Abstract
Objective
Infiltration of polymorphonuclear neutrophils into the lung is an important feature of ventilator-induced lung injury associated with pneumonia, but the mechanisms involved in neutrophil recruitment are poorly understood. Using Toll-like receptor 4 knockout (tl4r−/−) mice, we addressed the role of Toll-like receptor 4 signaling in mediating neutrophil recruitment and lung injury induced by lipopolysaccharide challenge coupled to lung hyperinflation.
Design
Experimental animal model.
Setting
University laboratory.
Subjects
tlr4−/− and wild-type C57BL/6 mice.
Interventions
Wild type or tlr4−/− mice were challenged by intratracheal instillation of lipopolysaccharide (0.3 mg/kg) for 2 h and then subjected to normal (7 ml/kg) or high (28 ml/kg) tidal volume ventilation for another 2 h. In other studies, neutrophils from wild type or tlr4−/− mice were pretreated with lipopolysaccharide for 30 min and then infused into the isolated lung preparation for 30 min while the lungs were ventilated with 25 cmH2O peak inspiratory pressure.
Measurements and Main Results
Lipopolysaccharide-challenged wild type mice ventilated with a 28 ml/kg tidal volume exhibited 12-fold increase in neutrophil sequestration, 18-fold increase in bronchoalveolar lavage neutrophil count, 6-fold increase in bronchoalveolar lavage albumin concentration, and 1.6-fold increase in lung water content compared to unchallenged mice exposed to normal tidal volume ventilation. However, tlr4−/− mice showed negligible neutrophil sequestration, microvascular barrier breakdown, or edema formation. Mechanical ventilation alone or combined with lipopolysaccharide caused activation of circulating neutrophils and pulmonary endothelium in wild type mice whereas this was prevented in tlr4−/− mice.
Conclusions
High tidal volume ventilation during pneumonia/sepsis induces lung neutrophil sequestration and injury via the Toll-like receptor 4-dependent signaling pathway. The results suggest an important role of Toll-like receptor 4 in the mechanism of lung neutrophil sequestration and acute lung injury when pneumonia/sepsis is coupled to lung hyperinflation.
Keywords: inflammation, Toll-like receptor 4, lipopolysaccharide, ventilator, neutrophils, lung injury
INTRODUCTION
Lung hyperinflation is an important contributing factor mediating ventilator-induced lung injury (VILI) (1) which is thought to account for as many as one-third of deaths attributed to acute lung injury (2). Several studies have convincingly shown that polymorphonuclear neutrophil (PMN) accumulation and activation of PMNs in lungs increase microvascular permeability associated with mechanical stretch in models of VILI (3–5). For example, hyaline membrane formation and elevated alveolar-capillary permeability induced by mechanical stretch were reduced in animals depleted of circulating PMNs (3,5). Although some studies showed that VILI can occur by a PMN-independent mechanism, even in these studies there was marked recruitment of PMNs at sites of injury, suggesting that the secondary phase of injury was mediated by PMNs (6). Thus, PMNs appear to play a central causative role in the development of VILI.
A recent study demonstrated that PMN sequestration in lungs secondary to stretch-induced inflammatory events is the result of circulating leukocyte stiffening mediated by an L-selectin-dependent mechanism (7). PMN recruitment in VILI may also be mediated by release of chemoattractants and expression of adhesion molecules in epithelial cells, endothelial cells, and alveolar macrophages (8). In addition, elevated alveolar pressure as a consequence of lung hyperinflation and resultant compression of alveolar capillaries may contribute to PMN sequestration (9,10). However, the molecular signaling mechanisms that recruit PMN during VILI have not yet been fully elucidated.
Toll-like receptor (TLR) 4 is a transmembrane protein expressed in many lung cell types, including endothelial and epithelial cells, PMNs, monocytes, macrophages, and dendritic cells (11,12). Although TLR4 is involved in the host-defense response, its activation can also contribute to inflammation by inducing the production of inflammatory cytokines and chemokines (11, 13–17). Recently, endothelial cell TLR4 was shown to be a key determinant of lipopolysaccharide (LPS)-induced PMN sequestration in lungs (18–21). Stimulation of TLR4 in endothelial cells induced rapid expression of cell surface adhesion proteins resulting in increased leukocyte rolling and firm adhesion (18). In response to LPS activation of TLR4, massive numbers of PMNs accumulated in lungs (14, 18, 22, 23). TLR4 null (tlr4−/−) mice, however, showed impaired PMN recruitment into the alveolar space (24). The present study was conducted to test the hypothesis that TLR4 mediates PMN recruitment and lung injury in response to LPS challenge coupled to lung hyperinflation. Using tlr4−/− mice, we demonstrate that TLR4 signaling activated by mechanical stretch and LPS is a critical determinant of PMN sequestration in lungs as a result of hyperinflation associated with pneumonia, and thus contributes to the mechanism of lung vascular injury and pulmonary edema formation.
MATERIALS AND METHODS
Animals
tlr4−/− mice and wild-type (tlr4+/+) C57BL/6 mice were used in all experiments. All animal studies were approved by the University of Illinois Institutional Animal Care and Use Committee.
Intact Mouse Protocols
We used an established mouse model of VILI as previously described (25, 26). Mice were anesthetized by intraperitoneal injection of 3 mg/kg xylazine and 75 mg/kg ketamine and suspended a 45° angle by the incisors from a rubber band attached to a Plexiglas support. A light source with two flexible fiber-optic arms allowed transillumination of the trachea just below the vocal cords. A metal “laryngoscope” was used to lift the lower jaw of the mouse and keep the mouth open and the tongue displaced to provide a clear view of the tracheal opening. A 2-cm-long PE-60 catheter attached to the hub of a needle was inserted ~3 mm into the trachea. LPS (0.3 mg/kg in 50 µl sterile 0.9% NaCl) or an equivalent volume of vehicle was instilled intratracheally via a cannula. A control experiment in which Evans blue-albumin solution dissolved in 50 μl 0.9% NaCl was instilled in mice was performed to confirm the uniform distribution of the instillation procedure. The mouse was then removed from the Plexiglas support and ventilated for 5 min at 120 breaths/min with a tidal volume of 200 μl and finally returned to its cage.
After 2 h of LPS or saline instillation, mice were re-anesthetized with ketamine (75 mg/kg) and ventilated for an additional 2 h with 1% isoflurane in room air to maintain anesthesia using either normal or high tidal volume. For normal volume ventilation, mice received 7 ml/kg tidal volume, a respiratory rate of 120 breaths/min, 0 cmH2O end-expiratory pressure, and FiO2 of 0.21 using a rodent ventilator (MiniVent, Harvard Biosciences; Holliston, MA). For high (28 ml/kg) tidal volume ventilation, the respiratory rate was reduced to 60 breaths/min and ~5 ml of dead space was added to the ventilator circuit to maintain arterial PCO2 between 35–45 mmHg. Body temperature was maintained between 37 and 38°C using a heating lamp. After 2 h of mechanical ventilation, various measurements were obtained. Age-matched tlr4+/+ and tlr4−/− mice were placed into the following four treatment groups: 1) saline instillation and low tidal volume; 2) saline instillation and high tidal volume; 3) LPS instillation and low tidal volume; and 4) LPS instillation and high tidal volume.
Lung Tissue Myeloperoxidase (MPO) Activity
Lung PMN sequestration was determined by measuring MPO activity of lung homogenates as described previously (27).
Quantification of PMN Activation
The degree of PMN activation in the circulation was quantified by assessing L-selectin and CD11b expression by flow cytometry (18). Briefly, an aliquote of blood (100 μl) was incubated with 1 μg of L-selectin and CD11b antibodies or nonspecific isotype-matched control antibody. Red blood cells were lysed, leukocytes were incubated with FITC-conjugated secondary antibody, and results expressed as arbitrary units of mean channel fluorescence (MCF).
Quantification of Endothelial Cell Activation
Expression of P-selectin was determined as a measure of endothelial activation using the dual-radiolabeled antibody technique as described (18,28). P-selectin rat anti-mouse mAb RB40.34 mAb and rat IgG1λ isotype-matched control mAb A110-1 were labeled with 125I (binding mAb) and 131I (non-binding mAb), respectively, using Iodogen beads (Pierce Chemical Co., Rockford, IL). Briefly, mice were intravenously injected with a mixture of 10 μg 125I-RB40.34 and variable amounts of 131I-A110-1 calculated to achieve a total injected 131I activity of 4~6×105 cpm in a volume of 200 μl. The antibodies were allowed to circulate for 5 min, and then a heparinized blood sample was obtained from a carotid artery catheter after which the mice were euthanatized by cervical dislocation and exsanguination. The lungs as well as heart, liver, kidney, stomach, mesentery, small intestine, large intestine, muscle, and skin were harvested, weighed, and counted to dertermine 131I and 125I activity in plasma and tissue samples. P-selectin expression was calculated by subtraction of the accumulated activity of nonspecific antibody (131I-A110-1) from accumulated activity of P-selectin antibody (125I-RB40.34) per gram wet weight of tissue.
Lung Histopathology
Lungs was fixed with 10% formalin via intratracheal injection for 1 h, and then embedded in paraffin. Four-micrometer-thick sections were stained with H&E and analyzed by light microscopy in a blinded fashion (27).
Isolation of Mouse PMNs
Peripheral blood PMNs were isolated as previously described (27,29). The exchange was performed by alternately withdrawing blood and infusing hetastarch solution in 0.2 ml aliquots via jugular vein catheter in ketamine/xylazine anesthetized mice. The leukocyte-rich suspension was layered on top of Ficoll-Pacque, and centrifuged for 20 min. The yield of PMNs obtained by our procedure is ~2 ×106 cells per mouse. The purity of isolated PMNs is >98% and viability is greater than 95% as determined by trypan blue exclusion.
Western Blot Analysis
Expression of TLR4 in lungs was determined by Western blot analysis as described (27, 29).
Isolated-perfused Lung Preparation
Mouse lungs were isolated and perfused as described (27, 29). Briefly, after the animals were anesthetized and ventilated, a thoracotomy was performed and pulmonary artery was cannulated in situ for perfusion of lungs with a Krebs-Henseleit/albumin solution and mounted on a perfusion apparatus. The composition of the solution was as follows: 118 mM NaCl, 4.7 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, 5.0 mM HEPES, 11 mM glucose, and 0.025 mM EDTA (pH 7.35–7.45), and BSA (5 g/100 ml). The lung preparation was perfused via the pulmonary artery at a constant flow (2 ml/min) and venous pressure (3 cmH2O), and pulmonary artery pressures of 8 ± 2 cm H2O. Pulmonary arterial pressure and lung weight were continuously monitored during experiments. Labtech software (Andover, MA) was used to control data acquisition and storage. At the end of experiments, lungs were weighed, dried, and reweighed. Wet/dry lung weight ratio was used as an index of lung water content.
Isolated-perfused Lung Protocols
The lungs were first perfused and ventilated for 15 min using normal (9 cmH2O) peak inspiratory pressure at respiratory rate of 40 breaths/min. PMNs were pretreated with LPS (0.5 μg/ml) for 30 min and then washed three times by suspension in fresh Hank’s balanced salt solution and centrifugation (total elapsed time, 30 min). Neutrophil viability was confirmed using trypan blue technique. PMN suspension (4×106 cells) was infused into the lungs at a rate of 2% perfusate flow for another 30 min via a side arm using a syringe pump while lungs were ventilated with 25 cmH2O peak inspiratory pressure. The following groups were studied using lungs and PMNs from tlr4+/+ and tlr4−/− mice: 1) Krebs solution in tlr4+/+ lungs (control); 2) tlr4+/+ PMNs in tlr4+/+ lungs; 3) tlr4−/− PMNs in tlr4+/+ lung; 4) Krebs solution in tlr4−/− lungs (control); and 5) tlr4+/+ PMNs in tlr4−/− lungs.
Microvessel Filtration Coefficient (Kf) Measurement
Kf was measured to determine pulmonary microvascular fluid permeability (27, 30). The rate of lung weight gain was obtained during a 20 min step increase in venous pressure (+6 cm H2O) and then normalized by the lung dry weight to determine Kf in units of ml/min/cmH2O/g dry lung. The analytic procedure used for computing Kf from recordings of lung wet weight was as described (27, 30).
Cytospin Analysis of Bronchoalveolar Lavage (BAL) Fluid
Cytospins of BAL fluid from the mice were prepared and stained, air dried, and coverslipped. An increase in BAL protein concentration was taken as a measure of increased permeability of the alveolar-capillary barriers (31).
Statistical Analysis
All data are expressed as mean ± standard error of mean. Statistical differences were assessed by ANOVA followed by post hoc analysis with Student-Newman-Keul test. Differences between treatment groups were considered significant at p < 0.05.
RESULTS
Deletion of TLR4 Prevents Hyperinflation-induced PMN Infiltration in Lungs
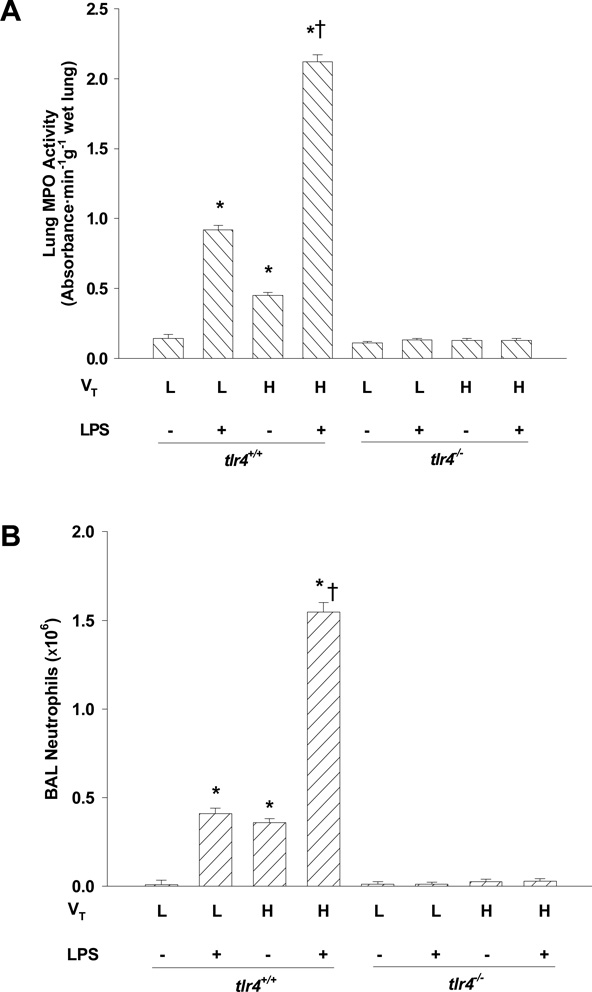
As measured by lung MPO activity (Fig. 1A) and PMN counts in BAL fluid (Fig. 1B), PMN infiltration in lungs was significantly increased in LPS-challenged tlr4+/+ mice ventilated with low (normal, 7 ml/kg) tidal volume compared to unchallenged mice. Although PMN infiltration in unchallenged mouse lungs was also increased by high tidal volume ventilation alone, this increase was enhanced markedly in the LPS-challenged mice. In tlr4−/− mice, surprisingly, there was no significant increase in PMN infiltration by any treatment (Figs. 1A and 1B) indicating TLR4 signaling plays a role in LPS- as well as hyperinflation-induced lung inflammation.
Figure 1.
Effects of lung hyperinflation in the presence and absence of LPS challenge on lung PMN accumulation in tlr4+/+ and tlr4−/− mice. (A) PMN sequestration in lungs as determined by myeloperoxidase (MPO) activity. (B) PMN recruitment into the alveolar space as determined by PMN count in BAL fluid. n=6-8 per group; *, p < 0.05 versus low tidal volume alone group in tlr4+/+ mice; †, p < 0.05 versus either high tidal volume alone group or low tidal volume plus LPS group. VT = tidal volume, L = low, H = high.
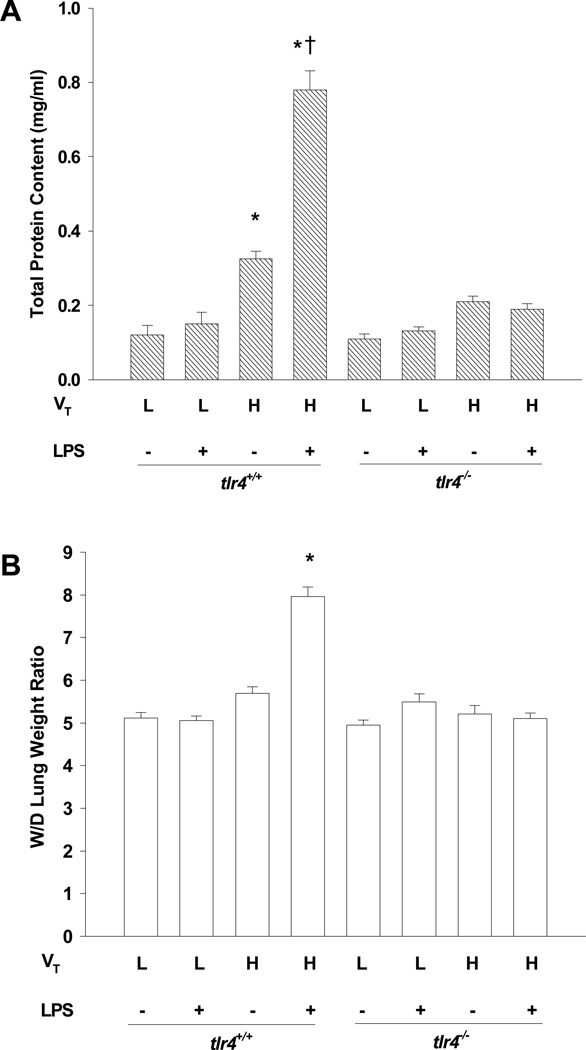
Deletion of TLR4 Reduces Hyperinflation-induced Increase in Lung Microvascular Permeability and Edema Formation
We assessed the integrity of the alveolar-capillary barrier and pulmonary microvascular liquid permeability by measuring the concentration of total protein in BAL fluid (31) and final wet-to-dry lung weight ratio, respectively (Figs. 2A and 2B). Normal tidal volume ventilation had no effect on protein permeability even in tlr4+/+ mice challenged with LPS, whereas high tidal volume ventilation alone increased permeability in tlr4+/+ mice (P< 0.05) but not in tlr4−/− mice (P > 0.05). The combination of high tidal volume ventilation and LPS induced a 6-fold increase in BAL protein and caused pulmonary edema only in tlr4+/+ mice. In tlr4−/− mice, there was no significant increase in protein permeability or wet-to-dry lung weight ratio following LPS challenge, ventilation with high tidal volume, or a combination of the two.
Figure 2.
Effects of lung hyperinflation in the presence and absence of LPS challenge on lung microvascular protein permeability and edema formation in tlr4+/+ and tlr4−/− mice. (A) Pulmonary vascular protein permeability as determined by protein concentration of BAL fluid. (B) Pulmonary edema formation measured by wet-to-dry (W/D) lung weight ratio for each experimental group. n=6-8 per group; *, p < 0.05 versus low tidal volume alone group in tlr4+/+ mice; †, p< 0.05 versus either high tidal volume alone group or low tidal volume plus LPS group. VT = tidal volume, L = low, H = high.
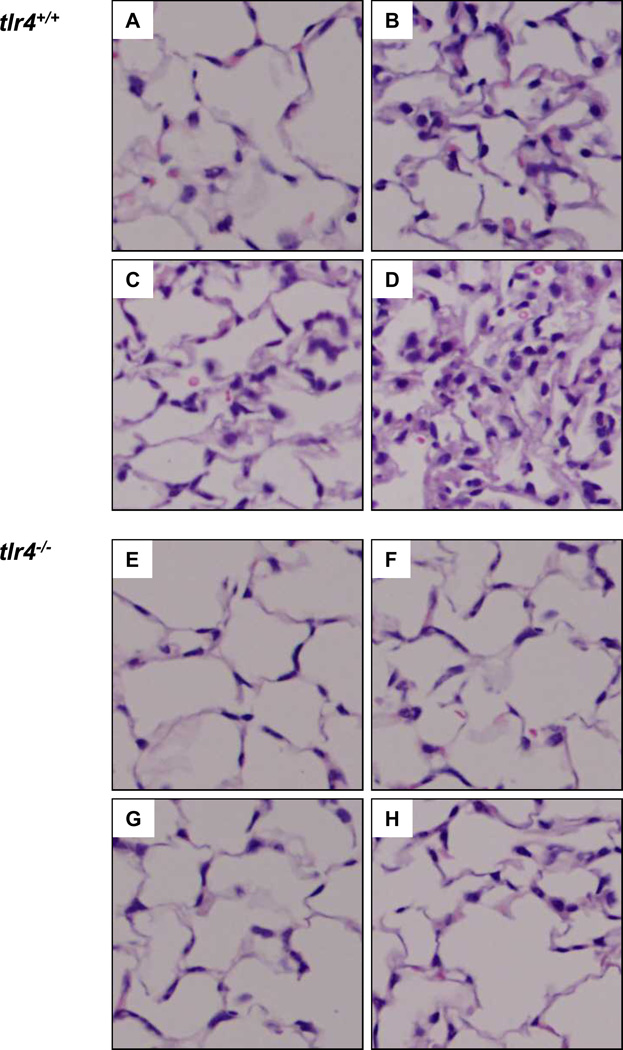
Reduction in Hyperinflation-induced Lung Tissue Injury in tlr4−/− Mice
Histopathological studies demonstrated normal lung structure and absence of pathology in lungs ventilated with a normal tidal volume of 7 ml/kg (Fig. 3A). However, in LPS-challenged tlr4+/+ mice, normal tidal volume ventilation induced PMN infiltration (Fig. 3B). High tidal volume ventilation alone caused bleeding and PMN infiltration in unchallenged tlr4+/+ mice (Fig. 3C), but in LPS challenged mice, there was more bleeding, greater PMN infiltration and edema, and septal thickening (Fig. 3D). In contrast, tlr4−/− mouse lungs had no PMN infiltration or bleeding in any of the treatment groups (Figs. 3E-3H).
Figure 3.
Histopathology of lungs following hyperinflation in presence and absence of LPS. Representative photomicrographs of H & E staining (20 ×) of lungs from: (A) tlr4+/+ + saline + low VT; (B) tlr4+/+ + LPS + low VT; (C) tlr4+/+ + saline + high VT; (D) tlr4+/+ + LPS + high VT; (E) tlr4−/− + saline + low VT; (F) tlr4−/− + LPS + low VT; (G) tlr4−/− + saline + high VT; (H) tlr4−/− + LPS + high VT. VT = tidal volume.
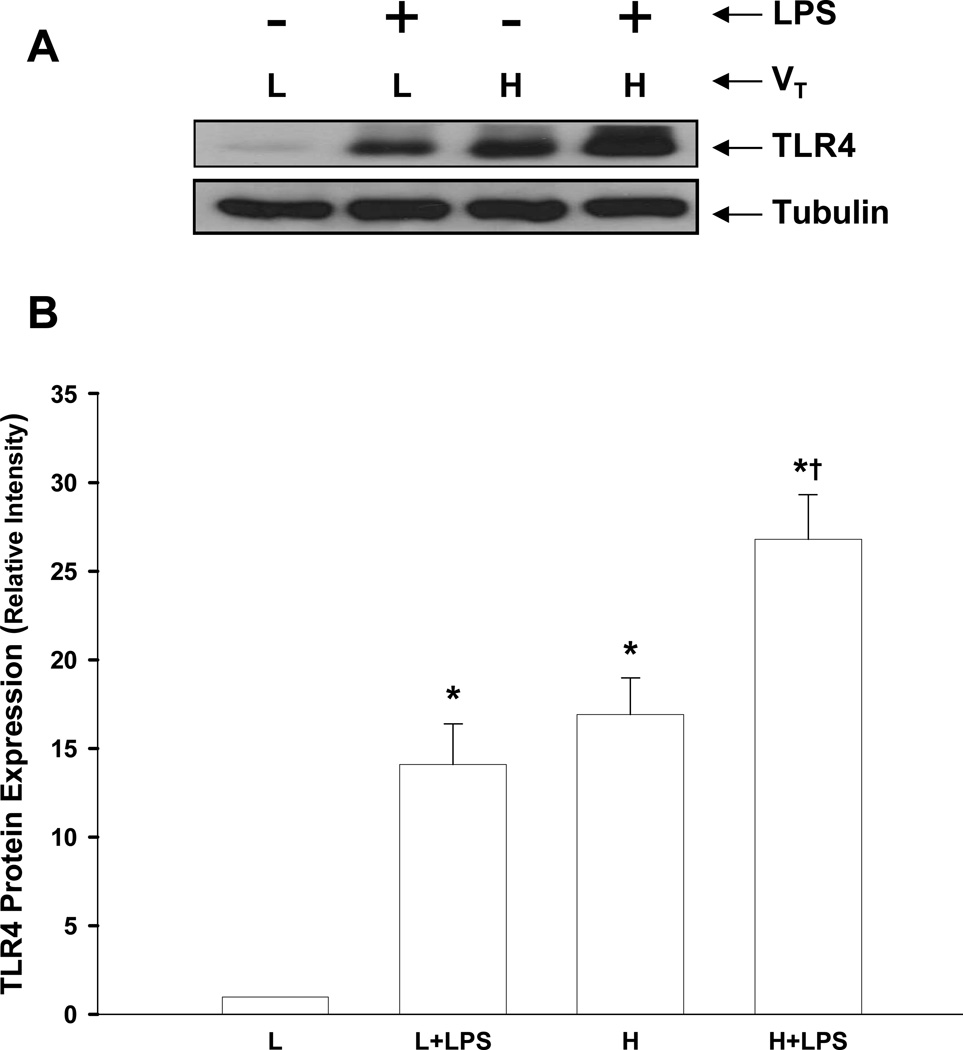
Increased Lung TLR4 Expression Following Lung Hyperinflation
We determined TLR4 expression in lungs of tlr4+/+ mice to address whether lung hyperinflation was a factor promoting expression of TLR4. High tidal volume ventilation alone or LPS treatment alone caused a significant increase in protein expression of TLR4 in lungs compared with normally ventilated mice. However, treatment with both high tidal volume ventilation and LPS further augmented TLR4 expression in lungs (Figs. 4A and 4B).
Figure 4.
Increased expression of TLR4 protein in lungs induced by lung hyperinflation. (A) TLR4 expression detected by Western blotting. Results shown are representative of 5 experiments. (B) Densitometric analysis of TLR4 in lung homogenates after different treatments. The density of proteins in normal ventilation alone (L) group was used as a standard (1 arbitrary unit) to compare relative densities in other groups. n=5/group. *, p < 0.05, compared to low tidal volume alone (L) group; †, p < 0.05 compared to normal ventilation plus LPS group (L + LPS) or high tidal volume ventilation alone (H) group. VT = tidal volume, L = low, H = high.
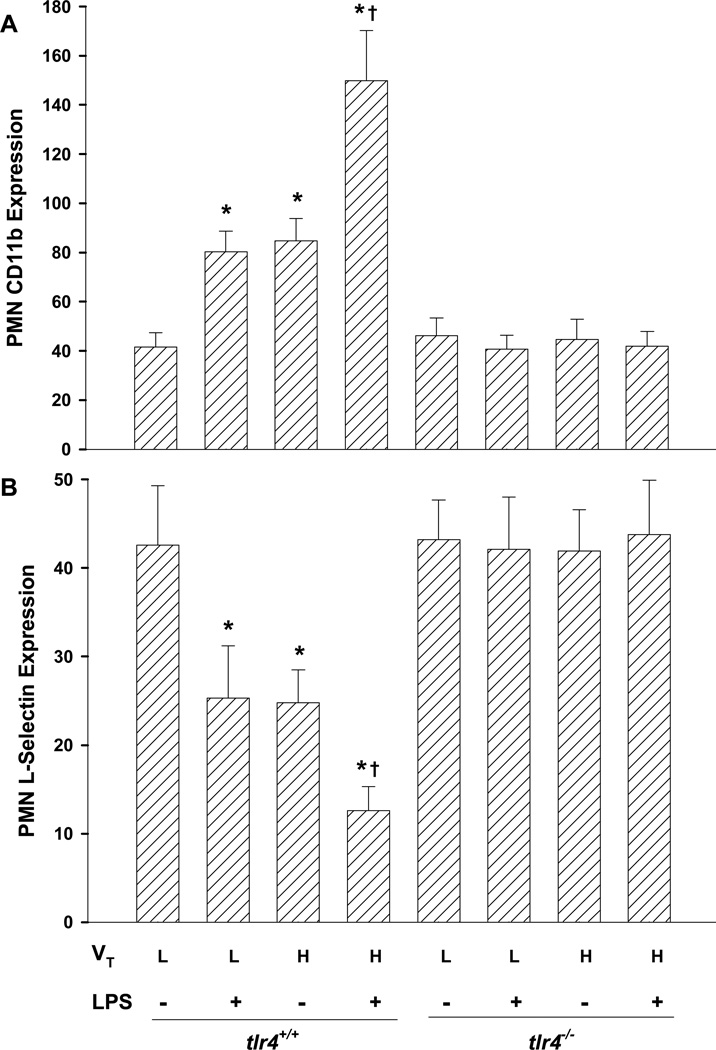
TLR4 Deletion Prevents Lung Hyperinflation-induced Activation of Circulating PMNs
To investigate mechanisms of PMN infiltration in lungs, we assessed PMN activation in vivo by measuring CD11b upregulation and L-selectin shedding (18). As shown in Figures 5A and 5B, unchallenged tlr4+/+ mouse PMNs expressed CD11b and L-selectin with MCF values of 42 ± 6, and 43 ± 7, respectively. High tidal volume ventilation alone or LPS treatment alone caused activation of circulating PMNs as indicated by greater CD11b and lower L-selectin expression. Further activation of circulating PMNs was found in high tidal volume-ventilated plus LPS-challenged tlr4+/+ mice. In tlr4−/− mice, however, there was no significant differences in CD11b or L-selectin expression between treatment groups (Figs. 5A and 5B).
Figure 5.
Augmented TLR4-dependent activation of neutrophils exposed to LPS and lung hyperinflation in vivo. Neutrophil CD11b (A) and L-selectin (B) expression were assessed by flow cytometry. Results are shown as arbitrary units of mean channel fluorescence (MCF). n=5/group. *, p < 0.05 compared to normal tidal volume (L); †, p < 0.05 compared to normal ventilation plus LPS group or high tidal volume ventilation alone group. VT = tidal volume, L = low, H = high.
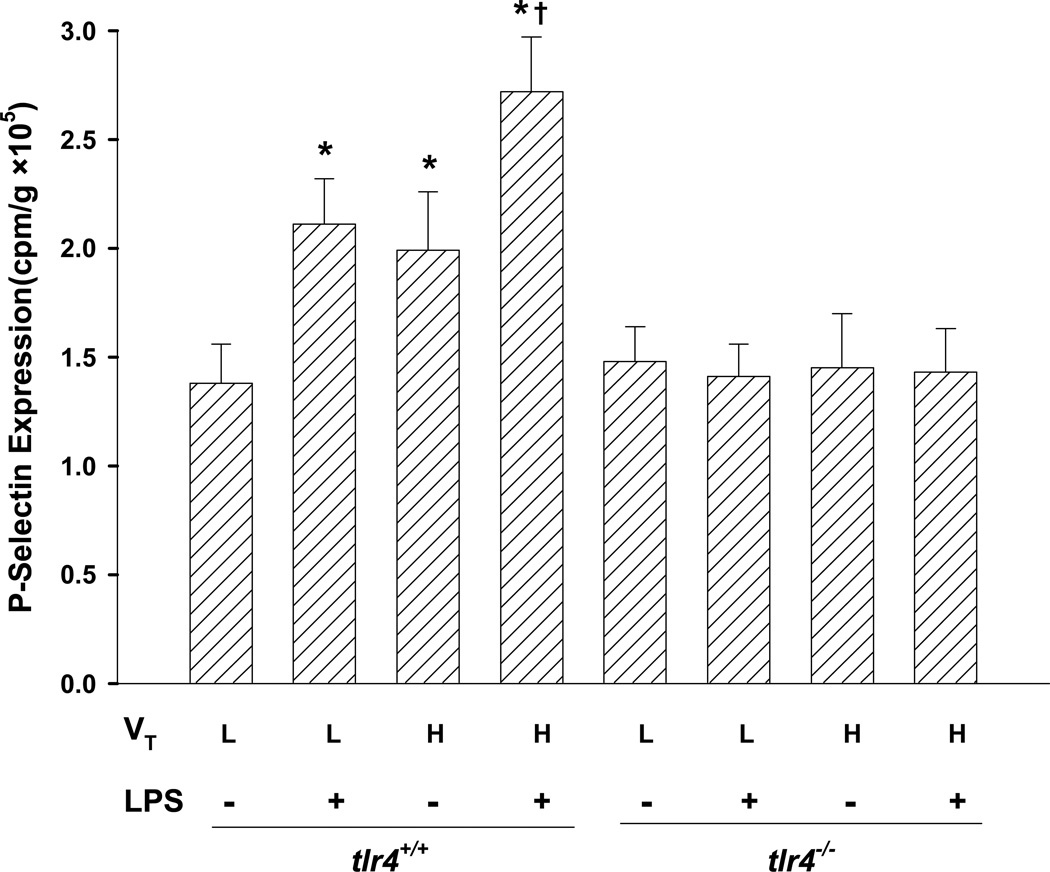
Deletion of TLR4 Prevents Lung Hyperinflation-induced Activation of Pulmonary Endothelium
We next examined expression of P-selectin as a measure of endothelial cell activation (18). Either LPS treatment alone or high tidal volume ventilation alone in tlr4+/+ mice significantly upregulated P-selectin expression, and high tidal volume ventilation significantly enhanced lung P-selectin expression in LPS-challenged tlr4+/+ mice. However, P-selectin expression was significantly reduced in lungs of tlr4−/− mice (Fig. 6). Furthermore, we observed significantly greater P-selectin expression in heart, liver, kidney, mesentery, small and large intestines, and stomach in tlr4+/+ compared to tlr4−/− mice (data not shown).
Figure 6.
Augmented in vivo TLR4-dependent activation of pulmonary vascular endothelium after exposure to LPS and lung hyperinflation. Expression of P-selectin was measured in lung tissue. Results are shown as the mean channel fluorescence (MCF). n=5/group. *, p < 0.05, compared to low tidal volume alone (L) group; †, p < 0.05 compared to normal ventilation plus LPS group (L + LPS) or high tidal volume ventilation alone (H) group. VT = tidal volume, L = low, H = high.
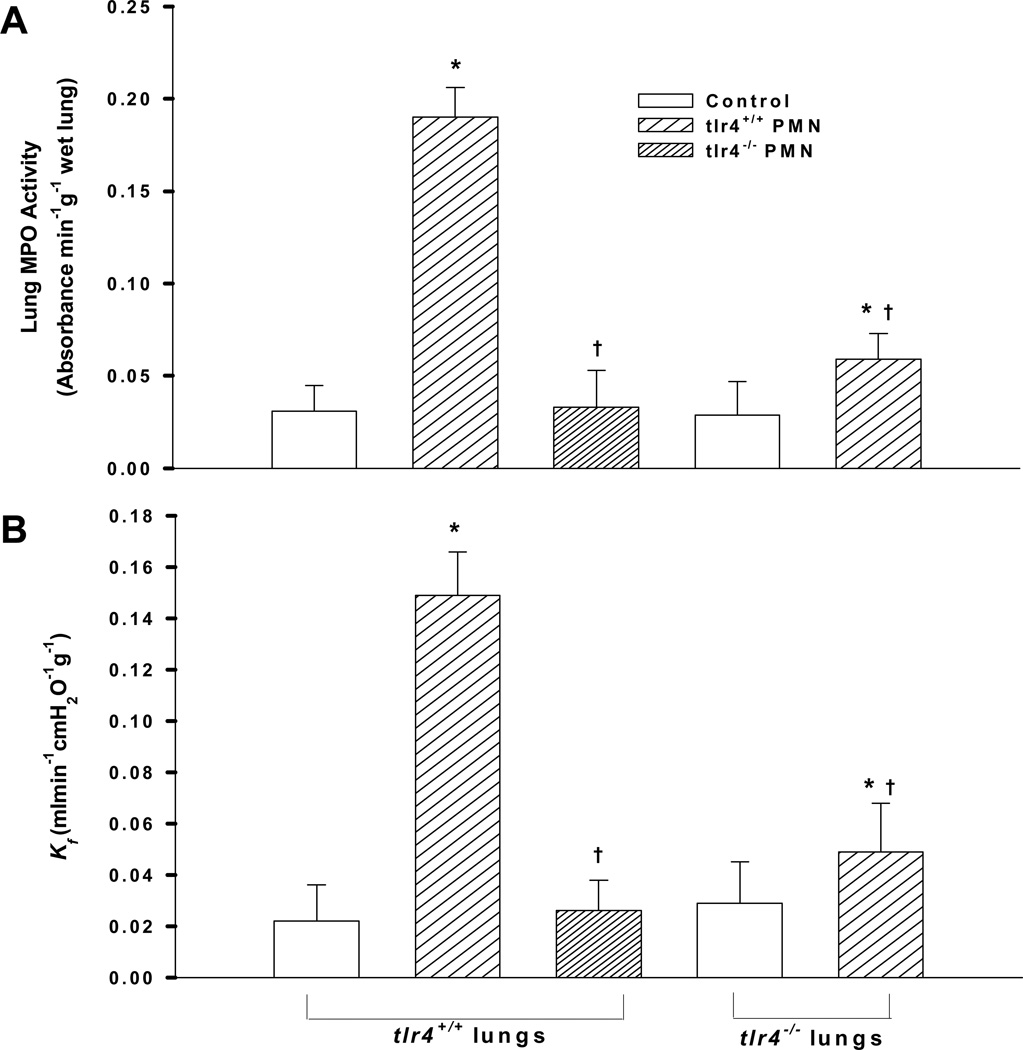
TLR4 Expression in PMNs and Lung Tissue are Both Required for Lung PMN Uptake and Vascular Injury Induced by Lung Hyperinflation
To address the role of PMN TLR4 vs. lung tissue TLR4 in the mechanism of lung PMN sequestration and vascular injury following lung hyperinflation (increase from 9 to 25 cmH2O peak inspiratory pressure), studies were made in Kreb’s-albumin perfused lungs to which PMNs were instilled. We compared the effects of adding tlr4+/+ vs. tlr4−/− PMNs to the perfusate on PMN uptake and lung vascular injury in isolated-perfused tlr4+/+ lungs. We observed that tlr4+/+ PMNs were actively recruited into lungs after high tidal volume ventilation, whereas this effect was markedly reduced when using tlr4−/− PMNs (Fig. 7A). Furthermore, tlr4+/+ PMNs caused an increase in pulmonary microvascular permeability (Kf) (Fig. 7B), whereas tlr4−/− PMNs had no effect. Next, using isolated-perfused tlr4−/− lungs, we addressed whether TLR4 expression in lung tissue plays a role in mediating PMN uptake and vascular injury. We observed that uptake of tlr4+/+ PMNs was reduced in tlr4−/− lungs and that this was coupled to a smaller increase in lung vascular permeability than in tlr4+/+ lungs (Figs. 7A and 7B).
Figure 7.
Effect of neutrophils (PMNs) and lung tissue TLR4 expression on (A) PMN infiltration (MPO activity) and (B) pulmonary microvessel permeability (Kf) in hyperinflated mouse lungs (see Methods for details). The peak inspiratory pressure was increased from 9 to 25 cmH2O to induce lung hyperinflation. PMNs (4×106 cells) from either tlr4+/+ or tlr4−/− mice were infused for 30 min (n= 6/group). MPO = myeloperoxidase; Kf = lung microvessel filtration coefficient. *, p < 0.05 versus respective control. †, p < 0.05 compared to tlr4+/+ PMNs and lungs.
DISCUSSION
Pulmonary inflammation in response to mechanical stretch induced by lung hyperinflation is the primary feature of VILI (32, 33). Mechanical ventilation resulting in lung stretch has been demonstrated to induce lung injury in animals with pre-existing pneumonia (34, 35). Infiltration of PMN into lungs plays a key role in the pathogenesis of VILI (4,7); however, the mechanisms responsible for recruitment of PMNs are poorly understood (8). TLR4 is critical for recognition of LPS/endotoxin by host cells and initiates cellular release of cytokines and chemokines (13–17). TLR4 also plays an important role in pulmonary recruitment of PMNs leading to acute lung injury (18). It is not clear, however, whether TLR4 signaling is involved in the regulation of the inflammatory response induced by mechanical stretch of lungs. In the present study, using a mouse model of genetic deletion of tlr4, we demonstrate that PMN recruitment and lung vascular injury induced by mechanical stretch alone or combined with LPS challenge is TLR4-dependent. Furthermore, TLR4 activation in both PMNs and lung stromal cells contributed to PMN infiltration and subsequent lung injury induced by lung hyperinflation.
Patients requiring mechanical ventilation in the presence of sepsis are at risk for VILI, which is characterized by increased pulmonary vascular permeability and inflammation (36). In fact several studies have demonstrated that in the presence of sepsis or pneumonia, mechanical ventilation augments the inflammatory response to LPS and promotes acute lung injury (25,34,35,37). In the present study, we showed that after LPS challenge, conventional mechanical ventilation at normal tidal volume increased PMN infiltration in lung, but had no effect on pulmonary vascular permeability or edema formation. However, high tidal volume ventilation induced marked increases in PMN infiltration, pulmonary vascular permeability, and lung edema formation in LPS challenged mice.
It is unclear how the interaction between LPS challenge and lung hyperinflation mediates lung PMN sequestration and vascular injury. One possibility is that lung stretch and LPS have a synergistic effect on the production of inflammatory cytokines and chemokines through activation of the transcription factors nuclear factor κB (NF-κB) and activator protein (AP)-1 (37). As described herein, increased PMN infiltration and lung injury induced by the combination of LPS and hyperinflation were abolished in tlr4−/− mice. Furthermore, Western blot analysis showed that like LPS treatment, high tidal volume ventilation alone significantly increased TLR4 protein expression in lungs as compared to normally ventilated mice. TLR4 expression was further enhanced in LPS challenged mice ventilated with high tidal volume, suggesting a role of mechanical stretch in inducing increased expression of TLR4 protein, presumably via de novo protein synthesis. Consistent with our findings, a recent study using TLR4 antibody in a rabbit model of VILI showed that TLR4 blockade reduces pulmonary inflammation caused by the combination of LPS and mechanical ventilation (21). Taken together, our findings demonstrate a critical role of TLR4 in mediating lung PMN uptake and inflammation induced by mechanical stretch of lungs during sepsis/pneumonia.
Interactions between PMNs and the endothelium are essential for PMN recruitment (38). Thus, we determined the activation states of PMNs and the endothelium in the in vivo lung hyperinflation model. We observed that either LPS or high tidal volume ventilation alone induced the activation of circulating PMN (assessed by decreased L-selectin and increased CD11b expression) and endothelial cells (assessed by increased P-selectin expression). The combination of LPS and mechanical ventilation with high tidal volume, however, greatly augmented these responses. Surprisingly, activation of PMNs and the endothelium were both abrogated in tlr4−/− mice, suggesting that TLR4 expressed in both PMNs and endothelium are critical determinants of lung PMN infiltration and injury. These findings are consistent with observations that TLR4 signaling induces upregulation of CD11b (39) and thus is essential for PMN adhesion and transendothelial PMN migration. PMN TLR4 has also been shown to regulate interaction of PMNs with endothelial cells and activation of endothelial cells (40). A study using chimeric mice (transferring bone marrow between tlr4+/+ and tlr4−/− mice) demonstrated that endothelial cell TLR4 rather than PMN TLR4 plays the dominant role in LPS-induced PMN sequestration in lungs (18). However, our study differs in that we examined the role of TLR4 in a model of lung hyperinflation-coupled pneumonia as compared to the previous study in which the effect of sepsis in the absence of hyperinflation was addressed.
To further characterize the contribution of cell-type specific expression of TLR4 to LPS and lung hyperinflation-induced PMN infiltration and lung injury, we infused LPS-pretreated PMNs in isolated-perfused mouse lungs using a “cross-matched” experimental design. We observed that tlr4+/+ PMNs were actively recruited into isolated-perfused tlr4+/+ lungs by high tidal volume ventilation, whereas this effect was markedly reduced upon perfusion of tlr4−/− PMNs. tlr4+/+ PMNs also caused an increase in pulmonary vascular permeability whereas tlr4−/− PMNs had no effect. These data suggest that PMN TLR4 plays an important role in high tidal volume-induced PMN sequestration and lung vascular injury. Similarly, tlr4+/+ PMN-induced infiltration and increase in lung vascular permeability were reduced in isolated-perfused tlr4−/− lungs. Taken together, these data suggest that PMN TLR4 as well as lung tissue TLR4 expression (i.e., TLR4 expressed in endothelial cells, epithelial cells, and macrophages) are likely to be involved in the “double hit” model of LPS/lung stretch-induced PMN infiltration and lung vascular injury. In the rat VILI model, macrophages were demonstrated to contribute to lung injury by facilitating the recruitment of PMNs into the air space (41), consistent with our findings that both PMN TLR4 expression and TLR4 expression in lung cells such as macrophages are important in the mechanism of PMN sequestration and lung vascular injury induced by lung hyperinflation.
The present study provides in vivo evidence of lung hyperinflation-induced TLR4 activation in the absence and presence of pneumonia. Our results suggest that high tidal volume mechanical ventilation activates the host innate immune system through TLR4 signaling which triggers the inflammatory response. Whether the observed effects of mechanical ventilation on TLR4 activation were due to a direct action of mechanical stretch on the pulmonary cells, i.e., endothelium and epithelium, or activation of other as yet undefined signaling pathways, remains to be determined. It is possible, for example, that release of endogenous ligands from damaged cells after lung stretch, such as heat shock protein (42,43), heparan sulfate, hyaluronan (44), high-mobility group box-1 (45,46), and fibronectin have the capacity to activate TLR4 (47). Another possibility is that endogenous ligands may induce TLR4 expression during lung hyperinflation, which may lead to the observed augmentation of LPS-induced TLR4 activation; thus, increased TLR4 expression may amplify the LPS-induced TLR4 activation response (48). In addition, a study in an in vivo mouse model indicated that acute lung injury induced by a high dose of intratracheal LPS (0.3 mg/kg) is partially mediated by CD14 (49). CD14 was also demonstrated to be highly expressed in alveolar macrophages isolated from over-ventilated rabbit lungs (50). Therefore, large tidal volume ventilation may "sensitize" alveolar macrophages to LPS by upregulation of CD14. Finally, it is possible that hyperinflation activates common downstream components of the TLR4 pathway such as NFκB or c-Jun N-terminal kinase leading to LPS-induced augmentation of the inflammatory response (33,26) seen in the present study.
CONCLUSIONS
In conclusion, we have identified TLR4 as a critical trigger of inflammatory injury caused by LPS and lung stretch induced by hyperinflation. Both PMN and lung stromal cell TLR4 expression contribute to pulmonary PMN infiltration and VILI. The results suggest an important role of lung innate immunity regulated by TLR4 in the mechanism of PMN sequestration and injury resulting from mechanical ventilation at high tidal volumes. These findings provide a rationale for designing therapeutic strategies directed against inappropriate TLR4 expression in ameliorating VILI.
ACKNOWLEDGMENTS
This work was supported by AHA 0730331N (GH), NIH HL71626 (RDM), and P01 HL60678 (ABM, RDM).
The authors thank David J. Visintine and Maricela Castellon (Departments of Pharmacology and Anesthesiology, University of Illinois College of Medicine) for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care. 2003;7:233–241. doi: 10.1186/cc1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Kotani M, Kotani T, Ishizaka A, et al. Neutrophil depletion attenuates interleukin-8 production in mild-overstretch ventilated normal rabbit. Crit Care Med. 2004;32:514–519. doi: 10.1097/01.CCM.0000110677.16968.E4. [DOI] [PubMed] [Google Scholar]
- 4.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110:1703–1716. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano T, Mori S, Cybulsky M, et al. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol. 1987;62:27–33. doi: 10.1152/jappl.1987.62.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg JM, Schiller HJ, Halter JM, et al. Alveolar instability causes early ventilatorinduced lung injury independent of neutrophils. Am J Respir Crit Care Med. 2004;169:57–63. doi: 10.1164/rccm.200304-544OC. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury S, Wilson MR, Goddard ME, et al. Mechanisms of early pulmonary neutrophil sequestration in ventilator-induced lung injury in mice. Am J Physiol. 2004;287:L902–L910. doi: 10.1152/ajplung.00187.2004. [DOI] [PubMed] [Google Scholar]
- 8.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11:82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Markos J, Doerschuk CM, English D, et al. Effect of positive end-expiratory pressure on leukocyte transit in rabbit lungs. J Appl Physiol. 1993;74:2627–2633. doi: 10.1152/jappl.1993.74.6.2627. [DOI] [PubMed] [Google Scholar]
- 10.Loick HM, Wendt M, Rotker J, et al. Ventilation with positive end-expiratory airway pressure causes leukocyte retention in human lung. J Appl Physiol. 1993;75:301–306. doi: 10.1152/jappl.1993.75.1.301. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Janeway CJ. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Akira S. Toll receptors and pathogen resistance Cell. Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 13.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 14.Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus Toll-like receptor 4 within microvessels. J Immunol. 2002;169:2111–2119. doi: 10.4049/jimmunol.169.4.2111. [DOI] [PubMed] [Google Scholar]
- 15.Kerfoot SM, Kubes P. Local coordination verses systemic disregulation: complexities in leukocyte recruitment revealed by local and systemic activation of TLR4 in vivo. J Leukoc Biol. 2005;77:862–867. doi: 10.1189/jlb.1004607. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 18.Andonegui G, Bonder CS, Green F, et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Zmijewski JW, Lorne E, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol. 2008;295:L497–L504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith LS, Kajikawa O, Elson G, et al. Effect of Toll-like receptor 4 blockade on pulmonary inflammation caused by mechanical ventilation and bacterial endotoxin. Exp Lung Res. 2008;34:225–243. doi: 10.1080/01902140802022492. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan BC, McIntyre RC, Jr, Moore EE, et al. Neutrophils mediate pulmonary vasomotor dysfunction in endotoxin-induced acute lung injury. J Trauma. 1997;42:391–396. doi: 10.1097/00005373-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Yipp BG, Andonegui G, Howlett CJ, et al. Profound differences in leukocyte-endothelial cell responses to lipopolysaccharide versus lipoteichoic acid. J Immunol. 2002;168:4650–4658. doi: 10.4049/jimmunol.168.9.4650. [DOI] [PubMed] [Google Scholar]
- 24.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 25.Altemeier WA, Matute-Bello G, Gharib SA, et al. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol. 2005;175:3369–3376. doi: 10.4049/jimmunol.175.5.3369. [DOI] [PubMed] [Google Scholar]
- 26.Li LF, Yu L, Quinn DA. Ventilation-induced neutrophil infiltration depends on c-Jun Nterminal kinase. Am J Respir Crit Care Med. 2004;169:518–524. doi: 10.1164/rccm.200305-660OC. [DOI] [PubMed] [Google Scholar]
- 27.Hu G, Ye RD, Dinauer MC, et al. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol. 2008;294:L178–L186. doi: 10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- 28.Eppihimer MJ, Wolitzky B, Anderson DC, et al. Heterogeneity of expression of E- and Pselectins in vivo. Circ Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Vogel SM, Schwartz DE, et al. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu N, Rahman A, Minshall RD, et al. ?2-Integrin blockade driven by E-selectin promoter prevents neutrophil sequestration and lung injury in mice. Circ Res. 2000;87:254–260. doi: 10.1161/01.res.87.3.254. [DOI] [PubMed] [Google Scholar]
- 31.Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996;270:L836–L845. doi: 10.1152/ajplung.1996.270.5.L836. [DOI] [PubMed] [Google Scholar]
- 32.Choi WI, Quinn DA, Park KM, et al. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2003;167:1627–1632. doi: 10.1164/rccm.200210-1216OC. [DOI] [PubMed] [Google Scholar]
- 33.Held HD, Boettcher S, Hamann L, et al. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-κB and is blocked by steroids. Am J Respir Crit Care Med. 2001;163:711–716. doi: 10.1164/ajrccm.163.3.2003001. [DOI] [PubMed] [Google Scholar]
- 34.Dhanireddy S, Altemeier WA, Matute-Bello G, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest. 2006;86:790–799. doi: 10.1038/labinvest.3700440. [DOI] [PubMed] [Google Scholar]
- 35.O'Mahony DS, Liles WC, Altemeier WA, et al. Mechanical ventilation interacts with endotoxemia to induce extrapulmonary organ dysfunction. Crit Care. 2006;10:R136. doi: 10.1186/cc5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc. 2007;4:77–84. doi: 10.1513/pats.200608-149JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altemeier WA, Matute-Bello G, Frevert CW, et al. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol. 2004;287:L533–L542. doi: 10.1152/ajplung.00004.2004. [DOI] [PubMed] [Google Scholar]
- 38.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Gao XP, Fan J, et al. LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am J Physiol. 2005;288:L655–L662. doi: 10.1152/ajplung.00327.2004. [DOI] [PubMed] [Google Scholar]
- 40.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank JA, Wray CM, McAuley DF, et al. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol. 2006;291:L1191–L1198. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- 42.Vreugdenhil HAE, Haitsma JJ, Jansen NJ, et al. Ventilator-induced heat shock protein 70 and cytokine mRNA expression in a model of lipopolysaccharide-induced lung inflammation. Intensive Care Med. 2003;29:915–922. doi: 10.1007/s00134-003-1747-6. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro SP, Rhee K, Tremblay L, et al. Heat stress attenuates ventilator-induced lung dysfunction in an ex vivo rat lung model. Am J Respir Crit Care Med. 2001;163:1451–1456. doi: 10.1164/ajrccm.163.6.9908076. [DOI] [PubMed] [Google Scholar]
- 44.Mascarenhas MM, Day RM, Ochoa CD, et al. Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol. 2004;30:51–60. doi: 10.1165/rcmb.2002-0167OC. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa EN, Ishizaka A, Tasaka S, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400–407. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 46.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 47.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 48.Zhai Y, Shen XD, O'Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 49.Jeyaseelan S, Chu HW, Young SK, et al. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun. 2005;73:1754–1763. doi: 10.1128/IAI.73.3.1754-1763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moriyama K, Ishizaka A, Nakamura M, et al. Enhancement of the endotoxin recognition pathway by ventilation with a large tidal volume in rabbits. Am J Physiol. 2004;286:L1114–L1121. doi: 10.1152/ajplung.00296.2003. [DOI] [PubMed] [Google Scholar]