Abstract
Objective
To determine whether patients with thin bone over the superior semicircular canal can develop signs or symptoms of superior canal dehiscence syndrome (SCDS).
Study Design
Retrospective case series
Setting
Tertiary referral center
Patients
All patients from our institution found to have thin but not frankly dehiscent bone over the superior canal despite symptoms and signs of SCDS.
Main outcome measures
Pre-operative CT imaging, symptoms, audiometry, vestibular evoked myogenic potentials (VEMP), and intraoperative electrocochleography (ECochG) results were reviewed. Symptoms were assessed at least 1 month post-operatively in all patients, and post-operative physiologic data are presented when available.
Results
10 patients (11 ears) had thin bone over the superior semicircular canal at surgery. All presented with autophony or sound- and/or pressure-induced vertigo, in addition to at least one physiologic measure consistent with SCDS. CT imaging was read as showing either dehiscence (36%) or marked thinning of bone overlying the affected canal (64%). Pre-operative median low-frequency air-bone gap (ABG) was elevated (10.9 dB, Interquartile range [IQR] 8.8–12.5), with 4 patients demonstrating negative bone conduction thresholds. Patients had elevated oVEMP amplitude (median 20.7, IQR 6.7–22.1) μV and ECochG SP/AP ratios (median 0.59, IQR 0.54–0.67). Post-operative ABG and SP/AP ratio decreased significantly compared to pre-operative values (p<0.05), and all patients reported symptomatic improvement.
Conclusions
Symptoms typical of SCDS can occur in cases with thin but not dehiscent bone. Surgical plugging or resurfacing can reduce symptoms in such cases.
Keywords: Superior Canal Dehiscence Syndrome, third mobile window
INTRODUCTION
In superior canal dehiscence syndrome (SCDS), an opening in the bone overlying the superior semicircular canal causes a spectrum of symptoms including bone conduction hyperacusis and vertigo elicited by loud sounds or Valsalva maneuvers (1,2). Physiological findings consistent with this syndrome include the presence of a low-frequency air-bone gap (ABG) on audiogram (3) and abnormalities in vestibular-evoked myogenic potentials (VEMP) including low cervical VEMP (cVEMP) thresholds and elevated ocular VEMP (oVEMP) peak-to-peak amplitudes (4,5). The current theory describing these abnormalities in SCDS is the “third mobile window” hypothesis, which suggests that acoustic energy transmitted to the oval window is partially shunted through this abnormally compliant “window” caused by a dehiscent semicircular canal (6,7). Recent studies have additionally identified an elevated summating potential (SP) to action potential (AP) ratio on electrocochleography (ECochG) in patients with SCDS (8,9).
Important to the diagnosis of SCDS in addition to the above clinical symptoms and physiological findings is the presence of a dehiscence on computed tomography (CT) imaging. With high-resolution imaging and reformation in the plane of the superior semicircular canal, however, CT imaging may misdiagnose the presence of a dehiscence (10). In particular, the limitations of resolution even with the best CT protocols may make thin remaining bone appear dehiscent. Furthermore, whether a third mobile window becomes symptomatic with thin or compliant bone present is currently unknown. When SCDS is properly diagnosed, surgical plugging of the dehiscent semicircular canal by a middle cranial fossa approach can result in both symptomatic improvements (11,12), and normalization of the above abnormal physiological tests (9,13,14). Variations on this surgical technique include resurfacing (rather than plugging) the canal or approaching it via the mastoid cavity (15–17). The purpose of this study was to review clinical characteristics of all diagnosed cases of SCDS performed at our institution in which at the time of middle cranial fossa approach, thin bone but not frank dehiscence was identified.
MATERIALS AND METHODS
Study Population
Clinical data from patients undergoing surgical repair of superior canal dehiscence via the middle cranial fossa approach from 1998 until July 2012 were reviewed retrospectively. Subjects were included in this study if the operative note reported that no dehiscence was seen at the time of surgery or that there was thinning of the bone over the superior semicircular canal. Patients were diagnosed with SCDS based on history and physical examination and at least one abnormal clinical physiologic test consistent with superior canal dehiscence syndrome, including vestibular-evoked myogenic potential (VEMP), low frequency ABG or sound and/or pressure induced eye movements. Patients must also have had evidence from high-resolution CT of a dehiscent superior semicircular canal or marked thinning of the bone over the superior semicircular canal on reformation in the plane of the affected canal and orthogonal to that plane. Reconstructions were from helical sections obtained at 0.5 mm collimation in the axial planes (18,19). The presence of other pathology associated with a third mobile window (enlarged vestibular aqueduct, other vestibular fistulae, etc.) was ruled out based on clinical examination and CT imaging. Of 269 patients diagnosed with SCDS at Johns Hopkins during the study period, 157 patients underwent surgical repair. A total of 10 patients (11 ears) were identified as having thin bone overlying the superior semicircular canal at the time of surgery and were included in this study. All patients involved in this study had agreed to undergo surgical repair of the affected semicircular canal. This study was a review of existing clinical data with patient identifiers removed. It qualified for exemption from an institutional review board protocol on the basis of United States Department of Health and Human Services criteria 45 CFR 46.101(b4). The Johns Hopkins Institutional Review Board approved this exemption.
Surgical Technique
The middle cranial fossa surgical approach was used in all patients, and intraoperative image guidance was used for all procedures to locate the affected canal (20). Plugging of the canal was performed in 8 affected ears, with the lumen being accessed by drilling open the thin bone overlying the affected canal for a length of 3–4 mm and gently but securely packing fascia strips, bone dust and bone chips inside the dehiscent canal to obliterate the canal lumen for 2–3 mm beyond either end of the dehiscence opening. Careful avoidance of unnecessary force on or suction near the membranous labyrinth was observed throughout the procedure. In all 11 procedures, the repair was covered with hydroxyapatite cement (Hydroset®, Stryker, Kalamazoo, Michigan) followed by a layer of fascia and fibrin glue. Resurfacing without plugging was performed in 3 ears. Intraoperative neural monitoring of the facial nerve, auditory brainstem response, electrocochleography (ECochG), and somatosensory evoked potentials was performed. All patients received dexamethasone (6 to 8 mg) three times per day for three doses. If post-operative clinical examination revealed no evidence of either sensorineural hearing loss by Weber tuning fork examination or labyrinthine hypofunction by horizontal head impulse testing, then steroids were tapered over five days. Patients with a tuning fork examination that lateralized to the non-surgical ear or with overt saccades during head impulse testing in the plane of the horizontal semicircular canal on the side of surgery had steroids tapered over a 10–14 day period.
Audiometry
All patients underwent audiometric testing prior to surgery and at least 1 month post-operatively. Pure-tone hearing thresholds were obtained using both air conduction (AC) at 250, 500, 1000, 2000, 4000 and 8000 Hz and bone conduction (BC) at 250, 500, 1000, 2000 and 4000 Hz. The audiometer was calibrated to permit accurate measurement of BC thresholds to −10 dB HL. Speech discrimination was also tested for all patients using the Northwestern University word list 6 (NU-6). The air-bone gap (ABG) was calculated by subtracting the BC threshold from the AC threshold at each frequency. The ABG was averaged for each audiogram over the lower frequencies including 250, 500, 1000 and 2000 Hz. A 4-tone pure tone average (PTA) using AC thresholds was calculated across 500, 1000, 2000 and 4000 Hz (21).
Vestibular-evoked Myogenic Potentials
VEMP testing was performed pre-operatively in all patients using either click stimulus cervical VEMPs or tone burst ocular VEMPs as previously described (22). Testing was performed in a semi-recumbent position, and responses were recorded with disposable silver/silver chloride electrodes. A commercial electromyographic (EMG) system was used (Medetec Synergy, software version 14.1, Care Fusion, Dublin, OH, USA) and AC stimuli were administered via headphones. Stimuli included 0.1 ms positive polarity clicks at a repetition rate of 5 per second for cVEMPs and 500-Hz, 125-dB sound pressure level (SPL) positive polarity tone bursts with 1 ms rise/fall time and 2 ms plateau and repetition rate of 5 per second for oVEMPs. EMG signals were amplified (2500 μV) and band pass filtered (20–2000 Hz). One hundred sweeps were averaged for each test. For cVEMP recordings, responses were rectified for baseline sternocleidomastoid muscle activity, and the lowest dB SPL at which a p13 and n23 response could be recorded was the threshold. For oVEMP testing, n10 amplitude was measured as the maximum negative voltage of the n10 potential. Ocular VEMP amplitudes in response to midline taps with a reflex hammer were recorded for subject 11 who had a middle-ear conductive hearing loss from prior stapedectomy.
Electrocochleography
Intraoperative ECochG was performed using gold foil tiptrodes (Etymotic Research Inc., Elk Grove, IL), which were placed adjacent to the tympanic membrane in the external auditory canal and stabilized at the foam tip of the insert audio transducer. Electrodes were introduced after general anesthesia was induced. Unfiltered clicks of 100-μs duration were presented at an intensity of 85 dB nHL. Two replications of averaged responses elicited by 1,500 clicks presented at a rate of 11.7 per second were obtained. Responses were band pass filtered (20–1500 Hz) and averaged, and the summating potential to action potential (SP/AP) ratio was calculated. SP/AP ratio of greater than 0.4 was defined as abnormal for purposes of this study, based on commonly used standards for clinical testing (23).
Data Analysis
Summary statistics were performed for demographics, ABG, PTA, VEMP amplitudes or thresholds, and SP/AP ratio for affected ears. Due to small n and non-normal distribution, non-parametric tests were use for all analyses. Wilcoxon matched pairs test was performed for differences between preoperative and postoperative continuous outcomes. Wilcoxon rank-sum test was used to compare pre-procedure ABG and SP/AP ratio to all intraoperative confirmed cases of SCDS at our institution from July 2008 to July 2012. Kruskal-wallis test was performed for between group comparisons of oVEMP peak-to-peak amplitude between subjects with thin bone, intraoperative confirmed dehiscence, and laboratory controls. Post-hoc comparisons were made with Wilcoxon rank-sum test. Associations were considered statistically significant for 2-sided statistics with a p-value < 0.05. All analyses were performed using Stata 12.0 (StataCorp, College Station, TX, USA).
RESULTS
Of 157 patients identified during the study period who had undergone surgical repair for SCDS by middle cranial fossa approach, 10 patients (11 ears, 7%) were found to have thinning of bone over the superior semicircular canal at the time of surgery without a frank dehiscence. The operative note described compliance of the thin bone overlying the superior semicircular canal in 3 of these cases. Eight affected ears underwent uncovering of the thin bone with both plugging and resurfacing of the superior semicircular canal, while 3 ears underwent resurfacing alone. One patient subsequently underwent contralateral surgery for symptoms of SCDS and was found to have thin bone during both procedures (subjects 3 and 5). In this individual, plugging and resurfacing was performed on the initial surgery, followed by resurfacing alone on the second side. High-resolution CT imaging as read by a neuroradiologist resulted in a radiologic misdiagnosis of a dehiscent semicircular canal on the affected side in 4 out of 11 cases (36%) and probable dehiscence in an additional case (9%). The CT images of remaining ears were read as having either equivocal dehiscence or thin bone overlying the superior semicircular canal. The attending surgeon interpreted the images as dehiscent in 5 cases (45%) and probably dehiscent in 3 additional cases (27%). Figure 1 demonstrates cases both of thin bone over the superior semicircular canal and of a case of overt dehiscence as found at surgery.
Figure 1.
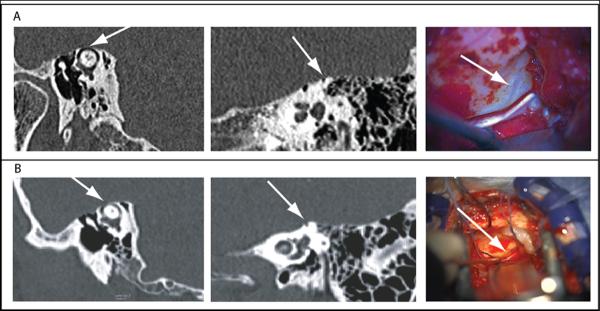
Example of CT imaging and intraoperative findings of left near dehiscence (figure A) and case of overt 2-mm left superior canal dehiscence (figure B). CT images show reformation in plane parallel to the superior semicircular canal and orthogonal to it. Arrows point to location of thin bone or dehiscence. Intraoperative microscopic views of dehiscence or near dehiscence are shown in the right panels. Photoshop filtering used to adjust blue content of entire intraoperative picture of near dehiscence to accentuate the blue-lining seen by eye at surgery.
Demographic information and presenting symptoms for the 10 patients (11 affected ears) are shown in table 1. The mean (SD) age at presentation was 41.6 (13.2) years. There were 8 (80%) women and 7 (64%) right ears. Presenting symptoms included vertigo or imbalance induced by straining or loud sounds (9 of 11 subjects), autophony (9 of 11), tinnitus (all affected ears, pulsatile in 10 of 11, “hissing” in one ear) and aural pressure or fullness (9 of 11). On clinical examination using Frenzel lenses, 4 subjects had slow-phase eye movements evoked by sound or pressure. Prior to presentation, 4 subjects had tympanostomy tubes placed (subjects 7,8, 9, 10). One had undergone stapedectomy (subject 11) with resultant hearing loss in the affected ear and without symptomatic relief. Three patients (subjects 1, 4, and 8) had comorbid migraine-related dizziness and were medically treated without improvement prior to undergoing surgery.
Table 1.
Pre-operative demographics and presenting symptoms
| Subject | Gender | Age (years) | Side | CT Interpretation | Sound/Pressure-induced Vertigo | Nystagmus | Pressure/ Fullness | Pulsatile Tinnitus | Autophony |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 34 | L | Bilateral dehiscence | yes | yes | yes | yes | no |
| 2 | M | 51 | R | Bilateral dehiscence | no | no | yes | no | yes |
| 3* | F | 19 | R | Bilateral thinning | yes | no | no | yes | no |
| 4 | F | 49 | L | L thinning/dehiscent | yes | no | yes | yes | yes |
| 5* | F | 20 | L | Bilateral thinning | no | no | yes | yes | yes |
| 6 | F | 47 | R | Possible R dehiscence | yes | no | yes | yes | yes |
| 7 | F | 49 | L | Not definite L | yes | yes | no | yes | yes |
| 8 | F | 55 | R | Probable R dehiscence | yes | no | yes | yes | yes |
| 9 | M | 55 | R | Not definite R | yes | yes | yes | yes | yes |
| 10 | F | 32 | R | R dehiscence | yes | yes | yes | yes | yes |
| 11 | F | 47 | R | Possible R dehiscence | yes | no | yes | yes | yes |
Presenting demographic information and symptoms for all subjects. CT interpretation is pre-operative interpretation by a neuroradiologist with reformation in the plane of the superior semicircular canal and orthogonal to that plane. Presenting symptoms include the presence of vertigo or disequilibrium induced by loud sounds or valsalva, nystagmus elicited by sound or pressure on clinical examination, pressure or fullness in the affected ear, pulsatile tinnitus, and autophony or internally transmitted sounds.
Pre-operative audiometry revealed a median low frequency ABG of 10.9 dB (IQR 8.8–12.5). This was significantly lower than those with an intraoperative confirmed dehiscence (median 18.8 dB, IQR 13.8–21.9, p<0.01). Four subjects (36%) had at least a negative 10 dB BC threshold for one or more frequencies on pre-operative audiometry. At greater than 1 month after surgery, the ABG of all subjects with thin bone had decreased to a median of 6.25 dB (IQR 5.0–8.8, p<0.05). There were no significant differences in median PTA at longer than 1 month post-surgery (p=0.08), however, two subjects (subjects 3 and 8) experienced greater than 20 dB increase in their AC PTA after the bone overlying the superior semicircular canal was uncovered and plugged. Subject 11 was excluded as an outlier from hearing analysis due to prior stapedectomy and resultant hearing loss.
VEMP results are shown in table 2. Pre-operative median cVEMP thresholds were 90 dB (IQR 75–95), with 3 out of 6 subjects with present cVEMP responses demonstrating reduced thresholds. Responses were absent in 3 subjects and could not be obtained due to discomfort in subject 2. Among the 5 subjects who underwent cVEMP testing before and after surgery, there were no significant differences in the thresholds of the affected ears post-operatively. For oVEMP testing, the median peak-to-peak amplitude for all subjects was 20.7 (IQR 6.7–22.1) μV. This was lower than those with intraoperative confirmed SCDS (median 45.6, IQR 32–67.8, p<0.01), but higher than laboratory controls (median 3.7, IQR 2.8–6.9, p<0.01). All four subjects with available post-operative tone burst oVEMP had a decline in peak-to-peak amplitude post-operatively.
Table 2.
Physiological test results for affected ear of each study participant.
| Pre-operative | Post-operative | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | ABG (dB HL) | PTA (dB HL) | oVEMP (uV) | cVEMP (dB HL) | SP/AP ratio | ABG (dB HL) | PTA (dB HL) | oVEMP (uV) | cVEMP (dB HL) | SP/AP ratio |
| 1 | 16.25 | 13.75 | -- | 75 | -- | 5 | 8.75 | -- | 75 | -- |
| 2 | 11.25 | 8.75 | 7.01 | refused | -- | 3.75 | 13.75 | refused | refused | -- |
| 3* | 16.25 | 5 | -- | 95 | 0.61 | 7.5 | 26.25 | 2.6 | 95 | 0.41 |
| 4 | 8.75 | 23.75 | 22.1 | -- | 0.57 | 2.5 | 26.25 | -- | -- | 0.35 |
| 5* | 10 | 3.75 | 20.7 | 85 | 0.38 | 8.75 | 5 | 14.8 | 85 | 0.36 |
| 6 | 12.5 | 8.75 | 18.9 | Absent | -- | 6.25 | 10 | -- | -- | -- |
| 7 | 11.25 | 32.5 | 6.7 | 100 | 0.67 | 7.5 | 36.25 | 5.7 | 95 | 0.47 |
| 8 | 7.5 | 20 | Absent | 95 | 0.88 | 10 | 67.5 | -- | -- | 0.40 |
| 9 | 5 | 45 | Absent | Absent | 0.54 | -- | -- | -- | -- | 0.39 |
| 10 | 10 | 8.75 | 21.3 | Absent | -- | 5 | 20 | 1.6 | Absent | -- |
| 11± | 31.25 | 51.25 | 25.2 | 75 | -- | 35 | 48.75 | 0.9 | 75 | -- |
Pre-operative and at least 1 month post-operative physiological values for the affected ear if available; ABG, air-bone gap, average air-bone gap of 250, 500,1000 and 2000 Hz; PTA, pure tone average, average of air-conduction thresholds at 500, 1000, 2000 and 4000 Hz; oVEMP, ocular vestibular-evoked myogenic potential; cVEMP, cervical vestibular-evoked myogenic potential, value=threshold for response; SP/AP, summating potential to action potential ratio on intraoperative electrocochleography,
indicates bilateral repair,
± subject had prior stapes surgery and subsequent hearing loss, thus forehead taps with reflex hammer used for oVEMP testing.
Intraoperative electrocochleography was performed in 9 of 11 subjects. Six out of 9 subjects had reproducible electrocochleography tracings before and after the procedure. The other 3 out of 9 subjects lacked reproducible tracings due to technical difficulties attributed to the operating room environment. Of those 6 subjects with reproducible recordings, the median pre-procedure SP/AP ratio was 0.59 (IQR 0.54–0.67), which decreased to 0.39 (IQR 0.36–0.43) at the conclusion of the case (p=0.03). There was a trend toward increased AP amplitude after plugging (p=0.058). The pre-procedure SP/AP ratio was not different from those with observed dehiscence during surgery (median 0.58, IQR 0.41–0.73, p=0.61). Five out of 6 subjects had SP/AP ratios greater than 0.4. Subject 5 had an SP/AP ratio below 0.4, and had no change after resurfacing. Subject 7 also was resurfaced alone and though the SP/AP ratio decreased, it did not normalize. All 4 affected ears with thin bone that was uncovered and plugged experienced decreases in their SP/AP ratio to below 0.4 at the conclusion of the case.
At least one month post-surgery, all patients reported improvement or resolution of at least one of their primary presenting symptoms. The majority of patients reported improvement or resolution of vertigo or disequilibrium induced by pressure or loud sounds (6 of 8 subjects), tinnitus (8 of 9 affected ears), and autophony in the affected ear (all ears) (Table 3). Three subjects experienced a temporary improvement, however, with two of these three subjects developing SCDS recurrence of their most bothersome symptoms after initial resolution (subjects 7 and 9) and a third (subject 3) developing symptoms in the contralateral ear. Of the 8 subjects whose thin bone was uncovered and plugged, 4 (50%) developed temporary vestibular hypofunction as evaluated by horizontal head impulse testing on post-operative day 1. Two subjects (subjects 3 and 10) developed a transient delayed facial nerve weakness and have since fully regained facial nerve function. Two subjects had moderate-to-severe sensorineural hearing loss (subjects 3 and 8). The first two subjects with thin bone who underwent plugging (subjects 1 and 2) were admitted within one week after discharge with post-operative nausea and dehydration. Of the 3 subjects who were resurfaced without plugging (subjects 5, 7 and 11), none developed post-operative complications; however, subject 7 developed symptom recurrence after temporary improvement.
Table 3.
Post-operative symptoms and surgical complications.
| Subject | Procedure | Vertigo | Pressure/ Fullness | Pulsatile Tinnitus | Autophony | Early Vestibular hypofunction | Post-surgical Complications |
|---|---|---|---|---|---|---|---|
| 1 | Plug+Resurface | Not noted | Recurred | Improved | NA | No | Readmission for nausea/dehydration |
| 2 | Plug+Resurface | Improved | Not noted | NA | Improved | No | Readmission for nausea/dehydration |
| 3* | Plug+Resurface | Improved | NA | Improved | NA | Yes | Delayed transient facial nerve weakness, SNHL |
| 4 | Plug+Resurface | Improved | Resolved | Resolved | Resolved | Yes | None |
| 5* | Resurface | NA | Not noted | Resolved | Resolved | No | None |
| 6 | Plug+Resurface | Not noted | Not noted | Resolved | Resolved | No | None |
| 7 | Resurface | Improved | NA | Recurred | Recurred | Yes | None |
| 8 | Plug+Resurface | Recurred | Improved | Resolved | Resolved | No | Delayed SNHL |
| 9 | Plug+Resurface | Recurred | Not noted | Not noted | Resolved | No | None |
| 10 | Plug+Resurface | Resolved | Not noted | Persistent | Improved | Yes | Delayed transient facial nerve weakness |
| 11 | Resurface | Improved | Resolved | not noted | Improved | No | None |
Post-surgical symptoms as obtained from medical records at least 1 month post-operatively and post-surgical complications. Symptoms are recorded as improved, resolved, recurred, not noted for absent data, or NA, not applicable if not present preoperatively. Procedure plug+resurface is defined as uncovering of thin bone and plugging and resurfacing the affected semicircular canal, and resurface as resurfacing alone without plugging. Early vestibular hypofunction is defined as overt saccade by horizontal head impulse testing on post-operative day 1 (This does not refer to permanent hypofunction). SNHL, sensorineural hearing loss with at least 20 dB increases in pure tone average.
indicates bilateral repair.
Discussion
In this study, we identified a cohort of patients with “near dehiscence” of the superior canal who had symptoms and physiological testing consistent with SCDS but who had thinning of bone overlying the superior semicircular canal found at the time of surgery. The thin bone found at surgery has the appearance of a surgically “blue-lined” canal (Figure 1). This case series identifies several clinical findings that may aid managing patients with an equivocal diagnosis of SCDS. Although patient symptoms in this series were consistent with the clinical SCD syndrome, physiological testing was either on the border of being normal or was abnormal in one of several tests performed. Furthermore, although the majority of patients with near dehiscence symptomatically improved after surgery, these patients may be at increased risk for post-surgical complications, particularly if the thin bone is uncovered and then plugged.
High-resolution CT imaging with reformation in the plane of the canal led the interpreting neuroradiologist to diagnose dehiscence or probable dehiscence in nearly half of cases in this series, while the surgeon diagnosed dehiscence or probable dehiscence in 72% of cases. Prior studies have identified limited abilities of both traditional temporal bone CT and high-resolution CT imaging to identify cases of SCD (24). A cadaveric study by Sequeira et al. found high-resolution CT can diagnose a dehiscence when thin bone is present (10). In the present study the radiographic interpretation of the CT identified a definite dehiscence in 4 cases (36%) and probable dehiscence in an additional case (9%). Thus, approximately one-third of cases of thin bone might be read as dehiscent. This is similar to the risk of misdiagnosis predicted by cadaveric studies (10,25).
CT scans from several patients in this series were correctly identified preoperatively as having thin bone on CT imaging. This has tended to occur in more recent cases and reflects, in part, the increasing experience we have gained in interpreting the CTs in light of surgical findings in previous cases. Although not previously reported, symptomatic improvement after surgery in the first cases incidentally discovered to have thin, compliant bone over the superior semicircular canal, suggested that pressure transmission through a near dehiscence might occur. Although the patients in this series did not have direct contact between the labyrinth and middle cranial fossa, bone overlying the superior semicircular canal was noted to be thin or abnormally compliant and could provide a low-impedance pathway for acoustic energy. Evidence from this cohort to support this hypothesis include the presence of an air-bone gap with bone conduction hyperacusis in some patients, as well as elevated oVEMP potentials compared with age-matched controls (although less elevated than in cases of frank dehiscence). Nearly all patients presented with auditory symptoms such as autophony and pulsatile tinnitus, symptoms that are more common in this series than previous reports of SCDS (26,27). These symptoms indicated abnormal transmission of internal sounds to the affected ear. Furthermore, the presence of vertigo or imbalance with loud sounds and Valsalva maneuvers in most patients suggested pressure changes across the labyrinth. That both these symptoms and objective physiologic tests such as the air-bone gap and SP/AP ratio improved after plugging or resurfacing the thin otic capsule in the majority of patients further supports the theory that pressure changes can be transmitted through a near dehiscent semicircular canal.
Electrocochleography was performed during surgery and available in 6 of the 11 cases. Interestingly, the majority of these cases with SC thinning did demonstrate an elevated SP/AP ratio that decreased after plugging and resurfacing the affected superior semicircular canal. Intraoperative correction of an elevated SP/AP ratio in cases of SCDS has recently been reported (8,9); however, the clinical implications of this change and the cause of an elevated ratio remain unclear. Arts et al. speculate that a dehiscence may cause a bias in the basilar membrane (8). Alternatively, we propose that the diversion of acoustic energy to the third mobile window could alter either or both the summating potential and compound action potential, leading to an elevated SP/AP ratio that normalizes after closing this compliant “window”. Arts et al. identified an increase in compound action potential amplitude after plugging the dehiscent superior canal. This series also identified a trend toward increased action potential amplitude after plugging. These findings may reflect alterations in pressure transmission away from the round window in cases of dehiscence or near dehiscence. Furthermore, recent work suggests sound sensitivity of saccular and utricular afferents (28,29). The contributions of saccular or utricular hair cells to the summating potential may help explain the elevated SP/AP ratio in these patients. A third mobile window that leads to pressure transmission across these structures could result in elevated summating potential that would decrease after repair of the dehiscence or near dehiscence. The influence of these organs on the summating potential in particular warrants further investigation.
There is reason to exercise caution in pursuing surgery in these cases. Although all patients noted initial improvement in at least one presenting symptom, 5 subjects (45%) had persistence or recurrence of at least one symptom at greater than one month after surgery. Furthermore, post-operative complications may have been higher than in our historical cases of frank dehiscence, but, with the small numbers involved, it is not clear if these differences are statistically significant. Superior canal dehiscence syndrome can present with a variety of symptoms that overlap with other otologic conditions (7). Some patients may also have comorbid disease such as migraine that can affect their recovery or could mimic symptoms of dehiscence altogether (27). It is therefore important to rule out or to treat other conditions with similar symptoms prior to considering surgery to plug or resurface a near dehiscence.
Patients should additionally be informed of the likelihood of finding a dehiscence at the time of surgery. While CT imaging was read as showing a dehiscence in a quarter of patients, VEMP testing may more reliably predict a near dehiscence. Ocular VEMP peak-to-peak amplitudes in particular fell between those cases of intraoperative confirmed dehiscence and normal controls. Air-bone gaps were also present on pre-operative audiometry in cases of near dehiscence, but were smaller than in cases of overt dehiscence (p<0.01), consistent with the finding that dehiscence size correlates with air-bone gap (30). While this study is underpowered to identify predictors of near dehiscence, results from this case series suggest that both pre-operative oVEMP peak-to-peak amplitude and air-bone gap may support clinicians in identifying a pre-operative near dehiscence.
The surgical approach in cases of near dehiscence may also influence outcome. We found that approximately half of subjects who underwent uncovering of a near dehiscence and plugging experienced a temporary labyrinthine hypofunction immediately after surgery, in addition to the expected deficiency in superior semicircular canal function. However, this rate of transient hypofunction is comparable to prior reports (31). This hypofunction did prompt a prolonged steroid taper in affected individuals. Two subjects who underwent uncovering of the dehiscence and plugging had moderate-to-severe sensorineural hearing loss, and 2 others experienced significant nausea to require admission for dehydration. The rate of moderate-to-severe hearing loss is higher in this series than prior reports (32,33). While the cause of this apparent increased complication rate is not clear, the complications could reflect trauma induced by opening a previously unexposed labyrinth. Image guidance was used during all procedures; however, there were two cases of delayed transient facial nerve palsy in this series. In one of these cases (subject 10) the navigation system post became dislodged, and navigation was not available for the remainder of the case. Subject 10 developed a House-Brackmann (HB) level V/VI on post-operative day 1 and subject 3 developed HB II/VI 10 days after surgery. Facial nerve function fully recovered in both cases. Though only 3 affected ears in this series underwent resurfacing of a near dehiscence without plugging, no complications were noted in the medical records of these subjects. One subject who underwent resurfacing, however, experienced recurrent symptoms 3 months after the procedure. Patients should be aware of potential increased surgical risk in these cases. Surgical navigation may be a particularly important consideration in cases of near dehiscence.
Limitations of this study include being a retrospective case series in which a surgical intervention was performed. As a result of not having non-surgical controls, definitive causal conclusions cannot be drawn regarding post-operative symptom improvement. The study is furthermore not powered to determine relative influence of surgical technique on post-operative outcomes. Since this is a retrospective series, there may be limitations in available data or reporting biases by treating physicians in the medical record. Future prospective studies investigating alternative treatment approaches for near dehiscence would be beneficial.
Conclusions
Patients with thin bone overlying the superior semicircular canal without overt dehiscence can present with symptoms of superior canal dehiscence syndrome. Furthermore, consistent with the third mobile window hypothesis, these cases of “near dehiscence” can have low-frequency air-bone gaps, elevated oVEMP amplitudes, and increased SP/AP ratios on electrocochleography. The mechanisms and frequency-dependence of these objective physiologic abnormalities in the presence of thin bone warrant additional study. Subjects with this “near dehiscence” syndrome generally do improve with surgery, but incomplete symptom relief and possible complications must be reviewed with patients. Air-bone gaps and oVEMPs that are intermediate between normal and frankly dehiscent SCD cases may indicate presence of near dehiscence.
Acknowledgements
The authors would like to thank Geraldine Zuniga for her help with providing Johns Hopkins laboratory data for ocular VEMPs and “Chely” Nirma Carballido Martinez for assistance with acquiring intraoperative electrocochleography data for review.
Funding provided by T32DC000027-22.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minor LB, Solomon D, Zinreich JS, et al. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–58. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Minor LB, Cremer PD, Carey JP, et al. Symptoms and signs in superior canal dehiscence syndrome. Ann N Y Acad Sci. 2001;942:259–73. doi: 10.1111/j.1749-6632.2001.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 3.Minor LB, Carey JP, Cremer PD, et al. Dehiscence of bone overlying the superior canal as a cause of apparent conductive hearing loss. Otol Neurotol. 2003;24:270–8. doi: 10.1097/00129492-200303000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Streubel SO, Cremer PD, Carey JP, et al. Vestibular-evoked myogenic potentials in the diagnosis of superior canal dehiscence syndrome. Acta Otolaryngol Suppl. 2001;545:41–9. doi: 10.1080/000164801750388090. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren SM, Aw ST, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials in superior canal dehiscence. J Neurol Neurosurg Psychiatry. 2008;79:559–68. doi: 10.1136/jnnp.2007.126730. [DOI] [PubMed] [Google Scholar]
- 6.Rosowski JJ, Songer JE, Nakajima HH, et al. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25:323–32. doi: 10.1097/00129492-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282–9. doi: 10.1097/mao.0b013e318161ab24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arts HA, Adams ME, Telian SA, et al. Reversible electrocochleographic abnormalities in superior canal dehiscence. Otol Neurotol. 2009;30:79–86. doi: 10.1097/MAO.0b013e31818d1b51. [DOI] [PubMed] [Google Scholar]
- 9.Adams ME, Kileny PR, Telian SA, et al. Electrocochleography as a diagnostic and intraoperative adjunct in superior semicircular canal dehiscence syndrome. Otol Neurotol. 2011;32:1506–12. doi: 10.1097/MAO.0b013e3182382a7c. [DOI] [PubMed] [Google Scholar]
- 10.Sequeira SM, Whiting BR, Shimony JS, et al. Accuracy of computed tomography detection of superior canal dehiscence. Otol Neurotol. 2011;32:1500–5. doi: 10.1097/MAO.0b013e318238280c. [DOI] [PubMed] [Google Scholar]
- 11.Crane BT, Minor LB, Carey JP. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118:1809–13. doi: 10.1097/MLG.0b013e31817f18fa. [DOI] [PubMed] [Google Scholar]
- 12.Crane BT, Lin FR, Minor LB, et al. Improvement in autophony symptoms after superior canal dehiscence repair. Otol Neurotol. 2010;31:140–6. doi: 10.1097/mao.0b013e3181bc39ab. [DOI] [PubMed] [Google Scholar]
- 13.Welgampola MS, Myrie OA, Minor LB, et al. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70:464–72. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- 14.Ward BK, Agrawal Y, Nguyen E, Della Santina CC, Francis HF, Limb CJ, Minor LB, Carey JP. Hearing Outcomes Following Surgical Plugging of the Superior Semicircular Canal by a Middle Cranial Fossa Approach. Otol Neurotol. 2012 doi: 10.1097/MAO.0b013e318268d20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlastarakos PV, Proikas K, Tavoulari E, et al. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: a meta-analysis of published interventional studies. Eur Arch Otorhinolaryngol. 2009;266:177–86. doi: 10.1007/s00405-008-0840-4. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal SK, Parnes LS. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008;29:363–7. doi: 10.1097/mao.0b013e3181616c9d. [DOI] [PubMed] [Google Scholar]
- 17.Fiorino F, Barbieri F, Pizzini FB, et al. A dehiscent superior semicircular canal may be plugged and resurfaced via the transmastoid route. Otol Neurotol. 2010;31:136–9. doi: 10.1097/MAO.0b013e3181b76b9e. [DOI] [PubMed] [Google Scholar]
- 18.Belden CJ, Weg N, Minor LB, et al. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology. 2003;226:337–43. doi: 10.1148/radiol.2262010897. [DOI] [PubMed] [Google Scholar]
- 19.Hirvonen TP, Weg N, Zinreich SJ, et al. High-resolution CT findings suggest a developmental abnormality underlying superior canal dehiscence syndrome. Acta Otolaryngol. 2003;123:477–81. doi: 10.1080/0036554021000028099. [DOI] [PubMed] [Google Scholar]
- 20.Crane BT, Carey JP, Minor LB. In: Otologic Surgery. Brackmann DE, Shelton C, Arriaga MA, editors. Elsevier; Philadelphia: 2010. pp. 507–18. [Google Scholar]
- 21.Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:176–8. doi: 10.1016/S0194-5998(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolis RH, Rieks D, Fournier EM, et al. Tympanic electrocochleography for diagnosis of Meniere's disease. Arch Otolaryngol Head Neck Surg. 1995;121:44–55. doi: 10.1001/archotol.1995.01890010032007. [DOI] [PubMed] [Google Scholar]
- 24.Tavassolie TS, Penninger RT, Zuniga MG, et al. Multislice computed tomography in the diagnosis of superior canal dehiscence: how much error, and how to minimize it? Otol Neurotol. 2012;33:215–22. doi: 10.1097/MAO.0b013e318241c23b. [DOI] [PubMed] [Google Scholar]
- 25.Carey JP, Minor LB, Nager GT. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg. 2000;126:137–47. doi: 10.1001/archotol.126.2.137. [DOI] [PubMed] [Google Scholar]
- 26.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–27. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 27.Niesten ME, McKenna MJ, Grolman W, et al. Clinical factors associated with prolonged recovery after superior canal dehiscence surgery. Otol Neurotol. 2012;33:824–31. doi: 10.1097/MAO.0b013e3182544c9e. [DOI] [PubMed] [Google Scholar]
- 28.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57:190–7. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curthoys IS, Vulovic V, Sokolic L, et al. Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res Bull. 2012;89:16–21. doi: 10.1016/j.brainresbull.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Chien WW, Janky K, Minor LB, et al. Superior canal dehiscence size: multivariate assessment of clinical impact. Otol Neurotol. 2012;33:810–5. doi: 10.1097/MAO.0b013e318248eac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal Y, Migliaccio AA, Minor LB, et al. Vestibular hypofunction in the initial postoperative period after surgical treatment of superior semicircular canal dehiscence. Otol Neurotol. 2009;30:502–6. doi: 10.1097/MAO.0b013e3181a32d69. [DOI] [PubMed] [Google Scholar]
- 32.Limb CJ, Carey JP, Srireddy S, et al. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969–80. doi: 10.1097/01.mao.0000235376.70492.8e. [DOI] [PubMed] [Google Scholar]
- 33.Ward BK, Agrawal Y, Nguyen E, et al. Hearing Outcomes After Surgical Plugging of the Superior Semicircular Canal by a Middle Cranial Fossa Approach. Otol Neurotol. 2012 doi: 10.1097/MAO.0b013e318268d20d. [DOI] [PMC free article] [PubMed] [Google Scholar]


