Abstract
Objective
The objective for this study was to investigate the effects of a high-fat diet supplemented with fish oil or olive oil on metabolic features associated with type 2 diabetes fed to C57BL/6J mice for an extended period.
Methods
Mice were fed one of four diets: a low-fat diet, a high-fat diet supplemented with lard, a high-fat diet supplemented with fish oil, or a high-fat diet supplemented with olive oil for 30 wk. Phenotypic and metabolic analysis were determined at 15 and 25–30 wk, thereby providing comparative analysis for weight gain, energy consumption, fat distribution, glucose and insulin tolerance, and hepatic/plasma lipid analysis.
Results
Mice fed a high-fat diet supplemented with fish oil had improved glucose tolerance after an extended period compared to mice fed a high-fat diet supplemented with lard. Moreover, mice fed a high-fat diet supplemented with fish oil diet had significantly decreased concentrations of liver cholesterol, cholesteryl ester, and triacylglycerol compared to mice fed a high-fat diet supplemented with either lard or olive oil.
Conclusion
Mice fed a high-fat diet supplemented with fish oil improved metabolic features associated with type 2 diabetes such as impaired glucose tolerance and hepatic steatosis.
Keywords: adipose, diabetes, inflammation, lipogenesis, mouse model, obesity
Introduction
The obesity pandemic serves as a major risk factor for chronic and life-threatening comorbidities, including hepatic steatosis, dyslipidemia, and type 2 diabetes (T2D) [1]. Recent genome-wide association studies have identified a number of obesity and T2D susceptibility genes, most of which are suspected to interact with environmental factors [2, 3]. It is therefore necessary to investigate how the fatty acid composition of high-fat diets influences the etiology and pathophysiology of these complex nutrition-related metabolic diseases.
In the last decade, appropriate mouse models fed a high-fat diet represent the best means for obtaining novel insight with regard to altered human metabolism and consequently obesity and T2D [4]. Studies have determined that wild-type C57BL/6J mice are genetically susceptible to obesity, impaired glucose tolerance, and T2D when a high-fat diet, but not a low-fat diet, and therefore represent an ideal animal model for investigating these metabolic diseases [5, 6]. Moreover, studies indicate that C57BL/6J mice are susceptible to the fatty acid composition of high-fat diets, resulting in the manifestation of altered disease phenotypes [7]. For instance, a high-fat diet supplemented with fish oil has been reported to reduce adiposity and body weight, along with lessening adipose inflammation and inhibiting lipid synthesis [8, 9].
The high-fat diets and time intervals for these studies varies widely, and despite using the C57BL/6J mouse model, the results vary as well. Therefore, the present study used C57BL/6J mice fed high-fat diets (a total of 45% kcal from fat) supplemented with 10% lard, 10% fish oil, or 10% olive oil, to investigate the health effects in relation to metabolic features associated with T2D.
Materials and methods
Mice
Wild-type male C57BL/6J weanling mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained at The University of New Mexico Health Sciences Center Animal Resources Facility according to Institutional Animal Care and Use Committee (IACUC) guidelines. The mice were housed (4 mice/cage) in a room maintained at 23–24°C, 31–32% humidity, with an alternating 12 h light and dark cycle, and fed diets with water ad libitum. The mice and food were weighed weekly up to 30 wks of age, after which mice were killed using CO2 asphyxiation.
Diets
The energy balanced diets: i) a low-fat diet (LF, D07021301), ii) a high-fat diet supplemented with lard (HF, D07021302), iii) a high-fat diet supplemented with fish oil (FO, D07021303), and iv) a high-fat diet supplemented with olive oil (OO, D07021304) were formulated and produced by Research Diets (New Brunswick, NJ). The detailed composition of these diets is provided in Table 1. The LF diet served as the negative control diet while the HF diet specifically designed to mimic the average western-type diet served as the positive control diet. It must be noted that the FO and OO diets still contain 75% lard as the HF diet, therefore in the FO and OO diets the fish oil or olive oil ingredient replaces 25% of the lard to sustain the same kcal% from fat in these high fat diets. The fatty acid profiles of these high fat diets, aside from LF diet, which was used as a negative control, does not differ (Supplementary Table 1).
Table 1.
Detailed composition of mouse diets
| Diet | LF | HF | FO | OO | ||||
|---|---|---|---|---|---|---|---|---|
| Macronutrients | Mass, % |
Energy, % |
Mass, % |
Energy, % |
Mass, % |
Energy, % |
Mass, % |
Energy, % |
| Protein | 19 | 20 | 24 | 20 | 24 | 20 | 24 | 20 |
| Carbohydrate | 67 | 70 | 41 | 35 | 41 | 35 | 41 | 35 |
| Fat | 4 | 10 | 24 | 45 | 24 | 45 | 24 | 45 |
| Fatty acid profile | % fat | % fat | % fat | % fat | ||||
| Saturated | 25.1 | 36.2 | 33.7 | 30.8 | ||||
| Monounsaturated | 34.7 | 45.3 | 39.6 | 51.0 | ||||
| Polyunsaturated | 40.2 | 18.5 | 26.7 | 18.2 | ||||
| Ingredient, g/4057 kcal | g | kcal | g | kcal | g | kcal | g | kcal |
| Casein, 80 Mesh | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn Starch | 427 | 1708 | 73 | 291 | 73 | 291 | 73 | 291 |
| Maltodextrin 10 | 100 | 400 | 100 | 400 | 100 | 400 | 100 | 400 |
| Sucrose | 173 | 691 | 173 | 691 | 173 | 691 | 173 | 691 |
| Cellulose, BW200 | 50 | 50 | 50 | 50 | ||||
| Fish Oil | 45 | 407 | ||||||
| Olive Oil | 45 | 407 | ||||||
| Soybean Oil | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 |
| Lard | 20 | 180 | 177 | 1598 | 132 | 1191 | 132 | 1191 |
| Mineral Mix S10026 | 10 | 10 | 10 | 10 | ||||
| DiCalcium Phosphate | 13 | 13 | 13 | 13 | ||||
| Calcium Carbonate | 5.5 | 5.5 | 5.5 | 5.5 | ||||
| Potassium Citrate, | 16.5 | 16.5 | 16.5 | 16.5 | ||||
| Vitamin Mix V10001 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2 | 2 | 2 | 2 | ||||
| Total | 1054 | 4057 | 858 | 4057 | 858 | 4057 | 858 | 4057 |
| Energy density, kcal/g | 3.85 | 4.73 | 4.73 | 4.73 | ||||
FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Glucose and insulin tolerance tests
Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed at 15 and 25 weeks of age, and 16 and 26 weeks of age, respectively, to determine the presence of glucose intolerance and insulin sensitivity as previously described [10]. The Precision Xtra Advanced Diabetes Management System (Abbott Diabetes Care Inc., Alameda, CA) was used to measure blood glucose.
Concentration of plasma components
The concentration of plasma insulin was determined using the Mouse High Range Insulin ELISA kit (ALPCO Immunoassays, Salem, NH). The concentration of plasma glucose, cholesterol, triacylglycerol, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were determined using the Infinity Glucose, Cholesterol, Triacylglycerol, ALT, and AST kits (Thermo Scientific, Middletown, VA). The concentration of inflammatory cytokines (IL-1β, IL-6, and TNF-α) and anti-inflammatory cytokines (IL-4, IL-10, and IL-14) were determined using appropriate mouse Ready-SET-Go cytokine kits (eBioscience, San Diego, CA). The homeostasis model assessment-insulin resistance index (HOMA-IR), homeostasis model assessment-β cell function percentile (HOMA-β%), and quantitative insulin sensitivity check index (QUICKIE), were calculated as previously described [11, 12].
Relative size of adipocytes
The relative size of adipocytes in adipose tissue was measured indirectly as the amount of DNA normalized to the weight of adipose tissue [10]. The concentration of DNA was determined using the DNA fluorescence quantification kit (Sigma, St. Louis, MO).
Concentration of liver lipids
The concentration liver lipids were determined after organic extraction, separation using TLC, and measuring the amounts of each lipid (unesterified cholesterol, cholesteryl ester, free fatty acids, and triacyglycerol) using enzymatic kits as previously described [13]. The concentration of liver lipids was normalized to the amount of liver protein that precipitated during organic extraction.
Fatty acid profile of liver lipids
The total lipids were extracted from liver using hexane/isopropanol (3:2, v/v) as previously described [13]. The dried lipid extract was transferred to a clean reaction vial using chloroform and the solvent was evaporated under N2 gas. Direct trans-esterification of the lipid extract was performed using 14% BF3 in methanol to prepare fatty acid methyl esters (FAMEs) as previously described [14]. FAMEs were separated by GLC using a HP5890 series II gas chromatograph (Hewlett-Packard, Wilmington, DE) equipped with a HP5972 mass detector and 15 m × 0.1 mm fused silica capillary column (Omegawax-100, 0.1 µm film thickness, Sigma, St. Louis, MO). The temperature program started with an initial temperature of 110°C, which was increased at 30°C/min to 260°C, followed by a 10 min isothermal period. Injector and detector temperatures were 250°C and 280°C, respectively. Helium was used as a carrier gas at constant flow rate of 0.5 ml/min. Individual fatty acids were identified using MS spectra and confirmed with known FAMEs standards (Sigma, St. Louis, MO). Pentadecanoic acid was used as an internal standard. Peak integration was performed using the MS ChemStation software version G1034C. A FAME mixture (Supelco 37 Comp. FAME Mix, Sigma, St. Louis, MO) was used as an external standard, which was analyzed directly to determine the response of each FAMEs relative to the pentadecanoic acid methyl ester.
RNA preparation and qRT-PCR
Total RNA was extracted from mouse livers using the RNeasy Mini Kit and treated with RNasefree DNase to remove contaminating DNA (Qiagen, Valencia, CA). Reverse transcription was performed using a TaqMan Reverse Transcription Reagents. The relative amounts of mRNA were measured using qRT-PCR. The TaqMan Gene Expression Assay, with inventory primer and probe for: Fas (Mm00662319_m1), Hmgcr (Mm01282499_m1), Il-1β (Mm01336189_m1), Il-6 (Mm00446190_m1), Npc1 (Mm00435283_m1), Pgc-1β (Mm00504720_m1), Srebp-1 (Mm00550338_m1), Srebp-2 (Mm01306292_m1), and Tnfα (Mm00443258_m1) was used with an ABI-PRISM Sequence Detection System (Applied Biosystems, Foster City, CA). The relative amounts of target mRNA was normalized to 18S rRNA (internal control).
Western blot analysis
The relative amounts of FAS, HMGCR, NPC1, PGC-1β, m-SREBP-1, and m-SREBP-2 were determined using Western blot analysis as previously described [13].
Statistical analysis
Statistical calculations were performed using StatView 5.0.1 (SAS Institute, Cary, NC). Quantitative data are represented as the mean ± SE within a group of mice. One-way ANOVA was used to determine significance among means for the groups of mice. Bonferroni/Dunn posthoc analysis was performed to determine significant differences between means (P < 0.05).
Results
Body, liver, and epididymal white adipose tissue weights
The body, liver, and epididymal white adipose tissue (EWAT) weights for mice fed the four diets were determined at 15 and 30 wk (Table 2). Mice fed HF, FO, and OO diets had significantly increased body, liver, and EWAT weights compared to mice fed the LF diet at 15 wk. Mice fed the FO diet had significantly decreased EWAT weights (12%) compared to mice fed the HF diet at this age. Similar to 15 wk, mice fed HF, FO, and OO diets had significantly increased body weights and liver weights compared to mice fed the LF diet at 30 wk. However, mice fed FO or OO diets had significantly increased liver weights (24%) compared to mice fed the HF diet at this age. Mice fed HF, FO, and OO diets had decreased EWAT weights (12%, 15%, and 24%, respectively) compared to mice fed the LF diet at 30 wk, which remained significant after adjustment for body weight. There were no significant differences in food consumption among mice fed HF, FO, and OO diets at 15 and 30 wk (data not shown). This shows that mice fed HF, FO, and OO diets had increased EWAT weights and body weights at 15 wk, but after extended feeding of these diets a redistribution of weight from EWAT to the liver was apparent.
Table 2.
Body, liver, and epididymal white adipose tissue weights
| Age | Diet | LF | HF | FO | OO |
|---|---|---|---|---|---|
| 15 weeks1 | Body weight, g | 27.4 ± 0.53b | 37.4 ± 0.48a | 38.7 ± 0.69a | 37.5 ± 0.66a |
| Liver weight, g | 1.11 ± 0.03b | 1.32 ± 0.05a | 1.41 ± 0.06a | 1.42 ± 0.05a | |
| mg/g body weight | 40.4 ± 0.87a | 35.0 ± 0.83b | 36.2 ± 1.21b | 37.7 ± 0.80ab | |
| EWAT weight, g | 0.71 ± 0.07c | 2.22 ± 0.05a | 1.95 ± 0.08b | 2.07 ± 0.08ab | |
| mg/g body weight | 25.4 ± 2.14c | 59.5 ± 1.19a | 50.6 ± 2.31b | 55.1 ± 1.68ab | |
| 30 weeks2 | Body weight, g | 34.3 ± 1.16b | 46.7 ± 0.95a | 48.7 ± 0.62a | 49.6 ± 0.86a |
| Liver weight, g | 1.37 ± 0.09c | 2.69 ± 0.22b | 3.35 ± 0.11a | 3.35 ± 0.12a | |
| mg/g body weight | 39.6 ± 1.42c | 56.9 ± 3.61b | 68.7 ± 1.51a | 67.5 ± 2.11a | |
| EWAT weight, g | 1.57 ± 0.08a | 1.38 ± 0.08ab | 1.34 ± 0.04ab | 1.20 ± 0.06b | |
| mg/g body weight | 45.7 ± 1.49a | 30.0 ± 2.29b | 27.6 ± 0.78b | 24.2 ± 1.38b | |
Data are represented as mean ± SE (1n = 20, 2n = 12) of mice fed the four diets at 15 and 30 wk. Labeled means in a row without a common letter differ (P < 0.05). EWAT, epididymal white adipose tissue; FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed on mice fed the four diets at 15, 25, and 26 wk (Figure 1). Mice fed HF, FO, or OO diets had significantly increased fasting blood glucose (0 min) and impaired glucose tolerance compared to mice fed the LF diet at both 15 wk and 25wk. However, at 25 wk mice fed the FO diet had significantly decreased glucose levels at later time points (60 and 120 min) compared to mice fed the HF diet, which was confirmed using AUC analysis. Finally, mice fed HF, FO, or OO diets had significantly impaired insulin sensitivity (15, 30, 60, and 120 min) compared to mice fed the LF at 26 wk. The glucose tolerance test clearly shows that mice fed the FO diet, but not the OO diet, had improved glucose tolerance which is a metabolic features associated with type 2 diabetes after extended feeding compared to mice fed the HF diet.
Fig. 1.
Glucose tolerance tests were performed on mice fed the four diets at 15 wk (A, D) and 25 wk (B, E), while insulin tolerance test were performed at 26 wk (C, F). Values are presented as mean ± SE (n = 10–15). Means without a common letter differ (P < 0.05); *P < 0.05 when compared to HF, FO, and OO; #P < 0.05 when compared to HF. FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Concentration of plasma components
The concentration of plasma glucose and insulin, lipids, liver enzymes, and inflammatory cytokines were determined at 30 wk (Table 3). Mice fed HF, FO, and OO diets had significantly increased plasma glucose and insulin compared to mice fed the LF diet. Consistent with these results, the calculated HOMAs of mice fed HF, FO, or OO diets indicated significantly increased insulin resistance. Although unexpected, mice fed the FO diet had significantly increased HOMA-IR and HOMA-β% and hence insulin resistance compared to mice fed the OO diet and HF and OO diets, respectively. The calculated QUICKIE of mice fed HF, FO, and OO diets confirmed decreased insulin sensitivity compared to mice fed the LF diet. With respect to the concentration of plasma lipids, mice fed HF, FO, or OO diets had significantly increased plasma total cholesterol compared to mice fed the LF diet. Among mice fed HF, FO, or OO diets, mice fed the OO diet had highest concentration of plasma total cholesterol. In contrast, there were no significant differences in the concentration of plasma triacylglycerol. In terms of liver enzymes, mice fed HF, FO, and OO diets had significantly increased plasma ALT compared to mice fed the LF diet, with the mice fed the OO diet having the highest levels of ALT. Similarly, mice fed the OO diet had significantly increased plasma AST compared to mice fed the LF, HF, and FO diets. Finally, there were no significant differences in the concentration of plasma inflammatory cytokines (IL-1β, IL-6, and TNF-α) and only trace amounts of anti-inflammatory cytokines (IL-4, IL-10, and IL-14) and therefore insufficient to quantitate. The mice fed HF, FO, or OO diets were generally characterized with measures of insulin resistance, liver damage and hypercholesterolemia, but neither hypertriacylglycerolemia or inflammation.
Table 3.
Concentration of plasma components
| Diet | LF | HF | FO | OO |
|---|---|---|---|---|
| Glucose, mmol/L | 8.03 ± 0.38b | 10.61 ± 0.36a | 10.42 ± 0.25a | 10.21 ± 0.39a |
| Insulin, nmol/L | 0.21 ± 0.04b | 0.79 ± 0.09a | 1.02 ± 0.11a | 0.71 ± 0.05a |
| HOMA-IR | 0.37 ± 0.09c | 1.72 ± 0.22ab | 2.30 ± 0.37a | 1.65 ± 0.16b |
| HOMA-β, % | 4.57 ± 1.03c | 10.20 ± 1.08b | 16.36 ± 1.37a | 10.55 ± 1.26b |
| QUICKIE | 0.49 ± 0.03a | 0.36 ± 0.01b | 0.33 ± 0.01b | 0.36 ± 0.01b |
| Triacylglycerol, mmol/L | 0.81 ± 0.04 | 0.82 ± 0.03 | 0.87 ± 0.04 | 0.86 ± 0.04 |
| Cholesterol, mmol/L | 3.50 ± 0.21c | 6.02 ± 0.32b | 6.33 ± 0.14ab | 6.94 ± 0.15a |
| ALT, U/L | 174 ± 26c | 262 ± 23b | 270 ± 18b | 365 ± 19a |
| AST, U/L | 176 ± 23b | 191 ± 23b | 170 ± 17b | 280 ± 223a |
| IL-1β, pg/mL | 42.4 ± 3.8 | 41.0 ± 10.3 | 40.7 ± 2.5 | 35.8 ± 2.4 |
| IL-6, pg/mL | 40.5 ± 3.6 | 33.5 ± 2.2 | 30.9 ± 1.8 | 33.0 ± 1.9 |
| TNF-α, pg/mL | 78.7 ± 16.3 | 92.8 ± 17.8 | 93.9 ± 15.4 | 100.9 ± 21.1 |
Data are represented as mean ± SE (n = 9–11) of mice at 30 wks. Labeled means in a row without a common letter differ (P < 0.05). FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Relative size of EWAT adipocytes
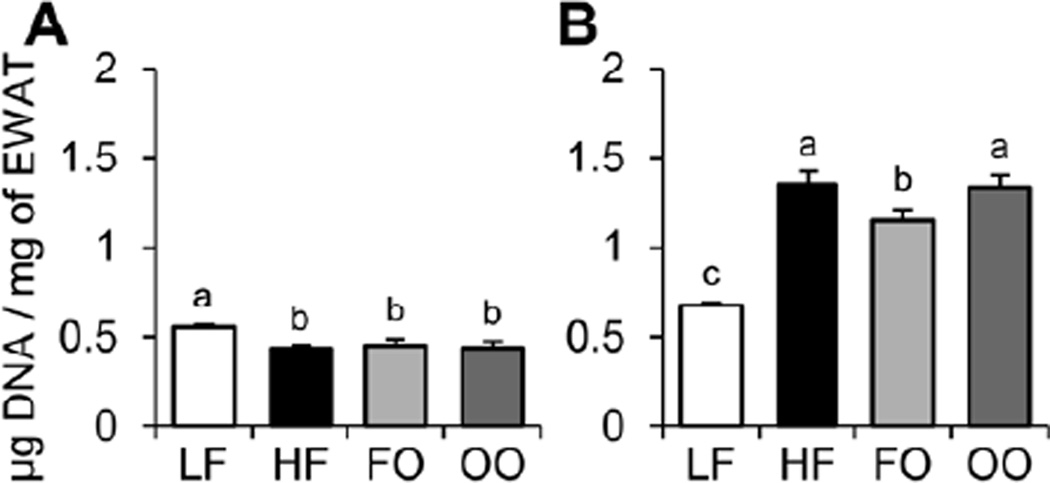
The relative size of EWAT adipocytes was determined at 15 and 30 wk (Figure 2). Mice fed HF, FO, or OO diets had modest but significantly increased EWAT adipocyte sizes compared to mice fed the LF diet at 15 wk. In contrast, mice fed HF, FO, or OO diets had significantly decreased EWAT adipocyte sizes compared to mice fed the LF diet at 30 wk. The mice fed HF, FO, or OO diets can be characterized with adipocyte hypertrophy at 15 wk, but adipocyte atrophy at 30 wk compared to mice fed the LF diet, while mice fed the FO diet showed significantly less adipocyte atrophy compared to mice fed HF and OO diets at 30 wk.
Fig. 2.
The relative size of EWAT adipocytes was determined for mice fed the four diets at 15 wk (A) and 30 wk (B) by measuring the amount of DNA normalized to weight of EWAT (µg DNA/mg EWAT). Values are presented as mean ± SE (n = 10–15). Means without a common letter differ (P < 0.05). EWAT, epididymal white adipose tissue; FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Concentration of liver lipids
The concentration of liver cholesterol, cholesteryl ester, and triacylglycerol was determined at 30 wk (Figure 3). Mice fed HF, FO, or OO diets had significantly increased liver cholesterol, cholesteryl ester, and triacylglycerol compared to mice fed the LF diet. The mice fed a FO diet had significantly decreased liver cholesterol (40%) and cholesteryl ester (41%) compared to mice fed the HF diet. This indicates that mice fed HF, FO, or OO diets were characterized with hepatic steatosis with modest but significantly decreased concentration of sterol (cholesterol and cholesteryl ester) for mice fed the FO diet.
Fig. 3.
The concentration of liver cholesterol (A), cholesteryl ester (B), and triacylglycerol (C) was determined for mice fed the four diets at 30 wk. Values are presented as mean ± SE (n = 10–15). Means without a common letter differ (P < 0.05). FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Fatty acid profile of liver lipids
The HF diet had the highest amounts of saturated FAs (C16:0, C18:0), while the FO diet contained significantly increased amounts of n-3 fatty acids (C20:5n3, C22:5n3, C22:6n3) not detected in other diets (Table 4). As expected, the OO diet had the highest amounts of monounsaturated C18:1. The liver fatty acid profile, again aside from a control LF diet, showed very similar results between HF and OO diet fed mice. The livers of mice fed the FO diet had significantly decreased amounts of monounsaturated FAs (C16:1, C18:1, C20:1) and significantly increased amounts n-3 FAs (C18:3n3, C20:5n3, C22:5n3, C22:6n3). Overall, supplementation of a high-fat diet with olive oil showed no significant difference in liver fatty acid profile, while supplementation with fish oil significantly boosted the amount of n-3 fatty acids within the livers of these mice.
Table 4.
Fatty acid profile of liver lipids.
| Fatty Acid | LF | HF | FO | OO |
|---|---|---|---|---|
| Saturated | ||||
| C14:0 | 1.23 ± 0.11 | 1.11 ± 0.05 | 1.07 ± 0.06 | 0.94 ± 0.07 |
| C16:0 | 22.18 ± 0.53 | 23.20 ± 0.66 | 22.95 ± 0.84 | 20.94 ± 0.72 |
| C17:0 | 0.32 ± 0.03b | 0.36 ± 0.03b | 0.48 ± 0.03a | 0.32 ± 0.02b |
| C18:0 | 4.88 ± 0.22ab | 4.38 ± 0.49b | 6.16 ± 0.51a | 4.33 ± 0.24b |
| C20:0 | 0.77 ± 0.08 | 0.65 ± 0.08 | 0.70 ± 0.06 | 0.62 ± 0.05 |
| C22:0 | 0.56 ± 0.07 | 0.52 ± 0.06 | ND | 0.44 ± 0.04 |
| C24:0 | 0.77 ± 0.11 | ND | ND | ND |
| Monounsaturated | ||||
| C16:1 | 6.73 ± 0.10a | 4.77 ± 0.57b | 2.84 ± 0.70b | 4.51 ± 0.16b |
| C18:1 | 36.40 ± 1.37a | 33.07 ± 2.59a | 22.90 ± 1.61b | 35.94 ± 1.21a |
| C20:1 | 1.59 ± 0.11a | 1.17 ± 0.08bc | 0.92 ± 0.05c | 1.43 ± 0.09ab |
| C24:1 | 0.42 ± 0.06 | ND | ND | ND |
| Polyunsaturated | ||||
| C18:2 | 8.90 ± 0.62b | 15.69 ± 1.34a | 15.74 ± 0.62a | 13.63 ± 0.76a |
| C18:3n6 | 0.49 ± 0.03 | 0.60 ± 0.05 | 0.46 ± 0.03 | 0.57 ± 0.04 |
| C20:2n6 | 0.42 ± 0.05 | 0.33 ± 0.13 | 0.41 ± 0.02 | 0.44 ± 0.02 |
| C20:3n9 | 0.61 ± 0.05a | 0.36 ± 0.09b | ND | 0.43 ± 0.04ab |
| C20:3n6 | 1.14 ± 0.02a | 1.06 ± 0.02b | 0.96 ± 0.02b | 1.20 ± 0.05a |
| C20:4n6 | 5.50 ± 0.57a | 5.10 ± 0.47a | 3.72 ± 0.27b | 5.84 ± 0.39a |
| n-3 Polyunsaturated | ||||
| C18:3n3 | 0.63 ± 0.04b | 0.74 ± 0.11b | 1.04 ± 0.03a | 0.66 ± 0.04b |
| C20:5n3 | ND | 0.26 ± 0.16b | 3.36 ± 0.73a | 0.42 ± 0.04b |
| C22:5n3 | 0.52 ± 0.06c | 0.94 ± 0.11b | 2.55 ± 0.08a | 0.93 ± 0.04b |
| C22:6n3 | 3.41 ± 0.35b | 3.56 ± 0.44b | 10.11 ± 0.74a | 4.04 ± 0.31b |
| Other/Unidentified | 2.53 ± 0.23 | 2.16 ± 0.25 | 3.62 ± 0.24 | 2.38 ± 0.16 |
Data are represented as wt/wt% with mean ± SE (n = 5). Means without a common letter differ, P ≤ 0.05. ND, not detected. FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
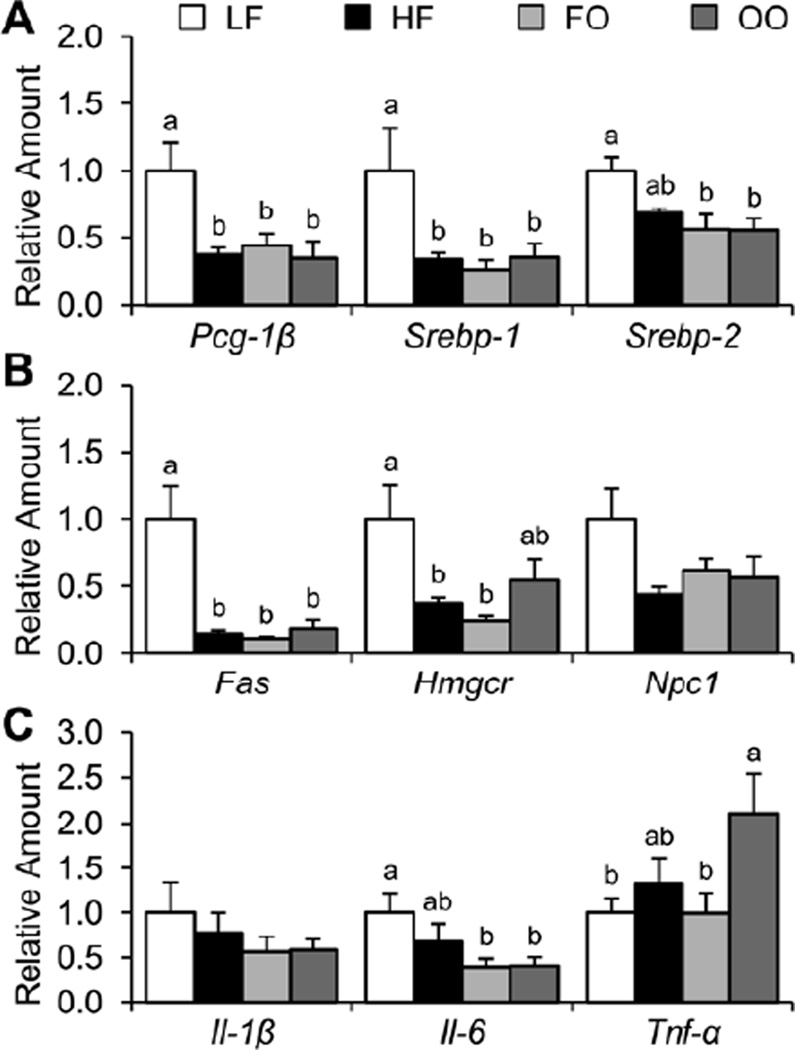
Relative amounts of liver transcription factor, target gene, and inflammatory cytokine mRNA
As shown in Figure 4, mice fed HF, FO, or OO diets had significantly decreased amounts of Pgc-1β and Srebp-1 mRNA compared to mice fed the LF diet. Moreover, mice fed FO or OO, but not HF diet had significantly decreased amounts of Srebp-2 mRNA compared to mice fed the LF diet. As expected, mice fed HF, FO, and OO diets with significantly decreased amounts of Pgc-1β and Srebp-1 mRNA which are transcription factors for the Fas gene had significantly decreased amounts of Fas mRNA. Moreover, consistent with mice fed HF, FO, and OO diets having decreased amounts of Srebp-1 and Srebp-2 mRNA, these mice had significantly decreased amounts of Hmgcr mRNA, with mice fed the OO diet having a significantly increased amount of Hmgcr mRNA compared to mice fed HF and FO diets. Although mice fed HF, FO, and OO diets had on average decreased amount of Npc1 mRNA compared to mice fed the LF diet, the differences were not statistically significant. With respect to the amounts of liver inflammatory cytokine, although mice fed FO and OO diets had significantly decreased amounts of Il-6 mRNA compared to mice fed the LF diet, there were no differences in the amounts of Il- 1β mRNA. Finally, mice fed the OO diet had significantly increased amounts of Tnf-α mRNA. Therefore, mice fed HF, FO, or OO diets were characterized with feedback inhibition of the SREBP pathway and downregulation of respective target gene mRNA.
Fig. 4.
Relative amounts of liver transcription factors (A), target genes (B), and inflammatory cytokines (C) mRNA were determined for mice fed the four diets at 30 wk. Values are presented as mean ± SE (n = 6–8). Means without a common letter differ (P < 0.05). FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
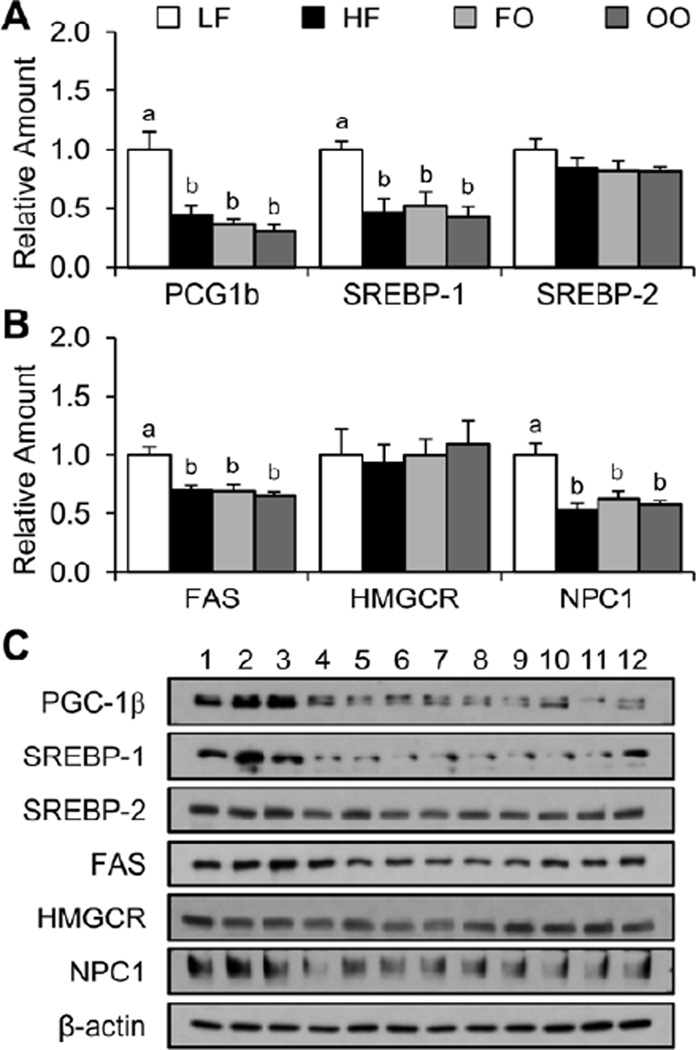
Relative amounts of liver transcription factor and target gene protein
As shown in Figure 5, mice fed HF, FO, or OO diets had significantly decreased amounts of PGC-1β and m-SREBP-1 protein compared to mice fed the LF diet. However, there was no significance difference in the amounts of m-SREBP-2 protein for mice fed the four diets. Consistent with results indicating that high-fat diets decreased the amounts of m-SREBP-1 but not m-SREBP-2 protein, the amounts of FAS but not HMGCR was significantly decreased compared to mice fed the LF diet. Finally, mice fed HF, FO, and OO diets had significantly decreased amounts of NPC1 protein compared to mice fed the LF diet.
Fig. 5.
Relative amounts of liver transcription factors (A) and target genes (B) protein were determined for mice fed the four diets at 30 wk. Representative Western blots are provided (C). Values are presented as mean ± SE (n = 6). Means without a common letter differ (P < 0.05). FO, high-fat diet supplemented with fish oil; HF, high-fat diet supplemented with lard; LF, low-fat diet; OO, high-fat diet supplemented with olive oil.
Discussion
The present study used C57BL/6J mice fed high-fat diets supplemented with 10% lard, 10% fish oil, or 10% olive oil, to investigate metabolic features associated with T2D. The results indicated that mice fed a high-fat diet supplemented with fish oil had improved glucose tolerance after extended feeding compared to mice fed a high-fat diet supplemented with lard. Moreover, mice fed a high-fat diet supplemented with fish oil diet had significantly decreased concentrations of liver lipids (cholesterol, cholesteryl ester, and triacylglycerol) compared to mice fed a high-fat diet supplemented with either lard or olive oil. Therefore, a high-fat diet supplemented with fish oil improved metabolic features associated with type 2 diabetes, including impaired glucose tolerance and hepatic steatosis.
The C57BL/6J mouse model has been used extensively to investigate nutrition-related weight gain and associated metabolic features. However, to our knowledge, the present study represents the first comparative analysis performed to investigate weight gain and metabolic features using this mouse model when fed a high-fat diet supplemented with moderate amounts of lipids from different sources over an extended period (15 and 30 wk). In brief, mice fed HF, FO, and OO diets had increased body weights at 15 and 30 wk compared to mice fed the LF diet, but there were no significant differences in body weights between mice fed the high-fat diets. This latter result contrasts previous studies suggesting that C57BL/6J mice fed a high-fat diet supplemented with fish oil have lessens energy balance [9, 15]. However, it must be noted that consistent with these studies our results indicated that mice fed the FO diet had significantly decreased EWAT weights (12%) compared to mice fed the HF diet at 15 wk, but not when extended to 30 wk. When mice were fed high-fat diets for an extended period (30 wk) the results indicated a significantly decreased EWAT weight, even compared to mice fed the LF diet. These results were verified upon examination of adipose tissue obtained from mice fed the high fats diets indicating decreased EWAT adipocyte size (adipocyte atrophy) at 30 wk. Moreover, the decrease in EWAT weight for mice fed the high-fat diets was inversely associated with an increase in liver weights at 30 wk. This redistribution of weight from EWAT to the liver may be explained by the development of EWAT insulin resistance characterized, in part, by enhanced lipolysis of adipose tissue triacyglycerol and transfer of free fatty acids to the liver [16, 17].
Although there were no significant differences in the body weight of mice fed high-fat diets supplemented with fatty acids from different sources, the results indicated that mice fed the FO diet had improved glucose tolerance compared to mice fed the HF diet at 30 wk. This result initially suggested that mice fed the FO diet had less insulin resistance. However, upon performing insulin tolerance tests and determining measures of insulin resistance (HOMA-IR, HOMA-β, and QUICKIE) it became obvious that mice fed the FO diet were not less insulin resistant but instead secreted more insulin which compensated for the impaired glucose tolerance, compared to mice fed the HF diet. This particular result is consistent with studies indicating that fish oil and other natural products such as glyceollin-containing fermented soybeans can improve pancreatic islet function by lessening the storage of triacylglycerol and thereby improve glucose-stimulated insulin secretion [18, 19]. Moreover, the increased concentration of n-3 FAs (C18:3n3, C20:5n3, C22:5n3, C22:6n3) in livers of mice fed the FO diet was inversely associated with the concentration of sterol (cholesterol and cholesteryl ester) in this tissue which may have also improved hepatic insulin signaling as recently shown by performing gastric bypass in a diet-induced rat model [20]. Similarly, other studies indicate that a high-fat diet supplemented with fish oil or eicosapentaenoic acid may improve glucose tolerance by decreasing adipose tissue lipogenesis and inflammation [8, 21]. In the present study, the expression of liver inflammatory cytokines (IL-1β, IL-6, and TNF-α) and the concentration of these cytokines in plasma of mice fed the FO diet were not significantly different compared to mice fed the HF diet. Moreover, the concentration of anti-inflammatory cytokines (IL-4, IL-10, and IL-14) in plasma of mice fed the different diets was present in only trace amounts and therefore insufficient to quantitate.
In conclusion, a high-fat diet supplemented with fish oil improved metabolic features associated with type 2 diabetes, including impaired glucose tolerance and hepatic steatosis, purportedly through improvement of pancreatic islet function.
Supplementary Material
Acknowledgements
This work was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) through grant number UL1 TR000041, the Diabetes Research Center at the University of Washington through grant number P30 DK017047 as an institutional affiliate and the Seattle Mouse Metabolic Phenotyping Center (MMPC) at the University of Washington through grant number U24 DK076126, a grant from the Dedicated Health Research Funds at the University of New Mexico School of Medicine, a grant from the Tohono O’odham Nation, and private donations for the investigation of childhood genetic and metabolic diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution: Conception and design of the study (DJ and WSG), generation, collection, assembly, analysis and/or interpretation of data (DJ, JJC, SLA, LMR, and WSG), drafting or revision of the manuscript (DJ, JJC, and WSG), and all authored approval the final version of the manuscript.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world - A growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen CH, Andersen G. Gene-environment interactions and obesity - Further aspects of genomewide association studies. Nutrition. 2009;25:998–1003. doi: 10.1016/j.nut.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 4.Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: A resource guide for complex trait analysis. Nat Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa S, Yasoshima A, Doi K, Nakagawa H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim. 2007;56:263–272. doi: 10.1538/expanim.56.263. [DOI] [PubMed] [Google Scholar]
- 6.Winzell MS, Ahren B. A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high-fat diet-induced obesity and leptin resistance in C57BL/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 8.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Kondo H, Hase T, Tokimitsu I, Murase T. Dietary fish oil upregulates intestinal lipid metabolism and reduces body weight gain in C57BL/6J mice. J Nutr. 2007;137:2629–2634. doi: 10.1093/jn/137.12.2629. [DOI] [PubMed] [Google Scholar]
- 10.Jelinek D, Millward V, Birdi A, Trouard TP, Heidenreich RA, Garver WS. Npc1 haploinsufficiency promotes weight gain and metabolic features associated with insulin resistance. Hum Mol Genet. 2010;20:312–321. doi: 10.1093/hmg/ddq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Garver WS, Jelinek D, Oyarzo JN, Flynn J, Zuckerman M, Krishnan K, et al. Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J Cell Biochem. 2007;101:498–516. doi: 10.1002/jcb.21200. [DOI] [PubMed] [Google Scholar]
- 14.Weston TR, Derner JD, Murrieta CM, Rule DC, Hess BW. Comparison of catalysts for direct transesterification of fatty acids in freeze-dried forage samples. Proc West Sec Am Soc Anim Sci. 2006;57:1–4. [Google Scholar]
- 15.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Vecka M, et al. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39:1177–1185. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 16.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, et al. Molecular evidence supporting the portal hypothesis: A causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol. 2005:E454–E461. doi: 10.1152/ajpendo.00203.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–761. doi: 10.2337/diabetes.51.3.755. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Kim DS, Kim JH, Kim JS, Kim HJ. Glyceollin-containing fermented soybeans improve glucose homeostasis in diabetic mice. Nutrition. 2012;28:204–211. doi: 10.1016/j.nut.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Pighin D, Karabatas L, Rossi A, Chicco A, Basabe JC, Lombardo YB. Fish oil affects pancreatic fat storage, pyruvate dehydrogenase complex activity, and insulin secretion in rats fed a sucrose-rich diet. J Nutr. 2003;133:4095–4101. doi: 10.1093/jn/133.12.4095. [DOI] [PubMed] [Google Scholar]
- 20.Bonhomme S, Guijarro A, Keslacy S, Goncalves CG, Suzuki S, Chen C, et al. Gastric bypass up-regulates insulin signaling pathway. Nutrition. 2011;27:73–80. doi: 10.1016/j.nut.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Muurling M, Mensink RP, Pijl H, Romijn JA, Havekes LM, Voshol PJ. A fish oil diet does not reverse insulin resistance despite decreased adipose tissue TNF-alpha protein concentration in ApoE-3 Leiden mice. J Nutr. 2003;133:3350–3355. doi: 10.1093/jn/133.11.3350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.