cells and DC and activation of the T cells, the asymmetric distribution of aPKC and Numb to the distal daughter was abrogated.
To assess whether the disruption of polarity by ATM treatment might correlate with altered T cell fate, we cultured OT-1 CD8+ T cells under conditions that induce effector or memory differentiation, using IL-2 and IL-15 as previously described (20). Memory T cells are characterised by their long-term homeostatic turnover, multipotency and rapid recall following secondary infection. However, they can also be more generally identified by the expression of specific surface markers (2). Following treatment with IL-2 and IL-15, naïve CD8+ T cells (CD62Lhi, CD44med, CD69−, CD25−, Fig. S8) developed into subsets characteristic of effector (CD62Llo, CD44hi, CD69med, CD25hi) and memory cells (CD62Lhi, CD44med, CD69lo, CD25med) respectively (Fig. 2C). Treatment of the T cell-DC conjugates with ATM 20 hours prior to first cell division had no effect on differentiation into effector T cells, as assessed by each of the four markers (Fig. 2C, compare first and second row). In contrast, following treatment with 40 µM ATM, the cells cultured in conditions designed to induce memory differentiation showed a shift towards a more effector-like phenotype with up-regulation of CD44 and down-regulation of CD62L (Fig. 2C, compare third and fourth row). No differences were observed for CD69 and CD25 expression between untreated and treated T cell-DC conjugates. These data suggest that ACD, regulated by the polarity network, might impact upon T cell fate decisions.
The polarity cue for ACD requires contact with the DC, but not sustained polarization of classic immunological synapse markers during mitosis
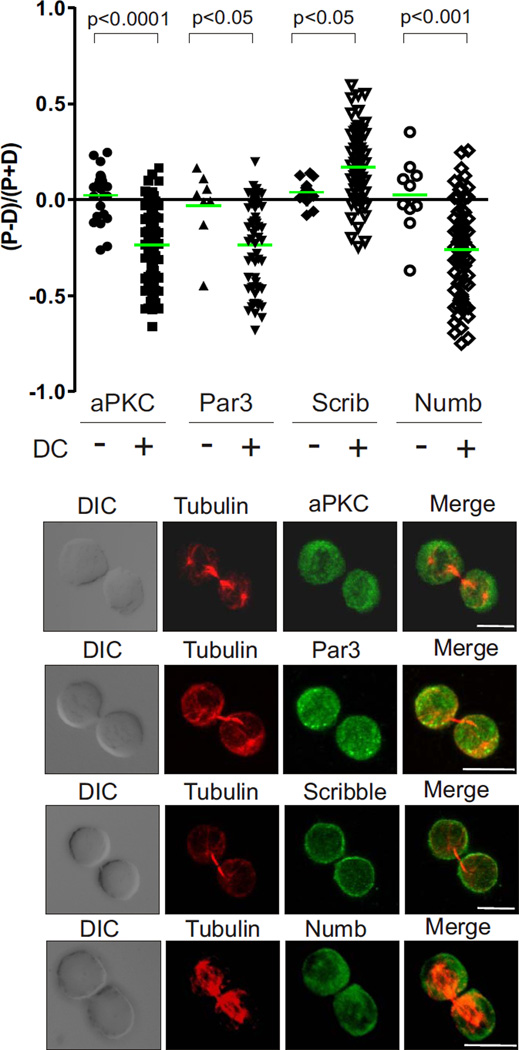
We next investigated how the polarity cue provided by the DC is transmitted to the dividing T cell. Cells such as the fertilized C. elegans zygote retain memory of a previous polarity cue, and in these cells polarity is maintained by proteins such as Par3 (14, 36). The asymmetry previously observed in mitotic cells separated from contact with antigen presenting cells suggests a similar possibility for T cells (4). Indeed, the recent identification of a molecule, CRTAM, that can interact with Scribble to sustain CD3/CD28-antibody mediated polarity after the cells have disengaged supports this notion (37). We therefore investigated the dependence of the asymmetric localization of aPKC, Par3, Scribble and Numb on the interaction with the antigen-presenting cell at the time of mitosis. The distribution of fluorescence in dividing T cells attached to a DC was compared to the distribution of fluorescence in the rare cells captured dividing while unattached to a DC. In the absence of DC, aPKC, Par3, Scribble and Numb were not polarized (Fig 3). This suggests that, where ACD is controlled by antigen presentation, memory of the contact is not sufficient for polarity at the time of division, and that contact with the DC is necessary not only to establish polarisation at the initiation of antigen presentation, but also to maintain this asymmetry through to the onset of mitosis.
Figure 3.
ACD of T cells requires contact with the antigen presenting cell. The ratio of proximal to distal polarization was assessed as in Fig 2 for aPKC (24 cells), Scribble (14 cells), PAR-3 (8 cells) and Numb (10 cells) in mitotic cells unattached to a DC (representative images below) compared to mitotic cells attached to a DC. Scale bars; 10 µm.
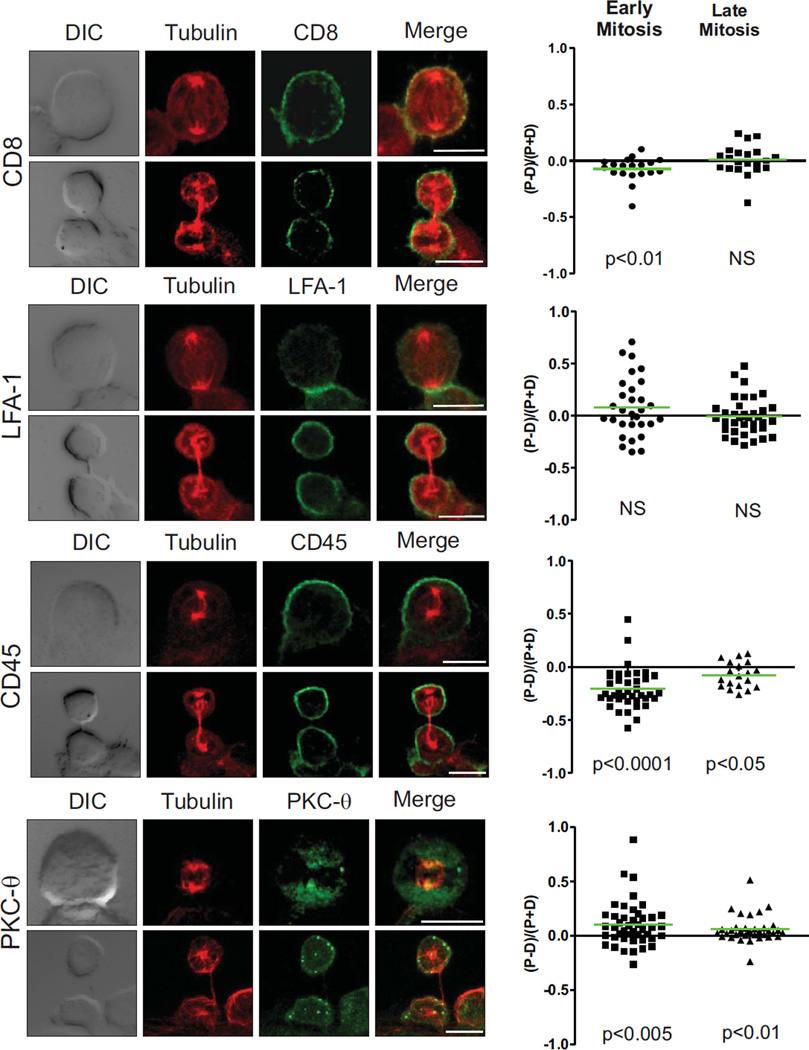
Antigen presentation initially involves the formation of an immunological synapse, with the recruitment of T Cell Receptor-associated signaling molecules and the microtubule organizing centre (MTOC) to the interface with the DC (38). We therefore determined whether proteins that are normally associated with the immunological synapse might transmit the polarity cue from the DC, by assessing whether they are also polarized to the interface in the dividing T cells. CD8 was not polarized to the proximal cell at either early or late mitosis, but showed localization to some distal cells in early mitosis. LFA-1 was enriched at the contact site in early mitosis but this did not result in significant polarization to the proximal cell. However, the synapse marker, PKCθ and distal pole marker, CD45, were significantly polarized in early mitosis (Fig. 4). The relatively even distribution of all these proteins at late mitosis suggests that, although the immunological synapse might play an important role in dictating the axis of polarity (perhaps related to the recruitment of the MTOC to the interface), differential inheritance of T Cell Receptor-associated signaling molecules is unlikely to be important for fate determination in this system.
Figure 4.
ACD of T cells requires contact with the DC, but not a sustained immunological synapse. The ratio of proximal to distal polarization was assessed as in Fig 2 for synapse and distal pole proteins CD8 (40 cells), LFA-1 (66 cells), PKCθ (79 cells) and CD45 (58 cells) (3 experiments). Representative images on left. Scale bars; 10 µm.
ACD of T cells utilizes conserved mechanisms to coordinate polarity with the orientation of the spindle
ACD requires not only polarization of proteins, but also alignment of the mitotic spindle with the axis of polarity (14, 15). In some instances, such as division of Drosophila male germ cells, the orientation of the mitotic spindle is defined by the polarization of the MTOC at interphase (39). After duplication, one centrosome remains anchored in this position by microtubules, and the other relocates to the opposite side of the nucleus (39, 40). The stable recruitment of the MTOC to the interface with the DC raised the possibility that it might also orientate the mitotic spindle during mitosis. However, staining of fixed, dividing T cell/DC conjugates for α-tubulin was not compatible with this, as the tubulin condensed in the centre of the cells before the centrosomes separated to opposite poles of the cell (Fig. 5A, 55 out of 60 cells at prophase were in the central third of the cell, with 5 slightly distal).
Figure 5.
ACD of T cells utilizes the Pins/G protein module to orient the spindle. OT-1 CD8+ T cells dividing whilst attached to a DC were (A) stained for tubulin and scored for orientation of the MTOC or (B) stained for tubulin and Pins to assess the ratio of proximal or distal polarization (3 experiments; 73 cells) or (C) Naive OT-T cells expressing GFP (n=75) or β-ARK-GFP (n=70) were incubated with DC and stained for tubulin to mark T cells in division. Image J software was used to draw a line through the axis of division based on the position of the centrosomes, using the DC interface as the horizontal axis and used to calculate the angle of spindle axis relative to the DC interface and plotted on the right (2 experiments, representative images on left). Scale bars; 10 µm.
An alternative means of dictating spindle orientation links Dlg to trimeric G protein signaling to coordinate the orientation of the spindle body with the axis of polarity (41). In Drosophila neuroblasts, Dlg can recruit Pins (Partner of Inscuteable, also known as LGN in mammals) (31, 42) which in turn reinforces polarity and orients the spindle of neuroblasts and mammalian neuronal precursors by binding to GαI (41, 43, 44). We found that Pins (45) was expressed in T cells (Fig S9), and polarized to the distal side of the asymmetrically dividing T cell (Fig. 5B). To assess whether the Pins/G protein pathway regulated spindle orientation in T cells, we attempted to disrupt G protein signaling by sequestering Gβγ proteins with overexpression of the β-adrenergic receptor kinase C-terminal domain (β-ARK) (16). β-ARK expression in asymmetrically dividing neural progenitors in the developing mouse neocortex disrupted the orientation of the mitotic spindle (46), and we assessed the effect on spindle orientation in T cells. We reconstituted mice with OT-1 hematopoietic stem cells transduced with a control GFP construct and the β-ARK-GFP construct. Analysis of the peripheral blood of reconstituted mice demonstrated that GFP+ T cells developed in these animals (Fig. S10). We then assessed spindle orientation, based on tubulin staining, of both GFP and β-ARKGFP transduced T cells dividing in contact with peptide-pulsed DCs (Fig. 5C). Of the control cells transfected with GFP, 75% showed an angle greater than 30 degrees from the DC interface, compatible with ACD. However, inhibition of G protein signaling significantly reduced the number of cells with an axis compatible with ACD (75% vs 30%), and the majority of the cells had a spindle almost parallel to the interface with the DC. These data combined indicate that CD8+ T cells not only utilize the evolutionarily conserved polarity network to polarize cell fate determinants, but also utilize the Pins/G protein module to align the mitotic spindle with the axis of polarity.
DISCUSSION
The question of whether memory cells arise in a linear developmental progression from effector cells, or whether certain progeny of an activated T cell have a predetermined propensity for differentiation into memory cells has generated much discussion (5, 47, 48). The latter model is supported by the discovery that the first daughters arising from activation of a naïve T cell could be segregated on the basis of cell surface markers to discriminate cells that have potential for memory differentiation (4). The observation of asymmetry during the first division also provided compelling evidence that ACD plays a role in this predetermination (4). Conversely, a recent study concluded that memory cells can arise from effector cells (identified by Granzyme B expression), providing support for the linear progression model (49). However, the system used did not exclude the possibility that transcription of Granzyme B mRNA might occur before the first division, followed by asymmetric polarization of cell fate determinants into the daughter cells. Resolution of this issue will depend upon continuing development of more sophisticated tools with which to dissect when, where and how the master regulators of cell fate are switched on. To test whether ACD can contribute to T cell fate decisions, we describe here a tractable system with which the early events in activation of naïve T cells can be monitored. We demonstrate that T cells undergo ACD, show evidence that ACD can dictate cell fate and identify conserved mechanisms by which ACD is controlled. Our study defines the key elements required for ACD: (1) the cue to dictate the axis of polarity; (2) asymmetry of proteins along this axis and (3) alignment of the mitotic spindle with the axis of polarity.
1. The polarity cue
Like Drosophila male germ cells and larval neuroblasts, T cells can utilize direct contact with an adjacent cell to orchestrate polarity throughout cell division (15). In the model we have studied, the cue comes from contact with the dendritic cell, but the immunological synapse does not seem to be involved per se by the time of division (as indicated by even distribution of TCR signaling components). It is possible that other cues, such as Notch ligands, Wnt signaling components or integrins might play a role in maintaining the axis of polarity until the point of division. Our data show that peptide-pulsed DC are capable of providing the cue, but the plethora of different cues that dictate polarity in T cells (50) suggests that other forms of antigen presentation, or other polarity cues such as chemokines, might dictate different forms of ACD in T cells, resulting in differing effects on cell fate determination. This concept is supported by observations that context can alter both the molecular distributions during ACD and the fate decisions of Drosophila neuroblasts and sensory organ precursors (51, 52). Further support comes from the differences in polarity observed between this system and the ex vivo system studied by Chang et al (4), which suggests that the ACD of Chang et al. is orchestrated by a cue other than the peptide-pulsed dendritic cells used in this study. The co-polarization of Numb and CD8 in mitotic cells activated by Listeria (compatible with Numb recruitment to the putative proximal cell) (4) suggests that Numb can orientate differently under different stimuli (eg. peptide dose versus in vivo infection) and that different polarity cues, by altering ACD, might regulate different fate outcomes
2. Maintaining polarity through division
The stable recruitment of the MTOC to the interface with the DC suggests that the polarity cue might be translated to the polarity network via microtubules (53). In Drosophila larval neuroblasts at metaphase, Dlg is transiently polarized by astral microtubules via the kinesin heavy chain, Khc-73 (42). In support of a similar mechanism in T cells, the mammalian homologue of Khc-73, GAKIN, is also localized by interactions with microtubules, and can interact with Dlg in T cells (54). These data, and analogies with other cell systems, indicate that the initial polarity triggered by TCR signaling is translated into stable polarity by antagonistic interactions between the Scribble and Par3 polarity complexes, that are maintained over the tens of hours until cell division. An involvement of the Par3 complex in this process is supported by the disruption in aPKC and Numb polarization upon treatment with ATM, which can functionally inhibit the Par3 complex by preventing interactions between Par6 and aPKC (35).
3. Alignment of the mitotic spindle
T cells differ from Drosophila male germ cells (53), in that the spindle orientation of T cells is not dictated by retention of the MTOC and centriole to the cell–cell interface, but is oriented de novo. This organisation is more similar to the first division of embryonic Drosophila neuroblasts (53), where it has been proposed that de novo establishment of the spindle might allow for flexibility in determining the proportion of cells undergoing ACD (14). By analogy, it is possible that differences in antigen presentation might allow finetuning of the immune response by regulating orientation of the spindle to dictate the proportion of cells undergoing ACD.
Our data also show that Pins localizes to the same daughter cell as the Par3 complex in the dividing T cells, suggesting that the interaction between the polarity proteins and spindle organisation in T cells is similar to ACD of Drosophila sensory organ precursors (55). Interestingly, these modules can cooperate or antagonize in different contexts (22), and it has been proposed that the proximity of Pins and Par3 dictates the different size of sensory organ precursor daughters (55). However, this does not seem to be the case in T cells, as the proximal and distal daughters were identical in size.
It is clear that different cell types, such as Drosophila neuronal and sensory organ precursors, utilize similar conserved polarity molecules, organized into different functional modules, to meet cell-specific requirements for ACD (15). The unique molecular processes regulating ACD of T cells described here suggests that T cells have adopted a number of these evolutionarily conserved mechanisms. It is likely that, as in other cell systems, the modules coordinating ACD will be arranged differently according to the context in which T cells divide, facilitating the highly regulated diversity that characterizes the immune system. The observations presented here suggest new approaches and tools that will enable the elucidation of the role of ACD in the generation of memory and effector precursor cells, and perhaps in other immune cell fate decisions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Cameron Nowell, Sarah Ellis, Anne Sossinka, Robert Lefkowitz, Fengwei Yu, Bill Chia, Patrick Metz, Vikrim Palanivel, Helena Richardson, Natasha Harvey, Alan Fields, Hideo Yagita and Sally Dunwoodie for technical assistance, reagents and helpful comments.
This work was supported by grants from the National Health and Medical Research Council, Human Frontier Science Program and the Australian Research Council.
REFERENCES
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell SM. Determination of T-cell fate by dendritic cells: a new role for asymmetric cell division? Immunol Cell Biol. 2008;86:423–427. doi: 10.1038/icb.2008.24. [DOI] [PubMed] [Google Scholar]
- 4.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 5.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 6.Davis DM. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9:543–555. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- 7.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 8.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T celldendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 9.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 11.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Spierings DC, Lemmens EE, Grewal K, Schoenberger SP, Green DR. Duration of CTL activation regulates IL-2 production required for autonomous clonal expansion. Eur J Immunol. 2006;36:1707–1717. doi: 10.1002/eji.200635929. [DOI] [PubMed] [Google Scholar]
- 13.Congdon KL, Reya T. Divide and conquer: how asymmetric division shapes cell fate in the hematopoietic system. Curr Opin Immunol. 2008;20:302–307. doi: 10.1016/j.coi.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- 15.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 17.Moriyama Y, Sekine C, Koyanagi A, Koyama N, Ogata H, Chiba S, Hirose S, Okumura K, Yagita H. Delta-like 1 is essential for the maintenance of marginal zone B cells in normal mice but not in autoimmune mice. Int Immunol. 2008;20:763–773. doi: 10.1093/intimm/dxn034. [DOI] [PubMed] [Google Scholar]
- 18.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 19.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, Murphy A, Ellis S, Smyth MJ, Kershaw MH, Darcy PK, Humbert PO, Russell SM. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henrickson SE, von Andrian UH. Single-cell dynamics of T-cell priming. Curr Opin Immunol. 2007;19:249–258. doi: 10.1016/j.coi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Round JL, Humphries LA, Tomassian T, Mittelstadt P, Zhang M, Miceli MC. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat Immunol. 2007;8:154–161. doi: 10.1038/ni1422. [DOI] [PubMed] [Google Scholar]
- 24.Xavier R, Rabizadeh S, Ishiguro K, Andre N, Ortiz JB, Wachtel H, Morris DG, Lopez-Ilasaca M, Shaw AC, Swat W, Seed B. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol. 2004;166:173–178. doi: 10.1083/jcb.200309044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soloff RS, Katayama C, Lin MY, Feramisco JR, Hedrick SM. Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol. 2004;173:3250–3260. doi: 10.4049/jimmunol.173.5.3250. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Hou KK, Piwnica-Worms H, Shaw AS. The polarity protein Par1b/EMK/MARK2 regulates T cell receptor-induced microtubule-organizing center polarization. J Immunol. 2009;183:1215–1221. doi: 10.4049/jimmunol.0803887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 28.Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161–173. doi: 10.1016/j.cell.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–170. doi: 10.1038/ncb922. [DOI] [PubMed] [Google Scholar]
- 32.Smith CA, Lau KM, Rahmani Z, Dho SE, Brothers G, She YM, Berry DM, Bonneil E, Thibault P, Schweisguth F, Le Borgne R, McGlade CJ. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. Embo J. 2007;26:468–480. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forestier J. Rheumatoid arthritis and its treatment by gold salts. Lancet. 1934;2:646–648. [Google Scholar]
- 34.Rau R. Have traditional DMARDs had their day? Effectiveness of parenteral gold compared to biologic agents. Clin Rheumatol. 2005;24:189–202. doi: 10.1007/s10067-004-0869-8. [DOI] [PubMed] [Google Scholar]
- 35.Stallings-Mann M, Jamieson L, Regala RP, Weems C, Murray NR, Fields AP. A novel small-molecule inhibitor of protein kinase Ciota blocks transformed growth of non-small-cell lung cancer cells. Cancer Res. 2006;66:1767–1774. doi: 10.1158/0008-5472.CAN-05-3405. [DOI] [PubMed] [Google Scholar]
- 36.Macara IG, Mili S. Polarity and differential inheritance--universal attributes of life? Cell. 2008;135:801–812. doi: 10.1016/j.cell.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh JH, Sidhu SS, Chan AC. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–859. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchman JJ, Tsai LH. Spindle regulation in neural precursors of flies and mammals. Nat Rev Neurosci. 2007;8:89–100. doi: 10.1038/nrn2058. [DOI] [PubMed] [Google Scholar]
- 42.Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Sans N, Wang PY, Du Q, Petralia RS, Wang YX, Nakka S, Blumer JB, Macara IG, Wenthold RJ. mPins modulates PSD-95 and SAP102 trafficking and influences NMDA receptor surface expression. Nat Cell Biol. 2005;7:1179–1190. doi: 10.1038/ncb1325. [DOI] [PubMed] [Google Scholar]
- 44.Bellaiche Y, Radovic A, Woods DF, Hough CD, Parmentier ML, O'Kane CJ, Bryant PJ, Schweisguth F. The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 2001;106:355–366. doi: 10.1016/s0092-8674(01)00444-5. [DOI] [PubMed] [Google Scholar]
- 45.Yu F, Morin X, Kaushik R, Bahri S, Yang X, Chia W. A mouse homologue of Drosophila pins can asymmetrically localize and substitute for pins function in Drosophila neuroblasts. J Cell Sci. 2003;116:887–896. doi: 10.1242/jcs.00297. [DOI] [PubMed] [Google Scholar]
- 46.Sanada K, Tsai LH. G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Feau S, Schoenberger SP. Immunology. Ex uno plura. Science. 2009;323:466–467. doi: 10.1126/science.1169409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dustin ML. Hunter to Gatherer and Back: Immunological Synapses and Kinapses as Variations on the Theme of Amoeboid Locomotion. Annu Rev Cell Dev Biol. 2008 doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 49.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell S. How polarity shapes the destiny of T cells. J Cell Sci. 2008;121:131–136. doi: 10.1242/jcs.021253. [DOI] [PubMed] [Google Scholar]
- 51.Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol. 2004;269:1–17. doi: 10.1016/j.ydbio.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 52.Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita YM, Fuller MT. Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol. 2008;180:261–266. doi: 10.1083/jcb.200707083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanada T, Lin L, Tibaldi EV, Reinherz EL, Chishti AH. GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J Biol Chem. 2000;275:28774–28784. doi: 10.1074/jbc.M000715200. [DOI] [PubMed] [Google Scholar]
- 55.Neumuller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–2699. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.